Abstract
Postmitotic neurons must survive for the entire life of the organism and be able to respond adaptively to adverse conditions of oxidative and genotoxic stress. Unrepaired DNA damage can trigger apoptosis of neurons which is typically mediated by the ataxia telangiectasia mutated (ATM) - p53 pathway. As in all mammalian cells, telomeres in neurons consist of TTAGGG DNA repeats and several associated proteins that form a nucleoprotein complex that prevents chromosome ends from being recognized as double strand breaks. Proteins that stabilize telomeres include TRF1 and TRF2, and proteins known to play important roles in DNA damage responses and DNA repair including ATM, Werner and the catalytic subunit of DNA-dependent protein kinase (DNA-PKcs). We have been performing studies of developing and adult neurons aimed at understanding the effects of global and telomere-directed DNA damage responses in neuronal plasticity and survival in the contexts of aging and neurodegenerative disorders. Deficits in specific DNA repair proteins, including DNA-PKcs and uracil DNA glycosylase (UDG), render neurons vulnerable to adverse conditions of relevance to the pathogenesis of neurodegenerative disorders such as Alzheimer’s disease and stroke. Similarly, early postmitotic neurons with reduced telomerase activity exhibit accentuated responses to DNA damage and are prone to apoptosis demonstrating a pivotal role for telomere maintenance in both mitotic cells and postmitotic neurons. Our recent findings suggest key roles for TRF2 in regulating the differentiation and survival of neurons. TRF2 promotes cell survival and differentiation by modulating DNA damage pathways, and gene expression. A better understanding of the molecular mechanisms by which neurons respond to global and telomere-specific DNA damage may reveal novel strategies for prevention and treatment of neurodegenerative disorders. Indeed, work in this and other laboratories has shown that dietary folic acid can protect neurons against Alzheimer’s disease by keeping homocysteine levels low and thereby minimizing the misincorporation of uracil into DNA in neurons.
Introduction
The nervous system consists of postmitotic neurons and mitotic glial cells. Because they are excitable cells with a high metabolic demand, neurons are subjected to higher levels of oxidative stress than many other cell types. Oxidative damage to DNA can trigger apoptosis of neurons, which typically occurs by a pathway involving ATM and p53 (Miller et al., 2000; Culmsee and Mattson, 2005). The sensitivity of neurons to impaired DNA damage responses is demonstrated by the neurological phenotypes in inherited human disorders caused by mutations in DNA repair proteins. Examples include ataxia telangiectasia (AT), in which cerebellar neurons degenerate, and Cockayne’s syndrome, in which multiple neural systems are damaged (Rotman and Shiloh, 1997; Brooks, 2002). Moreover, neurons in mice that are deficient in some DNA repair enzymes, such as DNA-PKcs and uracil DNA glycosylase (UDG), exhibit increased vulnerability to oxidative, metabolic and excitotoxic insults (Culmsee et al., 2001a; Kruman et al., 2004a). Other findings suggest that postmitotic neurons may undergo apoptosis when cell cycle events are aberrantly activated (Herrup et al., 2004). We have found that DNA damage can trigger cell cycle re-entry in terminally differentiated postmitotic neurons. Different genotoxic agents (etoposide, methotrexate, and homocysteine) induce activation of cell cycle-related pathways and apoptosis (Kruman et al., 2004b). Suppression of the function of ATM attenuates both cell cycle reentry and apoptosis triggered by DNA damage suggesting that cell cycle activation is a critical element of the apoptotic DNA damage response.
The ends of linear chromosomes in mammalian cells are stabilized by telomeres which consist of an array of (TTAGGG) repeats and associated non-histone proteins. This nucleoprotein complex forms a “cap” that prevents the chromosome ends from being recognized as double strand breaks (DSB), and also prevents telomere erosion and end-to-end fusion of chromosomes (Chan and Blackburn, 2004). Moreover, one universally conserved feature of telomeres is the repression of subtelomeric chromatin and local promoters, conferring a telomere position effect (TPE), which plays a pivotal role in epigenetic regulation of gene silencing (Perrod and Gasser, 2003; Duraisingh et al., 2005). Since the discovery of telomeres and telomerase three decades ago, the function of telomeres as a mitotic clock in proliferating cells has been supported by numerous studies which, collectively suggest a major role for telomeres in cancer and aging (reviewed by Blackburn et al., 2006). However, the roles of telomeres in non-dividing cells such as neurons remains obscure. Approximately 10% of the cells in the brain are postmitotic neurons and the remaining cells are mitotic (astrocytes, stem cells and microglia). Recent findings described below suggest that telomere-associated proteins may have extra-telomeric roles in DNA repair and chromatin reorganization in mammalian cells including neural cells.
The importance of telomere homeostasis in the nervous system is revealed by the presence of neuropathological alterations in human disorders caused by perturbed telomere function. In particular, telomere and subtelomere deletion and translocation rearrangements in chromosomes 1pter, 2qter, 4tpter, 5qter and 9qter are a cause of idiopathic mental retardation/developmental delay (Baker et al., 2002; Sogaard et al., 2005). Subtelomere deletion in chromosome 21 (21q22.2 to 21qter) is associated with cortical dysplasia in humans (Chen et al., 2004; Yao. et al, 2006). The neuronal cell adhesion molecule L1 (L1CAM) is essential in the development of the nervous system. The gene encoding L1CAM is located near the telomere of the long arm of the X chromosome in Xq28 (Fransen et al., 1997). Mutations in this single gene are responsible for CRASH syndrome, in which there are at least four overlapping clinical spectrums of neurological abnormalities including mental retardation. In addition, mutations in the telomere-associated proteins ATM, Werner and NBS1 (Nijmegen breakpoint syndrome 1) are responsible for human neurological disorders characterized by developmental abnormalities and increased cancers (Digweed et al., 1999; Opresko et al., 2003; McKinnon et al., 2004). Interestingly, telomere dysfunction in leukocytes was also reported in several age-related neurological alterations including Alzheimer’s disease (Zhang et al., 2003b) and dementia (Honig, 2006). A strong argument for the importance of telomeres in the nervous system is the prominent neurodegenerative symptoms seen in patients and mice with genetic defects in proteins associated with telomeres including ATM, Ku80 and NBS1 (Gu et al., 2000; Frappart et al., 2005; Dar et al., 2006). In this review we discuss the role of telomerase and telomere-associated proteins in neuronal plasticity and survival in the contexts of development and neurodegenerative disorders.
Telomerase and Neuronal Cell Death
Most cancer cells and all stem cells contain a ribonucleoprotein enzyme complex called telomerease, which is composed of telomerase reverse transcriptase (TERT), and a telomerase RNA component (TER) responsible for the elongation of telomeres which is critical for maintenance of genomic integrity. Like most somatic cells, fully differentiated neurons and astrocytes lack telomerase activity (Klapper et al., 2001; Flanary and Streit, 2004). However, when subject to oxidative, hypoxic or excitotoxic stress the expression of TERT and telomerase activity increases in astrocytes (Baek, et al, 2004), neurons (Kang et al., 2004) and/or microglia (Fu et al., 2002a; Flanary and Streit, 2005). Although the function of this stress-induced telomerase reactivation is not clear, a study suggests that it could be involved in DNA repair and chromatin remodeling (Masutomi et al., 2005). Indeed, emerging evidence suggests that telomerase has additional extratelomeric roles in mediating cell survival and anti-apoptotic functions against various cytotoxic stresses (Mattson et al., 2001; Chung et al., 2005).
