Abstract
We have recently shown in rats that cocaine-induced behavioral sensitization can be reversed by a 5-day treatment with ondansetron given 3.5 h after daily pergolide injections. In this study we further investigated the molecular/neurochemical alterations underlying cocaine sensitization and pergolide/ondansetron-mediated reversal. Results revealed that GAD65 and GAD67 are higher abundant in the NAc than that in the caudate and mPFC, while GABAA receptor α2 subunit level in the NAc shell is less abundant than that in the NAc core, mPFC and caudate. Cocaine sensitization leaded to 1) decrease in GAD67 expression, increase in total PKC ζ and phosphorylated PKC ζ/λ levels in the NAc core; 2) decrease in GAD67 and GABAA receptor α2 subunit expression, and increase in phosphorylated PKC ζ/λ levels in the NAc shell; 3) increase in GAD67 expression in the caudate. Importantly, pergolide/ondansetron treatment reversed these alterations. These results suggest that reversal of cocaine-induced behavioral sensitization is associated with reversal of region-specific changes in GABA function and PKC activity in the striatum.
Keywords: GAD65, GAD67, GABA, PKC, cocaine, pergolide, ondansetron, nucleus accumbens, caudate, behavioral sensitization
Introduction
In rodents, behavioral sensitization, as established by repeated administration of cocaine, has long been considered the reflection of intensification of cocaine craving in humans that characterizes addiction and promotes relapse [1, 2]. We have recently demonstrated in rats that sensitization established previously by 5 daily cocaine injections and a subsequent 9-day withdrawal period can be reversed by daily pergolide administration if each injection is followed 3.5 h later with the 5-HT3 antagonist ondansetron [3, 4]. Furthermore, similar treatment with ondansetron can decrease the break-point under progressive-ratio self-administration [5] and reduce cocaine intake under oral self-administration [6]. However, the molecular mechanisms of cocaine-induced behavioral sensitization and pergolide/ondansetron-induced behavioral reversal have not been elucidated.
Our previous studies have demonstrated that cocaine induces changes in expression and phosphorylated levels of the NR2B subunit of the NMDA receptors and the phosphorylated levels of GluR1 subunit of the AMPA receptors in the nucleus accumbens (NAc), and pergolide-ondansetron treatment normalizes these changes [3]. However, the mechanisms how a 5-HT3 receptor antagonist influences NAc dopamine and glutamate transmission are unclear. Previous studies revealed that 5-HT3 receptors in the NAc mediate the modulatory action of 5-HT3 antagonists on mesolimbic DA-mediated behaviors (e.g. locomotor activity) or accumbal DA outflow [7]. Microstructural research demonstrated that serotonergic cells typically contact GABAergic cells in the NAc, indicating that 5-HT3 receptors indirectly modulate DA via GABAergic contacts with DA cells bodies [8, 9]. Hence, the 5-HT3 receptor antagonist may also serve to influence synaptic plasticity of NMDA and AMPA receptors within NAc by normalizing GABA function, such as glutamic acid decarboxylase (GAD) expression and GABAA receptor responses.
GAD is present as two isoenzymes, GAD65 and GAD67, which are the products of two independently regulated genes [10]. In mammalian brain GAD catalyzes synthesis of the inhibitory neurotransmitter gamma-amino butyric acid (GABA) [11]. Notably, results from GAD65 gene knockout mice have revealed that deletion of GAD67 is lethal and brain GABA levels are reduced in adult heterozygous GAD67 mice, whereas brain GABA levels are normal in GAD65 knockout mice --suggesting that GAD67 is the primary rate-limiting enzyme regulating GABA levels under normal conditions [12, 13]. Thus, regulation of GAD67 expression may exert a more profound effect on GABA homeostasis and, possibly, be more response to exogenous agents.
