Abstract
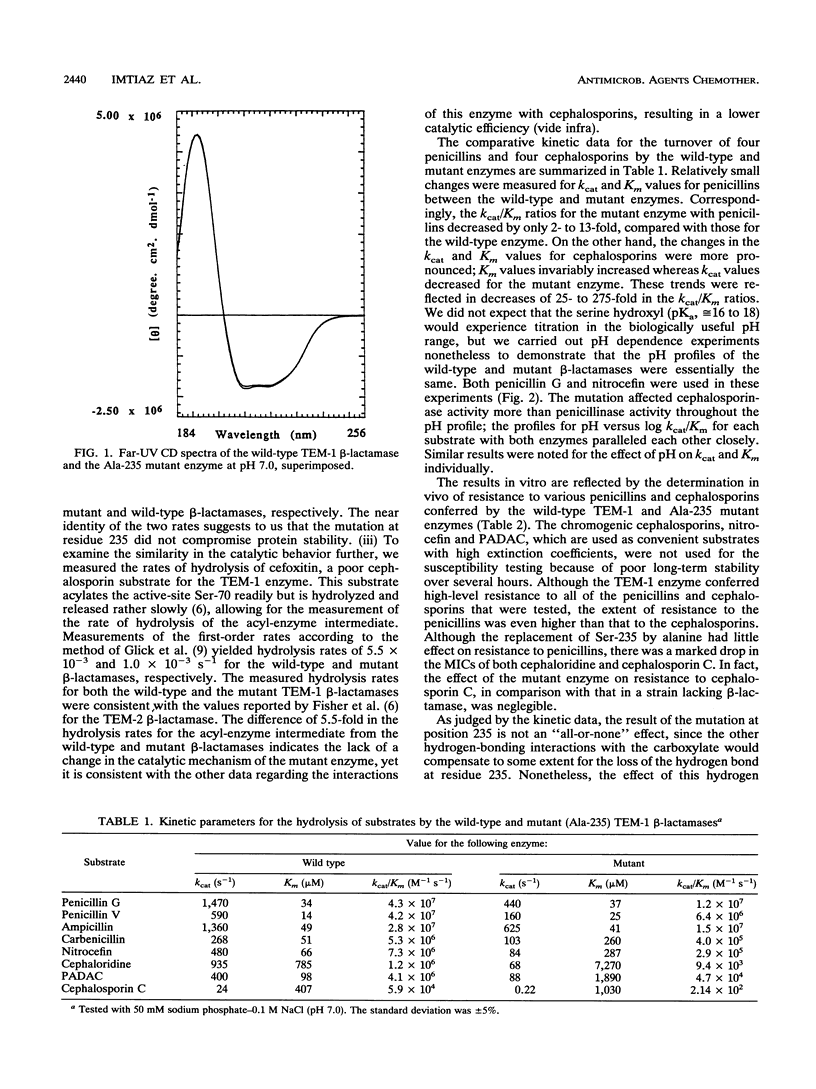
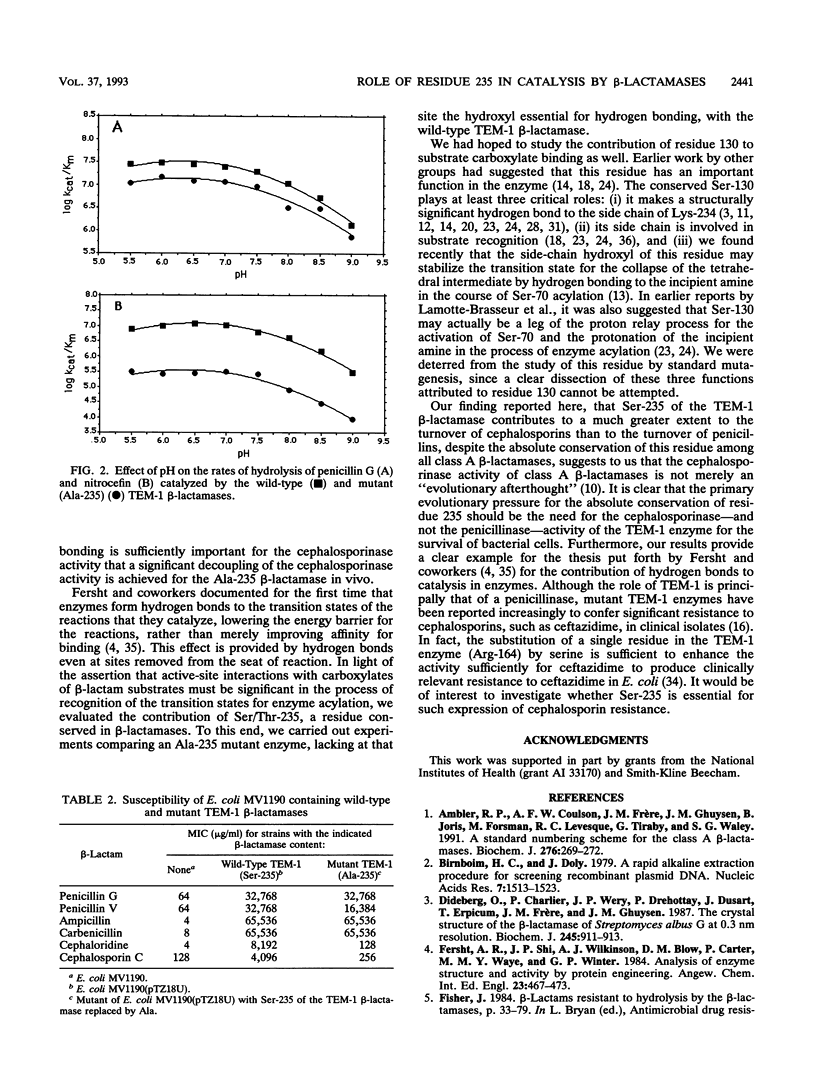
The role of Ser-235 in the catalytic mechanism of the TEM-1 beta-lactamase has been explored by the study of a mutant enzyme in which Ser-235 has been substituted by alanine (Ala-235 mutant enzyme). A comparative kinetic analysis of both the wild-type and the Ala-235 TEM-1 enzymes revealed little effect of this substitution of residue 235 on the turnover of penicillins but a greater effect on the turnover of cephalosporins. Susceptibility testing of Escherichia coli strains harboring the wild-type TEM-1 beta-lactamase and the Ala-235 mutant enzyme revealed an effect of the mutation similar to that observed in the enzymological studies. The MICs of two representative cephalosporins for the strain containing the mutant enzyme were much lower than those for the isogenic strain bearing the wild-type TEM-1 beta-lactamase. On the other hand, the strain with the mutant enzyme was still highly resistant to penicillins.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ambler R. P., Coulson A. F., Frère J. M., Ghuysen J. M., Joris B., Forsman M., Levesque R. C., Tiraby G., Waley S. G. A standard numbering scheme for the class A beta-lactamases. Biochem J. 1991 May 15;276(Pt 1):269–270. doi: 10.1042/bj2760269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birnboim H. C., Doly J. A rapid alkaline extraction procedure for screening recombinant plasmid DNA. Nucleic Acids Res. 1979 Nov 24;7(6):1513–1523. doi: 10.1093/nar/7.6.1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dideberg O., Charlier P., Wéry J. P., Dehottay P., Dusart J., Erpicum T., Frère J. M., Ghuysen J. M. The crystal structure of the beta-lactamase of Streptomyces albus G at 0.3 nm resolution. Biochem J. 1987 Aug 1;245(3):911–913. doi: 10.1042/bj2450911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher J., Belasco J. G., Khosla S., Knowles J. R. beta-Lactamase proceeds via an acyl-enzyme intermediate. Interaction of the Escherichia coli RTEM enzyme with cefoxitin. Biochemistry. 1980 Jun 24;19(13):2895–2901. doi: 10.1021/bi00554a012. [DOI] [PubMed] [Google Scholar]
- Glick B. R., Brubacher L. J., Leggett D. J. A graphical method for extracting rate constants from some enzyme-catalyzed reactions not monitored to completion. Can J Biochem. 1978 Nov;56(11):1055–1057. doi: 10.1139/o78-166. [DOI] [PubMed] [Google Scholar]
- Gutmann L., Kitzis M. D., Acar J. F. Evolution of enzymatic mechanisms of resistance among beta-lactam antibiotics. J Int Med Res. 1990;18 (Suppl 4):37D–47D. [PubMed] [Google Scholar]