By overexpressing, or knocking out the expression of the catalytic subunit of telomerase (TERT) in cultured neural tumor cells (PC12) and primary hippocampal neurons, we provided evidence that TERT can prevent neuronal apoptosis induced by cytotoxic stimuli in both types of cells (Fu et al., 1999, Fu et al., 2000). In mitotic cells, the catalytic (reverse transcriptase) activity of telomerase appears to be critical for its anti-apoptotic function. Thus, chemical inhibitors of telomerase activity increase the vulnerability of PC12 cells to apoptosis induced by oxidative stress and amyloid beta-peptide (Fu et al., 1999). The cell death-enhancing effect of telomerase inhibition could be abolished by an overexpression of Bcl-2 or by treatment with caspase inhibitors. Overexpression of wild-type TERT, but not TERT with a mutated reverse transcriptase domain, increases telomerase activity and protects HeLa cells against apoptosis that is induced by DNA-damaging agents (Zhang et al., 2003a). TERT suppresses apoptosis at an early step before the release of cytochrome c and apoptosis-inducing factor from mitochondria. A recent finding indicates that depletion of hTERT can alter the overall configuration of chromatin and suppresses the cell’s response to DNA double strand breaks (Masutomi et al., 2005). Interestingly, emerging findings suggest that TERT can prevent apoptosis of postmitotic neurons by a mechanism independent of telomerase activity. We found that antisense-mediated knockdown of TERT expression in early postmitotic embryonic neurons results in an increased vulnerability to apoptosis and excitotoxicity (Fu et al., 2000). Confirming, and extending on our findings, Kang et al. (2004), reports that neurons in the brain of transgenic mice overexpressing TERT are resistant to death induced by ischemia and excitotoxicity despite no effect of the ectopic TERT on telomerase activity in the brain cells.
Recent studies provide evidence that telomerase activity can be modulated by external signals in neural cells. We found that brain-derived neurotrophic factor (BDNF) upregulates telomerase in cultured hippocampal neurons by a mechanism involving activation of the Akt kinase (Fu et al., 2002b). Du et al. (2004), has similar findings showing that estrogen and raloxifene increase telomerase activity in PC12 cells by an Akt-mediated mechanism. In both of the latter studies, upregulation of telomerase is associated with cellular resistance to apoptosis.
Telomerase RNA (TER), which is expressed constitutively in many cell types including those lacking TERT (Paul-Samojedny et al., 2005), may also have roles in the nervous system. The mutations in TER cause defective telomere maintenance in stem cells of both humans and animal models. Ferron et al., reports that telomere attrition from telomere RNA knock out mice dramatically impair the in vitro proliferation of adult neural stem cells (NSCs), but not embryonic NSC isolated from the subventricular zone (SVZ) (Ferron et al., 2004), a brain region that contains NSC with telomerase activity in adult animals (Caporaso et al., 2003). Consistent with this, peripheral neuropathy in young patients with autosomal dominant inherited disease caused by mutations in TER (Ip et al., 2005). Therefore, both TERT and TER may play an important role in protecting neural cells against oxidative stress and DNA damage.
Telomere-Associated Proteins in DNA Damage Responses and Neuronal Plasticity
Nuclear DNA is tightly packaged by core histones and then folded into chromatin. In order to respond to different conditions (e.g., DNA damage, cell proliferation and differentiation, senescence and apoptosis), the modification of highly condensed chromatin domains is essential to allow DNA to be either more or less accessible to proteins involved in transcription, DNA replication and repair. The reorganization of chromatin is mediated by a large multi-subunit protein complex. Some of these chromatin modification complexes, as well as histone modification complexes, are functionally linked with transcription and DNA repair. For example, DNA damage induced phosphorylation of H2AX (γH2AX) appears instrumental not only in the engagement of the repair/check point machinery (d’Adda di Fagagna et al., 2003; Takai et al., 2003) but also in recruitment of chromatin remodeling and modifying complexes (Lydall and Whitehall, 2005). Several studies in yeast cells demonstrate that the NuA4HAT complex (Downs et al., 2004) and INO80 (a member of SWI/SNF superfamily) are associated with DSB foci (Tsukuda et al, 2005) in a γH2AX-dependent manner. Yeast cells lacking functional INO80 have transcriptional defects but are also hypersensitive to alkylating agents, UV and IR (Shen et al., 2003; Morrison et al., 2004). Therefore, depending upon the presence of γH2AX, both the NuA4HAT and INO80 complexes may be involved in the loosening of chromatin packaging around damaged DNA foci as well as helping DNA repair molecules gain access to the damaged DNA locus. As mentioned above, it has been proposed that in the presence of a DSB, telomerase is involved in alterations of the surrounding chromatin structure. As described below, growing evidence indicates that other telomere-associated proteins may also have similar extra-telomeric functions.
Telomeres are located in nuclear heterochromatin regions where they may silence nearby gene promoters through positional effects. Telomere position effect is the repression of nearby genes in an epigenetic manner and is maintained by telomere associated proteins. In contrast to the majority of genomic DNA, telomeres consist of a unique t-loop structure and specialized telomere-associated proteins (Fig.1) that conceal the single-stranded DNA 3′ overhang located at the ends of DNA chromosomes (de Lange, 2002 and de Lange, 2005). In mammalian cells, several telomere-associated proteins are identified by their ability to bind TTAGGG repeats and facilitate t-loop formation, including TRF1 (Bainchi et al., 1999), TRF2 (Court et al., 2005), Rap1 (Li et al., 2000) and POT1 (Lei et al., 2004). Growing evidence indicates that TRF2 and POT1 play a key role in maintaining telomere integrity. Disrupting either of these proteins from binding to telomeres using either a dominant negative molecule or small interfering RNAs triggers a DSB DNA damage response that results in the recruitment of various DNA repair proteins and enzymes (including ataxia telangiectasia mutated (ATM), γH2AX, 53BP1, MDC1, and NBS1 (d’Adda di Fagagna et al., 2003; Takai et al., 2003). This response can result in cell cycle arrest and senescence, or apoptosis (Karlseder et al., 1999, 2002; Campisi, 2005; Herbig et al., 2004).
Figure 1.
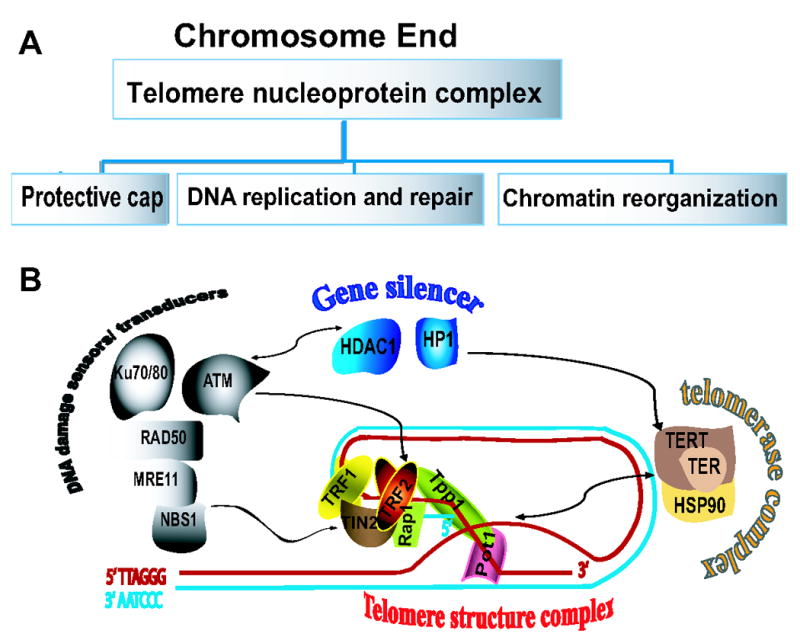
Telomeres are located in the constitutive heterochromatin region at the ends of chromosomes. They consist of G-rich noncoding repeating sequences and non-histone architectural proteins which form a complex t-loop structure also known as a telosome. The telosome prevents chromosome ends from being recognized as a double strand breaks (DSB). It also plays active roles in DNA replication, DNA repair and chromatin reorganization through telomere associated proteins (A). Telomere length can be increased by telomerase, which consists of telomerase reverse transcriptase (TERT), the telomerase RNA component (hTR) and heat-shock protein 90. Telomere tertiary structure is stabilized by telomere repeating factors (TRF1 and TRF2), TRF1 interacting nuclear protein 2(TIN2) and Rap1. In addition to maintenance of telomeres, several telomere associated proteins such as TERT and TRF2 cross-talk with DNA repair and chromatin remodeling protein complexes (B, black arrow). ATM and hTERT interact with chromatin silencing factors HDAC1 and HP1, respectively.