The strength of inhibitory synaptic currents is directly correlated with the number of synaptic GABAA receptor [14]. We recently demonstrated that chronic high dose METH regulates protein levels of GABAA receptor α2 subunit in the NAc and caudate, which was proposed to involve with METH sensitization and neurotoxicity [15]. Besides GABAA receptor expression, the changes in membrane GABAA receptor play main role in inhibitory synaptic currents. Recently, activation of PKC ζ has been demonstrated to regulate GABAA receptor phosphorylation and internalization [16]. The activity of atypical ζ isoform of PKC is under the control of phosphorylation at threonine 410 residue [17]. It is therefore important to determine the involvement of GAD65/67, GABAA receptor α2 subunit, PKC ζ, and the phosphorylation of PKC ζ/λ in cocaine sensitization and pergolide/ondansetron-mediated reversal.
Materials and methods
Animals and drugs
Male Sprague-Dawley rats (Charles River Laboratories, Raleigh, NC), initially weighing 150–200 g, were housed in pairs and experiments were conducted with an approved protocol by the Duke University Institutional Animal Care and Use Committee.
Cocaine HCl (NIDA, Bethesda, MD) was dissolved in 0.9% saline to a final concentration of 20 mg/ml. The ondansetron hydrochloride dihydrate solution (Duke Medical Center Pharmacy) was diluted with 0.9% saline to 0.2 mg/ml. Pergolide (Sigma, St. Louis, MO) was dissolved in 10% DMSO to 1 mg/ml, and further diluted 1:10 in saline before use.
Experimental groups
The animals were treated as previously described [3, 4]. Briefly, animals were ranked according to their responses to cocaine (7.5 mg/kg, s.c.) on day 1 and then divided into three groups. All three groups received 40 mg/kg/day cocaine (s.c.) for 4 consecutive days. Another three random groups received saline injections. All rats were then subjected 9 days of withdrawal. Starting on day 10 of withdrawal, one cocaine and one saline group were each given 0.1 mg/kg pergolide, followed 3.5 h later by saline injection for 5 consecutive days, termed C-P/S and S-P/S, respectively; one cocaine and one saline group were each given pergolide, followed 3.5 h later by 0.2 mg/kg ondansetron (s.c.) for 5 consecutive days, termed C-P/O and S-P/O, respectively; other cocaine (C-D/S) and saline groups (S-D/S) received parallel vehicle (1 % DMSO - 0.9 % saline, 1 ml/kg each, s.c.) injections. Following 9 days of a second withdrawal, all rats were challenged with 7.5 mg/kg cocaine (i.p.) on day 10. As either pergolide or ondansetron alone does not exert consistent effects on established cocaine sensitization and/or associated molecular markers (3, 5, 18), the data from these treatment groups were not included in the present study.
Behavioral assessment
The locomotor assessment and behavioral rating score of behavioral sensitization were performed as described [3, 4]. Behavioral sensitization established by 5 consecutive days of cocaine injections followed by a 9-day withdrawal period was reversed by pergolide/ondansetron treatment. These data are consistent with our previous studies [3, 4] and were not provided in the present study.
Brain dissection and protein measurement
Rats were euthanized 24 h after cocaine challenge. The NAc core, NAc shell and caudate were dissected separately and immediately frozen on dry ice. Samples were stored at − 80°C until protein extraction. Tissue samples were homogenized and prepared for Western blot as described [3, 4, 15]. Protein concentration was determined by using DC protein assay kit (Bio-Rad, Hercules, CA).
Western Blots
Western blot was performed as described [3, 4, 15]. The anti-GAD65 and anti-GAD67 antibodies (1:250 dilution) were purchased from BD Bioscience (San Jose, CA); the anti-PKC ζ antibody (1:200) was purchased from Santa Cruz Biotechnology (Santa Cruz, CA); the anti-PKC ζ/λ Thr410/403 antibody, which detects endogenous phosphorylated PKC ζ at threonine 410, and endogenous phosphorylated PKC λ at threonine 403 (1:1,000), and peroxidase-labeled secondary antibody (1:2,000) were purchased from Cell Signaling Technology (Danvers, MA). The blots were developed with chemiluminescent substrate (Santa Cruz Biotechnology). To control for loading efficiency, the blots for GAD65, GAD67, PKC ζ, and phospho-PKC ζ/λ (Thr410/403) were stripped and re-probed with α-tubulin antibody (1:1,000; Sigma). Expression of GAD65, GAD67, PKC ζ, and levels of phospho-PKC ζ/λ were evaluated relative to that for α-tubulin.
