Abstract
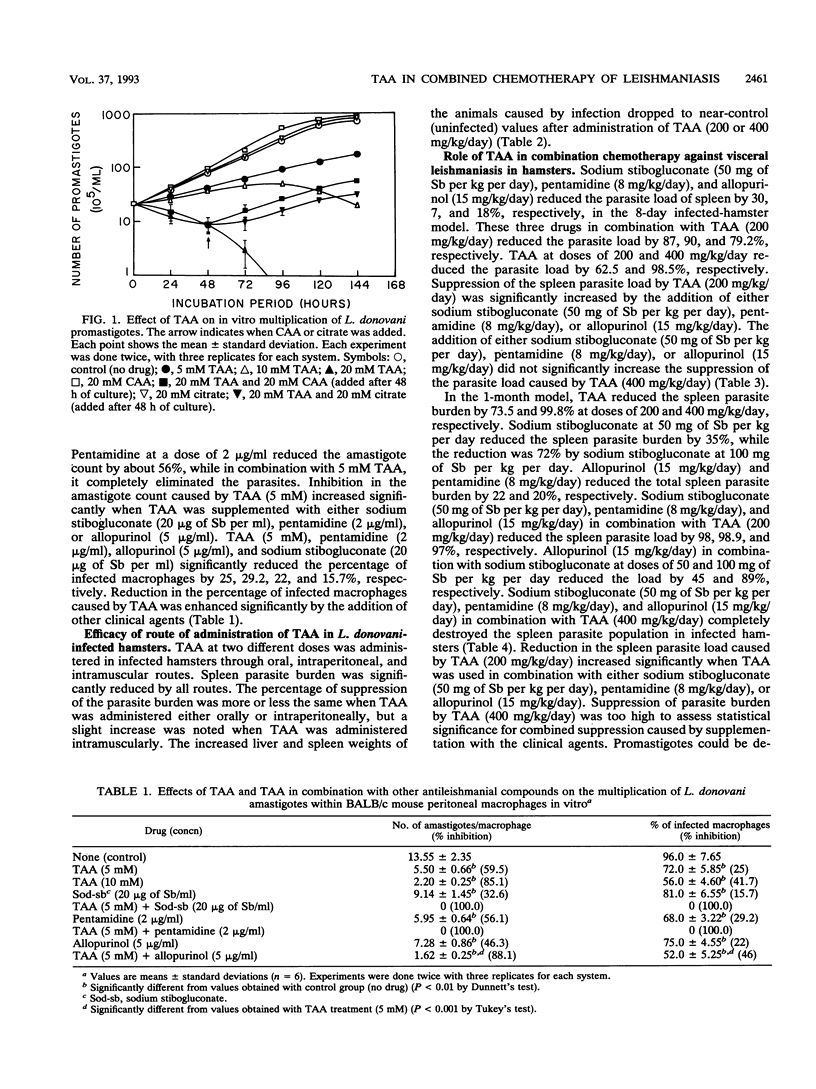
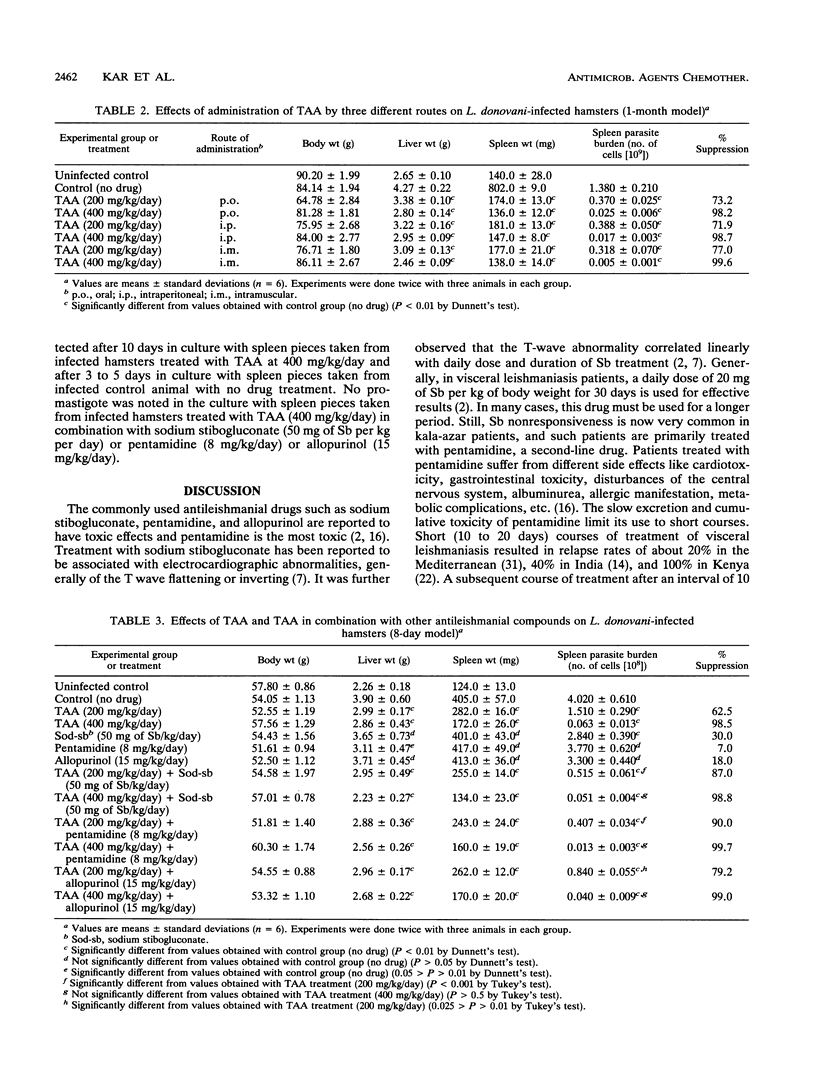
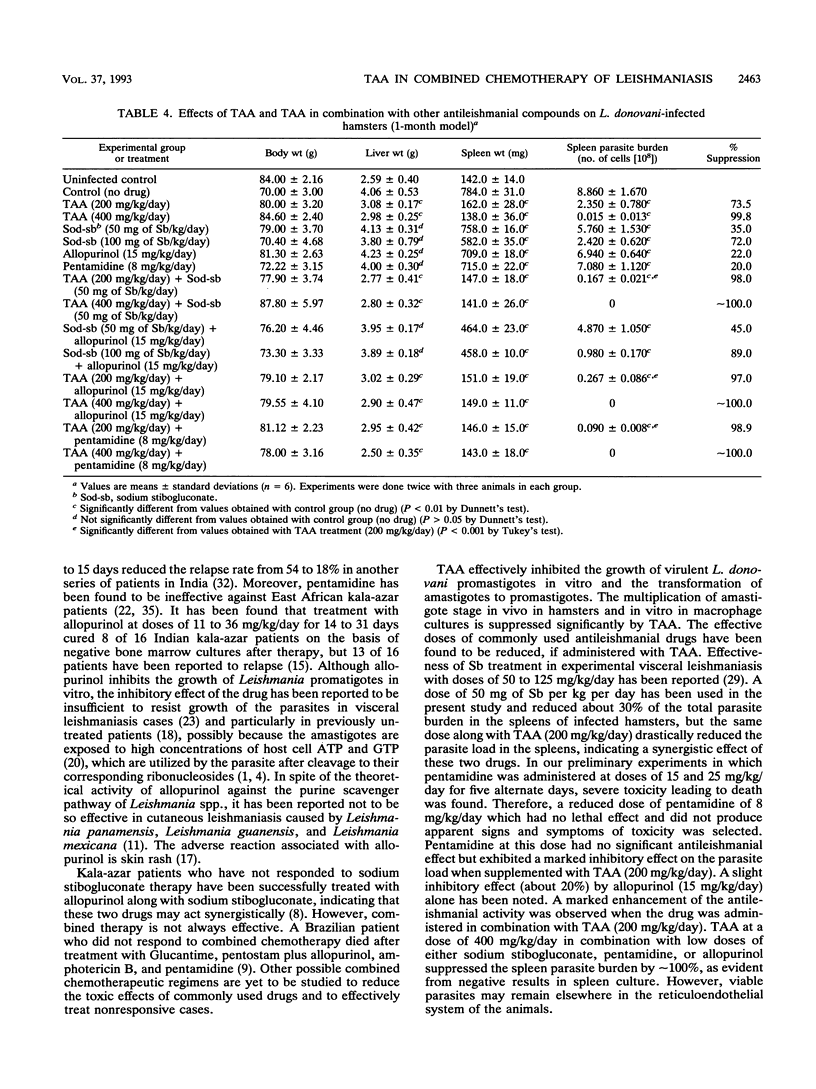
We previously reported the effectiveness of trans-aconitic acid (TAA) as an antileishmanial compound. Inhibitory effects of TAA along with other antileishmanial compounds on transformation and in vitro multiplication in macrophage cultures of Leishmania donovani have been assessed. The efficacy of TAA in combined chemotherapy of experimental visceral leishmaniasis has also been evaluated along with those of commonly used antileishmanial compounds such as sodium stibogluconate, pentamidine, and allopurinol. TAA (2 mM) inhibited transformation of L. donovani amastigotes to promastigotes by 95.2%, whereas in combination with pentamidine (5 micrograms/ml), allopurinol (10 micrograms/ml), and sodium stibogluconate (50 micrograms of Sb per ml), it inhibited transformation by about 100, 99, and 98.5%, respectively. Sodium stibogluconate (20 micrograms of Sb per ml), pentamidine (2 micrograms/ml), and allopurinol (5 micrograms/ml) suppressed the amastigote burden in peritoneal macrophage cultures from BALB/c mice by 32.6, 56.1, and 46.3%, respectively. When these three drugs were used along with TAA (5 mM), the parasite loads were reduced by 100, 100, and 88.1%, respectively. TAA (5 mM) alone suppressed the amastigote burden by 59.5%. In experimental visceral leishmaniasis in hamsters (1-month model), TAA at a dose of 200 mg/kg of body weight per day suppressed the spleen parasite load by 73.5%, and TAA in combination with sodium stibogluconate (50 mg of Sb per kg per day), pentamidine (8 mg/kg/day), and allopurinol (15 mg/kg/day) inhibited the spleen parasite load by 98, 98.9, and 97%, respectively. Individually, these three drugs inhibited the parasite load by 35, 20, and 22%, respectively. TAA (400 mg/kg/day) inhibited the spleen parasite load by 99.8%, but an inhibitory effect of approximately 100% was noted when TAA was supplemented with an antileishmanial drug. TAA was administered in experimental animals through oral, intraperitoneal, and intramuscular routes; the intramuscular route was most effective.