Abstract
The class II transactivator (CIITA) is a key regulatory factor for MHC class II expression. Here, we demonstrate that PKCδ plays an important role in regulating IFN-γ-inducible CIITA gene expression in macrophages. Inhibition of PKCδ by either a PKCδ inhibitor or a dominant negative (DN) mutant form of PKCδ led to down-regulation of CIITA expression. The decrease in CIITA expression by PKCδ inhibition was in part due to the reduced recruitment of serine 727-phosphorylated Stat1 and histone acetyltransferases to the CIITA promoter. As a result, IFN-γ induced histone acetylation at the CIITA promoter is also compromised. However, inhibition of PKCδ did not affect IRF-1 expression or IRF-1 binding to the CIITA promoter. Therefore, we report, for the first time, that PKCδ is an essential signaling molecule to achieve the maximal expression of CIITA in response to IFN-γ in macrophages. In addition, although IRF-1 is a key transcription factor to activate the IFN-γ inducible CIITA promoter, the effect of PKCδ on CIITA expression is mediated primarily by serine phosphorylation of Stat1.
Keywords: monocytes, macrophages, MHC, protein kinases, STAT1, transcription factors, gene regulation
1. Introduction
Proper MHC class II expression is essential to activate CD4 T cells and to mount an adaptive immune response. MHC class II genes are constitutively expressed in B cells and dendritic cells (DC) and can be induced in macrophages by IFN-γ (Steinman et al., 1999; Reith et al., 2005). Both constitutive and inducible MHC class II gene expression requires the class II transactivator (CIITA) (Reith et al., 2005; Steimle et al., 1993; Chang et al., 1996; LeibundGut-Landmann et al., 2004). CIITA is not a DNA binding protein. Instead, it acts as a scaffolding protein by interacting with itself and other transcriptional factors to activate the MHC class II promoter (Masternak et al., 2000; Sisk et al., 2001; Linhoff et al., 2001). CIITA transcription is controlled by at least three distinct promoters known as pI, pIII and pIV each of which generates unique CIITA transcripts (O’Keefe et al., 2001; Muhlethaler-Mottet et al., 1997; LeibundGut-Landmann et al., 2004). pI and pIII are active in DC, whereas pIII is used in B cells and plasmacytoid DC (Muhlethaler-Mottet et al., 1997; LeibundGut-Landmann et al., 2004). In macrophages, however, all three promoters can be induced by IFN-γ although the major form is transcribed from pIV (O’Keefe et al., 2001; Muhlethaler-Mottet et al., 1997; LeibundGut-Landmann et al., 2004). Thus, each CIITA promoter is used in a cell type-specific manner.
pIV is controlled by three major cis-acting elements: an IFN-γ activation sequence (GAS), an E box and an interferon regulatory factor (IRF) element, which bind the transcription factor Stat1, USF-1, and IRF-1, respectively (O’Keefe et al., 2001; Muhlethaler-Mottet et al., 1997; 1998). IFN-γ activates tyrosine kinase JAK1 and JAK2, which results in phosphorylation of Stat1. Activated Stat1 dimerizes and translocates to the nucleus, where it binds to the GAS element in pIV (Shuai et al., 1994; Wen et al., 1995; Vinkemeier et al., 1996; Mowen et al., 2000; Darnell, 1997). Stat1 also controls IRF-1 expression, which in turn activates the CIITA promoter (Piskurich et al., 1999; Morris et al., 2002). Therefore, IFN-γ mediated activation of pIV requires both Stat1 and IRF-1.
PKCs, serine/threonine-specific protein kinases, are known to regulate IFN-γ inducible expression of MHC class II (Smith et al., 1992; Giroux et al., 2003; Benveniste et al., 1991; Setterblad et al., 1998; Lee et al., 1995). Microinjection of PKC protein into peritoneal macrophages induced MHC class II expression (Smith et al., 1992). This induction was blocked by PKC inhibitors such as staurosporine, calphostin C, or H7 (Smith et al., 1992). MHC class II expression induced by IFN-γ in astrocytes is also down regulated when cells were treated with the pan-PKC inhibitor H7 (Benveniste et al., 1991). In the same study, the authors also showed that PMA treatment that activates PKC signaling does not affect MHC class II expression. In promyelocytic cells, however, PMA treatment reduced MHC class II expression by destabilizing CIITA mRNA (De Lerma Barbaro et al., 2005), suggesting a cell type specific effect of PKC. MHC class II expression requires CIITA and therefore PKC-mediated modulation of MHC class II is possibly due to a change in CIITA expression. Indeed, PKCα regulates IFN-γ-inducible expression of the CIITA gene via control of transactivation activity of IRF-1 (Giroux et al., 2003). Recently, we have demonstrated that constitutive expression of CIITA in B cells depends on PKCδ, which involves CREB phosphorylation and recruitment, but not regulation of IRF-1 (Kwon et al., 2006).
PKCδ is known to participate in both type I and II IFN signaling pathways (Uddin et al., 2002; Deb et al., 2003). PKCδ activated by type I IFN associates with Stat1, and is involved in phosphorylation of STAT1 on Serine 727 (Uddin et al., 2002). Furthermore, treating cells with a PKCδ inhibitor or a dominant negative mutant form of PKCδ prevents type I IFN-dependent gene transcription (Uddin et al., 2002). Similarly, PKCδ is activated by type II IFN, and phosphorylates Stat1(Ser727) in promyelocytic cells (Deb et al., 2003). Likewise, CaMKII expression is involved in serine phosphorylation of Stat1 in response to IFN-γ (Sun et al., 2005).
In this report, we demonstrate that PKCδ is essential for maximum IFN-γ inducible CIITA gene expression. PKCδ promoted the recruitment of serine phosphorylated Stat1 and histone acetyltransferases (HATs), but not IRF-1, to the CIITA promoter. As a result, histone acetylation of the CIITA promoter was enhanced by PKCδ. However, Stat1 dependent IRF-1 expression was not affected by PKCδ. The differential effect of PKCδ on CIITA and IRF-1 expression was at least partly due to a differential requirement for Stat1 serine phosphorylation. Activation of the CIITA promoter required both tyrosine and serine phosphorylation of Stat1, whereas serine phosphorylated Stat1 was not necessary to activate the IRF-1 expression. Together, PKCδ plays an important role in achieving the maximal induction of macrophage CIITA gene expression in response to IFN-γ.
2. Materials and Methods
2.1. Cells and Reagents
The murine macrophage cell line RAW264.7 and Stat1 deficient human fibrosarcoma cell line U3A were maintained in DMEM and 10% FBS and antibiotics. Bone marrow-derived macrophages were generated from C57BL/6 mice that were purchased from the Jackson Laboratory (Bar Harbor, ME). In brief, total BM cells depleted of RBC, T cells, B cells, and other MHC class II-positive cells were cultured 10 days in RPMI1640 supplemented with 5% FBS and 10 ng/ml murine rM-CSF.