Data analyses
The data are presented as means and standard errors of the mean and were analyzed using the Statistical Package for the Social Sciences, Version 14.0 (Chicago, IL). The results were analyzed using one-way ANOVA; post-hoc analyses were performed by Bonferroni comparisons. A p < 0.05 was considered statistically significant.
Results
Cocaine sensitization induces alterations in GAD67 expression in the NAc and caudate
GAD67 levels were significantly decreased in the NAc core and NAc shell in cocaine-sensitized (C-D/S) rats compared to that in the saline injected (S-D/S) rats. In contrast, GAD67 levels were significantly increased in the caudate of these same animals. Bonferroni tests demonstrated that in the NAc core the levels of GAD67 were decreased in the C-D/S group relative to those in the S-D/S group (Fig. 1A, p < 0.030). In the NAc shell, the levels of GAD67 are significantly lower in the C-D/S group than all other groups (Fig. 1B, ps < 0.047). Conversely, in the caudate, the levels of GAD67 are significantly higher in the C-D/S group than all other groups (Fig. 1C, ps < 0.013). The changes in GAD67 expression in the NAc shell and caudate were reversed by the pergolide/ondansetron treatment. However, no significant group differences in the GAD65 protein levels were observed in the NAc core, NAc shell and caudate (data not shown).
Figure 1. Expression of enzyme GAD67 in the NAc and caudate.
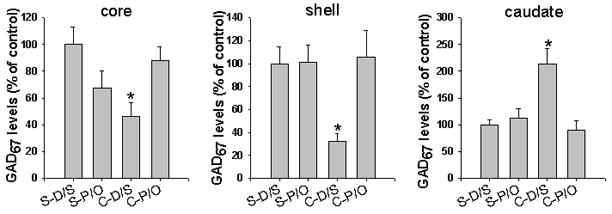
Blots were scanned and the densities under the peaks corresponding to total immunoreactivity of GAD67 and α-tubulin were determined. See Methods for descriptions of the experimental groups. *, p < 0.05, cocaine (C-D/S) vs saline group (S-D/S) in the core and vs all other groups in the shell and caudate; N= 6 rats/group.
Distribution of GAD65 and GAD67 in the brain
We compared the striatal GAD65 and GAD67 expression with mPFC in the native rats. The GAD65 protein level in the caudate is similar to that in the mPFC; however, its level in the NAc core and NAc shell is 1.41- and 1.61-fold higher than that in the mPFC respectively (Fig. 2A & 2B). The GAD67 levels in the caudate, NAc core and NAc shell are 1.55-, 5.62-, and 7.96-fold higher than that in the mPFC respectively (Fig. 2A & 2C). These results demonstrated that striatum is plenty in GAD65 and GAD67 expression.
Figure 2. Distribution of GAD65 and GAD67 in the brain.
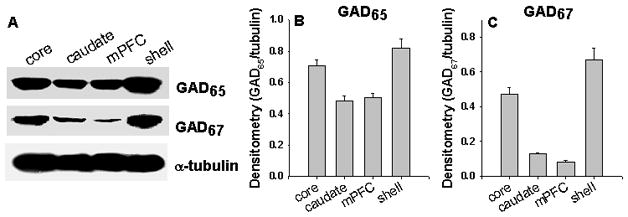
A, Representative Western blot of GAD65, GAD67 and α-tubulin expression. Each lane represents a different region (10 μg protein/lane). B & C, Blots were scanned, and the relative density of GAD65 and GAD67 immunoreactivity to that of α-tubulin was determined for each sample; N= 6 rats/group.
