Abstract
According to Cloninger’s model, Type I alcoholics are thought to be innately vulnerable to anxiety and depression. In contrast, Type-II alcoholics are thought to have increased likelihood of antisocial personality disorder (ASPD) and reduced anxiety. However, allostatic activations of stress, anxiety and dysphoria may be a common thread in alcohol use disorders (AUD). Our aim was to find commonalities and differences in temperament of alcoholics with and without ASPD in three diverse populations. By sib-sib comparisons, we also evaluated the extent to which the temperament traits were moderated by familial factors including inheritance. We compared harm avoidance (HA), novelty seeking (NS), and reward dependence (RD) in alcoholics with ASPD, alcoholics without ASPD and controls. Correlations for each temperament dimension were evaluated in pairs of siblings concordant and discordant for AUD. Participants were derived from three independent populations: Finnish Caucasians (N=453, men = 100%, including a sample of alcoholic criminals), a Plains American Indian community sample (N=378; men = 42%) and a subset of the familial and predominantly Caucasian COGA sample (N=967, men = 47%). In all three populations both alcoholics with and without ASPD were higher in HA than controls. The increase of HA among alcoholics as compared to controls ranged from 54% to 12%. In two populations (COGA and Finns), NS was highest in alcoholics with ASPD, intermediate in alcoholics without ASPD, and lowest in controls. HA levels were correlated in sib-pairs concordant (either affected or unaffected) for AUD but not in discordant pairs. In conclusions, despite cultural diversity and different modes of ascertainment we found a consistent pattern of elevated HA in all groups of alcoholics, including alcoholics with ASPD. Even in alcoholics with long-term exposure to the anxiogenic effects of repeated cycles of alcohol withdrawal, genetic and other familial influences seem to play a role in moderating anxiety.
Keywords: Harm Avoidance, Novelty Seeking, Alcohol Use Disorders, ASPD
1. Introduction
Alcohol Use Disorders (alcohol dependence and abuse (AUD)) are common and heterogeneous diseases. According to the National Epidemiological Survey on Alcohol and Related Conditions the 12-month prevalence of AUD in the U.S. is 8.6% (Grant et al., 2004a). Alcoholics differ in patterns of psychiatric comorbidity, personality profiles, age at onset, familiality and other measurable features. Psychiatric diseases commonly associated with AUD can be broadly divided into internalizing disorders (e.g. depression and anxiety) and externalizing disorders (e.g. antisocial personality disorder (ASPD), conduct disorder, attention deficit hyperactivity disorder) (Grant et al., 2004a; Grant et al., 2004b; Kessler et al;1997).
Personality features have been used to identify subtypes of alcoholics that are clinically, and perhaps etiologically, more homogeneous. According to Cloninger’s original psychobiological model (Cloninger et al.,1987), personality varies along three dimensions: novelty seeking (NS), harm avoidance (HA) and reward dependence (RD). These dimensions are commonly assessed via the Tridimensional Personality Questionnaire (TPQ) or the Temperament and Character Inventory (TCI). HA is related to behavioral inhibition and pessimistic anticipation of negative events. Individuals high in HA are cautious, tense, shy, apprehensive, inhibited and fearful. NS is a tendency to react with intense excitement to novelty or potential rewards. Individuals with high NS are characterized by frequent exploratory activity, impulsivity, irritability, and avoidance of monotony and frustrations. RD is related to reward signals, particularly those associated with social interactions. High RD individuals are sentimental, empathic, socially attached and dependent on others’ approval. These dimensions are normally distributed and causally independent but interactive (Cloninger et al.,1987). The heritabilities of the dimensions are moderate: HA, 0.41–0.55; NS, 0.34–0.55; and RD, 0.38– 0.56 (Ando et al., 2004; Heath et al., 1994; Keller et al., 2005; Stallings et al., 1996) Cloninger’s system represented an attempt to use dimensional personality measures reflecting neurobiological processes to predict categorically defined behaviors (Cloninger et al., 1987). Thus, a combination of low RD, low HA and high NS was hypothesized to lead to ASPD, consistent with the conceptualization that individuals with ASPD are aggressive/impulsive and also have low emotionality/detachment. NS and HA have been reported to discriminate between internalizing and externalizing disorders (Copeland et al., 2004).
