Abstract
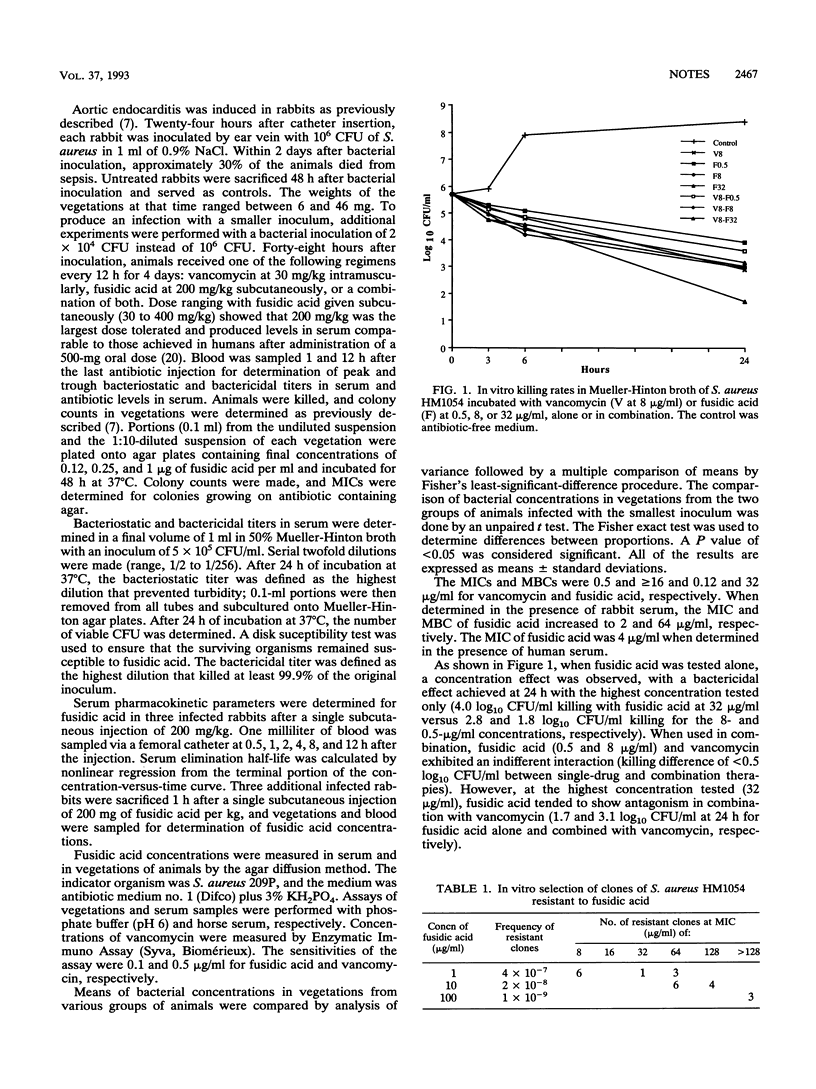
The usefulness of fusidic acid, alone or combined with vancomycin, was investigated for the therapy of experimental endocarditis caused in rabbits by a methicillin-resistant strain of Staphylococcus aureus. In vitro killing curves showed an indifferent interaction between the two antibiotics. In vivo, vancomycin alone was as effective as a vancomycin-fusidic acid combination (P < 0.05 versus control animals). No resistance to fusidic acid emerged during combination therapy. Fusidic acid alone was not effective. Resistance emerged in 5 of 12 animals treated with fusidic acid alone and was responsible for antibacterial failure. Fusidic acid alone was effective (P < 0.001) and did not select resistant strains if therapy was started when animals retained a smaller inoculum. We concluded that the vancomycin-fusidic acid combination exhibited no advantage over vancomycin alone in this model.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ackerman B. H., Vannier A. M., Eudy E. B. Analysis of vancomycin time-kill studies with Staphylococcus species by using a curve stripping program to describe the relationship between concentration and pharmacodynamic response. Antimicrob Agents Chemother. 1992 Aug;36(8):1766–1769. doi: 10.1128/aac.36.8.1766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayer A. S., Lam K. Efficacy of vancomycin plus rifampin in experimental aortic-valve endocarditis due to methicillin-resistant Staphylococcus aureus: in vitro-in vivo correlations. J Infect Dis. 1985 Jan;151(1):157–165. doi: 10.1093/infdis/151.1.157. [DOI] [PubMed] [Google Scholar]
- Faber M., Rosdahl V. T. Susceptibility to fusidic acid among Danish Staphylococcus aureus strains and fusidic acid consumption. J Antimicrob Chemother. 1990 Feb;25 (Suppl B):7–14. doi: 10.1093/jac/25.suppl_b.7. [DOI] [PubMed] [Google Scholar]
- Fantin B., Carbon C. In vivo antibiotic synergism: contribution of animal models. Antimicrob Agents Chemother. 1992 May;36(5):907–912. doi: 10.1128/aac.36.5.907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fantin B., Leclercq R., Arthur M., Duval J., Carbon C. Influence of low-level resistance to vancomycin on efficacy of teicoplanin and vancomycin for treatment of experimental endocarditis due to Enterococcus faecium. Antimicrob Agents Chemother. 1991 Aug;35(8):1570–1575. doi: 10.1128/aac.35.8.1570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foldes M., Munro R., Sorrell T. C., Shanker S., Toohey M. In-vitro effects of vancomycin, rifampicin, and fusidic acid, alone and in combination, against methicillin-resistant Staphylococcus aureus. J Antimicrob Chemother. 1983 Jan;11(1):21–26. doi: 10.1093/jac/11.1.21. [DOI] [PubMed] [Google Scholar]