Cocaine sensitization decreases GABAA receptor α2 subunit expression in the NAc shell
The levels of GABAA receptor α2 subunit in the NAc core, caudate, and mPFC are equally higher (~2.48-fold) than that in the NAc shell (Fig. 3A & B). Bonferroni tests demonstrated that the levels of GABAA receptor α2 subunit in the NAc shell were significantly attenuated in the C-D/S group relative to those of all other groups (Fig. 3C, ps < 0.035). No changes in GABAA receptor α2 subunit levels were observed in the NAc core and caudate following establishment of cocaine sensitization (data not shown). These data demonstrate a brain-region specific alteration in GABAA receptor α2 subunit levels in the NAc shell, which is associated with cocaine sensitization.
Figure 3. Expression of GABAA receptor α2 subunit in the NAc shell.
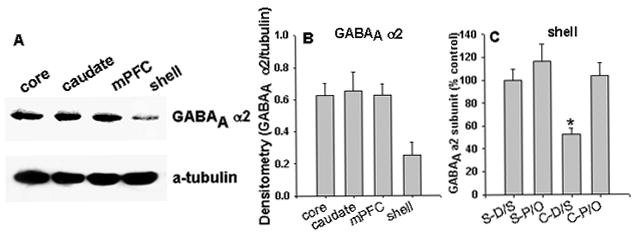
A, Representative Western blot of GABAA receptor α2 subunit and α-tubulin expression. Each lane represents a different region (10 μg protein/lane). B, Blots were scanned, and the relative density of GABAA receptor α2 subunit immunoreactivity to that of α-tubulin was determined for each sample. C, The GABAA receptor α2 subunit expression was quantified by Western blot assay. *, p < 0.05, C-D/S vs all other groups; N= 6 rats/group.
Cocaine sensitization increases PKC ζ expression and PKC ζ/λ phosphorylation in the NAc
In the NAc core, phospho-PKC ζ/λ levels were significantly increased in the sensitized (C-D/S) group compared to those of all other groups (Fig. 4A, ps < 0.05), as well as, PKC ζ expression was significantly increased in the C-D/S group compared to those of all other groups (Fig. 4B, ps < 0.005). However, the ratio of phospho-PKC ζ/λ to total PKC ζ has no changes (Fig. 4C). In the NAc shell, phospho-PKC ζ/λ levels were significantly increased in the C-D/S group compared to those of all other groups (Fig. 4D, ps < 0.05), whereas, the total PKC ζ has no changes (Fig. 4E), but the ratio of phospho-PKC ζ/λ to PKC ζ levels were significantly increased in the C-D/S group compared to those of all other groups (Fig. 4F, ps < 0.05). No significant treatment group differences in PKC ζ and phospho-PKC ζ/λ levels were detected among cocaine-sensitized and saline-control rats in the caudate (data not shown).
Figure 4. Expression of total PKC ζ and phospho-PKC ζ/λ (Thr410/403) levels in the NAc.
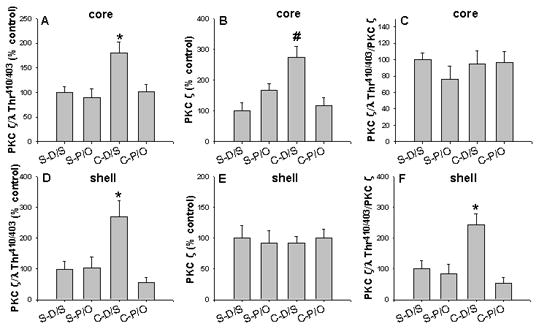
Blots were scanned and the densities of phospho-PKC ζ/λ (Thr410/403), total PKC ζ, and α-tubulin were determined. *, p < 0.05, cocaine (C-D/S) vs all other groups; #, p < 0.005, cocaine (C-D/S) vs all other groups; N= 6 rats/group.