The application of Cloninger’s model of personality to AUD (Cloninger et al., 1981) led to the identification of two subtypes. Type 1 is late in onset and was described as having a temperament profile of high HA, high RD and low NS. Type II is early in onset, associated with ASPD, and was described as having a temperament profile of high NS, low RD and low HA. Genetic liability is usually considered to be stronger for Type II (Cloninger et al., 1981). However, a later study found that heritability of AUD with internalizing features was higher (Prescott et al., 2005). Although other classifications of AUD identify more than two subtypes (Del Boca et al., 1996; Windle & Scheidt, 2004; Zucker, 1986), all typologies generally agree that two main paths lead to addiction: one through the internalizing psychopathology and the other through the externalizing domain. Externalizing individuals are hypothesized to be more likely to take substances experimentally and less able to delay reward or resist positive reinforcement (Cloninger et al., 1981). Internalizing individuals are hypothesized to use drugs to relieve affective disturbances (Mulder, 2002; Zucker, 1986). However, previous studies have shown that alcoholics with early onset and antisocial behavior are actually higher in both externalizing and internalizing symptoms (Babor et al., 1992; McGue et al., 1997; Windle & Scheidt, 2004).
Externalizing as well as internalizing symptoms can be a consequence of alcohol use. Alcohol is disinhibiting such that approximately half of violent acts, and many other antisocial behaviors, occur while individuals are under the influence (Brewer et al., 2005). On the other hand, allostatic activation of the brain stress axis is an important mechanism of addiction to alcohol and other drugs, leading to persistent affective disturbances which increase the risk of relapse and rapid reinstatement (Roberts & Koob, 1997).
Twin studies and longitudinal studies have explored the question of whether associations of alcoholism to externalizing and internalizing disorders are primary or secondary. Twin studies reveal shared genetic influences between AUD, other addictions, externalizing disorders and NS (Fu et al., 2002; Kendler et al., 2003; Krueger et al., 2002; Young et al., 2000). In longitudinal studies, impulsivity, sensation-seeking and behavioral disinhibition predicted subsequent substance use (Sher et al., 2000). On the other hand, results from twin studies evaluating evidence for shared genetic influences between alcoholism and internalizing disorders are more controversial (Kendler et al., 1993; Kendler et al., 2003; Prescott et al., 2000). However, panic disorder and social phobia predict subsequent alcohol problems in adolescents and young adults (Zimmermann et al., 2003). Also, twin studies have demonstrated genetic correlations between HA and internalizing disorders (Ono et al., 2002). Finally, internalizing psychopathology may be a more important predisposing factor in women than men (Dell’Osso et al., 2002; Prescott et al., 1997).
The aim of the present study was to investigate commonalities and differences in temperament of alcoholics with and without ASPD in three independent samples. The populations we used were diverse in ethnic and gender composition, cultural background, and mode of ascertainment (e.g. community-ascertained as well as treatment-ascertained and criminal alcoholics). These differences created samples that were differentially enriched for subtypes of AUD. Associations between temperament and AUD obtained in cross-sectional studies are difficult to interpret from a cause-effect perspective. However, the family-design of one of the three datasets allowed some exploration of the nature of the links between temperament and alcoholism that we observed across all three samples. Using sib-pairs concordant and discordant for AUD, we tested whether temperament features in alcoholics, particularly anxiety, are traits that are only secondary to AUD, or moderated by familial factors that include inheritance.
2. Method
2.1. Samples
Participants were derived from three independent samples: Finnish Caucasians, Plains American Indians and a publicly available subset of the Collaborative Study on the Genetics of Alcoholism (COGA) dataset that is predominantly Caucasian American.
a) Finns (N=453)
This sample consists of criminal alcoholics and controls collected from the same Caucasian source population. Alcoholics were 217 unrelated male criminals who underwent forensic psychiatric examinations at the time of their initial incarceration. Controls were 236 unrelated male volunteers who were recruited by advertisements in local newspapers and were compensated for their participation. For a more detailed description see Lappalainen et al. (1998).