Murine and human rIFN-γ was obtained from BD Pharmingen (San Diego, CA), and Murine rM-CSF was from R&D Systems (Minneapolis, MN). Antibodies against CIITA, PKCδ, IRF1, and HA were purchased from Santa Cruz (Santa Cruz, CA), and anti-RFX5 was obtained from Rockland, INC (Gilbertsville, PA). Antibodies used for flow cytometry, FITC-conjugated MHC class I (H-2Kb, clone AF6-88.5), and PE-conjugated MHC class II (I-Ab, clone AF6-120.1) were obtained from BD Biosciences. Antibodies against phospho-PKCδ (Thr505) and phospho-PKCδ (Ser623), phospho-Stat1(Tyr701) and phospho-Stat1(Ser727), and Stat1 were from Cell Signaling Technology (Berverly, MA). For the ChIP assay, anti-Stat1, IRF-1, CBP, and p300 antibodies were purchased from Santa Cruz, and histone H4 was obtained from Upstate Biotechnology (Lake Placid, NY). The PKC inhibitor, Ro-31-8225, Go-6976, and Rottlerin were obtained from Cal Biochem (La Jolla, CA).
2.2. Plasmid and Transfections
Wild type (WT) and the dominant negative (DN) mutant form of PKCδ have been described previously (Giroux et al., 2003; Soh and Weinstein, 2003). To generate stable cell lines expressing WT or DN PKCδ, RAW264.7 cells were transfected with WT, DN PKCδ or the empty vector, and selected with G418. The 1.1 kb pIV-driven luciferase plasmid and MHC class II Eα driven luciferase reporter plasmid were described previously (Sisk et al., 2003; Yao et al., 2006). The IRF-1 promoter construct that contains the GAS and NF-κB site (0.7kb) was kindly provided by Dr. Richard Pine (Public Health Research Institute, New York, NY) (Pine, 1997). Transient transfection to RAW264.7 cells was performed with 2 μg of the luciferase reporter (5×105cells) using the lipofectamine method (Invitrogen, Carlsbad, CA). After 36 hrs of transfection, IFN-γ (10ng/ml) was added with or without Rottlerin for additional 10 hrs. In some experiments, the reporter was co-transfected with WT or DN PKCδ for 48hrs followed by IFN-γ treatment for 12hrs. WT, Stat1(Y701F), and Stat1 (S727A) constructs were as described (Zhang et al., 2005). U3A cells were transfected with the reporter together with WT, Stat1 (Y701F), Stat1 (S727A) or the empty vector (EV) using the calcium phosphate method. Two days later, the cells were treated with human IFN-γ for 12hrs. Cell lysates were prepared to measure the luciferase activity as described (Sisk et al, 2003). Luciferase activity was normalized with CMV promoter driven β-galactosidase expression. The results were expressed as relative luciferase activity.
2.3. RT-PCR and Quantitative real-time PCR
Preparation of total RNA, cDNA synthesis, and PCR was conducted as described (Gourley et al., 2002). The following primers were used: type IV CIITA (5′-GCATGCCCGAACCTGCGCTGA-3′, and 5′-GGCCATCTTGGGCCTCTAGCT-3′), and ICSBP-1 (5′-GAATTCCGAAAGGATGTGGA-3′ and 5′-GATGCCAGTGA-ATGGGTCTT-3′). The primers used for IRF-1, MHC class II (I-Eαd) and HPRT were described previously (Yao et al., 2006).
Quantitative real-time PCR was performed by the comparative threshold cycle (ΔCT) method and normalized to GAPDH. The primers used for type IV CIITA and GAPDH were as described (Yao et al., 2006).
2.4. FACS analysis
Cells were preincubated 15 min with the anti-FcγR mAb 2.4G2 to block non-specific binding before staining with Abs against MHC class I and class II for 30 min at 4°C. Flow cytometric analysis was performed using FACS Calibur and analyzed using Cell Quest software (BD Biosciences).
2.5. Immunoblotting & Immunoprecipitation
Total cell lysates were used for immunoblotting as previously described (Sisk et al., 2003). To assess CIITA expression, 50 μg of proteins from cell lysates were subjected to SDS-PAGE on 6% slab gels. The same membrane was used to detect RFX5. To assess phosphorylation of PKCδ, 500 μg of cell lysates were immunoprecipitated with an anti-PKCδ antibody followed by western blotting with an antibody recognizing PKCδ (Thr 505) or PKCδ (Ser 623). The same membrane was then stripped and reprobed with an anti-PKCδ antibody. The level of Stat1 phosphorylation was assessed with anti-pStat1(Tyr701) or -pStat1 (Ser 727) using SDS-page on 8% gels. The membrane was reprobed with anti-Stat1 antibody to detect total Stat1.
2.6. Chromatin Immunoprecipitation
Chromatin immunoprecipitation (ChIP) assay was performed as described (Yao et al., 2005). Approximately 1×107 cells were used for each chromatin preparation. The chromatin sample from cells cross linked with formaldehyde and sonicated was precleared with salmon-sperm DNA and protein A sepharose. Two percent of the sample was used for input. One-seventh of the sample (1.5×106 cells) was used for immunoprecipitation with the antibody recognizing IRF-1, Stat1, pStat1(Tyr701), pStat1(Ser 727), CBP and p300 or acetylated histone H4. Immune complexes were collected with protein A sepharose beads and eluted. After reversal crosslinks and digestion of proteins with proteinase K, the DNA was purified by phenol/chloroform extraction. PCR was performed using primers designed to amplify the 180 bp fragment that contains GAS and E box of the type IV CIITA promoter(Yao et al., 2006).
3. Results
3.1. PKCδ regulates CIITA gene transcription in response to IFN-γ
PKCα is known to regulate IFN-γ inducible expression of the CIITA gene (Giroux et al., 2003). However, we have previously observed that PKCδ, not PKCα, is necessary to maintain CIITA expression in B cells (Kwon et al., 2006). Based on these observations, we investigated the role of PKC members in CIITA gene regulation in macrophages. To do this, we treated RAW264.7 cells with IFN-γ alone or in the presence of a PKC inhibitor with different specificities; RO-31-8425 for PKCα, β, γ, and ε; GO-6976 for PKCα; Rottlerin for PKCδ. We used the concentration of each inhibitor that corresponds to IC50 based on the manufacture’s specification. As expected, IFN-γ treated cells expressed a high level of CIITA protein (Fig. 1A). Each inhibitor diminished the level of CIITA to a different degree, suggesting that all three are involved in CIITA expression. However, among the three inhibitors tested, the PKCδ inhibitor Rottlerin was most potent in preventing the induction, indicating that PKCδ plays a major role in regulating IFN-γ inducible expression of CIITA. Rottlerin treatment showed little effect on the level of RFX5 that is another critical transcription factor for MHC class II expression (Fig. 1A). The decrease in CIITA protein by the PKCδ inhibitor was coincident with reduced CIITA mRNA (Fig. 1B).
Figure 1.