Discussion
In rodents, repeated administration of cocaine results in progressive augmentation of locomotor and stereotypical behaviors [1, 2]. These behavioral changes are accompanied by molecular/cellular alterations even after long-term withdrawal [19]. Previous studies suggest that 5-HT3 receptors in the NAc mediate the modulatory action of 5-HT3 antagonists on mesolimbic DA-mediated locomotor activity [7]. Our studies also demonstrated that behavioral sensitization, established by repeated cocaine treatments (5 or 7 daily injections at 40 mg/kg/day, s.c.) and a subsequent 7- or 9-day withdrawal period, is reversed by daily cocaine plus ondansetron [5] or pergolide plus ondansetron injections [3, 4] if the 5-HT3 antagonist is given 3.5 h after each cocaine or pergolide injection. In the present study, pergolide/ondansetron also reverses cocaine sensitization associated changes in the expression of GAD67, GABAA receptor α2 subunit, and PKC ζ, and PKC ζ phosphorylation in the NAc. However, intra-NAc administration of 5-HT3 antagonists has no effects on morphine-induced accumbal DA release [20] and amphetamine-induced hyperactivity [21]. These contradictory results raise the possibility that the NAc 5-HT3 receptor control of accumbal DA release or mesolimbic DA-mediated locomotor activity requires the pre-activation of the dopaminergic neurons.
Cellular GABA is compartmentalized into a vesicular pool and a cytoplasmic pool [22]. The two GAD isoforms, GAD65 and GAD67 expressed in GABAergic neurons, revealed differential regulation on GABA vesicular and cytoplasmic pools [23]. Studies in GAD65 knockout mice demonstrated that GAD67 is important for controlling the cytoplasmic pool, as well as, to a large extend the vesicular pool of GABA. Conversely, GAD65 appears to regulate primarily the vesicular pool, especially under conditions of sustained synaptic activity [23]. Thus, any agents that influence GAD67 expression will affect overall GABA levels and, thereby regulate striatal GABA function. In the present study, chronic high dose cocaine treatment and withdrawal have no effect on GAD65 expression, but they attenuated the expression of GAD67 in the NAc core and shell, and increased GAD67 expression in the caudate. Pergolide/ondansetron treatment reversed cocaine behavioral sensitization accompanied with the reversal of GAD67 levels (Fig. 1). This implies that adaptation in GAD67 expression in the NAc and caudate are involved in the cocaine sensitization, as well as, modulation of 5-HT3 receptor function can affect striatal GABAergic transmission.
Besides the GABA levels, the strength of inhibitory synaptic currents is directly correlated with the number of synaptic GABAA receptors [14]. The majority of GABAA receptors contain a single type of γ2 subunit together with α- and β-subunit variants [15]. In the present study, chronic cocaine treatment decreased GABAA receptor α2 subunit expression in the NAc shell (Fig. 3). We thereby think that the composition of GABAA receptor is associated with cocaine sensitization. However, the membrane GABAA receptors directly mediate the inhibitory synaptic currents. Previous studies linked the PKC-regulated GABA receptor trafficking to the behavioral sensitization induced by amphetamine [24]. Activation of PKC can modulate GABAA receptors by inducing their internalization in various types of neurons and in cell lines expressing these receptors [25]. Our data revealed that cocaine sensitization increases PKC ζ activity in the NAc core and shell, but not in the caudate (Fig. 4). Thus, we speculate that activation of PKC ζ underlying chronic cocaine sensitization may increase the internalization of GABAA receptors in the NAc. 5-HT3 receptor antagonist, ondansetron given 3.5 h after pergolide injection reversed PKC ζ activation in the NAc. Altogether, the possible mechanisms might be associated with cocaine-induced release of 5-HT in the NAc that acts upon post-synaptic 5-HT3 receptors, which decreases GABA synthesis and downregulates GABAA receptor expression or increases PKC-mediated GABAA receptor internalization; thereby, mediating local stimulatory actions of 5-HT.