- George R. H. The influence of protein binding on the antistaphylococcal activity of antibiotics. J Antimicrob Chemother. 1986 Apr;17(4):539–540. doi: 10.1093/jac/17.4.539. [DOI] [PubMed] [Google Scholar]
- Güttler F., Tybring L., Engberg-Pedersen H. Interaction of albumin and fusidic acid. Br J Pharmacol. 1971 Sep;43(1):151–160. doi: 10.1111/j.1476-5381.1971.tb07164.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maple P. A., Hamilton-Miller J. M., Brumfitt W. World-wide antibiotic resistance in methicillin-resistant Staphylococcus aureus. Lancet. 1989 Mar 11;1(8637):537–540. doi: 10.1016/s0140-6736(89)90076-7. [DOI] [PubMed] [Google Scholar]
- Menday A. P., Marsh B. T. Intravenous fusidic acid ('Fucidin') in the management of severe staphylococcal infections: a review of 46 cases. Curr Med Res Opin. 1976;4(2):132–138. doi: 10.1185/03007997609109293. [DOI] [PubMed] [Google Scholar]
- Pearson R. D., Steigbigel R. T., Davis H. T., Chapman S. W. Method of reliable determination of minimal lethal antibiotic concentrations. Antimicrob Agents Chemother. 1980 Nov;18(5):699–708. doi: 10.1128/aac.18.5.699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips I., King A., Gransden W. R., Eykyn S. J. The antibiotic sensitivity of bacteria isolated from the blood of patients in St Thomas' Hospital, 1969-1988. J Antimicrob Chemother. 1990 Apr;25 (Suppl 100):59–80. doi: 10.1093/jac/25.suppl_c.59. [DOI] [PubMed] [Google Scholar]
- Portier H. A multicentre, open, clinical trial of a new intravenous formulation of fusidic acid in severe staphylococcal infections. J Antimicrob Chemother. 1990 Feb;25 (Suppl B):39–44. doi: 10.1093/jac/25.suppl_b.39. [DOI] [PubMed] [Google Scholar]
- Rolinson G. N., Sutherland R. The binding of antibiotics to serum proteins. Br J Pharmacol Chemother. 1965 Dec;25(3):638–650. doi: 10.1111/j.1476-5381.1965.tb01788.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shanson D. C. Clinical relevance of resistance to fusidic acid in Staphylococcus aureus. J Antimicrob Chemother. 1990 Feb;25 (Suppl B):15–21. doi: 10.1093/jac/25.suppl_b.15. [DOI] [PubMed] [Google Scholar]
- Taburet A. M., Guibert J., Kitzis M. D., Sorensen H., Acar J. F., Singlas E. Pharmacokinetics of sodium fusidate after single and repeated infusions and oral administration of a new formulation. J Antimicrob Chemother. 1990 Feb;25 (Suppl B):23–31. doi: 10.1093/jac/25.suppl_b.23. [DOI] [PubMed] [Google Scholar]