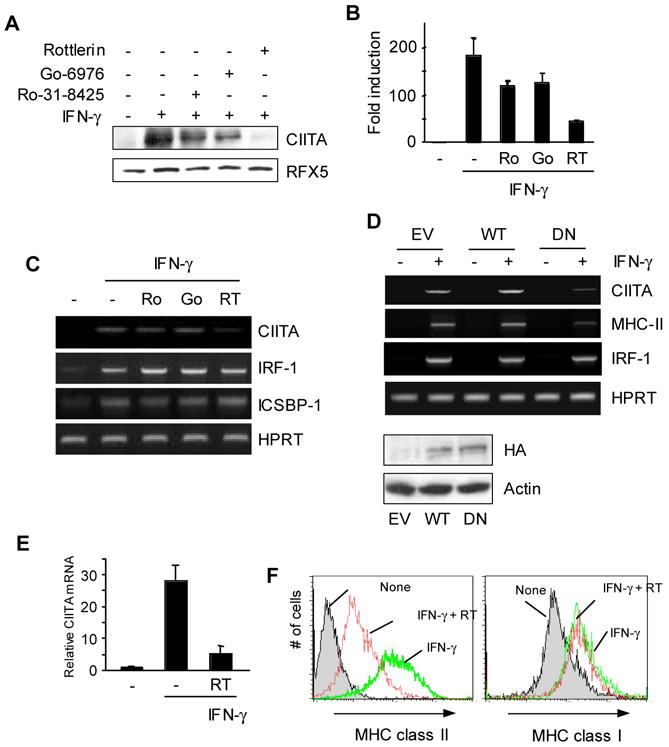
PKCδ regulates IFN-γ inducible expression of CIITA in macrophages. (A–C) RAW264.7 cells were pre-treated with each PKC inhibitor: Ro-31-8425 (500nM), Go-6976 (200nM), Rottlerin (5 μM), or DMSO for 30 min followed by IFN-γ for 8hrs. (A) Cell lysates were prepared to do immunoblot using the antibody as indicated. 50 μg of total lysates was loaded on a 6% gel. The same membrane was stripped and re-probed with an anti-RFX5 antibody. (B) RNA was prepared from RAW264.7 cells treated with PKC inhibitors as in (A). Type IV CIITA mRNA levels were determined by qRT-PCR. qRT-PCR data were normalized against mRNA levels of the GAPDH gene. Data are means ± SE of three independent experiments. (C) Other IFN-γ-inducible genes were not affected by PKCδ inhibition. RAW264.7 cells were treated with PKC inhibitors as in (A). mRNA was analyzed using RT-PCR for each gene as indicated. (D) DN PKCδ expressing cells have reduced levels of CIITA and MHC class II mRNA. RAW264.7 cells that were stably transfected with the empty vector (EV), wild type (WT), or a dominant negative (DN) mutant form of PKCδ were treated with IFN-γ for 12hrs. Expression of transfected PKCδ was determined by immunoblotting with an anti-HA antibody. (E) BM macrophages from C57BL/6 mice were pretreated with Rottlerin (5μM) for 30 min, followed by IFN-γ stimulation for 6 hrs. Type IV CIITA mRNA levels were measured by qRT-PCR. The relative mRNA levels were normalized to the GAPDH gene. (F) BM macrophages were pretreated with Rottlerin for 30 min, followed by IFN-γ stimulation for 24 h. Cell surface MHC class II and class I expression was analyzed by flow cytometry. Data are representative (A, C, D, and F) or means ± SE (B and E) of at least two independent experiments.
We next investigated whether PKCδ inhibition affects expression of other IFN-γ inducible genes. For this, we compared the levels of two additional IFN-γ inducible genes, IRF-1 and ICSBP-1 (Yao et al., 2005). As demonstrated in Figure 1C, expression of these genes was not influenced by PKCδ inhibition, suggesting that PKCδ does not affect all IFN-γ inducible gene expression in macrophages.
To further confirm the effect of Rottlerin on CIITA expression, we generated RAW264.7 stable cell lines that express a wild type (WT) or a dominant negative mutant (DN) form of PKCδ (Soh and Weinstein, 2003). As a control, cells transfected with the empty vector (EV) was generated. Consistent with the effect of Rottlerin treatment, the induction of CIITA expression by IFN-γ was decreased in cells expressing DN but not WT PKCδ (Fig. 1D). As a consequence, the mRNA level of MHC class II was also decreased (Fig. 1D). However, IRF-1 mRNA levels were comparable among the three cell lines (Fig. 1D). The expression of both WT and DN PKCδ was determined by an immunoblot using an anti-HA antibody since the two PKCδ constructs have a HA-tag (Fig. 1D, bottom group).
We further investigated whether PKCδ is also important for CIITA expression in bone marrow derived macrophages (BMMP) that were prepared as described in the Materials and Methods. Consistent with the data from RAW264.7 cells, IFN-γ inducible expression of the CIITA gene was greatly diminished when BMMP were treated with IFN-γ together with Rottlerin (Fig. 1E). As a consequence, the level of MHC class II on the cell surface was also decreased (Fig. 1F, left panel). However, Rottlerin did not affect MHC class I expression (Fig 1F, right panel). Together, PKCδ is critical for the maximal induction of CIITA and MHC class II expression in response to IFN-γ in macrophages.
3.2. CIITA promoter pIV activity is controlled by PKCδ
Next, we asked whether PKCδ regulates the promoter activity of the CIITA gene. In IFN-γ treated macrophages, the major form of CIITA mRNA is transcribed from pIV (O’Keefe et al, 2001; Muhlethaler-Mottet et al., 1997; LeibundGut-Landmann et al., 2004). Therefore, we used a luciferase reporter driven by the 1.1 kb fragment of CIITA promoter pIV (pIV-luciferase) for transient transfection assays in RAW264.7 cells. To test the effect of PKCδ, we employed two complementary approaches: Rottlerin treatment and co-transfection with DN PKCδ. We transfected RAW264.7 cells with the pIV-luciferase reporter, divided into three groups, and then treated with DMSO, IFN-γ, or IFN-γ together with Rottlerin. pIV activity was enhanced by IFN-γ but the induction was decreased in cells treated with the PKCδ inhibitor (Fig. 2A, left). We next tested the promoter activity of the MHC class II gene with the notion that the decreased CIITA level would lead to low MHC class II promoter activity. Indeed, MHC class II promoter activity was down-regulated by treatment of the PKCδ inhibitor (Fig. 2A, middle). However, IRF-1 promoter activity was comparable under all conditions, supporting the specificity of PKCδ-mediated regulation of CIITA (Fig. 2, right).
Figure 2.
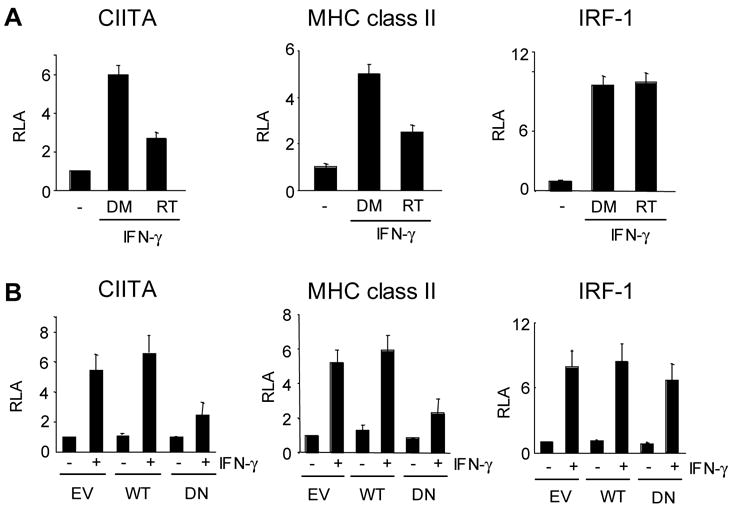
PKCδ regulates CIITA and MHC class II promoters but not IRF-1 promoter. (A) The effects of Rottlerin on the indicated promoter activity. RAW264.7 cells were transiently transfected with the luciferase reporter for 36 hrs. Cells then were divided into three, treated with none, IFN-γ with DMSO (DM), or IFN-γ with Rottlerin (RT) for 10hrs. (B) RAW264.7 cells were transfected with the indicated luciferase reporter along with the empty vector (EV), wild type (WT) or DN (DN) PKCδ. Two days after the transfection, cells were treated with IFN-γ for 12 hrs. Cell lysates were prepared to assess the luciferase activity. Luciferase activity in each group was normalized by β-galactosidase activity. The results were expressed as relative luciferase activity (RLA) against the value of the DMSO treatment or the empty vector transfection. Transfections were performed in duplicate and data are means ± SE of at least three independent experiments.