b) Plains American Indians (N=378; women 217, men 161)
Community volunteers were recruited from a Plains Indian tribe living in rural Oklahoma. Probands were initially ascertained at random from the tribal register, and the families of alcoholic probands were extended. Although most participants derived from one large, multigenerational pedigree, the average sharing of descent was only 0.3%. The average Plains Indian ancestry in our sample was 87% (S.D. 21%); however the median and modal values were 100%. For a more detailed description see Enoch et al. (2006).
c) Collaborative Study on the Genetics of Alcoholism (COGA) (N=967, men = 452, women = 515)
The present study includes a publicly available subset of the Wave I COGA sample, a family dataset collected at six centers within the U.S. Alcohol dependent probands were ascertained from consecutive admissions to treatment facilities. The ascertainment criteria included a minimum of two affected relatives. Both alcoholic and nonalcoholic members of families are included here; thus, all subjects were related to at least one other participant in the sample. In the present analysis, 78% of subjects were Caucasian, 12% were African American, 7% Hispanic and 3% had other ethnic origins. A detailed description of this dataset can be found at the website (http://www.niaaa.nih.gov/ResearchInformation/ExtramuralResearch/SharedResources/projcoga.htm).
Informed consent was obtained according to human research protocols approved by the human research committees of the University of Helsinki for the Finnish sample, the Institutional review boards at all six centers involved for the COGA sample, and the National Institute on Alcohol Abuse and Alcoholism (NIAAA) for the Plains Indian samples. The Plains Indian Tribal Council also gave their approval.
2.2. Psychiatric Assessment
All analyses used lifetime psychiatric diagnoses that were based on DSM-IIIR criteria and assessed using semi-structured psychiatric interviews. The Schedule for Affective Disorders and Schizophrenia-Lifetime Version (SADS-L) (Endicott et al., 1978) was used in the Plains Indian sample. The Semi-Structured Assessment for the Genetics of Alcoholism (SSAGA) (Nurnberger et al., 1994) was used in the COGA sample. The Structured Clinical Interview for DSM-IIIR (Spitzer et al., 1990a; Spitzer et al., 1990b; Spitzer et al., 1990c) was used in the Finnish sample.
Validity of standard criteria for psychiatric diagnosis can be problematic within special cultural groups. Therefore, provisions were taken within the Plain Indians sample to limit diagnostic errors due to culture-specific phenomena (Greene et al., 2003). SADS-L interviews were administered by a clinical social worker (B.A.) who has extensive experience in the tribal customs and culture. The blind-rated DSM-III-R diagnoses yielded acceptable rates of agreement (kappa coefficient ranging from 0.63 to 1.00).
Each population was divided into three groups: alcohol use disorders with comorbid antisocial personality disorder (AUD + ASPD), alcohol use disorders without antisocial personality disorder (AUD − ASPD) and controls (no AUD, no ASPD). AUD was defined as either alcohol dependence or abuse. Age and gender distributions for alcoholics with and without ASPD and controls within each population are shown in Table 1. Proportions of participants with alcohol dependence among individuals with AUD were: 0.82 in the Finnish sample, 0.98 in COGA and 0.95 in the Plains sample.
Table 1.
Gender distribution, mean age at time of evaluation and age at onset of alcohol use disorders (AUD) in alcoholics with ASPD (AUD + ASPD), alcoholics without ASDP (AUD − ASPD) and controls (C), across the three populations. SE = standard error; SD=Standard Deviation, ASPD=antisocial personality disorder.
| FINNS | COGA | Plains Indians | |||||||
|---|---|---|---|---|---|---|---|---|---|
| AUD + ASPD | AUD − ASPD | C | AUD + ASPD | AUD − ASPD | C | AUD + ASPD | AUD − ASPD | C | |
| N | 109 | 108 | 236 | 88 | 410 | 469 | 45 | 175 | 157 |
| Men % | 100 | 100 | 100 | 89 | 61 | 26 | 78 | 49 | 26 |
| Mean age (SD) | 30.6(10.2) | 35.2(9.9) | 31.7(8.6) | 35.4(9.6) | 38.8(13.1) | 44.1(16.9) | 44.8(10.2) | 41.9(11.9) | 43.3(16.4) |
| Mean age at onset (SE) | 19.4(0.5) | 22.7(0.5) | 19.8(0.7) | 23.7(0.3) | 20.2(0.6) | 21.5(1.1) | |||
2.3. Temperament Assessment
Temperament was assessed using the Tridimensional Personality Questionnaire (TPQ). The TPQ is divided into three scales (harm avoidance (HA), novelty seeking (NS) and reward dependence (RD) each of which is sub-divided into four subscales. HA subscales are anticipatory worry (HA1), fear of uncertainty (HA2), shyness (HA3) and fatigability (HA4). NS subscales are: exploratory excitability (NS1), impulsivity (NS2), extravagance (NS3) and disorderliness (NS4). RD subscales are sentimentality (RD1), persistence (RD2), attachment (RD3), and dependence (RD4) (Cloninger et al., 1987). TPQ subscales scores were unavailable for the COGA population.
