Abstract
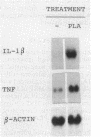
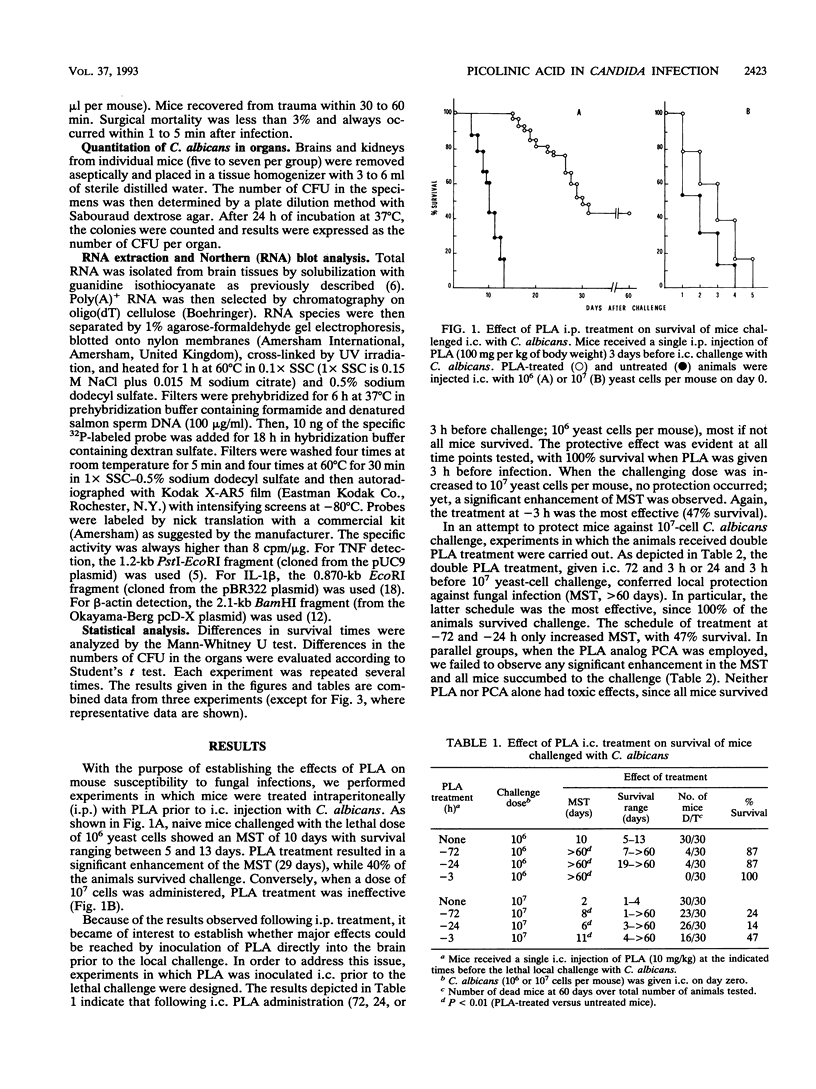
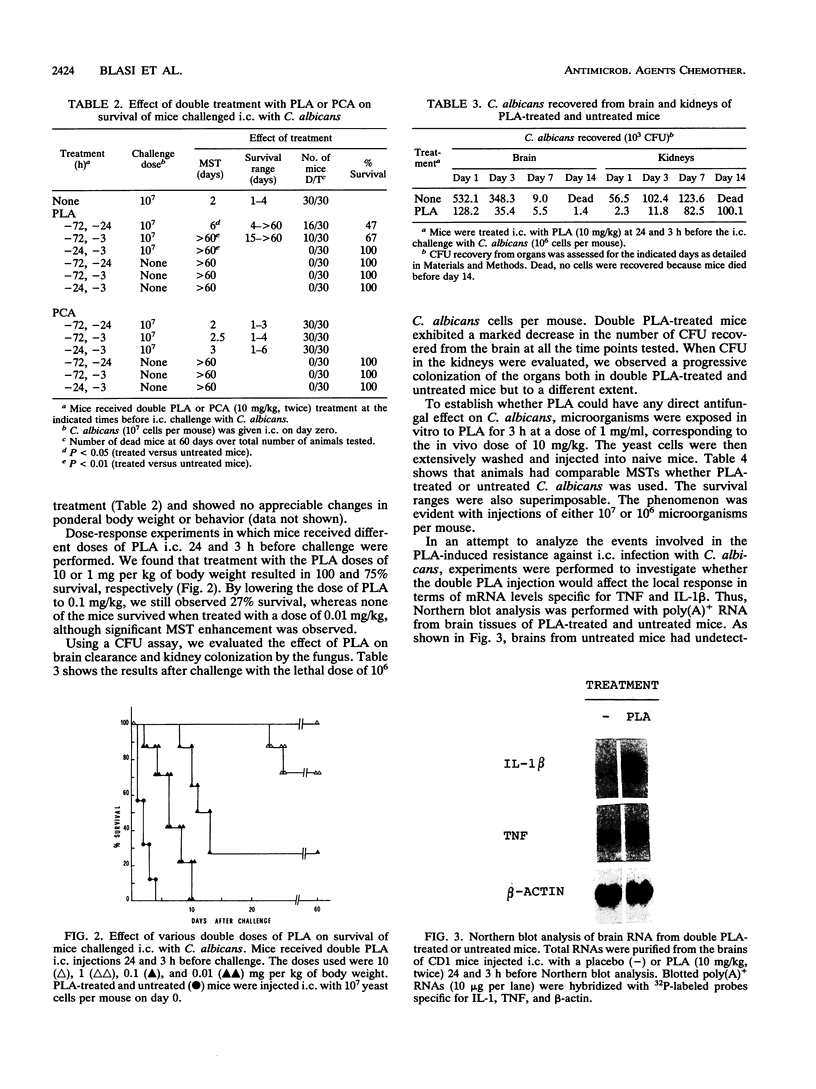
We have studied the effects of picolinic acid (PLA), a product of tryptophan degradation, on mouse susceptibility to intracerebral infection with Candida albicans. We show that intraperitoneal administration of PLA significantly enhances the median survival time of mice inoculated with the lethal challenge. Furthermore, intracerebral administration of this agent induces a protective state against the local lethal infection, the phenomenon depending upon the administration schedule and doses of PLA employed. According to survival data, yeast growth in the brain as well as yeast colonization of the kidneys are drastically reduced in PLA-treated mice compared with those for untreated controls. Northern (RNA) blot analysis of brain tissues demonstrates that mRNA levels specific for tumor necrosis factor and interleukin 1 are augmented and induced, respectively, after inoculation of PLA. These results indicate that PLA has a protective effect likely involving elicitation of a cytokine response in vivo against fungal infections.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Blasi E., Barluzzi R., Bocchini V., Mazzolla R., Bistoni F. Immortalization of murine microglial cells by a v-raf/v-myc carrying retrovirus. J Neuroimmunol. 1990 May;27(2-3):229–237. doi: 10.1016/0165-5728(90)90073-v. [DOI] [PubMed] [Google Scholar]
- Blasi E., Farinelli S., Varesio L., Bistoni F. Augmentation of GG2EE macrophage cell line-mediated anti-Candida activity by gamma interferon, tumor necrosis factor, and interleukin-1. Infect Immun. 1990 Apr;58(4):1073–1077. doi: 10.1128/iai.58.4.1073-1077.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blasi E., Mazzolla R., Barluzzi R., Mosci P., Bartoli A., Bistoni F. Intracerebral transfer of an in vitro established microglial cell line: local induction of a protective state against lethal challenge with Candida albicans. J Neuroimmunol. 1991 Jun;32(3):249–257. doi: 10.1016/0165-5728(91)90195-d. [DOI] [PubMed] [Google Scholar]
- Bocchini V., Rebel G., Massarelli R., Schuber F., Muller C. D. Latex beads phagocytosis capacity and ecto-NAD+ glycohydrolase activity of rat brain microglia cells in vitro. Int J Dev Neurosci. 1988;6(6):525–534. doi: 10.1016/0736-5748(88)90060-3. [DOI] [PubMed] [Google Scholar]