To further confirm the effect of the inhibitor treatment, we co-transfected the reporter with either wild type or DN PKCδ. Similar to the inhibitor treated cells, luciferase activity expressed by the CIITA and MHC class II promoter was reduced when cells were co-transfected with DN but not WT PKCδ (Fig. 2B, left and middle). However, the IRF-1 promoter activity was not affected by DN PKCδ (Fig. 2B, right).
3.3. PKCδ regulates the recruitment of serine phosphorylated Stat1 and HATs, but not IRF-1, to the CIITA promoter
Key transcription factors necessary for IFN-γ inducible expression of the CIITA gene are Stat1 and IRF-1 (Muhlethaler-Mottet et al., 1998; Piskurich et al., 1999). Therefore, we investigated whether the inhibition of PKCδ affects the recruitment of Stat1 and IRF-1. To do this, we first examined the binding of Stat1 and IRF-1 to the CIITA promoter in RAW264.7 cells upon IFN-γ stimulation using a chromatin immunoprecipitation (ChIP) assay. Figure 3A showed that Stat1 was bound to the CIITA promoter prior to IFN-γ stimulation and its binding was enhanced by IFN-γ, maintained at an elevated level up to 4hrs, and then declined (Fig. 3A, first panel). In contrast, IRF-1 binding was detectable 1 hr after IFN-γ treatment and maintained throughout the time points tested (Fig. 3A second panel). In addition, in agreement with the published data (Lee et al., 1995), IFN-γ treatment enhanced histone acetylation of the CIITA promoter (Fig. 3A, third panel). Therefore, IFN-γ-mediated signaling induces the binding of Stat1 and IRF-1 to the CIITA promoter, which accompanies histone acetylation.
Figure 3.
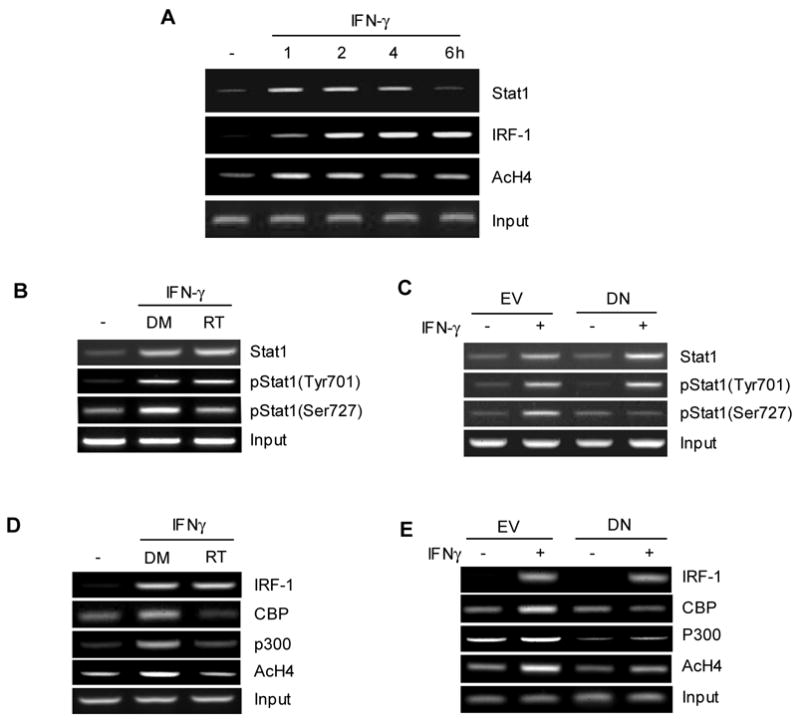
PKCδ is necessary to recruit serine phosphorylated Stat1, CBP and p300 to the CIITA promoter to enhance histone acetylation. (A) Kinetics of Stat1 and IRF-1 recruitment to the CIITA promoter, and histone acetylation upon IFN-γ treatment. IFN-γ was added to RAW264.7 cells for the indicated time period. ChIP assays were performed with the antibody as indicated and PCR was carried out with the primers to detect the binding to CIITA pIV promoter. (B and D) RAW264.7 cells were treated with Rottlerin or DMSO for 1 hr prior to the treatment with IFN-γ for additional 2 hrs. (C and E ) RAW264.7 cells that stably expressing the empty vector (EV) or DN PKCδ (DN) were treated with IFN-γ for 2 hrs. ChIP assays were performed with the indicated antibody followed by PCR to measure the binding to the CIITA promoter. Input shows 2% of total cell lysates. Data are representative of at least three independent experiments.
We next investigated the effect of PKCδ on Stat1 recruitment to the CIITA promoter. Based on the kinetics shown in Figure 3A, we pretreated RAW264.7 cells with Rottlerin or DMSO followed by IFN-γ stimulation for 2 hrs. ChIP assays were performed with the antibodies specific to Stat1 phosphorylated at tyrosine 701 [pStat1(Tyr701)], serine 727 [pStat1(Ser727)], or total Stat1. The ChIP results showed that Rottlerin treatment diminished binding of serine but not tyrosine phosphorylated Stat1 or total Stat1 to the CIITA promoter (Fig. 3B). Consistent with these results, RAW264.7 cells expressing DN PKCδ showed decreased binding of pStat1(Ser727) but not others after IFN-γ induction (Fig. 3C). Therefore, PKCδ appears to regulate the recruitment of serine phosphorylated Stat1 to the CIITA in macrophages in response to IFN-γ stimulation.
IRF-1 is an additional critical transcriptional factor for the activation of the IFN-γ inducible CIITA promoter. When we compared IRF-1 recruitment to the CIITA promoter, IRF-1 binding was comparable with and without PKCδ inhibition (Fig. 3D). Next, we examined the co-activators CBP and p300 that possess histone acetyltransferase (HAT) activity (Utley et al., 1998; Korzus et al., 1998) and are known to interact with pStat1(ser727) (Sun et al., 2005; Zhang et al., 2005). It is possible that reduced pStat1(ser727) on the CIITA promoter could have resulted in less efficient HAT recruitment. Indeed, both CBP and p300 binding was compromised in the presence of Rottlerin (Fig. 3D, second and third panel) or DN PKCδ (Fig. 3E, second and third panel), which accompanied the decreased levels of histone acetylation (fourth panel in Fig 3D and E). These data suggest that PKCδ regulates the recruitment of pStat1(ser727) and HATs, but not IRF-1, to the CIITA promoter.