2.4. Age at onset
In the COGA and Finnish samples, age of onset was defined as the age at which subjects met full DSM-IIIR criteria for alcohol dependence. In the Plains sample, age at onset was evaluated for each person as the time when a problematic pattern of drinking started, regardless of whether subjects fulfilled DSM-IIIR criteria for an AUD. Problematic patterns included binge drinking every weekend or maintenance of drinking almost every day. Mean age at onset for alcoholics with and without ASPD is shown in table 1.
2.5. Statistical analyses
All statistical analyses were conducted separately within each sample.
Analysis of Covariance (ANCOVA) was used to compare mean TPQ scores between AUD + ASPD, AUD − ASPD and controls. Age and gender were used as covariates.
When significant differences emerged, post hoc comparisons of pairs of groups were performed using Tukey-Kramer honestly significant difference (HSD) test.
If ANCOVA was significant for a TPQ scale, the same analyses, ANCOVA and if necessary HSD, were conducted for the corresponding subscales to identify characteristics that differed between groups more precisely.
In the Plains Indian population, the fraction of genes shared between any two individuals through common descent was calculated for all possible pairs (related and unrelated) using SAGE. The average sharing of descent was only 0.3% which is equivalent to the degree of relatedness that lies between second cousins once removed and third cousins and indicates that most pairs of individuals in this population have a very low degree of relationship. We were therefore able to undertake group analyses that assume independence of individuals.
In the COGA sample, differences in TPQ scores between the three diagnostic categories might be reduced because the control group was entirely composed of relatives of alcoholics. However, we were still able to detect differences in temperament features between alcoholics and their non-alcoholic relatives.
To better understand the origin of changes in temperament features in alcoholics, we compared sib-sib correlations for HA, NS and RD between pairs of COGA siblings concordant or discordant for AUD. The COGA sample included 105 families and 619 siblings. A total of 901 sib-pairs were generated computing all the possible combinations of pairs within each family. For a family including N siblings the number of all possible pairs is given by the binomial formula: N!/[2! (N-2)!]. Sib-pairs were divided into three groups: concordant, affected (AUD/AUD); concordant, unaffected (no-AUD/no-AUD), and discordant (AUD/no-AUD).
Analyses were performed with JMP statistical software v5.1 (SAS Institute, Cary, NC, USA). The criterion for significance was p<0.05.
3. Results
Alcoholics with ASPD made up approximately half of the alcoholics in the Finnish sample and approximately one fifth of the alcoholics in both the COGA and Plains populations (Table 1). Age at onset of AUD or heavy drinking was similar across the three samples (table 1) and converged to 19–20 years for alcoholics with ASPD, and to 21–24 years for other alcoholics. Alcoholics with ASPD had an earlier onset than alcoholics without ASPD in the Finnish (F =21.90, df=1, p<0.0001) and COGA (F =25.03, df=1, P<0.0001) populations, whereas only a trend was found among Plains Indians. Lifetime rates of major depression and anxiety disorders were variable across populations (Table 1). Lowest rates were found in the Finns, whereas highest rates were reported among Plains Indians.
3.1. HA, NS and RD in controls compared to alcoholics with and without ASPD
HA
HA was elevated in alcoholics compared to controls consistently across the three populations (Finns, F= 56.18, df=2, p = 2e−22, COGA, F = 4.22, df=2, p=0.01, Plains Indians, F= 4.38, df=4, p=0.01). As shown, in Figure 1, age and sex-adjusted HA was 12% to 50% higher in alcoholics as compared to controls. Post hoc analyses indicated there was no significant difference in HA between alcoholics with and without ASPD they were both equivalently higher in HA than controls (Finns, t= −1.36, p = 0.17; COGA, t = 0.32, p=0.75; Plains Indians, t= 0.69, p=0.48).
