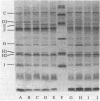
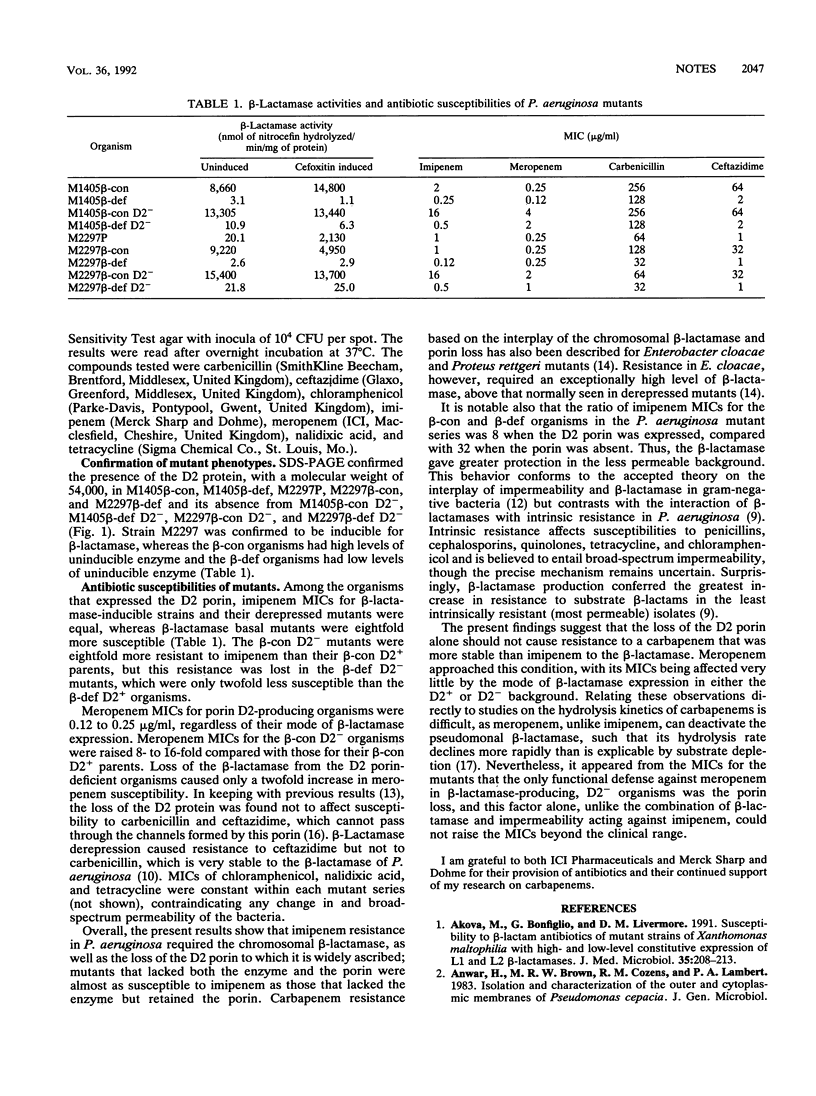
Abstract
Mutational loss of the D2 porin causes imipenem resistance in Pseudomonas aeruginosa. It was found that this mechanism could function only when the chromosomal beta-lactamase was expressed. Mutants lacking both the beta-lactamase and the D2 porin were almost as susceptible as those that lacked the beta-lactamase but retained the porin. Thus, imipenem resistance reflected an interplay of the enzyme and impermeability, not either factor alone. These findings suggest that the activity of a carbapenem more beta-lactamase stable than imipenem should be less affected by the porin loss. Meropenem approached this behavior.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akova M., Bonfiglio G., Livermore D. M. Susceptibility to beta-lactam antibiotics of mutant strains of Xanthomonas maltophilia with high- and low-level constitutive expression of L1 and L2 beta-lactamases. J Med Microbiol. 1991 Oct;35(4):208–213. doi: 10.1099/00222615-35-4-208. [DOI] [PubMed] [Google Scholar]
- Ashby J., Kirkpatrick B., Piddock L. J., Wise R. The effect of imipenem on strains of Enterobacteriaceae expressing Richmond & Sykes class I beta-lactamases. J Antimicrob Chemother. 1987 Jul;20(1):15–22. doi: 10.1093/jac/20.1.15. [DOI] [PubMed] [Google Scholar]
- Bush K. Classification of beta-lactamases: groups 2c, 2d, 2e, 3, and 4. Antimicrob Agents Chemother. 1989 Mar;33(3):271–276. doi: 10.1128/aac.33.3.271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Büscher K. H., Cullmann W., Dick W., Opferkuch W. Imipenem resistance in Pseudomonas aeruginosa resulting from diminished expression of an outer membrane protein. Antimicrob Agents Chemother. 1987 May;31(5):703–708. doi: 10.1128/aac.31.5.703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curtis N. A., Eisenstadt R. L., Rudd C., White A. J. Inducible type I beta-lactamases of gram-negative bacteria and resistance to beta-lactam antibiotics. J Antimicrob Chemother. 1986 Jan;17(1):51–61. doi: 10.1093/jac/17.1.51. [DOI] [PubMed] [Google Scholar]