Increasing evidence indicates that TRF2 is an important protein that directly connects telomere maintenance with other chromosomal functions. TRF2 associates with a wide spectrum of proteins and enzymes, and suppresses their function in various genetic processes such as DNA replication and DNA repair (Table 1), implicating TRF2 in a new functional role as a component of the DNA repair system. A recent report shows that TRF2 migrates rapidly to sites of DSB after laser microbeam irradiation (Bradshaw et al., 2005). Further evidence indicates that non-telomeric phosphorylated TRF2 serves as an early recruiting molecule in DNA repair (Tanaka et al., 2005). The available information on TRF2 suggests roles for this protein in chromatin reorganization, telomere maintenance and DNA repair. The latter possibility is supported by evidence demonstrating an association of TRF2 with autophosphorylated ATM. ATM is a kinase that plays multiple roles in activation of signal transduction pathways (Burma et al., 2001) and in regulation of chromatin modification enzymes (with or without DNA damage; Bakkenist and Kastan, 2003), by interacting with the histone deacetylase HDAC1 (Kim et al., 1999) and autodegradation of the E3 ubiquitin ligase COP1 after DNA damage (Dornan et al., 2006). Since TRF2 associates with ATM at its autophosphorylation site (Karlseder et al., 2004 ), TRF2 is likely involved in chromatin remodeling as we have observed that TRF2 regulates the genetic program that controls the differentiation of neurons (Zhang et al., unpublished data).
Table 1.
Protein and enzymes associated with TRF2a, telomeraseb and TRF1c
| Protein | Function | Interactions | References |
|---|---|---|---|
| ATM a | sensor of DSB DNA repair pathway | phosphorylates TRF1; TRF2 binds and inhibits ATM at Ser-1981 autophosphorylation site |
Kishi et al., 2001
Karlseder et al., 2004 |
| Ku 70 a | NHEJ, recruiting DNA-PK | Ku 70 interacts with TRF2 | Song et al., 2000 |
| Ku 70/80b | NHEJ, recruiting DNA-PK | Ku70/80 associates physically with telomerase through interaction with hTERT | Chai et al., 2002 |
| NBS1 a | recombinational DNA repair sensor | interaction with TRF2 in S-phase; play role in intra-S phase checkpoint | Zhu et al., 2000b |
| WRN a | helicase, exonuclease | interaction with TRF2 | Opresko et al., 2002, 2003, 2004 |
| BLM a | helicase | interaction with TRF2 | Opresko et al., 2002 |
| PARP-2 a | base excision repair | PARP-2 physically binds to TRF2 with high affinity; single strand break repair | Dantzer et al., 2004 |
| OZF (ZNF146) | Kruppel protein, with 10 zinc finger motifs | interacts with hRap1 | Antoine et al., 2005 |
| DNA polymerase βa | base excision repair and meiosis | interacts with TRF2 | Fotiadou et al., 2004 |
| HP1α, β b | heterochromatin protein | Interfere hTERT-telomere interaction | Sharma et al., 2003 |
| HSP90b | chaperone | present in telomerase complex | Boltze et al., 2003 |
| dyskerinb | small nucleolar ribonucleoprotein | found in the telomerase complex | Mason et al., 2003 |
| VPARPb | component of vault ribonucleoprotein; subcelluar transport | binds to telomerase associated protein 1 (TEP1) | Liu et al., 2004 |
| TEP1b | component both in Vault RNA and telomerease RNA | associated with hTR | Poderycki et al., 2005 |
| Tankyrasesc | role in mitosis | possitive regulate telomere length | Seimiya et al., 2002 |
| ERCC1/XPF a | structure-specific endonuclease | ERCC1/XPF associates with TRF2 at telomere | Zhu et al., 2003 |
We elucidate the effects of telomere dysfunction on proliferating neural cells (NSC, neuroblastoma cells and astrocytes) and postmitotic neurons (primary hippocampal neurons) by overexpressing a dominant negative form of TRF2 (DN-TRF2). In the mitotic astrocytes, NSC and neuroblastoma cells, DN-TRF2 expression results in the activation of ATM and γH2AX, and stabilization of p53 and p21 resulting in cell cycle arrest and senescence (Zhang et al., 2006). However, in the postmitotic neurons, although DN-TRF2 causes ATM activation and the accumulation of γH2AX at telomeres, the cells do not undergo senescence or apoptosis, and they do not exhibit p53 stabilization (Fig. 2, Zhang et al., 2006). These results show that telomere DNA damage mediated activation of ATM and H2AX has different effects in mitotic neural cells and postmitotic neurons. The function of activated ATM and H2AX in response to telomere damage in neurons is unknown, but may be involved in chromatin remodeling. This hypothesis is supported by the observation that TRF2 dysfunction-induced damage to telomeres can promote differentiation of proliferating neuronal cells as well as postmitotic neurons, as indicated by increases in neurite outgrowth and the expression of ion channels and cytoskeletal proteins characteristic of mature neurons (Zhang et al., 2006). We are therefore testing the hypothesis that TRF2 plays a role in neuronal differentiation.
Figure 2.
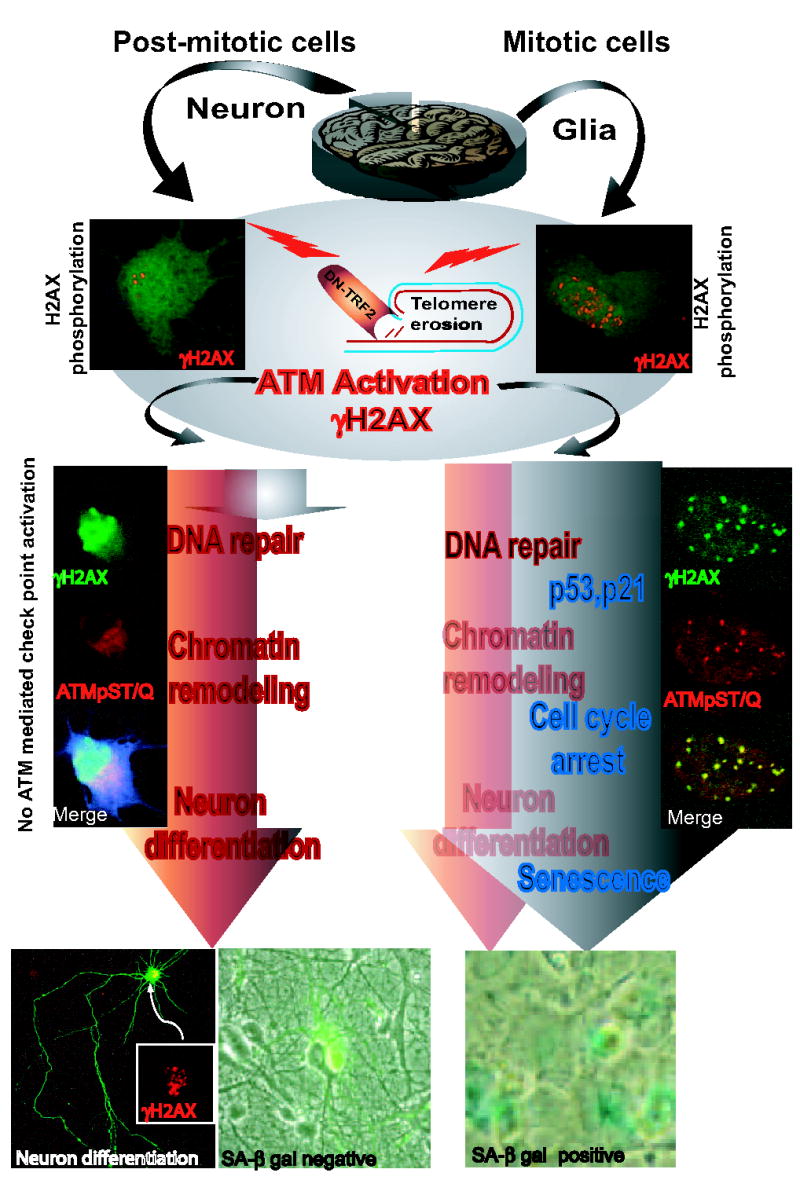
Differential effect of telomere dysfunction/damage on mitotic neural cells and postmitotic neurons. The activation of ATM and phosphorylated H2AX (γH2AX) (insert photograph on top shows the presence of γH2AX foci) is an early response to telomeric DNA damage observed in both glia and neurons after introduction of dominant negative TRF2 (DN-TRF2) mutant. However, several events downstream of the initial DNA damage response including cell cycle checkpoint activation (insert photograph in the middle shows ATM-mediated check point activation detected using an antibody that recognizes ATM substrate proteins phosphoryated on common core motif SQ or TQ, such as p53-S15 and Chk2-T68). The insert photograph at the bottom shows negative and positive (SA-β-gal) staining in neurons and astrocytes, respectively, indicating that senescence-like changes were only observed in cycling glial cells, but not in non-cycling neurons. However, telomere damage-induced neuronal differentiation was observed in both mitotic and post-mitotic neural cells regardless the activation of cell cycle checkpoint (bottom left panel, arrow and insert box shows the co-existence of γH2AX activation and differentiation in postmitotic neurons).