Figure 1.
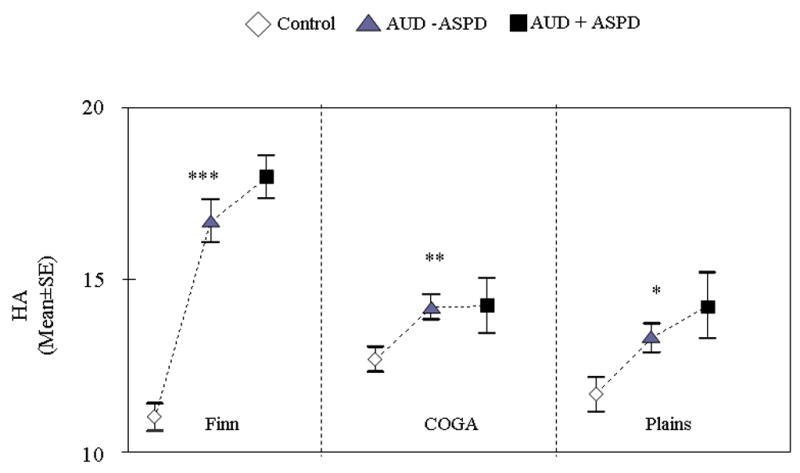
HA scores in controls, alcoholics with ASPD (AUD + ASPD), and alcoholics without ASPD (AUD − ASPD) across the three samples: Finns (N=439), COGA (N=804) and Plains (N=351) (ANCOVA, p value reported are global p value). ***=p<0.0001; **=p<0.01; *=p<0.05. AUD=alcohol use disorder; ASPD= antisocial personality disorder.
In Finns, all HA subscales were higher among both antisocial and non-antisocial alcoholics as compared to controls. Among Plains Indians, H3 was the only subscale that significantly differed between alcoholics and controls (Table 2). HA subscale scores were not analyzed in the COGA population because they were not available
Table 2.
TPQ subscale scores in controls, alcoholics with ASPD (AUD + ASPD), and alcoholics without ASPD (AUD − ASPD) in the Finnish (N=439) and Plains Indian populations (N=351) (ANCOVA). HA=harm avoidance, NS=novelty seeking, RD= reward dependence; AUD=alcohol use disorder; ASPD= antisocial personality disorder
| Finns | |||||
|---|---|---|---|---|---|
| Subscales: | C | AUD − ASPD | AUD+ ASPD | F | p= |
| HA1 (Anticipatory worry) | 3.00 (0.14) | 4.61 (0.22) | 5.12 (0.21) | 41.32 | 3.71e−17 |
| HA2 (Fear of uncertainty) | 3.31 (0.12) | 4.39 (0.18) | 4.16 (0.18) | 15.22 | 4.07e−07 |
| HA3 (Shyness) | 2.30 (0.13) | 3.82 (0.19) | 4.56 (0.19) | 54.47 | 7.18e−22 |
| HA4 (Fatigability) | 2.41 (0.15) | 3.88 (0.23) | 4.14 (0.22) | 26.93 | 9.37e−12 |
| NS1 (Exploratory excitability) | 4.94 (0.11) | 4.43 (0.17) | 4.54 (0.17) | 3.78 | 0.02 |
| NS2 (Impulsiveness) | 3.58 (0.14) | 4.31(0.21) | 5.09(0.21) | 19.04 | 1.18e−08 |
| NS3 (Extravagance) | 4.26 (0.10) | 4.99(0.16) | 4.81 (0.17) | 9.06 | 0.0001 |
| NS4 (Disorderliness) | 4.29 (0.11) | 4.88 (0.18) | 5.89 (0.18) | 28.19 | 3.09e−12 |
| RD1 (Sentimentality) | 2.52 (0.08) | 2.82 (0.13) | 2.57 (0.13) | 1.79 | 0.17 |
| RD2 (Persistence) | 3.84 (0.12) | 4.28 (0.19) | 3.98 (0.18) | 1.83 | 0.16 |
| RD3 (Attachment) | 7.28 (0.16) | 6.33 (0.25) | 5.55 (0.25) | 17.4 | 5.36e−08 |
| RD4 (Dependence) | 3.01 (0.09) | 2.66 (0.14) | 2.38 (0.13) | 8.2 | 0.0003 |