- Caput D., Beutler B., Hartog K., Thayer R., Brown-Shimer S., Cerami A. Identification of a common nucleotide sequence in the 3'-untranslated region of mRNA molecules specifying inflammatory mediators. Proc Natl Acad Sci U S A. 1986 Mar;83(6):1670–1674. doi: 10.1073/pnas.83.6.1670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Collins J. J., Alder C. R., Fernandez-Pol J. A., Court D., Johnson G. S. Transient growth inhibition of Escherichia coli K-12 by ion chelators: "in vivo" inhibition of ribonucleic acid synthesis. J Bacteriol. 1979 Jun;138(3):923–932. doi: 10.1128/jb.138.3.923-932.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans G. W., Johnson P. E. Characterization and quantitation of a zinc-binding ligand in human milk. Pediatr Res. 1980 Jul;14(7):876–880. doi: 10.1203/00006450-198007000-00007. [DOI] [PubMed] [Google Scholar]
- Fernandez-Pol J. A. Iron: possible cause of the G1 arrest induced in NRK cells by picolinic acid. Biochem Biophys Res Commun. 1977 Sep 9;78(1):136–143. doi: 10.1016/0006-291x(77)91231-1. [DOI] [PubMed] [Google Scholar]
- Fernandez-Pol J. A. Transition metal ions induce cell growth in NRK cells synchronized in G1 by picolinic acid. Biochem Biophys Res Commun. 1976 May 23;76(2):413–419. doi: 10.1016/0006-291x(77)90740-9. [DOI] [PubMed] [Google Scholar]
- Fortnagel P., Freese E. Inhibition of aconitase by chelation of transition metals causing inhibition of sporulation in Bacillus subtilis. J Biol Chem. 1968 Oct 25;243(20):5289–5295. [PubMed] [Google Scholar]
- Gunning P., Ponte P., Okayama H., Engel J., Blau H., Kedes L. Isolation and characterization of full-length cDNA clones for human alpha-, beta-, and gamma-actin mRNAs: skeletal but not cytoplasmic actins have an amino-terminal cysteine that is subsequently removed. Mol Cell Biol. 1983 May;3(5):787–795. doi: 10.1128/mcb.3.5.787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heyes M. P., Quearry B. J., Markey S. P. Systemic endotoxin increases L-tryptophan, 5-hydroxyindoleacetic acid, 3-hydroxykynurenine and quinolinic acid content of mouse cerebral cortex. Brain Res. 1989 Jul 3;491(1):173–179. doi: 10.1016/0006-8993(89)90101-7. [DOI] [PubMed] [Google Scholar]
- Heyes M. P., Rubinow D., Lane C., Markey S. P. Cerebrospinal fluid quinolinic acid concentrations are increased in acquired immune deficiency syndrome. Ann Neurol. 1989 Aug;26(2):275–277. doi: 10.1002/ana.410260215. [DOI] [PubMed] [Google Scholar]
- Johnson G. S., Fernandez-Pol J. A. NRK cells synchronized in G1 by picolinic acid are super-sensitive to prostaglandin E1 stimulation. FEBS Lett. 1977 Mar 1;74(2):201–204. doi: 10.1016/0014-5793(77)80846-6. [DOI] [PubMed] [Google Scholar]
- Leuthauser S. W., Oberley L. W., Oberley T. D. Antitumor activity of picolinic acid in CBA/J mice. J Natl Cancer Inst. 1982 Jan;68(1):123–126. [PubMed] [Google Scholar]
- Lipton S. A., Hickey W. F., Morris J. H., Loscalzo J. Candidal infection in the central nervous system. Am J Med. 1984 Jan;76(1):101–108. doi: 10.1016/0002-9343(84)90757-5. [DOI] [PubMed] [Google Scholar]