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akiyama H. J., Haight R. D. Interaction of Leishmania donovani and hamster peritoneal macrophages. A phase-contrast microscopical study. Am J Trop Med Hyg. 1971 Jul;20(4):539–545. doi: 10.4269/ajtmh.1971.20.539. [DOI] [PubMed] [Google Scholar]
- Berman J. D. Chemotherapy for leishmaniasis: biochemical mechanisms, clinical efficacy, and future strategies. Rev Infect Dis. 1988 May-Jun;10(3):560–586. doi: 10.1093/clinids/10.3.560. [DOI] [PubMed] [Google Scholar]
- Berman J. D., Gallalee J. V., Best J. M. Sodium stibogluconate (Pentostam) inhibition of glucose catabolism via the glycolytic pathway, and fatty acid beta-oxidation in Leishmania mexicana amastigotes. Biochem Pharmacol. 1987 Jan 15;36(2):197–201. doi: 10.1016/0006-2952(87)90689-7. [DOI] [PubMed] [Google Scholar]
- Bhattacharya A., Janovy J. Leishmania donovani: autoradiographic evidence for molecular exchanges between parasites and host cells. Exp Parasitol. 1975 Jun;37(3):353–360. doi: 10.1016/0014-4894(75)90003-x. [DOI] [PubMed] [Google Scholar]
- Brun R., Schönenberger M. Stimulating effect of citrate and cis-Aconitate on the transformation of Trypanosoma brucei bloodstream forms to procyclic forms in vitro. Z Parasitenkd. 1981;66(1):17–24. doi: 10.1007/BF00941941. [DOI] [PubMed] [Google Scholar]
- Chulay J. D., Spencer H. C., Mugambi M. Electrocardiographic changes during treatment of leishmaniasis with pentavalent antimony (sodium stibogluconate). Am J Trop Med Hyg. 1985 Jul;34(4):702–709. doi: 10.4269/ajtmh.1985.34.702. [DOI] [PubMed] [Google Scholar]
- Chunge C. N., Gachihi G., Muigai R., Wasunna K., Rashid J. R., Chulay J. D., Anabwani G., Oster C. N., Bryceson A. D. Visceral leishmaniasis unresponsive to antimonial drugs. III. Successful treatment using a combination of sodium stibogluconate plus allopurinol. Trans R Soc Trop Med Hyg. 1985;79(5):715–718. doi: 10.1016/0035-9203(85)90200-7. [DOI] [PubMed] [Google Scholar]
- Ghosh A. K., Bhattacharyya F. K., Ghosh D. K. Leishmania donovani: amastigote inhibition and mode of action of berberine. Exp Parasitol. 1985 Dec;60(3):404–413. doi: 10.1016/0014-4894(85)90047-5. [DOI] [PubMed] [Google Scholar]