| Plains | |||||
| Subscales: | C | AUD − ASPD | AUD+ ASPD | F | P= |
| HA1 (Anticipatory worry) | 2.63 (0.18) | 2.94 (0.16) | 3.39 (0.34) | 2.08 | 0.13 |
| HA2 (Fear of uncertainty) | 3.92 (0.14) | 4.34 (0.13) | 4.17 (0.27) | 2.41 | 0.09 |
| HA3 (Shyness) | 2.31 (0.16) | 2.80 (0.14) | 3.38 (0.31) | 5.35 | 0.005 |
| HA4 (Fatigability) | 2.83 (0.20) | 3.19 (0.18) | 3.29 (0.39) | 1.02 | 0.36 |
NS
Significant differences between groups were found in the COGA (F = 38.64, df=2, p=7.7e−17) and Finnish (F = 31.42, df=2, p=1.8e−13) populations (Figure 2). Post hoc comparisons showed a significant stepwise increase; NS was highest in alcoholics with ASPD, intermediate in alcoholics without ASPD and lowest in controls. NS was 24% and 34% higher in alcoholics with ASPD than controls in the Finn and COGA samples respectively. On the other hand, in the Plains Indian sample, NS was similar across the three diagnostic categories and close overall to the NS scores of COGA controls.
Figure 2.
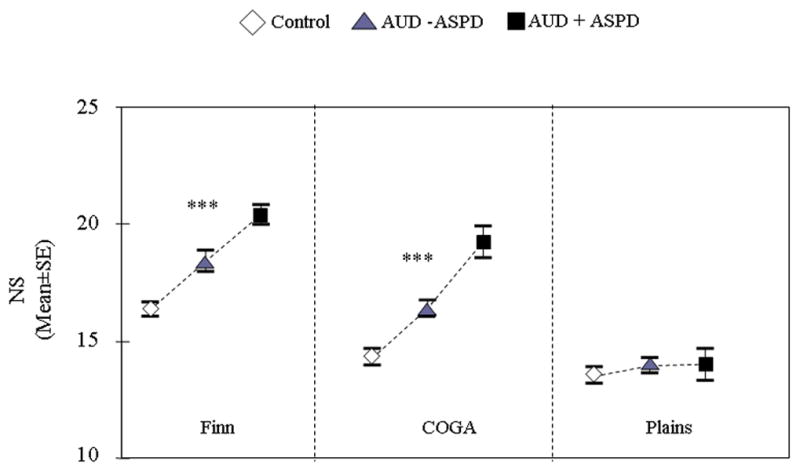
NS scores in controls, alcoholics with ASPD (AUD + ASPD), and alcoholics without ASPD (AUD − ASPD) across the three samples: Finns (N=439), COGA (N=804) and Plains (N=351) (ANCOVA, p value reported are global p value). ***=p<0.0001; **=p<0.01; *=p<0.05. AUD=alcohol use disorder; ASPD= antisocial personality disorder.
NS subscales were analyzed only in the Finns (see Table 2) because NS subscales were unavailable for the COGA dataset. Significant differences were found for all NS subscales. Only NS2 and NS4 scales discriminated significantly between each group; scores were highest among antisocial alcoholics, intermediate in non-antisocial alcoholics and lowest among controls
RD
significantly differentiated the diagnostic groups only within the Finnish population (F = 8.82, df=2, p= 0.0002) (Figure 3). Alcoholics with ASPD scored lower than both other alcoholics and controls, and these last two groups did not differ significantly one from the other. The signal came from RD3 and RD4 (see table 2).
Figure 3.