At the embryonic stages of neural development, the occurrence of programmed cell death in a subset of neurons is well established. The proliferating NSC as well as newly generated postmitotic neurons may be particularly vulnerable to DNA damage as indicated by the presence of large-scale double-stranded DNA breaks and cell death in early development of nervous system (Gilmore et al, 2000). Based on the results described above, we hypothesize that during development of the nervous system DNA damage will recruit telomere associated proteins, such as telomerase and TRF2, to non-telomeric regions of chromosomes where they may function in DNA repair and chromatin remodeling. Displacement of TRF2 from telomeres may also relieve the telomere positional effect on nearby genes (Ai et al., 2002), including those that encode proteins involved in neurogenesis (Fransen et al., 1997). Indeed, we obtain evidence for an association between DNA damage and neuronal differentiation (Fig. 2). In yeast there is an increase of subtelomeric cell wall gene expression (a tolerance response) when the silencing effect of telomeres is reduced by stress and cytotoxins (Ai et al., 2002). During neurogenesis, the alteration of telomeric silencing patterns might be correlated with changes in transcription patterns. Telomeres are replicated in the late S-phase. The general presumption is that early S-phase replication is a necessary condition for transcription to take place, and that late-replication genes are assembled into transcriptionally inactive chromatin. Recent findings demonstrate that, in mouse neuronal differentiation, some neuroectoderm-specific genes switch from late- to early replication and become transcriptionally activated (Hiratani et al., 2004).
Alterations of telomere topology may also occur in response to environmental changes. Telomeres of yeast (Taddei et al., 2004) and pathogens such as malaria (Freitas-Junior et al., 2005), are normally clustered in foci attached to the nuclear envelop; however, in response to changes in cellular energy levels telomere clustering enhances (Hediger et al., 2002). Although telomere clustering is not normally found in mammalian telomeres, which seem to be randomly positioned throughout the interphase nucleus, close apposition of telomeres can be observed in non-cycling mammalian cells (Taddei et al., 2004).
A better understanding of telomere maintenance in NSC and postmitotic neurons will help us to further unravel the mechanisms of neural survival and differentiation in neurogenesis, and may aid in the development of novel strategies for manipulating NSC for the treatment of neurological disorders.
DNA and Telomere Damage in the Pathogenesis of Alzheimer’s Disease
Alzheimer’s disease (AD) is an age-related neurodegenerative disorder characterized by the progressive accumulation of amyloid beta-peptides, and associated degeneration and death of neurons located in brain regions involved with learning, memory and emotional behaviors (Mattson, 2004). Increased damage to DNA occurs in neurons during normal aging and, to a greater extent, in neurodegenerative disorders such as AD. There are several examples of evidence showing that DNA damage and impaired DNA repair contribute to neuronal death in AD. For example, levels of DNA repair enzymes (e.g., Parp-1, DNA-PK, Ku) are altered in tissue samples from affected brain regions of AD patients (Davydov et al., 2003), and there is a deficiency of the Mre11 DNA repair complex in AD brains (Jacobsen et al., 2004). Levels of oxidized DNA bases are greater in tissue samples from affected brain regions of AD patients compared to the same brain regions of age-matched normal subjects (Wang et al., 2005), and this oxidative DNA damage appears to occur early in the AD disease process and, therefore, may play a key role in the death of neurons (Wang et al., 2006). Levels of p53 also increase in tissue samples from affected brain regions of AD patients (de la Monte et al., 1997), and p53 inhibitors protect neurons from being killed by amyloid beta-peptide (Culmsee et al., 2001b).
Genetic and environmental factors that cause or affect the risk of AD prove to have an impact on DNA damage responses and neuronal vulnerability in AD animal and cell culture models. For example, presenilin-1 mutations that cause early-onset inherited AD are shown to increase the vulnerability of neurons to DNA damage-induced death (Chan et al., 2002). The hypersensitivity to DNA damage is correlated with elevated intracellular calcium levels and calpain activity, induction of p53, and mitochondrial membrane depolarization. Epidemiological and experimental studies show that an increased uracil misincorporation has a role in causing AD pathogenesis. Data from the Framingham study establishes that elevated plasma homocysteine levels is an independent risk factor for AD (Seshadri et al., 2002). Homocysteine can impair one-carbon metabolism resulting in methionine depletion and uracil misincorporation into DNA (Mattson and Shea, 2003). When cultured hippocampal neurons are exposed to homocysteine they are more vulnerable to being killed by amyloid beta-peptide, apparently as a result of impaired DNA repair (Kruman et al., 2002). Dietary folic acid deficiency is one cause of elevated homocysteine levels. APP mutant mice develop amyloid deposits in their hippocampus, but do not suffer loss of neurons. However, APP mutant mice that are maintained on a folic acid deficient diet, hippocampal pyramidal neurons exhibit impaired DNA repair and degenerate (Kruman et al., 2002). These findings provide an example of how DNA repair mechanisms can be influenced by dietary factors and reveal a novel approach for reducing the risk of AD.
The possibility that telomere damage/dysfunction plays a role in AD merits investigation based upon several recent observations. Lymphocytes from AD patients exhibit telomere shortening and altered telomerase activity compared to lymphocytes from age-matched control subjects (Panossian et al., 2003; Zhang et al., 2003). In addition, cell culture studies show that TERT can protect neurons from being killed by amyloid beta-peptide (Zhu et al., 2000a) and DNA-damaging agents (Lu et al., 2001). These findings suggest an important role for telomere damage in the death of neurons that occurs in AD.
Conclusions
Emerging findings suggest that telomerase and the telomere-associated protein TRF2 play important roles in the process of neurogenesis. Proliferating NSC have high levels of telomerase to maintain telomeres and promote cell survival. Telomerase levels decrease precipitously in neurons soon after they differentiate from NSC, which may contribute to the vulnerability of newly generated neurons to apoptosis. TRF2 appears to play roles in the responses of NSC and postmitotic neurons to DNA damage, not only at the telomere, but elsewhere in the nucleus. There are important links between telomere-associated proteins and DNA repair pathways, most notably the ATM pathway. Studies of cell culture and animal models, and of human disorders, suggest the potential for targeting of telomere-associated molecules as a therapeutic approach for neurological disorders.