- Herzberg O., Moult J. Bacterial resistance to beta-lactam antibiotics: crystal structure of beta-lactamase from Staphylococcus aureus PC1 at 2.5 A resolution. Science. 1987 May 8;236(4802):694–701. doi: 10.1126/science.3107125. [DOI] [PubMed] [Google Scholar]
- Herzberg O. Refined crystal structure of beta-lactamase from Staphylococcus aureus PC1 at 2.0 A resolution. J Mol Biol. 1991 Feb 20;217(4):701–719. doi: 10.1016/0022-2836(91)90527-d. [DOI] [PubMed] [Google Scholar]
- Jacob-Dubuisson F., Lamotte-Brasseur J., Dideberg O., Joris B., Frère J. M. Arginine 220 is a critical residue for the catalytic mechanism of the Streptomyces albus G beta-lactamase. Protein Eng. 1991 Oct;4(7):811–819. doi: 10.1093/protein/4.7.811. [DOI] [PubMed] [Google Scholar]
- Jacob F., Joris B., Dideberg O., Dusart J., Ghuysen J. M., Frère J. M. Engineering a novel beta-lactamase by a single point mutation. Protein Eng. 1990 Oct;4(1):79–86. doi: 10.1093/protein/4.1.79. [DOI] [PubMed] [Google Scholar]
- Jacoby G. A., Medeiros A. A. More extended-spectrum beta-lactamases. Antimicrob Agents Chemother. 1991 Sep;35(9):1697–1704. doi: 10.1128/aac.35.9.1697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juteau J. M., Billings E., Knox J. R., Levesque R. C. Site-saturation mutagenesis and three-dimensional modelling of ROB-1 define a substrate binding role of Ser130 in class A beta-lactamases. Protein Eng. 1992 Oct;5(7):693–701. doi: 10.1093/protein/5.7.693. [DOI] [PubMed] [Google Scholar]
- Knowles J. R. Tinkering with enzymes: what are we learning? Science. 1987 Jun 5;236(4806):1252–1258. doi: 10.1126/science.3296192. [DOI] [PubMed] [Google Scholar]
- Knox J. R., Moews P. C. Beta-lactamase of Bacillus licheniformis 749/C. Refinement at 2 A resolution and analysis of hydration. J Mol Biol. 1991 Jul 20;220(2):435–455. doi: 10.1016/0022-2836(91)90023-y. [DOI] [PubMed] [Google Scholar]
- Kunkel T. A. Rapid and efficient site-specific mutagenesis without phenotypic selection. Proc Natl Acad Sci U S A. 1985 Jan;82(2):488–492. doi: 10.1073/pnas.82.2.488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunkel T. A., Roberts J. D., Zakour R. A. Rapid and efficient site-specific mutagenesis without phenotypic selection. Methods Enzymol. 1987;154:367–382. doi: 10.1016/0076-6879(87)54085-x. [DOI] [PubMed] [Google Scholar]