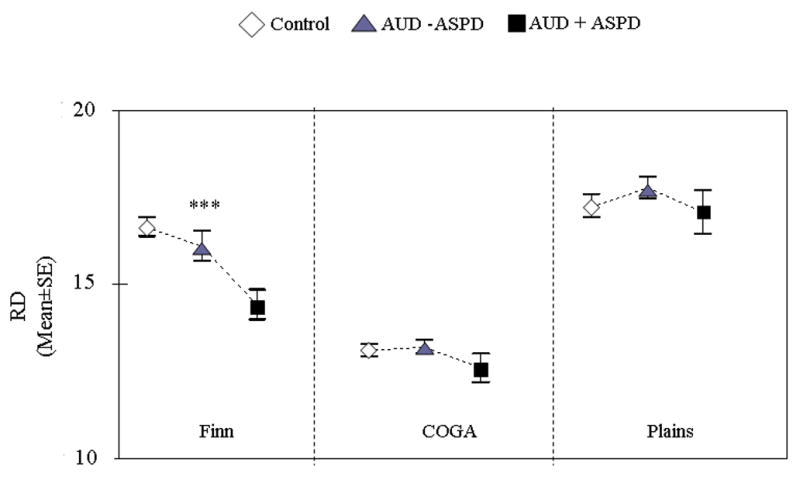
RD scores in controls, alcoholics with ASPD (AUD + ASPD), and alcoholics without ASPD (AUD − ASPD) across the three samples: Finns (N=439), COGA (N=804) and Plains (N=351) (ANCOVA, p value reported are global p value). ***=p<0.0001; **=p<0.01; *=p<0.05. AUD=alcohol use disorder; ASPD= antisocial personality disorder.
3.2. Correlations for temperament within sib pairs concordant and discordant for AUD
HA, NS and RD correlations between siblings concordant or discordant for AUD are shown in table 3. For HA, sib-pair correlations were approximately 0.20 for both AUD/AUD (p<0.0001) and no-AUD/no-AUD pairs (p=0.06, ns). In contrast, among pairs discordant for AUD diagnosis (AUD/-no AUD), HA of the two siblings did not correlate. For NS and RD, sib-pair correlations were lower and non-significant (except for RD in the AUD/no-AUD group).
Table 3.
Correlations of HA, NS and RD scores between siblings concordant or discordant for alcohol use disorders (AUD) in the COGA population. AUD/AUD= both siblings are affected by AUD; AUD/no-AUD= one sibling is affected by AUD and the other is unaffected; No-AUD/No-AUD= both siblings are unaffected.
| Type of sibling pair | N of pairs | r | P< | |
|---|---|---|---|---|
| HA | AUD/AUD | 357 | 0.23 | 0.0001 |
| AUD/no-AUD | 404 | 0.07 | ns | |
| No-AUD/No-AUD | 140 | 0.18 | 0.06 | |
| NS | AUD/AUD | 357 | 0.08 | ns |
| AUD/no-AUD | 404 | 0.08 | ns | |
| No-AUD/No-AUD | 140 | 0.08 | ns | |
| RD | AUD/AUD | 357 | 0.09 | ns |
| AUD/no-AUD | 404 | 0.17 | 0.03 | |
| No-AUD/No-AUD | 140 | 0.13 | ns |
4. Discussion
In this study we found that HA was significantly elevated in both alcoholics with and without ASPD across all three independent and diverse populations. The increase in HA among alcoholics as compared to controls ranged from 12% to 54%.