Acknowledgments
This work was supported by the National Institute on Aging Intramural Research Program.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ai W, Bertram PG, Tsang CK, Chan TF, Zheng XF. Regulation of subtelomeric silencing during stress response. Mol Cell. 2002;10:1295–305. doi: 10.1016/s1097-2765(02)00695-0. [DOI] [PubMed] [Google Scholar]
- Antoine K, Ferbus D, Kolahgar G, Prosperi MT, Goubin G. Zinc finger protein overexpressed in colon carcinoma interacts with the telomeric protein hRap1. J Cell Biochem. 2005;95:763–768. doi: 10.1002/jcb.20487. [DOI] [PubMed] [Google Scholar]
- Baek S, Bu Y, Kim H, Kim H. Telomerase induction in astrocytes of Sprague-Dawley rat after ischemic brain injury. Neurosci Lett. 2004;363:94–6. doi: 10.1016/j.neulet.2004.03.059. [DOI] [PubMed] [Google Scholar]
- Baker E, Hinton L, Callen DF, Altree M, Dobbie A, Eyre HJ, Sutherland GR, Thompson E, Thompson P, Woollatt E, Haan E. Study of 250 children with idiopathic mental retardation reveals nine cryptic and diverse subtelomeric chromosome anomalies. Am J Med Genet. 2002;107:285–93. doi: 10.1002/ajmg.10159. [DOI] [PubMed] [Google Scholar]
- Bakkenist CJ, Kastan MB. DNA damage activates ATM through intermolecular autophosphorylation and dimer dissociation. Nature. 2003;421:499–506. doi: 10.1038/nature01368. [DOI] [PubMed] [Google Scholar]
- Bianchi A, Stansel RM, Fairall L, Griffith JD, Rhodes D, de Lange T. TRF1 binds a bipartite telomeric site with extreme spatial flexibility. EMBO J. 1999;18:5735–5744. doi: 10.1093/emboj/18.20.5735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blackburn EH, Greider CW, Szostak JW. Telomeres and telomerase: the path from maize, Tetrahymena and yeast to human cancer and aging. Nat Med. 2006;12:1133–8. doi: 10.1038/nm1006-1133. [DOI] [PubMed] [Google Scholar]
- Boltze C, Lehnert H, Schneider-Stock R, Peters B, Hoang-Vu C, Roessner A. HSP90 is a key for telomerase activation and malignant transition in pheochromocytoma. Endocrine. 2003;22:193–201. doi: 10.1385/ENDO:22:3:193. [DOI] [PubMed] [Google Scholar]
- Bradshaw PS, Stavropoulos DJ, Meyn MS. Human telomeric protein TRF2 associates with genomic double-strand breaks as an early response to DNA damage. Nat Genet. 2005;37:193–7. doi: 10.1038/ng1506. [DOI] [PubMed] [Google Scholar]
- Brooks PJ. DNA repair in neural cells: basic science and clinical implications. Mutat Res. 2002;509:93–108. doi: 10.1016/s0027-5107(02)00222-1. [DOI] [PubMed] [Google Scholar]
- Burma S, Chen BP, Murphy M, Kurimasa A, Chen DJ. ATM phosphorylates histone H2AX in response to DNA double-strand breaks. J Biol Chem. 2001;276:42462–42467. doi: 10.1074/jbc.C100466200. [DOI] [PubMed] [Google Scholar]
- Campisi J. Senescent cells, tumor suppression, and organismal aging: good citizens, bad neighbors. Cell. 2005;120:513–522. doi: 10.1016/j.cell.2005.02.003. [DOI] [PubMed] [Google Scholar]
- Caporaso GL, Lim DA, Alvarez-Buylla A, Chao MV. Telomerase activity in the subventricular zone of adult mice. Mol Cell Neurosci. 2003;23:693–702. doi: 10.1016/s1044-7431(03)00103-9. [DOI] [PubMed] [Google Scholar]
- Chai W, Ford LP, Lenertz L, Wright WE, Shay JW. Human Ku70/80 associates physically with telomerase through interaction with hTERT. J Biol Chem. 2002;277:47242–47247. doi: 10.1074/jbc.M208542200. [DOI] [PubMed] [Google Scholar]
- Chan SL, Culmsee C, Haughey N, Klapper W, Mattson MP. Presenilin-1 mutations sensitize neurons to DNA damage-induced death by a mechanism involving perturbed calcium homeostasis and activation of calpains and caspase-12. Neurobiol Dis. 2002;11:2–19. doi: 10.1006/nbdi.2002.0542. [DOI] [PubMed] [Google Scholar]
- Chan SR, Blackburn EH. Telomeres and telomerase. Philos Trans R Soc Lond B Biol Sci. 2004;359:109–121. doi: 10.1098/rstb.2003.1370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen CP, Lin SP, Chern SR, Lee CC, Huang JK, Wang W, Liao YW. De novo satellited 21q associated with corpus callosum dysgenesis, colpocephaly, a concealed penis, congenital heart defects, and developmental delay. Genetic counseling. 2004;15:437–42. [PubMed] [Google Scholar]
- Chung HK, Cheong C, Song J, Lee HW. Extratelomeric functions of telomerase. Curr Mol Med. 2005;5:233–241. doi: 10.2174/1566524053586635. [DOI] [PubMed] [Google Scholar]
- Court R, Chapman L, Fairall L, Rhodes D. How the human telomeric proteins TRF1 and TRF2 recognize telomeric DNA: a view from high-resolution crystal structures. EMBO Rep. 2005;6:39–45. doi: 10.1038/sj.embor.7400314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Culmsee C, Bondada S, Mattson MP. Hippocampal neurons of mice deficient in DNA-dependent protein kinase exhibit increased vulnerability to DNA damage, oxidative stress and excitotoxicity. Mol Brain Res. 2001a;87:257–262. doi: 10.1016/s0169-328x(01)00008-0. [DOI] [PubMed] [Google Scholar]
- Culmsee C, Zhu X, Yu QS, Chan SL, Camandola S, Guo Z, Greig NH, Mattson MP. A synthetic inhibitor of p53 protects neurons against death induced by ischemic and excitotoxic insults, and amyloid beta-peptide. J Neurochem. 2001b;77:220–228. doi: 10.1046/j.1471-4159.2001.t01-1-00220.x. [DOI] [PubMed] [Google Scholar]
- Culmsee C, Mattson MP. p53 in neuronal apoptosis. Biochem Biophys Res Commun. 2005;331:761–777. doi: 10.1016/j.bbrc.2005.03.149. [DOI] [PubMed] [Google Scholar]
- d’Adda di Fagagna F, Reaper PM, Clay-Farrace L, Fiegler H, Carr P, Von Zglinicki T, Saretzki G, Carter NP, Jackson SP. A DNA damage checkpoint response in telomere-initiated senescence. Nature. 2003;426:194–198. doi: 10.1038/nature02118. [DOI] [PubMed] [Google Scholar]
- Dantzer F, Giraud-Panis MJ, Jaco I, Ame JC, Schultz I, Blasco M, Koering CE, Gilson E, Menissier-de Murcia J, de Murcia G, Schreiber V. Functional interaction between poly(ADP-Ribose) polymerase 2 (PARP-2) and TRF2: PARP activity negatively regulates TRF2. Mol Cell Biol. 2004;24:1595–1607. doi: 10.1128/MCB.24.4.1595-1607.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dar I, Biton S, Shiloh Y, Barzilai A. Analysis of the ataxia telangiectasia mutated-mediated DNA damage response in murine cerebellar neurons. J Neurosci. 2006;26:7767–7774. doi: 10.1523/JNEUROSCI.2055-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davydov V, Hansen LA, Shackelford DA. Is DNA repair compromised in Alzheimer’s disease? Neurobiol Aging. 2003;24:953–968. doi: 10.1016/s0197-4580(02)00229-4. [DOI] [PubMed] [Google Scholar]
- De la Monte SM, Sohn YK, Wands JR. Correlates of p53- and Fas (CD95)-mediated apoptosis in Alzheimer’s disease. J Neurol Sci. 1997;152:73–83. doi: 10.1016/s0022-510x(97)00131-7. [DOI] [PubMed] [Google Scholar]