3.4. PKCδ is necessary for the maximal level of Stat1 serine phosphorylation in macrophages in response to IFN-γ
The diminished binding ability of pStat1(Ser727) to the CIITA promoter upon inhibition of PKCδ signal in RAW264.7 cells suggests that PKCδ may be responsible for Stat1 serine phosphorylation in macrophages. Consistent with this notion, Stat1 serine phosphorylation by PKCδ has been observed in promyelocytic cells (Deb et al., 2003). Therefore, we investigated the phosphorylation status of PKCδ in RAW264.7 cells. RAW264.7 cells were treated with IFN-γ and cell lysates were prepared. We then performed immunoprecipitation to enrich PKCδ followed by western blotting to detect phosphorylated PKCδ using an antibody recognizing phosphorylation at the serine or threonine residue. As shown in Figure 4A, both threonine and serine residues were phosphorylated by IFN-γ within 5 min and the signal was sustained up to 20 min. Therefore, IFN-γ activates PKCδ in macrophages.
Figure 4.
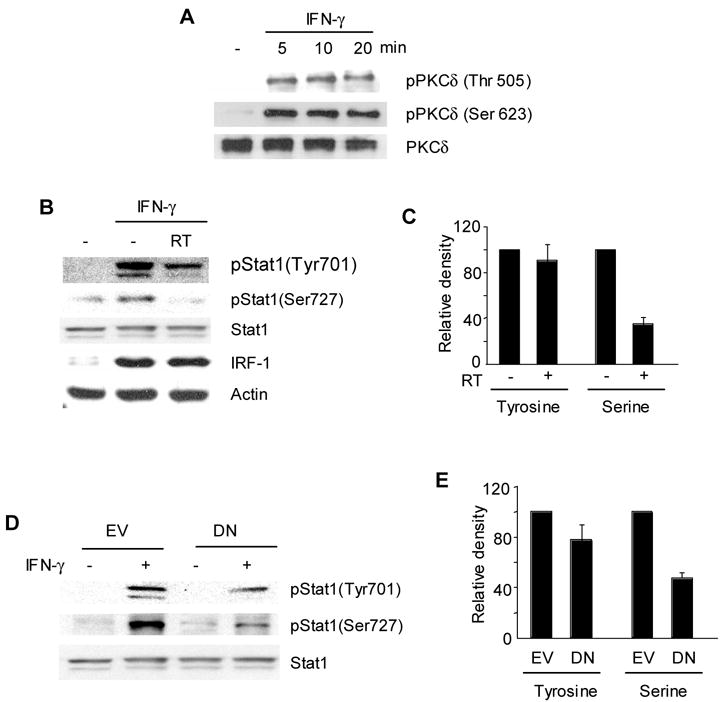
PKCδ is responsible for Stat1 serine phosphorylation upon IFN-γ stimulation. (A) IFN-γ phosphorylates PKCδ in RAW264.7 cells. RAW264.7 cells were treated with IFN-γ as indicated. Immunoprecipitation was performed with an anti-PKCδ antibody and then subjected to immunoblotting to detect phosphorylation of PKCδ with an antibody recognizing phospho-PKCδ (Thr 505) or phospho-PKCδ (Ser 623). The same membrane was stripped and used to detect total PKCδ. (B) RAW264.7 cells were treated with Rottlerin for 1hr prior to the treatment with IFN-γ for 30 min. Immunoblot was performed using the antibody as indicated. To assess phosphorylation of Stat1, 15 μg of total cell lysates were loaded on an 8 % gel. The same membrane was used to detect serine phosphorylated Stat1 [pStat1(Ser727)], tyrosine phosphorylated Stat1 [Stat1(Tyr701)], and followed by total Stat1. IRF-1 protein expression was assessed with an anti-IRF-1 antibody and the amount of actin is shown as a loading control. (C) Relative intensity of pStat1. (D) DN PKCδ prevents serine phosphorylation of Stat1. (E) Relative intensity of pStat1. RAW264.7 cells expressing the empty vector (EV) or DN PKCδ (DN) were treated with or without IFN-γ for 30 min. Immunoblot and densitometric analysis was performed the same way as in (B) and (C). Relative intensity of pStat1 was determined by a densitometric analysis. The intensity of pStat1 in cells treated IFN-γ alone was set at 100%. Data are means and standard errors of three independent experiments.
Next, we investigated whether PKCδ regulates Stat1 phosphorylation. RAW264.7 cells were treated with IFN-γ or IFN-γ together with Rottlerin followed by immunoblot to assess the amount of pStat1(Tyr701) and pStat1(Ser727). IFN-γ treatment induced tyrosine phosphorylation of both p91 and p84 forms of Stat1 evidenced by two bands with different mobility (Fig. 4B, top panel). There was a minimal change in Stat1 tyrosine phosphorylation by PKCδ inhibition, whereas the induction of serine phosphorylation was substantially decreased in cells treated with Rottlerin (Fig. 4B and 4C). Again, IRF-1 protein expression was induced by IFN-γ and the level was not affected by PKCδ inhibitor (Fig. 4B, fourth panel). When we compared Stat1 phosphorylation in RAW264.7 cells expressing DN PKCδ, a similar pattern of inhibition was observed (Fig. 4D and 4E). Thus, PKCδ participates in Stat1 phosphorylation at serine 727 in macrophages upon IFN-γ stimulation.
3.5. Differential susceptibility of Stat1 phosphorylation for CIITA and IRF-1 gene expression
We showed different susceptibilities between CIITA and IRF-1 expression to PKCδ inhibition. In addition, expression of IRF-1 in macrophages after IFN-γ treatment is known to be independent of PKCα (Giroux et al., 2003). Therefore, we considered the possibility that pStat1(Ser727) is required for CIITA but not IRF-1 expression. To test this possibility, we utilized two Stat1 mutants: Y701F and S727A that cannot be phosphorylated at tyrosine 701 and serine 727, respectively (Zhang et al., 2005). We transfected the pIV-luciferase reporter with the Stat1 mutant to Stat1 null U3A cells (McKendry et al., 1991). We found that co-transfection of wild type Stat1 but not mutants activated the CIITA promoter in the presence of IFN-γ (Fig. 5A). In contrast, IRF-1 promoter activity was enhanced by the wild type as well as Stat1(S727A) in response to IFN-γ (Fig. 5B). Therefore, CIITA expression requires Stat1 that is phosphorylated at both tyrosine and serine residues, whereas the IRF-1 promoter can be activated in the absence of serine phosphorylated Stat1.
Figure 5.
