Abstract
Because proinflammatory cytokines are markedly elevated during H5N1 influenza virus infection, the “cytokine storm” is hypothesized to be the main cause of mortality. Here, we demonstrate that mice deficient in the hallmark inflammatory cytokines TNF-α, IL-6, or CC chemokine ligand 2 succumb to infection with A/Vietnam/1203/04 (H5N1) virus, as do wild-type mice treated with glucocorticoids for suppression of cytokines. Because cytokine inhibition does not protect against death, therapies that target the virus rather than cytokines may be preferable.
Highly pathogenic H5N1 avian influenza virus has caused >150 human deaths since 2003 in Asia, Africa, and Europe, where it continues to circulate in wild and domestic poultry. Its mortality rate in humans is >50%, with clinical manifestations that include fever, diarrhea, viral pneumonia, encephalitis, and acute respiratory distress syndrome (1–10). Lymphopenia and high levels of inflammatory cytokines have also been reported (1, 2, 5, 7), and laboratory studies have revealed high cytokine induction. Elevated levels of proinflammatory cytokines, including TNF-α, IL-6, and CC chemokine ligand 2 (CCL2), have been detected in human cells and mice infected with highly pathogenic H5N1 influenza virus (2, 10–14). A high cytokine response was observed in mice and macaques inoculated with the 1918 pandemic strain of influenza (15–17). However, the significance of the elevated proinflammatory cytokine response (“cytokine storm”) in the pathogenesis of H5N1 remains to be determined.
Because of the pronounced elevation of cytokines during H5N1 infection, cytokine storm has been widely hypothesized to be the main cause of pathology and ultimately of death. The cytokines' multiple functions potentially allow them to affect host survival both positively and negatively. They promote lymphocyte activation and infiltration of sites of infection and exert direct antiviral effects. However, during a cytokine storm in which there is a dsyregulation of cytokines, their effects (e.g., excessive induction of apoptosis) may become excessive and harmful.
We investigated whether inhibition of the cytokine response is sufficient to protect against death caused by A/Vietnam/1203/04 (H5N1) virus isolated from a recent human fatality. These studies were performed in mice, which have been well established as a model that has H5N1 replication sites, H5N1-induced cytokine production, and H5N1 pathogenicity similar to that in humans (11, 13). Furthermore, transgenic technology allowed us to examine mice genetically deficient in specific cytokines. The results show that mice deficient in the inflammatory cytokines TNF-α, IL-6, or CCL2 succumb to infection with A/Vietnam/1203/04, as do wild-type mice treated with the cytokine inhibitor glucocorticoids.
Results
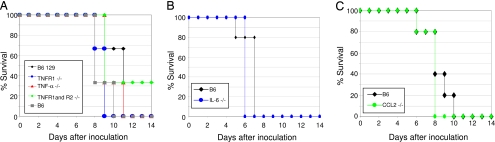
TNF-α is a proinflammatory molecule that induces diverse cellular responses and is proposed to be the primary architect of lymphocyte-mediated lung injury during influenza virus infection (18). To determine whether TNF-α contributes to the high morbidity and mortality caused in mammals by avian H5N1 virus infection, we inoculated mice genetically deficient in TNF-α or its receptors with A/Vietnam/1203/04 virus. Mortality (Fig. 1A) and weight loss (Table 1) were similar in wild-type mice and mice deficient in TNF-α. We then repeated these tests in mice deficient in the TNF receptor 1 (TNFR1) receptor or in both the TNFR1 and TNFR2 receptors. These mice had mortality and morbidity similar to that observed in TNF-α-deficient and wild-type mice (Fig. 1A and Table 1). Therefore, the absence of TNF-α or of responsiveness to TNF-α does not reduce the morbidity and mortality caused by H5N1 virus infection.
Fig. 1.
Mortality and morbidity of mice deficient in TNF-α, IL-6, and CCL2 after inoculation with A/Vietnam/1203/04 influenza virus. Percent survival of mice deficient in TNF-α, TNFR1, or TNFR1 and R2 (A); IL-6 (B); and CCL2 (C).