- Guderian R. H., Chico M. E., Rogers M. D., Pattishall K. M., Grogl M., Berman J. D. Placebo controlled treatment of Ecuadorian cutaneous leishmaniasis. Am J Trop Med Hyg. 1991 Jul;45(1):92–97. doi: 10.4269/ajtmh.1991.45.92. [DOI] [PubMed] [Google Scholar]
- Hart D. T., Coombs G. H. Leishmania mexicana: energy metabolism of amastigotes and promastigotes. Exp Parasitol. 1982 Dec;54(3):397–409. doi: 10.1016/0014-4894(82)90049-2. [DOI] [PubMed] [Google Scholar]
- Hart D. T., Vickerman K., Coombs G. H. Respiration of Leishmania mexicana amastigotes and promastigotes. Mol Biochem Parasitol. 1981 Nov;4(1-2):39–51. doi: 10.1016/0166-6851(81)90027-x. [DOI] [PubMed] [Google Scholar]
- Jha T. K. Evaluation of allopurinol in the treatment of kala-azar occurring in North Bihar, India. Trans R Soc Trop Med Hyg. 1983;77(2):204–207. doi: 10.1016/0035-9203(83)90071-8. [DOI] [PubMed] [Google Scholar]
- Jha T. K. Evaluation of diamidine compound (pentamidine isethionate) in the treatment resistant cases of kala-azar occurring in North Bihar, India. Trans R Soc Trop Med Hyg. 1983;77(2):167–170. doi: 10.1016/0035-9203(83)90058-5. [DOI] [PubMed] [Google Scholar]
- Kager P. A., Rees P. H., Manguyu F. M., Bhatt K. M., Wellde B. T., Hockmeyer W. T., Lyerly W. H., Jr Clinical, haematological and parasitological response to treatment of visceral leishmaniasis in Kenya. A study of 64 patients. Trop Geogr Med. 1984 Mar;36(1):21–35. [PubMed] [Google Scholar]
- Kager P. A., Rees P. H., Wellde B. T., Hockmeyer W. T., Lyerly W. H. Allopurinol in the treatment of visceral leishmaniasis. Trans R Soc Trop Med Hyg. 1981;75(4):556–559. doi: 10.1016/0035-9203(81)90198-x. [DOI] [PubMed] [Google Scholar]
- Kar K., Mukerji K., Naskar K., Bhattacharya A., Ghosh D. K. Leishmania donovani: a chemically defined medium suitable for cultivation and cloning of promastigotes and transformation of amastigotes to to promastigotes. J Protozool. 1990 Jul-Aug;37(4):277–279. doi: 10.1111/j.1550-7408.1990.tb01147.x. [DOI] [PubMed] [Google Scholar]