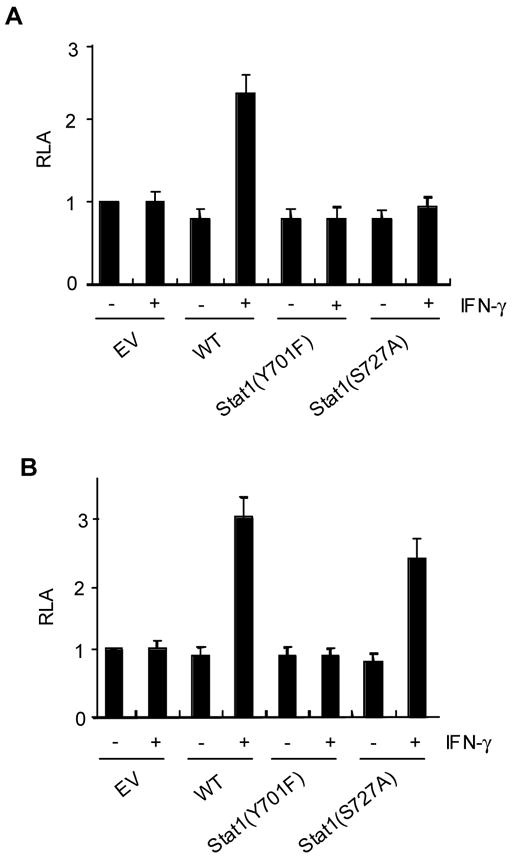
CIITA expression requires Stat1 that is phosphorylated at both tyrosine and serine residues. U3A cells were transiently transfected with the luciferase reporter driven by the CIITA (A) or IRF-1 promoter (B) together with wild type Stat1 (WT), Stat1(Y701F), and Stat1(S727A). EV indicates the empty vector. Two days after the transfection, human IFN-γ was added and cultured for additional 12hrs. Transfections were performed in duplicate and data are means ± SE of at least three independent experiments.
4. Discussion
Several studies have shown that PKC regulates IFN-γ-inducible MHC class II expression in macrophages and astrocytes but underlying mechanisms for PKC action was not well understood (Giroux et al., 2003; Benveniste et al., 1991; Lee et al., 1995). IFN-γ inducible expression of MHC class II depends on CIITA and therefore the effect of PKC is most likely on CIITA. Indeed, in the current study, we have shown that PKCδ is an important signaling molecule for IFN-γ inducible expression of CIITA determined by treating cells with PKCδ inhibitor or expressing DN PKCδ. We also have shown previously that PKCδ but not PKCα, is essential for constitutive expression of CIITA and MHC class II in B cells (Kwon et al., 2006). Therefore, among different isoforms of PKC, PKCδ appears to be the major form regulating CIITA gene expression.
Although PKCδ regulates both constitutive and IFN-γ inducible expression of the CIITA gene, the underlying mechanism is distinct. In B cells, PKCδ is important for CREB phosphorylation (Kwon et al., 2006), whereas PKCδ inhibition had little effect on CREB phosphorylation in macrophages (data not shown). Moreover, PKC activation by PMA in the absence of IFN-γ signaling was not sufficient to activate CIITA expression in macrophages (data not shown). Therefore, the role of PKCδ seems to augment IFN-γ signaling to further enhance CIITA expression. In contrast, B cells express more CIITA upon PMA treatment, presumably due to enhanced CREB phosphorylation (Kwon et al., 2006). It is not surprising that the molecular mechanisms governing CIITA expression by PKCδ is different between B cells and macrophages since the promoter responsible for constitutive (pIII) or IFN-γ inducible (pIV) expression has different cis-acting elements and require different transcription factors (Muhlethaler-Mottet et al., 1997; 1998). Indeed, CREB and Stat1 are necessary for constitutive and IFN-γ inducible expression of the CIITA gene, respectively. Accordingly, the effect of PKCδ signaling is exerted through different transcription factors in B cells and macrophages.
The activation of the IFN-γ inducible CIITA promoter requires Stat1 and IRF-1 (Muhlethaler-Mottet et al., 1997; 1998; Piskurich et al., 1999; Morris et al., 2002). Based on our data and published studies, IFN-γ stimulation activates PKCδ, which in turn leads phosphorylation of Stat1 at serine 727. Stat1 phosphorylated at both tyrosine and serine residues following JAK and PKCδ signaling can efficiently interact with CBP/p300, resulting in the induction of overall histone acetylation of the CIITA promoter. In agreement with the studies that pStat1(Ser727) interacts with HATs (Sun et al., 2005; Zhang et al., 2005), serine phosphorylated Stat1 was responsible for bringing CBP/p300 to the CIITA promoter evidenced by the diminished binding of CBP/p300 when serine phosphorylation of Stat1 was reduced by inhibiting PKCδ (Fig. 3 and 4). pStat1(Ser727) is also shown to recruit MCM5 in IFN-γ induced transcriptional activation (Snyder et al., 2005). MCM5 is a DNA replication factor and the interaction of pStat1(Ser727) with MCM5 is essential for Stat1-mediated gene activation (Zhang et al., 1998). Interestingly, when MCM5 expression was reduced by siRNA, IFN-γ inducible expression of CIITA was impaired, indicating a role of MCM5 in CIITA expression (Snyder et al., 2005). It is tempting to speculate that decreased serine phosphorylation of Stat1 by PKCδ inhibition also results in an inefficient recruitment of MCM5 to the CIITA promoter. Therefore, pStat1(Ser727) is essential for the maximal expression of the CIITA gene, which is controlled by PKCδ.
The differential effect of PKCδ on CIITA and IRF-1 expression is at least partly contributed by the differential requirement of Stat1 phosphorylation. Our data showed that the CIITA expression requires Stat1 phosphorylated at both tyrosine and serine residues, whereas IRF-1 expression can be achieved in the absence of serine phosphorylated Stat1 (Fig. 5). In line of our observations, the inhibition of PKCα did not affect IRF-1 expression in response to IFN-γ in macrophages (Giroux et al., 2003). Moreover, treating cells with either an activator or an inhibitor of PKC did not affect IFN-γ inducible expression of IRF-1 in human epidermal keratinocytes (Nakanishi et al., 1997). These studies support the notion that pStat1(ser727) is less critical for IRF-1 expression by stimulation of IFN-γ. However, other studies have reported that IRF-1 expression requires pSTAT1(ser727) (Kovarik et al., 2001; Ramsauer et al., 2002; Varinou et al., 2003). Although the sources of discrepancy between two types of results are not clear at the moment, in all studies, IRF-1 expression was induced by IFN-γ in the absence of serine phosphorylation of Stat1, albeit at a low level. Notably, Varinou et al. showed that the expression of several IFN-γ inducible genes including IRF-1 was down-regulated in macrophages that were prepared from the mice expressing only a serine 727-alanine mutant of Stat1 (Stat1S727A) (Varinou et al., 2003). However, the IRF-1 levels in mutant mice after IFN-γ stimulation were still higher than the basal levels, implying that tyrosine phosphorylated Stat1 can activate IRF-1 expression. Together, in the absence of Stat1 serine 727 phosphorylation, IRF-1 expression can be induced and, more importantly, IRF-1 binding to the CIITA promoter is not compromised in macrophages.
In sum, our current study demonstrates that the maximal induction of CIITA expression by IFN-γ signaling requires PKCδ. Activated PKCδ by IFN-γ stimulation results in phosphorylation of serine 727 of Stat1, and enhances the recruitment of HATs to the CIITA promoter. Together with the role of PKCδ in the maintenance of CIITA expression in B cells, PKCδ is an essential signaling molecule regulating CIITA and MHC class II expression.
Acknowledgments
We thank Drs. Richard Pine, Jae-Won Soh, Wei Li, and Mark Kaplan for providing reagents, helpful discussions and critical reading the manuscript.