Most (90–95%) of the striatum is comprised of GABAergic medium sized spiny projection neurons, which predominantly send their axons to the ventral tegmental area and ventral pallidum, two regions are thought to be involved in the rewarding effects and reinforcing properties of most drugs of abuse [26]. Thus, disinhibitory NAc GABA function may mediate drug addiction through potentiation of dopamine and glutamate transmission in the NAc projection regions [15]. GABA neuron is therefore a mediator for that 5-HT3 receptor antagonist influences downstream dopamine and glutamate transmission. Upregulation of inhibitory GABA tone in the caudate has been proposed to be involved in the METH-induced neurotoxicity in this brain region through activation of the corticothalamostriatal pathway [15, 27]. Although neurotoxicity is not associated with our cocaine regimen in the present study (data not shown), chronic high dose cocaine leaded to an increase in GAD67 expression in the caudate. We thereby think that increased GABA release in the caudate can activate GABAA receptors in the substantial nigra, subsequently increase corticostriatal glutamate release via decreasing GABAergic nigrothalamic activity [15]. Thus, the cocaine sensitization-induced glutamate release in the caudate may result in synaptic plasticity, but not toxicity.
Local circuit neurons, which make up approximately 5–10% of all accumbal neurons, manufacture either acetylcholine or GABA [28, 29]. These interneurons provide some input to the projection neurons [28]. Especially, the glutamate terminals contain GABAB receptors and are regulated by local release of GABA [29]. Our previous studies demonstrated that chronic cocaine-induced sensitization leads to increase in phosphorylation of the GluR1 subunit of the AMPA receptors in the NAc core and shell, and increase in total NR2B subunit of NMDA receptors [3]. We thereby postulate that the cocaine-induced changes in GABA synthesis and PKC-mediated GABAA receptor trafficking in the NAc may disinhibite GABAergic function, subsequently regulate the activation of glutamate receptors locally.
In conclusion, modulation of 5-HT3 receptor function under activation of dopamine neurons can reverse cocaine sensitization through regulating dopamine and glutamate transmission in the striatum and striatal GABAergic neuron projection areas indirectly by disinhibiting NAc GABAergic function and upregulating GABA function in the caudate. Thus, establishment of inhibitory GABA tone in the NAc by over-expressing GAD67 or inhibition of it in the caudate by silencing GAD67 expression may be effective in reversing cocaine addiction.
Acknowledgments
Supported by grants from the National Institute of Drug Abuse (DA-10327 and DA-14323 to E.H.E., DA021185–01 to X.Z.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Wolf ME. The role of excitatory amino acids in behavioral sensitization to psychomotor stimulants. Prog Neurobiol. 1998;54:679–720. doi: 10.1016/s0301-0082(97)00090-7. [DOI] [PubMed] [Google Scholar]
- 2.Robinson TE, Berridge KC. The neural basis of drug craving: an incentive-sensitization theory of addiction. Brain Res Rev. 1993;18:247–291. doi: 10.1016/0165-0173(93)90013-p. [DOI] [PubMed] [Google Scholar]
- 3.Zhang X, Lee TH, Davidson C, Lazarus C, Wetsel WC, Ellinwood EH. Reversal of cocaine-induced behavioral sensitization and associated phosphorylation of NR2B and GluR1 subunits of NMDA and AMPA receptors. Neuropsychopharmacology. 2006 doi: 10.1038/sj.npp.1301101. Epub ahead. [DOI] [PubMed] [Google Scholar]