Table 1.
Percent weight loss in cytokine-deficient and wild-type mice 5 days after inoculation
| Mouse strain | N | Weight loss, % |
|---|---|---|
| B6 129 | 3 | 17.1 ± 2.2 |
| B6 | 3 | 15.2 ± 3.7 |
| TNF-α−/− (B6 129) | 3 | 11.1 ± 8.5 |
| TNFR1−/− (B6) | 3 | 14.8 ± 2.1 |
| TNFR1 and TNFR2−/− (B6 129) | 3 | 9.1 ± 7.1 |
| B6 | 5 | 19.2 ± 2.1 |
| IL-6−/− (B6) | 5 | 15.7 ± 3.6 |
| B6 | 5 | 21.3 ± 4.9 |
| CCL2−/− (B6) | 5 | 24.7 ± 0.9 |
Number of mice per experiment is N. Values for percent weight loss are shown as mean ± SE.
Another prominent inflammatory cytokine, IL-6, has been reported to be elevated during H5N1 virus infection (11, 19). After we inoculated IL-6-deficient and wild-type mice with A/Vietnam/1203/04 virus, they lost a significant but similar amount of weight (Table 1), and all died by day 7 after inoculation (Fig. 1B). Therefore, IL-6 deficiency is not sufficient to reduce the morbidity and mortality of H5N1 virus infection in mice.
Also of interest are chemoattractant cytokines, or chemokines, which recruit leukocytes to infection sites. The chemokine CCL2, also termed monocyte chemoattractant protein-1, is produced at high levels after H5N1 virus infection (11, 19). We inoculated wild-type and transgenic CCL2-deficient mice with A/Vietnam/1203/04 virus. Weight change (Table 1) and mortality (Fig. 1C) were similar in the two sets of mice. Taken together, our results showed that deficiency of any of the key inflammatory cytokines (TNF-α, IL-6, or CCL2) alone is not sufficient to protect mammalian hosts from death caused by highly pathogenic H5N1 virus.
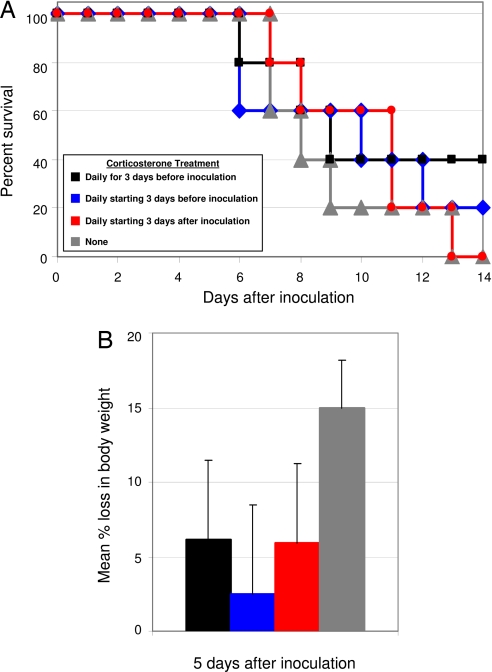
Because no single cytokine was implicated in the lethality of the virus, we next tested the effect of glucocorticoids, which are steroids with diverse functions, including cytokine suppression (20). Wild-type B6 mice were administered corticosterone, the natural murine glucocorticoid, for 3 days before inoculation with A/Vietnam/1203/04, daily starting 3 days before inoculation, or daily starting 3 days after inoculation. Untreated mice had significant weight loss as early as 3 days after inoculation and began to succumb to infection 7 days after inoculation (Fig. 2). The morbidity of treated mice was not significantly different from the untreated mice (Fig. 2B). Mortality rates were similar in all groups of mice, regardless of treatment (Fig. 2A). Therefore, glucocorticoid treatment does not reduce the lethality of infection with highly pathogenic H5N1 virus.
Fig. 2.