Abbreviation used in this paper
- DC
dendritic cell
- IRF-1
interferon regulatory factor-1
- PKC
protein kinase C
- HAT
histone acetyltransferases
- DN
dominant negative
- ChIP
chromatin immunoprecipitation
- qRT-PCR
quantitative real-time PCR
- HPRT
hypoxantine guanine phosphoribosyl transferase
Footnotes
This work is supported in part by National Institute of Health grant AI53556 (to C-H. C.).
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Benveniste EN, Vidovic M, Panek RB, Norris JG, Reddy AT, Benos DJ. Interferon-gamma-induced astrocyte class II major histocompatibility complex gene expression is associated with both protein kinase C activation and Na+ entry. J Biol Chem. 1991;266:18119–18126. [PubMed] [Google Scholar]
- Chang CH, Guerder S, Hong SC, van Ewijk W, Flavell RA. Mice lacking the MHC class II transactivator (CIITA) show tissue-specific impairment of MHC class II expression. Immunity. 1996;4:167–178. doi: 10.1016/s1074-7613(00)80681-0. [DOI] [PubMed] [Google Scholar]
- Darnell JE., Jr STATs and gene regulation. Science. 1997;277:1630–1635. doi: 10.1126/science.277.5332.1630. [DOI] [PubMed] [Google Scholar]
- De Lerma Barbaro A, Procopio FA, Mortara L, Tosi G, Accolla RS. The MHC class II transactivator (CIITA) mRNA stability is critical for the HLA class II gene expression in myelomonocytic cells. Eur J Immunol. 2005;35:603–611. doi: 10.1002/eji.200425378. [DOI] [PubMed] [Google Scholar]
- Deb DK, Sassano A, Lekmine F, Majchrzak B, Verma A, Kambhampati S, Uddin S, Rahman A, Fish EN, Platanias LC. Activation of protein kinase C delta by IFN-gamma. J Immunol. 2003;171:267–273. doi: 10.4049/jimmunol.171.1.267. [DOI] [PubMed] [Google Scholar]
- Giroux M, Schmidt M, Descoteaux A. IFN-gamma-induced MHC class II expression: transactivation of class II transactivator promoter IV by IFN regulatory factor-1 is regulated by protein kinase C-alpha. J Immunol. 2003;171:4187–4194. doi: 10.4049/jimmunol.171.8.4187. [DOI] [PubMed] [Google Scholar]
- Gourley TS, Patel DR, Nickerson K, Hong SC, Chang CH. Aberrant expression of Fas ligand in mice deficient for the MHC class II transactivator. J Immunol. 2002;168:4414–4419. doi: 10.4049/jimmunol.168.9.4414. [DOI] [PubMed] [Google Scholar]
- Korzus E, Torchia J, Rose DW, Xu L, Kurokawa R, McInerey EM, Mullen M, Glass CK, Rosenfeld MG. Transcription factor-specific requirements for coactivators and their acetyltransferase functions. Science. 1998;279:703–707. doi: 10.1126/science.279.5351.703. [DOI] [PubMed] [Google Scholar]
- Kovarik P, Mangold M, Ramsauer K, Heidari H, Steinborn R, Zotter A, Levy DE, Muller M, Decker T. Specificity of Signaling by Stat1 depends on SH2 and C-terminal domains that regulates Ser 727 phosphorylation, differentially affecting specific target gene expression. EMBO J. 2001;20:91–100. doi: 10.1093/emboj/20.1.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwon MJ, Soh JW, Chang CH. Protein Kinase C delta is essential to maintain CIITA gene expression in B cells. J Immunol. 2006;177:950–956. doi: 10.4049/jimmunol.177.2.950. [DOI] [PubMed] [Google Scholar]
- Lee YJ, Panek RB, Huston M, Benveniste EN. Role of protein kinase C and tyrosine kinase activity in IFN-gamma-induced expression of the class II MHC gene. Am J Physiol. 1995;68:C127–137. doi: 10.1152/ajpcell.1995.268.1.C127. [DOI] [PubMed] [Google Scholar]
- LeibundGut-Landmann S, Waldburger JM, Reis e Sousa C, Acha-Orbea H, Reith W. MHC class II expression is differentially regulated in plasmacytoid and conventional dendritic cells. Nat Immunol. 2004;5:899–908. doi: 10.1038/ni1109. [DOI] [PubMed] [Google Scholar]
- LeibundGut-Landmann S, Waldburger JM, Krawczyk M, Otten LA, Suter T, Fontana A, Acha-Orbea H, Reith W. Mini-review: Specificity and expression of CIITA the master regulator of MHC class II genes. Eur J Immunol. 2004;34:1513–1525. doi: 10.1002/eji.200424964. [DOI] [PubMed] [Google Scholar]
- Linhoff MW, Harton JA, Cressman DE, Martin BK, Ting JP. Two distinct domains within CIITA mediate self-association: involvement of the GTP-binding and leucine-rich repeat domains. Mol Cell Biol. 2001;21:3001–3011. doi: 10.1128/MCB.21.9.3001-3011.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masternak K, Muhlethaler-Mottet A, Villard J, Zufferey M, Steimle V, Reith W. CIITA is a transcriptional coactivator that is recruited to MHC class II promoters by multiple synergistic interactions with an enhanceosome complex. Genes Dev. 2000;14:1156–1166. [PMC free article] [PubMed] [Google Scholar]
- McKendry R, John J, Flavell D, Muller M, Kerr IM, Stark GR. High-frequency mutagenesis of human cells and characterization of a mutant unresponsive to both alpha and gamma interferons. Proc Natl Acad Sci U S A. 1991;88:11455–11459. doi: 10.1073/pnas.88.24.11455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris AC, Beresford GW, Mooney MR, Boss JM. Kinetics of a gamma interferon response: expression and assembly of CIITA promoter IV and inhibition by methylation. Mol Cell Biol. 2002;22:4781–4791. doi: 10.1128/MCB.22.13.4781-4791.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mowen K, David M. Regulation of STAT1 nuclear export by Jak1. Mol Cell Biol. 2000;20:7273–7281. doi: 10.1128/mcb.20.19.7273-7281.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muhlethaler-Mottet A, Otten LA, Steimle V, Mach B. Expression of MHC class II molecules in different cellular and functional compartments is controlled by differential usage of multiple promoters of the transactivator CIITA. EMBO J. 1997;16:2851–2860. doi: 10.1093/emboj/16.10.2851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muhlethaler-Mottet A, Di Berardino W, Otten LA, Mach B. Activation of the MHC class II transactivator CIITA by interferon-gamma requires cooperative interaction between Stat1 and USF-1. Immunity. 1998;8:157–166. doi: 10.1016/s1074-7613(00)80468-9. [DOI] [PubMed] [Google Scholar]
- Nakanishi G, Fujimoto w, Arata J. IRF-1 expression in normal epidermal keratinocytes. Arch Dermatol Res. 1997;289:415–420. doi: 10.1007/s004030050214. [DOI] [PubMed] [Google Scholar]