- 4.Zhang X, Mi J, Wetsel WC, Davidson C, Xiong X, Chen Q, Lee TH, Ellinwood EH. PI3 kinase is involved in cocaine behavioral sensitization and its reversal with brain area specificity. Biochem Biophys Res Commun. 2006;340:1144–1150. doi: 10.1016/j.bbrc.2005.12.114. [DOI] [PubMed] [Google Scholar]
- 5.Davidson C, Lee TH, Xiong Z, Ellinwood EH. Ondansetron given either in the acute or chronic withdrawal from repeated cocaine sensitization dosing regimens reverses the expression of sensitization and inhibits self-administration. Neuropsychopharmacology. 2002;27:542–553. doi: 10.1016/S0893-133X(02)00336-6. [DOI] [PubMed] [Google Scholar]
- 6.Davidson C, Lazarus C, Lee TH, Ellinwood EH. Ondansetron, given during the acute cocaine withdrawal, attenuates oral cocaine self-administration. Eur J Pharm. 2004;503:99–102. doi: 10.1016/j.ejphar.2004.09.041. [DOI] [PubMed] [Google Scholar]
- 7.De Deurwaerdere P, Moison D, Navailles S, Porras G, Spampinato U. Regionally and functionally distinct serotonin3 receptors control in vivo dopamine outflow in the rat nucleus accumbens. J Neurochem. 2005;94:140–149. doi: 10.1111/j.1471-4159.2005.03174.x. [DOI] [PubMed] [Google Scholar]
- 8.Tecott LH, Maricq AV, Julius D. Nervous system distribution of the serotonin 5-HT3 receptor mRNA. Proc Natl Acad Sci U S A. 1993;90:1430–1434. doi: 10.1073/pnas.90.4.1430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ricci LA, Stellar JR, Todtenkopf MS. Subregion-specific down-regulation of 5-HT3 immunoreactivity in the nucleus accumbens shell during the induction of cocaine sensitization. Pharmacol Biochem Behav. 2004;77:415–422. doi: 10.1016/j.pbb.2003.10.005. [DOI] [PubMed] [Google Scholar]
- 10.Lindefors N. Dopaminergic regulation of glutamic acid decarboxylase mRNA expression and GABA release in the striatum: a review. Prog Neuropsychopharmacol Biol Psychiatry. 1993;17:887–903. doi: 10.1016/0278-5846(93)90018-n. [DOI] [PubMed] [Google Scholar]
- 11.Soghomonian JJ, Martin DL. Two isoforms of glutamate decarboxylase: why? Trends Pharmacol Sci. 1998;19:500–505. doi: 10.1016/s0165-6147(98)01270-x. [DOI] [PubMed] [Google Scholar]
- 12.Asada H, Kawamura Y, Maruyama K, Kume H, Ding R, Ji FY, Kanbara N, Kuzume H, Sanbo M, Yagi T, Obata K. Mice lacking the 65 kDa isoform of glutamic acid decarboxylase (GAD65) maintain normal levels of GAD67 and GABA in their brains but are susceptible to seizures. Biochem Biophys Res Commun. 1996;229:891–895. doi: 10.1006/bbrc.1996.1898. [DOI] [PubMed] [Google Scholar]
- 13.Asad H, Kawamura Y, Maruyama K, Kume H, Ding RG, Kanbara N, Kuzume H, Sanbo M, Yagi T, Obata K. Cleft palate and decreased brain gamma-aminobutyric acid in mice lacking the 67-kDa isoform of glutamic acid decarboxylase. Proc Natl Acad Sci U S A. 1997;94:6496–6499. doi: 10.1073/pnas.94.12.6496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Luscher B, Keller CA. Regulation of GABAA receptor trafficking, channel activity, and functional plasticity of inhibitory synapses. Pharmacol Ther. 2004;102:195–221. doi: 10.1016/j.pharmthera.2004.04.003. [DOI] [PubMed] [Google Scholar]
- 15.Zhang X, Lee TH, Xiong X, Chen Q, Davidson C, Wetsel WC, Ellinwood EH. Methamphetamine induces long-term changes in GABA(A) receptor alpha2 subunit and GAD(67) expression. Biochem Biophys Res Commun. 2006;351:300–305. doi: 10.1016/j.bbrc.2006.10.046. [DOI] [PubMed] [Google Scholar]