Effect of glucocorticoid treatment on mice inoculated with A/Vietnam/1203/04. (A and B) Percent survival (A) and mean percent weight loss (B) ± SE 5 days after inoculation. n = 5 mice per group.
Discussion
These results demonstrate that inhibition of the cytokine response to infection with highly pathogenic H5N1 influenza virus is not sufficient to protect mammalian hosts from death. Mice deficient in the inflammatory cytokines TNF-α, IL-6, or CCL2 had similar morbidity and mortality rates to wild-type mice after infection with A/Vietnam/1203/04. Furthermore, treatment of mice glucocorticoid did not protect mice against the highly pathogenic H5N1 virus. These results have relevance to worldwide public health, and implications for clinical therapy investigations and for laboratory studies of the pathogenesis of H5N1 infection.
There is intense interest in the question whether H5N1 avian influenza viruses, and similarly the 1918 Spanish influenza virus, exert their lethal effects by inducing a cytokine storm. Several studies have reported the significant increase in proinflammatory cytokines after H5N1 infections (2, 10–14). The scientific and clinical communities are questioning whether inhibition of the inflammatory cytokine response might offer a lifesaving therapy for humans with H5N1 infection.
The data we report here addressed this question by demonstrating that genetic deficiencies in or chemical suppression of the inflammatory cytokines do not protect mice against a highly lethal human H5N1 isolate. Although these findings are negative in nature, they are crucially needed at this time to guide the search for effective therapy for human H5N1 infection. These results refute the popular paradigm that the cytokine storm is the cause of death during H5N1 infection.
The contribution of individual inflammatory cytokines to the morbidity and mortality caused by H5N1 was not apparent in our studies using mice genetically deficient in these cytokines. The inflammatory cytokines TNF-α, IL-6, and CCL2 either are not involved in a destructive cytokine response or are redundant to other factors elicited during such a response. The latter possibility is relevant because of the pleiotropic functions of the cytokines. Dissecting the contribution of each cytokine to factors, other than weight loss and mortality, is necessary. Clues to the roles of these inflammatory cytokines in H5N1 will be gained from determining the mechanisms of induction of these cytokines, the cellular sources of these proinflammatory factors, and their effects on viral and host factors.
We previously showed that the lethality of A/Vietnam/1203/04 virus in mammalian hosts is associated with viral factors that include the polymerase genes (21). Mutations in these viral genes were associated with decreased viral replication and increased host survival. Thus, inhibition of the H5N1 polymerase activity has the potential to be a therapeutic target. In contrast, inhibiting the host cytokine response is not sufficient to reduce morbidity and lethality of the viral infection. Taken together, these data suggest that early inhibition of viral replication is more promising than inhibition of the cytokine response in promoting host survival of H5N1 influenza virus infection. It is important to note that, for certain antiviral drugs, there is a narrow time frame during which their administration will be effective. Thus, continued research on targeting host responses to H5N1 is justifiable.
Materials and Methods
Generation of Recombinant Viruses by Reverse Genetics.
All experiments were performed in approved biosafety level 3+ laboratories. A/Vietnam/1203/04 (H5N1) was obtained from World Health Organization collaborating laboratories and grown in allantoic cavities of 10-day-old embryonated chicken eggs. RT-PCR was used to amplify the eight viral genes, and viral cDNAs were inserted into dual-promoter plasmid pHW2000 (22). The plasmids were sequenced, and QuikChange Site-Directed Mutagenesis kits (Stratagene, La Jolla, CA) were used to generate coding sequences in plasmids identical to PCR fragment sequences. Recombinant viruses were generated by DNA transfection of MDCK/293 T cells. Transfection supernatant was injected into 10-day-old embryonated chicken eggs, and virus stock was prepared, sequenced, and titrated.
Sequence Analysis.
The viral RNA was isolated from allantoic fluid by using RNA isolation kit (RNeasy; Qiagen, Valencia, CA). RT-PCR was performed by using the previously described universal primer set for influenza A (23). Sequencing was done at the St. Jude Children's Research Hospital's Hartwell Center for Bioinformatics and Biotechnology by using Big Dye Terminator (version 3) chemistry and synthetic oligonucleotides. The Applied Biosystems (Foster City, CA) 3700 DNA Analyzers were used to evaluate samples.