- Hancock R. E., Carey A. M. Outer membrane of Pseudomonas aeruginosa: heat- 2-mercaptoethanol-modifiable proteins. J Bacteriol. 1979 Dec;140(3):902–910. doi: 10.1128/jb.140.3.902-910.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knott-Hunziker V., Petursson S., Waley S. G., Jaurin B., Grundström T. The acyl-enzyme mechanism of beta-lactamase action. The evidence for class C Beta-lactamases. Biochem J. 1982 Nov 1;207(2):315–322. doi: 10.1042/bj2070315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Livermore D. M., Davy K. W. Invalidity for Pseudomonas aeruginosa of an accepted model of bacterial permeability to beta-lactam antibiotics. Antimicrob Agents Chemother. 1991 May;35(5):916–921. doi: 10.1128/aac.35.5.916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livermore D. M., Yang Y. J. Beta-lactamase lability and inducer power of newer beta-lactam antibiotics in relation to their activity against beta-lactamase-inducibility mutants of Pseudomonas aeruginosa. J Infect Dis. 1987 Apr;155(4):775–782. doi: 10.1093/infdis/155.4.775. [DOI] [PubMed] [Google Scholar]
- Nikaido H., Normark S. Sensitivity of Escherichia coli to various beta-lactams is determined by the interplay of outer membrane permeability and degradation by periplasmic beta-lactamases: a quantitative predictive treatment. Mol Microbiol. 1987 Jul;1(1):29–36. doi: 10.1111/j.1365-2958.1987.tb00523.x. [DOI] [PubMed] [Google Scholar]
- Quinn J. P., Dudek E. J., DiVincenzo C. A., Lucks D. A., Lerner S. A. Emergence of resistance to imipenem during therapy for Pseudomonas aeruginosa infections. J Infect Dis. 1986 Aug;154(2):289–294. doi: 10.1093/infdis/154.2.289. [DOI] [PubMed] [Google Scholar]
- Raimondi A., Traverso A., Nikaido H. Imipenem- and meropenem-resistant mutants of Enterobacter cloacae and Proteus rettgeri lack porins. Antimicrob Agents Chemother. 1991 Jun;35(6):1174–1180. doi: 10.1128/aac.35.6.1174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richmond M. H., Sykes R. B. The beta-lactamases of gram-negative bacteria and their possible physiological role. Adv Microb Physiol. 1973;9:31–88. doi: 10.1016/s0065-2911(08)60376-8. [DOI] [PubMed] [Google Scholar]
- Trias J., Nikaido H. Outer membrane protein D2 catalyzes facilitated diffusion of carbapenems and penems through the outer membrane of Pseudomonas aeruginosa. Antimicrob Agents Chemother. 1990 Jan;34(1):52–57. doi: 10.1128/aac.34.1.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y. J., Livermore D. M. Interactions of meropenem with class I chromosomal beta-lactamases. J Antimicrob Chemother. 1989 Sep;24 (Suppl A):207–217. doi: 10.1093/jac/24.suppl_a.207. [DOI] [PubMed] [Google Scholar]