- Lamotte-Brasseur J., Dive G., Dideberg O., Charlier P., Frère J. M., Ghuysen J. M. Mechanism of acyl transfer by the class A serine beta-lactamase of Streptomyces albus G. Biochem J. 1991 Oct 1;279(Pt 1):213–221. doi: 10.1042/bj2790213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamotte-Brasseur J., Jacob-Dubuisson F., Dive G., Frère J. M., Ghuysen J. M. Streptomyces albus G serine beta-lactamase. Probing of the catalytic mechanism via molecular modelling of mutant enzymes. Biochem J. 1992 Feb 15;282(Pt 1):189–195. doi: 10.1042/bj2820189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leigh D. A., Bradnock K., Marriner J. M. Augmentin (amoxycillin and clavulanic acid) therapy in complicated infections due to beta-lactamase producing bacteria. J Antimicrob Chemother. 1981 Mar;7(3):229–236. doi: 10.1093/jac/7.3.229. [DOI] [PubMed] [Google Scholar]
- Mead D. A., Szczesna-Skorupa E., Kemper B. Single-stranded DNA 'blue' T7 promoter plasmids: a versatile tandem promoter system for cloning and protein engineering. Protein Eng. 1986 Oct-Nov;1(1):67–74. doi: 10.1093/protein/1.1.67. [DOI] [PubMed] [Google Scholar]
- Moellering R. C., Jr Beta-lactamase inhibition: therapeutic implications in infectious diseases--an overview. Rev Infect Dis. 1991 Jul-Aug;13 (Suppl 9):S723–S726. [PubMed] [Google Scholar]
- Moews P. C., Knox J. R., Dideberg O., Charlier P., Frère J. M. Beta-lactamase of Bacillus licheniformis 749/C at 2 A resolution. Proteins. 1990;7(2):156–171. doi: 10.1002/prot.340070205. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strynadka N. C., Adachi H., Jensen S. E., Johns K., Sielecki A., Betzel C., Sutoh K., James M. N. Molecular structure of the acyl-enzyme intermediate in beta-lactam hydrolysis at 1.7 A resolution. Nature. 1992 Oct 22;359(6397):700–705. doi: 10.1038/359700a0. [DOI] [PubMed] [Google Scholar]
- Vieira J., Messing J. Production of single-stranded plasmid DNA. Methods Enzymol. 1987;153:3–11. doi: 10.1016/0076-6879(87)53044-0. [DOI] [PubMed] [Google Scholar]
- Warshel A., Levitt M. Theoretical studies of enzymic reactions: dielectric, electrostatic and steric stabilization of the carbonium ion in the reaction of lysozyme. J Mol Biol. 1976 May 15;103(2):227–249. doi: 10.1016/0022-2836(76)90311-9. [DOI] [PubMed] [Google Scholar]
- Weber D. A., Sanders C. C., Bakken J. S., Quinn J. P. A novel chromosomal TEM derivative and alterations in outer membrane proteins together mediate selective ceftazidime resistance in Escherichia coli. J Infect Dis. 1990 Aug;162(2):460–465. doi: 10.1093/infdis/162.2.460. [DOI] [PubMed] [Google Scholar]
- Zafaralla G., Manavathu E. K., Lerner S. A., Mobashery S. Elucidation of the role of arginine-244 in the turnover processes of class A beta-lactamases. Biochemistry. 1992 Apr 21;31(15):3847–3852. doi: 10.1021/bi00130a016. [DOI] [PubMed] [Google Scholar]