- De Lange T. Protection of mammalian telomeres. Oncogene. 2002;21:532–540. doi: 10.1038/sj.onc.1205080. [DOI] [PubMed] [Google Scholar]
- De Lange T. Shelterin: the protein complex that shapes and safeguards human telomeres. Genes Dev. 2005;19:2100–2110. doi: 10.1101/gad.1346005. [DOI] [PubMed] [Google Scholar]
- Dornan D, Shimizu H, Mah A, Dudhela T, Eby M, O’rourke K, Seshagiri S, Dixit VM. ATM engages autodegradation of the E3 ubiquitin ligase COP1 after DNA damage. Science. 2006;313:1122–1126. doi: 10.1126/science.1127335. [DOI] [PubMed] [Google Scholar]
- Downs JA, Allard S, Jobin-Robitaille O, Javaheri A, Auger A, Bouchard N, Kron SJ, Jackson SP, Cote J. Binding of chromatin-modifying activities to phosphorylated histone H2A at DNA damage sites. Mol Cell. 2004;16:979–90. doi: 10.1016/j.molcel.2004.12.003. [DOI] [PubMed] [Google Scholar]
- Du B, Ohmichi M, Takahashi K, Kawagoe J, Ohshima C, Igarashi H, Mori-Abe A, Saitoh M, Ohta T, Ohishi A, Doshida M, Tezuka N, Takahashi T, Kurachi H. Both estrogen and raloxifene protect against ß-amyloid-induced neurotoxicity in estrogen receptor -transfected PC12 cells by activation of telomerase activity via Akt cascade. J Endocrinol. 2004;183:05–615. doi: 10.1677/joe.1.05775. [DOI] [PubMed] [Google Scholar]
- Duraisingh MT, Voss TS, Marty AJ, Duffy MF, Good RT, Thompson JK, Freitas-Junior LH, Scherf A, Crabb BS, Cowman AF. Heterochromatin silencing and locus repositioning linked to regulation of virulence genes in Plasmodium falciparum. Cell. 2005;121:1–2. doi: 10.1016/j.cell.2005.01.036. [DOI] [PubMed] [Google Scholar]
- Digweed M, Reis A, Sperling K. Nijmegen breakage syndrome: consequences of defective DNA double strand break repair. Bioessays. 1999;21:649–656. doi: 10.1002/(SICI)1521-1878(199908)21:8<649::AID-BIES4>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- Ferron S, Mira H, Franco S, Cano-Jaimez M, Bellmunt E, Ramirez C, Farinas I, Blasco MA. Telomere shortening and chromosomal instability abrogates proliferation of adult but not embryonic neural stem cells. Development. 2004;131:4059–4070. doi: 10.1242/dev.01215. [DOI] [PubMed] [Google Scholar]
- Flanary BE, Streit WJ. Progressive telomere shortening occurs in cultured rat microglia, but not astrocytes. Glia. 2004;45:75–88. doi: 10.1002/glia.10301. [DOI] [PubMed] [Google Scholar]
- Flanary BE, Streit WJ. Effects of axotomy on telomere length, telomerase activity, and protein in activated microglia. J Neurosci Res. 2005;82:160–71. doi: 10.1002/jnr.20636. [DOI] [PubMed] [Google Scholar]
- Fotiadou P, Henegariu O, Sweasy JB. DNA polymerase beta interacts with TRF2 and induces telomere dysfunction in a murine mammary cell line. Cancer Res. 2004;64:3830–3837. doi: 10.1158/0008-5472.CAN-04-0136. [DOI] [PubMed] [Google Scholar]
- Fransen E, Van Camp G, Vits L, Willems PJ. L1-associated diseases: clinical geneticists divide, molecular geneticists unite. Human molecular genetics. 1997;6:1625–32. doi: 10.1093/hmg/6.10.1625. [DOI] [PubMed] [Google Scholar]
- Frappart PO, Tong WM, Demuth I, Radovanovic I, Herceg Z, Aguzzi A, Digweed M, Wang ZQ. An essential function for NBS1 in the prevention of ataxia and cerebellar defects. Nat Med. 2005;11:538–544. doi: 10.1038/nm1228. [DOI] [PubMed] [Google Scholar]
- Freitas-Junior LH, Hernandez-Rivas R, Ralph SA, Montiel-Condado D, Ruvalcaba-Salazar OK, Rojas-Meza AP, Mancio-Silva L, Leal-Silvestre RJ, Gontijo AM, Shorte S, Scherf A. Telomeric heterochromatin propagation and histone acetylation control mutually exclusive expression of antigenic variation genes in malaria parasites. Cell. 2005;121:25–36. doi: 10.1016/j.cell.2005.01.037. [DOI] [PubMed] [Google Scholar]
- Fu W, Begley JG, Killen MW, Mattson MP. Anti-apoptotic role of telomerase in pheochromocytoma cells. J Biol Chem. 1999;274:7264–7271. doi: 10.1074/jbc.274.11.7264. [DOI] [PubMed] [Google Scholar]
- Fu W, Killen M, Culmsee C, Dhar S, Pandita TK, Mattson MP. The catalytic subunit of telomerase is expressed in developing brain neurons and serves a cell survival-promoting function. J Mol Neurosci. 2000;14:3–15. doi: 10.1385/JMN:14:1-2:003. [DOI] [PubMed] [Google Scholar]
- Fu W, Lee J, Guo Z, Mattson MP. Seizures and tissue injury induce telomerase in hippocampal microglial cells. Exp Neurol. 2002a;178:294–300. doi: 10.1006/exnr.2002.8030. [DOI] [PubMed] [Google Scholar]
- Fu W, Lu C, Mattson MP. Telomerase mediates the cell survival-promoting actions of brain-derived neurotrophic factor and secreted amyloid precursor protein in developing hippocampal neurons. J Neurosci. 2002b;22:10710–10719. doi: 10.1523/JNEUROSCI.22-24-10710.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilmore EC, Nowakowski RS, Caviness VS, Jr, Herrup K. Cell birth, cell death, cell diversity and DNA breaks: how do they all fit together? Trends Neurosci. 2000;23:100–105. doi: 10.1016/s0166-2236(99)01503-9. [DOI] [PubMed] [Google Scholar]
- Gu Y, Sekiguchi J, Gao Y, Dikkes P, Frank K, Ferguson D, Hasty P, Chun J, Alt FW. Defective embryonic neurogenesis in Ku-deficient but not DNA-dependent protein kinase catalytic subunit-deficient mice. Proc Natl Acad Sci USA. 2000;97:2668–2673. doi: 10.1073/pnas.97.6.2668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hediger F, Neumann FR, Van Houwe G, Dubrana K, Gasser SM. Live imaging of telomeres: yKu and Sir proteins define redundant telomere-anchoring pathways in yeast. Curr Biol. 2002;12:2076–89. doi: 10.1016/s0960-9822(02)01338-6. [DOI] [PubMed] [Google Scholar]
- Herbig U, Jobling WA, Chen BP, Chen DJ, Sedivy JM. Telomere shortening triggers senescence of human cells through a pathway involving ATM, p53, and p21(CIP1), but not p16(INK4a) Mol Cell. 2004;14:501–513. doi: 10.1016/s1097-2765(04)00256-4. [DOI] [PubMed] [Google Scholar]
- Herrup K, Neve R, Ackerman SL, Copani A. Divide and die: cell cycle events as triggers of nerve cell death. J Neurosci. 2004;24:9232–39. doi: 10.1523/JNEUROSCI.3347-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiratani I, Leskovar A, Gilbert DM. Differentiation-induced replication-timing changes are restricted to AT-rich/long interspersed nuclear element (LINE)-rich isochores. Proc Natl Acad Sci U S A. 2004;101:16861–6. doi: 10.1073/pnas.0406687101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honig LS, Schupf N, Lee JH, Tang MX, Mayeux R. Shorter telomeres are associated with mortality in those with APOE epsilon4 and dementia. Ann Neurol. 2006;60:181–7. doi: 10.1002/ana.20894. [DOI] [PubMed] [Google Scholar]