- 16.Brandon N, Jovanovic J, Moss S. Multiple roles of protein kinases in the modulation of gamma-aminobutyric acid(A) receptor function and cell surface expression. Pharmacol Ther. 2002;94:113–122. doi: 10.1016/s0163-7258(02)00175-4. [DOI] [PubMed] [Google Scholar]
- 17.Liu WS, Heckman CA. The sevenfold way of PKC regulation. Cell Signal. 1998;10:529–542. doi: 10.1016/s0898-6568(98)00012-6. [DOI] [PubMed] [Google Scholar]
- 18.Malcolm R, Kajdasz DK, Herron J, Anton RF, Brady KT. A double-blind, placebo-controlled outpatient trial of pergolide for cocaine dependence. Drug Alcohol Depend. 2000;60:161–168. doi: 10.1016/s0376-8716(99)00151-9. [DOI] [PubMed] [Google Scholar]
- 19.Wolf ME. Addiction: making the connection between behavioral changes and neuronal plasticity in specific pathways. Mol Interv. 2002;2:146–157. doi: 10.1124/mi.2.3.146. [DOI] [PubMed] [Google Scholar]
- 20.Imperato A, Angelucci L. 5-HT3 receptors control dopamine release in the nucleus accumbens of freely moving rats. Neurosci Lett. 1989;101:214–217. doi: 10.1016/0304-3940(89)90533-8. [DOI] [PubMed] [Google Scholar]
- 21.Gillies DM, Mylecharane EJ, Jackson DM. Effects of 5-HT3 receptor-selective agents on locomotor activity in rats following injection into the nucleus accumbens and the ventral tegmental area. Eur J Pharmacol. 1996;303:1–12. doi: 10.1016/0014-2999(96)00028-3. [DOI] [PubMed] [Google Scholar]
- 22.Akbarian S, Huang HS. Molecular and cellular mechanisms of altered GAD1/GAD67 expression in schizophrenia and related disorders. Brain Res Brain Res Rev. 2006;52:293–304. doi: 10.1016/j.brainresrev.2006.04.001. [DOI] [PubMed] [Google Scholar]
- 23.Tian N, Petersen C, Kash S, Baekkeskov S, Copenhagen D, Nicoll R. The role of the synthetic enzyme GAD65 in the control of neuronal gamma-aminobutyric acid release. Proc Natl Acad Sci U S A. 1999;96:12911–12916. doi: 10.1073/pnas.96.22.12911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kantor L, Gnegy ME. Protein kinase C inhibitors block amphetamine-mediated dopamine release in rat striatal slices. J Pharmacol Exp Ther. 1998;284:592–598. [PubMed] [Google Scholar]
- 25.Browman KE, Kantor L, Richardson S, Badiani A, Robinson TE, Gnegy ME. Injection of the protein kinase C inhibitor Ro31–8220 into the nucleus accumbens attenuates the acute response to amphetamine: tissue and behavioral studies. Brain Res. 1998;814:112–119. doi: 10.1016/s0006-8993(98)01040-3. [DOI] [PubMed] [Google Scholar]
- 26.Heimer L. A new anatomical framework for neuropsychiatric disorders and drug abuse. Am J Psychiatry. 2003;160:1726–1739. doi: 10.1176/appi.ajp.160.10.1726. [DOI] [PubMed] [Google Scholar]
- 27.Timmerman W, Westerink BH. Electrical stimulation of the substantia nigra reticulata: detection of neuronal extracellular GABA in the ventromedial thalamus and its regulatory mechanism using microdialysis in awake rats. Synapse. 1997;26:62–71. doi: 10.1002/(SICI)1098-2396(199705)26:1<62::AID-SYN7>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- 28.Meredith GE. The synaptic framework for chemical signaling in nucleus accumbens. Ann N Y Acad Sci. 1999;877:140–156. doi: 10.1111/j.1749-6632.1999.tb09266.x. [DOI] [PubMed] [Google Scholar]
- 29.Kelley AE. Functional specificity of ventral striatal compartments in appetitive behaviors. Ann N Y Acad Sci. 1999;877:71–90. doi: 10.1111/j.1749-6632.1999.tb09262.x. [DOI] [PubMed] [Google Scholar]