- O’Keefe GM, Nguyen VT, Ping Tang LL, Benveniste EN. IFN-gamma regulation of class II transactivator promoter IV in macrophages and microglia: involvement of the suppressors of cytokine signaling-1 protein. J Immunol. 2001;166:2260–2269. doi: 10.4049/jimmunol.166.4.2260. [DOI] [PubMed] [Google Scholar]
- Pine R. Convergence of TNFalpha and IFNgamma signalling pathways through synergistic induction of IRF-1/ISGF-2 is mediated by a composite GAS/kappaB promoter element. Nucleic Acids Res. 1997;25:4346–4354. doi: 10.1093/nar/25.21.4346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piskurich JF, Linhoff MW, Wang Y, Ting JP. Two distinct gamma interferon-inducible promoters of the major histocompatibility complex class II transactivator gene are differentially regulated by STAT1, interferon regulatory factor 1, and transforming growth factor beta. Mol Cell Biol. 1999;19:431–440. doi: 10.1128/mcb.19.1.431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramsauer K, Sadzak I, Porras A, Pilz A, Nebreda AR, Decker T, Kovarik P. p38 MAPK enhances STAT1-dependent transcription independently of Ser-727 phosphorylation. Proc Natl Acad Sci U S A. 2002;99:12859–12864. doi: 10.1073/pnas.192264999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reith W, LeibundGut-Landmann S, Waldburger JM. Regulation of MHC class II gene expression by the class II transactivator. Nat Rev Immunol. 2005;5:793–806. doi: 10.1038/nri1708. [DOI] [PubMed] [Google Scholar]
- Setterblad N, Onyango I, Pihlgren U, Rask L, Andersson G. The role of protein kinase C signaling in activated DRA transcription. J Immunol. 1998;161:4819–4824. [PubMed] [Google Scholar]
- Shuai K, Horvath CM, Huang LH, Qureshi SA, Cowburn D, Darnell JE., Jr Interferon activation of the transcription factor Stat91 involves dimerization through SH2-phosphotyrosyl peptide interactions. Cell. 1994;76:821–828. doi: 10.1016/0092-8674(94)90357-3. [DOI] [PubMed] [Google Scholar]
- Sisk TJ, Nickerson K, Kwok RP, Chang CH. Phosphorylation of class II transactivator regulates its interaction ability and transactivation function. Int Immunol. 2003;15:1195–1205. doi: 10.1093/intimm/dxg116. [DOI] [PubMed] [Google Scholar]
- Sisk TJ, Roys S, Chang CH. Self-association of CIITA and its transactivation potential. Mol Cell Biol. 2001;21:4919–4928. doi: 10.1128/MCB.21.15.4919-4928.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith MR, Ramsburg EA, Kung HF, Durum SK. Components of the protein kinase C pathway induce Ia expression after injection into macrophages. J Immunol. 1992;149:1304–1310. [PubMed] [Google Scholar]
- Snyder M, He W, Zhang JJ. The DNA replication factor MCM5 is essential for Stat1-mediated transcriptional activation. Proc Natl Acad Sci U S A. 2005;102:14539–14544. doi: 10.1073/pnas.0507479102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soh JW, I, Weinstein B. Roles of specific isoforms of protein kinase C in the transcriptional control of cyclin D1 and related genes. J Biol Chem. 2003;278:34709–34716. doi: 10.1074/jbc.M302016200. [DOI] [PubMed] [Google Scholar]
- Steimle V, Otten LA, Zufferey M, Mach B. Complementation cloning of an MHC class II transactivator mutated in hereditary MHC class II deficiency (or bare lymphocyte syndrome) Cell. 1993;75:135–146. [PubMed] [Google Scholar]
- Steinman RM, Inaba K, Turley S, Pierre P, Mellman I. Antigen capture, processing, and presentation by dendritic cells: recent cell biological studies. Hum Immunol. 1999;60:562–567. doi: 10.1016/s0198-8859(99)00030-0. [DOI] [PubMed] [Google Scholar]
- Sun W, Xu W, Snyder M, He W, Ho H, Ivashkiv LB, Zhang JJ. The conserved Leu-724 residue is required for both serine phosphorylation and co-activator recruitment for Stat1-mediated transcription activation in response to interferon-gamma. J Biol Chem. 2005;280:41844–41851. doi: 10.1074/jbc.M505797200. [DOI] [PubMed] [Google Scholar]
- Uddin S, Sassano A, Deb DK, Verma A, Majchrzak B, Rahman A, Malik AB, Fish EN, Platanias LC. Protein kinase C-delta (PKC-delta) is activated by type I interferons and mediates phosphorylation of Stat1 on serine 727. J Biol Chem. 2002;277:14408–14416. doi: 10.1074/jbc.M109671200. [DOI] [PubMed] [Google Scholar]
- Utley RT, Ikeda K, Grant PA, Cote J, Steger DJ, Eberharter A, John S, Workman JL. Transcriptional activators direct hisone acetyltransferase complexes to nucleosomes. Nature. 1998;394:498–502. doi: 10.1038/28886. [DOI] [PubMed] [Google Scholar]
- Varinou L, Ramsauer K, Karaghiosoff M, Kolbe T, Pfeffer K, Muller M, Decker T. Phosphorylation of the Stat1 transactivation domain is required for full-fledged IFN-gamma-dependent innate immunity. Immunity. 2003;19:793–802. doi: 10.1016/s1074-7613(03)00322-4. [DOI] [PubMed] [Google Scholar]
- Vinkemeier U, Cohen SL, Moarefi I, Chait BT, Kuriyan J, Darnell JE., Jr DNA binding of in vitro activated Stat1 alpha, Stat1 beta and truncated Stat1: interaction between NH2-terminal domains stabilizes binding of two dimers to tandem DNA sites. EMBO J. 1996;15:5616–5626. [PMC free article] [PubMed] [Google Scholar]
- Wen Z, Zhong Z, Darnell JE., Jr Maximal activation of transcription by Stat1 and Stat3 requires both tyrosine and serine phosphorylation. Cell. 1995;82:241–250. doi: 10.1016/0092-8674(95)90311-9. [DOI] [PubMed] [Google Scholar]
- Yao Y, Xu Q, Kwon MJ, Matta R, Liu Y, Hong SC, Chang CH. ERK and P38 MAPK signaling pathways negatively regulate CIITA gene expression in dendritic cells and macrophages. J Immunol. 2006;177:70–76. doi: 10.4049/jimmunol.177.1.70. [DOI] [PubMed] [Google Scholar]
- Yao Y, Li W, Kaplan MH, Chang CH. Interleukin (IL)-4 inhibits IL-10 to promote IL-12 production by dendritic cells. J Exp Med. 2005;201:1899–1903. doi: 10.1084/jem.20050324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang JJ, Zhao Y, Chait BT, Lathem WW, Ritzi M, Knippers R, Darnell JE., Jr Ser727-dependent recruitment of MCM5 by Stat1alpha in IFN-gamma-induced transcriptional activation. EMBO J. 1998;17:6963–6971. doi: 10.1093/emboj/17.23.6963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Takami K, Lo MS, Huang G, Yu Q, Roswit WT, Holtzman MJ. Modification of the Stat1 SH2 domain broadly improves interferon efficacy in proportion to p300/CREB-binding protein coactivator recruitment. J Biol Chem. 2005;280:34306–34315. doi: 10.1074/jbc.M503263200. [DOI] [PubMed] [Google Scholar]