Infection and Glucocorticoid Treatment of Mice.
Seven- to 10-week-old mice were lightly anesthetized with isoflurane and inoculated intranasally with 103 50% egg infective dose of infectious A/Vietnam/1203/04 virus in 50 μl of PBS. Male C57BL/6J, B6129SF2/J, B6.129Tnfrsf1atm1Mak/J, B6;129S6-Tnftm1Gkl/J, B6;129S-Tnfrsf1atm1Imx, Tnfrsf1btm1Imx/J, B6.129S2-Il6tm1Kopf/J, and B6.129S4-Ccl2tm1Rol/J mice were purchased from The Jackson Laboratory (Bar Harbor, ME). Weight, clinical signs of infection, and survival were recorded. Glucocorticoid-treated mice received 30 μg/ml corticosterone (Sigma, St. Louis, MO) in drinking water as previously described (24) 3 days before infection, 3 days prior and throughout infection, or 3 days after infection with A/Vietnam/1203/04. The levels of drinking water were monitored for intake of glucocorticoids.
Acknowledgments
We thank Scott Krauss for scientific assistance, Sharon Naron for editorial assistance, and Patrick Seiler for technical support. We also thank The Hartwell Center for Bioinformatics and Biotechnology and the Animal Resources Center at St. Jude Children's Research Hospital. This study was supported by National Institutes of Health Grants AI95357, CA21765, and National Institute of Allergy and Infectious Diseases Center of Excellence, and by the American Lebanese Syrian Associated Charities.
Abbreviations
- CCL2
CC chemokine ligand 2
- TNFR
TNF receptor.
Footnotes
The authors declare no conflict of interest.
References
- 1.Tran TH, Nguyen TL, Nguyen TD, Luong TS, Pham PM, Nguyen VC, Pham TS, Vo CD, Le TQ, Ngo TT, et al. N Engl J Med. 2004;350:1179–1188. doi: 10.1056/NEJMoa040419. [DOI] [PubMed] [Google Scholar]
- 2.Cheung CY, Poon LL, Lau AS, Luk W, Lau YL, Shortridge KF, Gordon S, Guan Y, Peiris JS. Lancet. 2002;360:1831–1837. doi: 10.1016/s0140-6736(02)11772-7. [DOI] [PubMed] [Google Scholar]
- 3.Beigel JH, Farrar J, Han AM, Hayden FG, Hyer R, de Jong MD, Lochindarat S, Nguyen TK, Nguyen TH, Tran TH, et al. N Engl J Med. 2005;353:1374–1385. doi: 10.1056/NEJMra052211. [DOI] [PubMed] [Google Scholar]
- 4.Yuen KY, Chan PK, Peiris M, Tsang DN, Que TL, Shortridge KF, Cheung PT, To WK, Ho ET, Sung R, et al. Lancet. 1998;351:467–471. doi: 10.1016/s0140-6736(98)01182-9. [DOI] [PubMed] [Google Scholar]
- 5.Wong SS, Yuen KY. Chest. 2006;129:156–168. doi: 10.1378/chest.129.1.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chan PK. Clin Infect Dis. 2002;34(Suppl 2):S58–S64. doi: 10.1086/338820. [DOI] [PubMed] [Google Scholar]
- 7.Chotpitayasunondh T, Ungchusak K, Hanshaoworakul W, Chunsuthiwat S, Sawanpanyalert P, Kijphati R, Lochindarat S, Srisan P, Suwan P, Osotthanakorn Y, et al. Emerg Infect Dis. 2005;11:201–209. doi: 10.3201/eid1102.041061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.To KF, Chan PK, Chan KF, Lee WK, Lam WY, Wong KF, Tang NL, Tsang DN, Sung RY, Buckley TA, et al. J Med Virol. 2001;63:242–246. doi: 10.1002/1096-9071(200103)63:3<242::aid-jmv1007>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- 9.Uiprasertkul M, Puthavathana P, Sangsiriwut K, Pooruk P, Srisook K, Peiris M, Nicholls JM, Chokephaibulkit K, Vanprapar N, Auewarakul P. Emerg Infect Dis. 2005;11:1036–1041. doi: 10.3201/eid1107.041313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.de Jong MD, Bach VC, Phan TQ, Vo MH, Tran TT, Nguyen BH, Beld M, Le TP, Truong HK, Nguyen VV, et al. N Engl J Med. 2005;352:686–691. doi: 10.1056/NEJMoa044307. [DOI] [PubMed] [Google Scholar]