- Ip P, Knight R, Dokal I, Manzur AY, Muntoni F. Peripheral neuropathy--a novel finding in dyskeratosis congenita. Eur J Paediatr Neurol. 2005;9:85–9. doi: 10.1016/j.ejpn.2005.01.007. [DOI] [PubMed] [Google Scholar]
- Jacobsen E, Beach T, Shen Y, Li R, Chang Y. Deficiency of the Mre11 DNA repair complex in Alzheimer’s disease brains. Mol Brain Res. 2004;128:1–7. doi: 10.1016/j.molbrainres.2004.05.023. [DOI] [PubMed] [Google Scholar]
- Kang HJ, Choi YS, Hong SB, Kim KW, Woo RS, Won SJ, Kim EJ, Jeon HK, Jo SY, Kim TK, Bachoo R, Reynolds IJ, Gwag BJ, Lee HW. Ectopic expression of the catalytic subunit of telomerase protects against brain injury resulting from ischemia and NMDA-induced neurotoxicity. J Neurosci. 2004;24:280–1287. doi: 10.1523/JNEUROSCI.4082-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karlseder J, Broccoli D, Dai Y, Hardy S, de Lange T. p53- and ATM-dependent apoptosis induced by telomeres lacking TRF2. Science. 1999;283:1321–25. doi: 10.1126/science.283.5406.1321. [DOI] [PubMed] [Google Scholar]
- Karlseder J, Hoke K, Mirzoeva OK, Bakkenist C, Kastan MB, Petrini JH, de Lange T. The telomeric protein TRF2 binds the ATM kinase and can inhibit the ATM-dependent DNA damage response. PLoS Biol. 2004;2:E240. doi: 10.1371/journal.pbio.0020240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karlseder J, Smogorzewska A, de Lange T. Senescence induced by altered telomere state, not telomere loss. Science. 2002;295:2446–2449. doi: 10.1126/science.1069523. [DOI] [PubMed] [Google Scholar]
- Kim GD, Choi YH, Dimtchev A, Jeong SJ, Dritschilo A, Jung M. Sensing of ionizing radiation-induced DNA damage by ATM through interaction with histone deacetylase. J Biol Chem. 1999;274:31127–30. doi: 10.1074/jbc.274.44.31127. [DOI] [PubMed] [Google Scholar]
- Kishi S, Zhou XZ, Ziv Y, Khoo C, Hill DE, Shiloh Y, Lu KP. Telomeric protein Pin2/TRF1 as an important ATM target in response to double strand DNA breaks. J Biol Chem. 2001;276:29282–29291. doi: 10.1074/jbc.M011534200. [DOI] [PubMed] [Google Scholar]
- Klapper W, Shin T, Mattson MP. Differential regulation of telomerase activity and TERT expression during brain development in mice. J Neurosci Res. 2001;64:252–260. doi: 10.1002/jnr.1073. [DOI] [PubMed] [Google Scholar]
- Kruman II, Kumaravel TS, Lohani A, Pedersen WA, Cutler RG, Kruman Y, Haughey N, Lee J, Evans M, Mattson MP. Folic acid deficiency and homocysteine impair DNA repair in hippocampal neurons and sensitize them to amyloid toxicity in experimental models of Alzheimer’s disease. J Neurosci. 2002;22:1752–1762. doi: 10.1523/JNEUROSCI.22-05-01752.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kruman II, Schwartz E, Kruman Y, Cutler RG, Zhu X, Greig NH, Mattson MP. Suppression of uracil-DNA glycosylase induces neuronal apoptosis. J Biol Chem. 2004a;27:43952–960. doi: 10.1074/jbc.M408025200. [DOI] [PubMed] [Google Scholar]
- Kruman II, Wersto RP, Cardozo-Pelaez F, Smilenov L, Chan SL, Chrest FJ, Emokpae R, Jr, Gorospe M, Mattson MP. Cell cycle activation linked to neuronal cell death initiated by DNA damage. Neuron. 2004b;41:549–561. doi: 10.1016/s0896-6273(04)00017-0. [DOI] [PubMed] [Google Scholar]
- Lei M, Podell ER, Cech TR. Structure of human POT1 bound to telomeric single-stranded DNA provides a model for chromosome end-protection. Nat Struct Mol Biol. 2004;11:1223–1229. doi: 10.1038/nsmb867. [DOI] [PubMed] [Google Scholar]
- Li B, Oestreich S, de Lange T. Identification of human Rap1: implications for telomere evolution. Cell. 2000;101:471–83. doi: 10.1016/s0092-8674(00)80858-2. [DOI] [PubMed] [Google Scholar]
- Liu Y, Snow BE, Kickhoefer VA, Erdmann N, Zhou W, Wakeham A, Gomez M, Rome LH, Harrington L. Vault poly(ADP-ribose) polymerase is associated with mammalian telomerase and is dispensable for telomerase function and vault structure in vivo. Mol Cell Biol. 2004;24:5314–5323. doi: 10.1128/MCB.24.12.5314-5323.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu C, Fu W, Mattson MP. Telomerase protects developing neurons against DNA damage-induced cell death. Dev Brain Res. 2001;131:167–171. doi: 10.1016/s0165-3806(01)00237-1. [DOI] [PubMed] [Google Scholar]
- Lydall D, Whitehall S. Chromatin and the DNA damage response. DNA Repair (Amst) 2005;4:1195–207. doi: 10.1016/j.dnarep.2005.06.007. Review. [DOI] [PubMed] [Google Scholar]
- Mason PJ. Stem cells, telomerase and dyskeratosis congenita. Bioessays. 2003;25:126–133. doi: 10.1002/bies.10229. [DOI] [PubMed] [Google Scholar]
- Masutomi K, Possemato R, Wong JM, Currier JL, Tothova Z, Manola JB, Ganesan S, Lansdorp PM, Collins K, Hahn WC. The telomerase reverse transcriptase regulates chromatin state and DNA damage responses. Proc Natl Acad Sci U S A. 2005;102:8222–7. doi: 10.1073/pnas.0503095102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattson MP. Pathways towards and away from Alzheimer’s disease. Nature. 2004;430:631–639. doi: 10.1038/nature02621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattson MP, Fu W, Zhang P. Emerging roles for telomerase in regulating cell differentiation and survival: a neuroscientist’s perspective. Mech Ageing Dev. 2001;122:659–671. doi: 10.1016/s0047-6374(01)00221-4. [DOI] [PubMed] [Google Scholar]
- Mattson MP, Shea TB. Folate and homocysteine metabolism in neural plasticity and neurodegenerative disorders. Trends Neurosci. 2003;26:137–146. doi: 10.1016/S0166-2236(03)00032-8. [DOI] [PubMed] [Google Scholar]
- McKinnon PJ. ATM and ataxia telangiectasia. EMBO Rep. 2004;5:772–776. doi: 10.1038/sj.embor.7400210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller FD, Pozniak CD, Walsh GS. Neuronal life and death: an essential role for the p53 family. Cell Death Differ. 2000;7:880–888. doi: 10.1038/sj.cdd.4400736. [DOI] [PubMed] [Google Scholar]
- Morrison AJ, Highland J, Krogan NJ, Arbel-Eden A, Greenblatt JF, Haber JE, Shen X. INO80 and gamma-H2AX interaction links ATP-dependent chromatin remodeling to DNA damage repair. Cell. 2004;119:767–75. doi: 10.1016/j.cell.2004.11.037. [DOI] [PubMed] [Google Scholar]
- Opresko PL, Cheng WH, von Kobe C, Harrigan JA, Bohr VA. Werner syndrome and the function of the Werner protein; what they can teach us about the molecular aging process. Carcinogenesis. 2003;24:791–802. doi: 10.1093/carcin/bgg034. [DOI] [PubMed] [Google Scholar]
- Opresko PL, Otterlei M, Graakjaer J, Bruheim P, Dawut L, Kolvraa S, May A, Seidman MM, Bohr VA. The Werner syndrome helicase and exonuclease cooperate to resolve telomeric D loops in a manner regulated by TRF1 and TRF2. Mol Cell. 2004;14:763–774. doi: 10.1016/j.molcel.2004.05.023. [DOI] [PubMed] [Google Scholar]