- MAY E. L., MEHLER A. H. Studies with carboxyl-labeled 3-hydroxyanthranilic and picolinic acids in vivo and in vitro. J Biol Chem. 1956 Nov;223(1):449–455. [PubMed] [Google Scholar]
- Mazzolla R., Barluzzi R., Romani L., Mosci P., Bistoni F. Anti-Candida resistance in the mouse brain and effect of intracerebral administration of interleukin 1. J Gen Microbiol. 1991 Aug;137(8):1799–1804. doi: 10.1099/00221287-137-8-1799. [DOI] [PubMed] [Google Scholar]
- Murphy J. W. Immunity to fungi. Curr Opin Immunol. 1989;2(3):360–367. doi: 10.1016/0952-7915(89)90142-8. [DOI] [PubMed] [Google Scholar]
- Pendlebury W. W., Perl D. P., Munoz D. G. Multiple microabscesses in the central nervous system: a clinicopathologic study. J Neuropathol Exp Neurol. 1989 May;48(3):290–300. doi: 10.1097/00005072-198905000-00006. [DOI] [PubMed] [Google Scholar]
- Plata-Salaman C. R. Immunomodulators and feeding regulation: a humoral link between the immune and nervous systems. Brain Behav Immun. 1989 Sep;3(3):193–213. doi: 10.1016/0889-1591(89)90036-6. [DOI] [PubMed] [Google Scholar]
- Rapoport M. I., Beisel W. R. Studies of tryptophan metabolism in experimental animals and man during infectious illness. Am J Clin Nutr. 1971 Jul;24(7):807–814. doi: 10.1093/ajcn/24.7.807. [DOI] [PubMed] [Google Scholar]
- Rogers T. J., Balish E. Immunity to Candida albicans. Microbiol Rev. 1980 Dec;44(4):660–682. doi: 10.1128/mr.44.4.660-682.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruffmann R., Welker R. D., Saito T., Chirigos M. A., Varesio L. In vivo activation of macrophages but not natural killer cells by picolinic acid (PLA). J Immunopharmacol. 1984;6(4):291–304. doi: 10.3109/08923978409028605. [DOI] [PubMed] [Google Scholar]
- Schwarcz R., Whetsell W. O., Jr, Mangano R. M. Quinolinic acid: an endogenous metabolite that produces axon-sparing lesions in rat brain. Science. 1983 Jan 21;219(4582):316–318. doi: 10.1126/science.6849138. [DOI] [PubMed] [Google Scholar]
- Varesio L., Clayton M., Blasi E., Ruffman R., Radzioch D. Picolinic acid, a catabolite of tryptophan, as the second signal in the activation of IFN-gamma-primed macrophages. J Immunol. 1990 Dec 15;145(12):4265–4271. [PubMed] [Google Scholar]
- Waage A., Halstensen A., Shalaby R., Brandtzaeg P., Kierulf P., Espevik T. Local production of tumor necrosis factor alpha, interleukin 1, and interleukin 6 in meningococcal meningitis. Relation to the inflammatory response. J Exp Med. 1989 Dec 1;170(6):1859–1867. doi: 10.1084/jem.170.6.1859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wannemacher R. W., Jr Key role of various individual amino acids in host response to infection. Am J Clin Nutr. 1977 Aug;30(8):1269–1280. doi: 10.1093/ajcn/30.8.1269. [DOI] [PubMed] [Google Scholar]