- Looker D. L., Marr J. J., Berens R. L. Mechanisms of action of pyrazolopyrimidines in Leishmania donovani. J Biol Chem. 1986 Jul 15;261(20):9412–9415. [PubMed] [Google Scholar]
- MANSON-BAHR P. E. East African kalazar with special reference to the pathology, prophylaxis and treatment. Trans R Soc Trop Med Hyg. 1959 Mar;53(2):123–137. doi: 10.1016/0035-9203(59)90060-4. [DOI] [PubMed] [Google Scholar]
- MILLER R. E., CANTOR S. M. Aconitic acid, a by-product in the manufacture of sugar. Adv Carbohydr Chem. 1951;6:231–249. doi: 10.1016/s0096-5332(08)60069-7. [DOI] [PubMed] [Google Scholar]
- Marr J. J., Berens R. L., Nelson D. J., Krenitsky T. A., Spector T., LaFon S. W., Elion G. B. Antileishmanial action of 4-thiopyrazolo (3.4-d) pyrimidine and its ribonucleoside. Biological effects and metabolism. Biochem Pharmacol. 1982 Jan 15;31(2):143–148. doi: 10.1016/0006-2952(82)90203-9. [DOI] [PubMed] [Google Scholar]
- Meade J. C., Glaser T. A., Bonventre P. F., Mukkada A. J. Enzymes of carbohydrate metabolism in Leishmania donovani amastigotes. J Protozool. 1984 Feb;31(1):156–161. doi: 10.1111/j.1550-7408.1984.tb04307.x. [DOI] [PubMed] [Google Scholar]
- Misra S., Sanyal T., Sarkar D., Bhattacharya P. K., Ghosh D. K. Evaluation of antileishmanial activity of trans-aconitic acid. Biochem Med Metab Biol. 1989 Dec;42(3):171–178. doi: 10.1016/0885-4505(89)90052-2. [DOI] [PubMed] [Google Scholar]
- Raether W., Seidenath H., Loewe H. Action of p-(4-amidino-phenoxy)-benzaldehyde-p-amidino-phenylhydrazone dihydrochloride on Leishmania donovani infections in the golden hamster. Ann Trop Med Parasitol. 1978 Dec;72(6):543–547. doi: 10.1080/00034983.1978.11719358. [DOI] [PubMed] [Google Scholar]
- Sarkar S., Mukherjee T., Patra P., Bhaduri A. Antileishmanial activity of hamycin: a polyene antibiotic. Biochem Biophys Res Commun. 1992 Jan 15;182(1):86–91. doi: 10.1016/s0006-291x(05)80115-9. [DOI] [PubMed] [Google Scholar]
- Simon M. W., Martin E., Mukkada A. J. Evidence for a functional glyoxylate cycle in the leishmaniae. J Bacteriol. 1978 Sep;135(3):895–899. doi: 10.1128/jb.135.3.895-899.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]