- Opresko PL, von Kobbe C, Laine JP, Harrigan J, Hickson ID, Bohr VA. Telomere-binding protein TRF2 binds to and stimulates the Werner and Bloom syndrome helicases. J Biol Chem. 2002;277:41110–19. doi: 10.1074/jbc.M205396200. [DOI] [PubMed] [Google Scholar]
- Panossian LA, Porter VR, Valenzuela HF, Zhu X, Reback E, Masterman D, Cummings JL, Effros RB. Telomere shortening in T cells correlates with Alzheimer’s disease status. Neurobiol Aging. 2003;24:77–84. doi: 10.1016/s0197-4580(02)00043-x. [DOI] [PubMed] [Google Scholar]
- Paul-Samojedny M, Witek A, Samojedny A, Witkowska A, Wilczok T. Human telomerase RNA as endogenous control in endometrial tissue. Int J Gynecol Cancer. 2005;15:343–8. doi: 10.1111/j.1525-1438.2005.15227.x. [DOI] [PubMed] [Google Scholar]
- Perrod S, Gasser SM. Long-range silencing and position effects at telomeres and centromeres: parallels and differences. Cellular and molecular life sciences. 2003;60:2303–18. doi: 10.1007/s00018-003-3246-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poderycki MJ, Rome LH, Harrington L, Kickhoefer VA. The p80 homology region of TEP1 is sufficient for its association with the telomerase and vault RNAs, and the vault particle. Nucleic Acids Res. 2005;33:893–902. doi: 10.1093/nar/gki234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rotman G, Shiloh Y. Ataxia-telangiectasia: is ATM a sensor of oxidative damage and stress? Bioessays. 1997;19:911–17. doi: 10.1002/bies.950191011. [DOI] [PubMed] [Google Scholar]
- Seimiya H, Smith S. The telomeric poly(ADP-ribose) polymerase, tankyrase 1, contains multiple binding sites for telomeric repeat binding factor 1 (TRF1) and a novel acceptor, 182-kDa tankyrase-binding protein (TAB182) J Biol Chem. 2002;277:14116–14126. doi: 10.1074/jbc.M112266200. [DOI] [PubMed] [Google Scholar]
- Seshadri S, Beiser A, Selhub J, Jacques PF, Rosenberg IH, D’Agostino RB, Wilson PW, Wolf PA. Plasma homocysteine as a risk factor for dementia and Alzheimer’s disease. N Engl J Med. 2002;346:476–483. doi: 10.1056/NEJMoa011613. [DOI] [PubMed] [Google Scholar]
- Sharma GG, Hwang KK, Pandita RK, Gupta A, Dhar S, Parenteau J, Agarwal M, Worman HJ, Wellinger RJ, Pandita TK. Human heterochromatin protein 1 isoforms HP1(Hsalpha) and HP1(Hsbeta) interfere with hTERT-telomere interactions and correlate with changes in cell growth and response to ionizing radiation. Mol Cell Biol. 2003;23:8363–376. doi: 10.1128/MCB.23.22.8363-8376.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen X, Xiao H, Ranallo R, Wu WH, Wu C. Modulation of ATP-dependent chromatin-remodeling complexes by inositol polyphosphates. Science. 2003;299:112. doi: 10.1126/science.1078068. [DOI] [PubMed] [Google Scholar]
- Sogaard M, Tumer Z, Hjalgrim H, Hahnemann J, Friis B, Ledaal P, Pedersen VF, Baekgaard P, Tommerup N, Cingoz S, Duno M, Brondum-Nielsen K. Subtelomeric study of 132 patients with mental retardation reveals 9 chromosomal anomalies and contributes to the delineation of submicroscopic deletions of 1pter, 2qter, 4pter, 5qter and 9qter. BMC medical genetics. 2005;6:21. doi: 10.1186/1471-2350-6-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song K, Jung D, Jung Y, Lee SG, Lee I. Interaction of human Ku70 with TRF2. FEBS Lett. 2000;48:81–85. doi: 10.1016/s0014-5793(00)01958-x. [DOI] [PubMed] [Google Scholar]
- Taddei A, Hediger F, Neumann FR, Gasser SM. The function of nuclear architecture: a genetic approach. Annu Rev Genet. 2004;38:305–45. doi: 10.1146/annurev.genet.37.110801.142705. [DOI] [PubMed] [Google Scholar]
- Takai H, Smogorzewska A, de Lange T. DNA damage foci at dysfunctional telomeres. Curr Biol. 2003;13:549–1556. doi: 10.1016/s0960-9822(03)00542-6. [DOI] [PubMed] [Google Scholar]
- Tanaka H, Mendonca MS, Bradshaw PS, Hoelz DJ, Malkas LH, Meyn MS, Gilley D. DNA damage-induced phosphorylation of the human telomere-associated protein TRF2. Proc Natl Acad Sci U S A. 2005;102:15539–44. doi: 10.1073/pnas.0507915102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsukuda T, Fleming AB, Nickoloff JA, Osley MA. Remodelling at a DNA double-strand break site in Saccharomyces cerevisiae. Nature. 2005;438:379–83. doi: 10.1038/nature04148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Markesbery WR, Lovell MA. Increased oxidative damage in nuclear and mitochondrial DNA in mild cognitive impairment. J Neurochem. 2006;96:825–832. doi: 10.1111/j.1471-4159.2005.03615.x. [DOI] [PubMed] [Google Scholar]
- Wang J, Xiong S, Xie C, Markesbery WR, Lovell MA. Increased oxidative damage in nuclear and mitochondrial DNA in Alzheimer’s disease. J Neurochem. 2005;93:953–962. doi: 10.1111/j.1471-4159.2005.03053.x. [DOI] [PubMed] [Google Scholar]
- Yao G, Chen XN, Flores-Sarnat L, Barlow GM, Palka G, Moeschler JB, McGillivray B, Morse RP, Korenberg JR. Deletion of chromosome 21 disturbs human brain morphogenesis. Genet Med. 2006;8:1–7. doi: 10.1097/01.gim.0000195892.60506.3f. [DOI] [PubMed] [Google Scholar]
- Zhang J, Kong Q, Zhang Z, Ge P, Ba D, He W. Telomere dysfunction of lymphocytes in patients with Alzheimer disease. Cogn Behav Neurol. 2003b;16:70–176. doi: 10.1097/00146965-200309000-00004. [DOI] [PubMed] [Google Scholar]
- Zhang P, Chan SL, Fu W, Mendoza M, Mattson MP. TERT suppresses apoptotis at a premitochondrial step by a mechanism requiring reverse transcriptase activity and 14-3-3 protein-binding ability. FASEB J. 2003a;17:767–769. doi: 10.1096/fj.02-0603fje. [DOI] [PubMed] [Google Scholar]
- Zhang P, Furukawa K, Opresko PL, Xu X, Bohr VA, Mattson MP. TRF2 dysfunction elicits DNA damage responses associated with senescence in proliferating neural cells and differentiation of neurons. J Neurochem. 2006 doi: 10.1111/j.1471-4159.2006.03779.x. [DOI] [PubMed] [Google Scholar]
- Zhu H, Fu W, Mattson MP. The catalytic subunit of telomerase protects neurons against amyloid beta-peptide-induced apoptosis. J Neurochem. 2000a;75:117–124. doi: 10.1046/j.1471-4159.2000.0750117.x. [DOI] [PubMed] [Google Scholar]
- Zhu XD, Kuster B, Mann M, Petrini JH, de Lange T. Cell-cycle-regulated association of RAD50/MRE11/NBS1 with TRF2 and human telomeres. Nat Genet. 2000b;25:347–352. doi: 10.1038/77139. [DOI] [PubMed] [Google Scholar]
- Zhu XD, Niedernhofer L, Kuster B, Mann M, Hoeijmakers JH, de Lange T. ERCC1/XPF removes the 3′ overhang from uncapped telomeres and represses formation of telomeric DNA-containing double minute chromosomes. Mol Cell. 2003;12:1489–98. doi: 10.1016/s1097-2765(03)00478-7. [DOI] [PubMed] [Google Scholar]