- 11.Xu T, Qiao J, Zhao L, Wang G, He G, Li K, Tian Y, Gao M, Wang J, Wang H, et al. Am J Respir Crit Care Med. 2006;174:1011–1017. doi: 10.1164/rccm.200511-1751OC. [DOI] [PubMed] [Google Scholar]
- 12.Chan MC, Cheung CY, Chui WH, Tsao SW, Nicholls JM, Chan YO, Chan RW, Long HT, Poon LL, Guan Y, et al. Respir Res. 2005;6:135. doi: 10.1186/1465-9921-6-135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Szretter KJ, Gangappa S, Lu X, Smith C, Shieh WJ, Zaki S, Sambhara S, Tumpey TM, Katz JM. J Virol. 2007;81:2736–2744. doi: 10.1128/JVI.02336-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lee DC, Cheung CY, Law AH, Mok CK, Peiris M, Lau AS. J Virol. 2005;79:10147–10154. doi: 10.1128/JVI.79.16.10147-10154.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kash JC, Tumpey TM, Proll SC, Carter V, Perwitasari O, Thomas MJ, Basler CF, Palese P, Taubenberger JK, Garcia-Sastre A, et al. Nature. 2006;443:578–581. doi: 10.1038/nature05181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kobasa D, Jones SM, Shinya K, Kash JC, Copps J, Ebihara H, Hatta Y, Kim JH, Halfmann P, Hatta M, et al. Nature. 2007;445:319–323. doi: 10.1038/nature05495. [DOI] [PubMed] [Google Scholar]
- 17.Kash JC, Basler CF, Garcia-Sastre A, Carter V, Billharz R, Swayne DE, Przygodzki RM, Taubenberger JK, Katze MG, Tumpey TM. J Virol. 2004;78:9499–9511. doi: 10.1128/JVI.78.17.9499-9511.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hussell T, Pennycook A, Openshaw PJ. Eur J Immunol. 2001;31:2566–2573. doi: 10.1002/1521-4141(200109)31:9<2566::aid-immu2566>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 19.de Jong MD, Simmons CP, Thanh TT, Hien VM, Smith GJ, Chau TN, Hoang DM, Chau NV, Khanh TH, Dong VC, et al. Nat Med. 2006;12:1203–1207. doi: 10.1038/nm1477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Almawi WY, Beyhum HN, Rahme AA, Rieder MJ. J Leukocyte Biol. 1996;60:563–572. doi: 10.1002/jlb.60.5.563. [DOI] [PubMed] [Google Scholar]
- 21.Salomon R, Franks J, Govorkova EA, Ilyushina NA, Yen HL, Hulse-Post DJ, Humberd J, Trichet M, Rehg JE, Webby RJ, et al. J Exp Med. 2006;203:689–697. doi: 10.1084/jem.20051938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hoffmann E, Neumann G, Kawaoka Y, Hobom G, Webster RG. Proc Natl Acad Sci USA. 2000;97:6108–6113. doi: 10.1073/pnas.100133697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hoffmann E, Stech J, Guan Y, Webster RG, Perez DR. Arch Virol. 2001;146:2275–2289. doi: 10.1007/s007050170002. [DOI] [PubMed] [Google Scholar]
- 24.Ruzek MC, Pearce BD, Miller AH, Biron CA. J Immunol. 1999;162:3527–3533. [PubMed] [Google Scholar]