Our finding of increased HA among alcoholics with ASPD as well as the strongest association between HA and AUD among Finnish criminal alcoholics is to some extent counterintuitive and appears to contradict Cloninger’s typology. However, previous studies also have found high levels of internalizing symptomathology among alcoholics with comorbid ASPD (Babor et al., 1992; McGue et al., 1997; Widle & Scheidt, 2004). Although Babor’s Type A/B alcoholics largely resemble Cloninger’s type I/II, these two classificatory schemes differ in the attribution of internalizing symptomathology. Type B alcoholics are characterized by antisocial behaviors but also display anxious/depressive symptoms. Further, among the four types identified by Widle & Scheidt (2004) the chronic ASPD alcoholics, characterized by the highest level of adult antisocial behavior, displayed an increase in negative mood. Finally, high rates of comorbidity between anxiety disorders and ASPD have been reported by epidemiological studies (Goodwin et al., 2003)
Our study partially supports the discriminant validity of NS previously reported for alcoholic subtypes (Howard et al., 1997; Zimmermann et al., 2003). NS was a significant predictor of AUD in two of the three populations analyzed (COGA and Finns). The association between alcoholism and NS was not entirely accounted for by ASPD. In fact, NS distinguished alcoholics with ASPD from alcoholics without ASPD as well as the latter group from controls (figure 2). The relation between alcoholism and NS was not found among Plains Indians. In this population NS was not associated with AUD and, even more surprising, with ASPD. In addition, alcoholics with ASPD did not have an earlier onset than alcoholics without ASPD as found in the COGA and Finnish samples (see table 1). This is strange considering that the relationship between antisocial behavior and early onset, as well as the one between ASPD and NS, is well documented and consistent across the different approaches used to sub-classify alcoholics (Windle & Scheidt, 2004; Cloninger et al., 1981). It is important to consider that environmental or biological factors particular to Native Americans may alter relationships of NS to alcoholism and ASPD seen in other populations. Further, although provisions were taken to avoid diagnostic errors due to culture-specific phenomena, standard criteria used for ASPD diagnosis might be particularly problematic within the Native American socio-cultural context. Finally, age at onset data in the Plains sample may be difficult to interpret in comparison to the Finnish and COGA data, as criteria for early onset were different for this dataset
Inconsistent results were found for RD, in line with the mixed findings previously reported by other cross-sectional studies (Mulder, 2002). Significant differences emerged only in the Finnish population. Alcoholics with comorbid ASPD scored lower in RD than the other two groups and were characterized by the lowest social attachment. A possible explanation for this result is that low RD might identify antisocial alcoholics who more resemble the traditional psychopath profile characterized by low emotionality/detachment (Cleckley, 1976) and that are likely to be more represented in a sample of criminal alcoholics such as the Finns.
The present study, along with previous work (Babor et al., 1992; Windle & Scheidt, 2004; Zimmermann et al., 2004) showing higher prevalence of internalizing symptoms in alcoholics with ASPD, partially challenges the personality structure of alcohol subtypes hypothesized by Cloninger (1995). Alcoholics with ASPD tend to have both high HA and high NS as opposed to a profile of high NS and low HA. The associations of HA and NS with AUD observed in this study cannot be interpreted from a cause-effect perspective. However, if one assumes that temperament is a risk factor for AUD, this result might indicate that NS and HA influence the development of AUD in an additive way. Thus, individuals who are both high in HA and NS may have the highest risk. Another powerful model that may account for the increase in anxiety (HA) in alcoholics of all types is the allostatic activation of the brain stress axis (Roberts & Koob, 1997). In this model, repeated exposures to alcohol result in long-lasting, trait-like activations of stress/anxiety such that new stresses or alcohol cues are likely to lead to relapse and rapid reinstatement. In this model, preexisting anxiety/dysphoria and preexisting differences in stress resiliency play a role in vulnerability, but the addictive process itself homogenizes the clinical population in terms of higher anxiety and dysphoria. The cross-sectional nature of this study hinders the ability to discriminate whether the elevated anxiety in the alcoholics is primary or secondary. However, the family structure of the COGA population, comprised of alcoholic probands and their affected and unaffected relatives, allowed some exploration of the origin of the link between temperament traits and AUD. Since siblings share aspects of their environment as well as on average 50% of their genes, the existence of a correlation for a trait between pairs of siblings indicates the action of familial influences.
The HA correlations of approximately 0.2 that we observed between sibpairs are in line with correlation previously reported for fraternal twins in twin studies (Ando et al., 2004; Heath et al., 1994). Interestingly, HA correlations were the highest in pairs of sibling concordant for AUD diagnosis (0.23). Taking into account that among alcoholics HA is likely to measure a mixture of innate temperament and secondary effects of addiction, the evidence here suggest that both these components might be subject to familial/genetic influence. Consistently, genetic variation at GABRA2 has been recently associated with AUD moderated by HA in this Finnish and Plains Indian population (Enoch et al., 2006). The complete lack of correlation for HA in pairs of siblings discordant for AUD diagnosis (0.07) as compared to concordant pairs (0.23 and 0.18 for AUD/AUD and no-AUD/no-AUD pairs respectively) may support a genetic link between AUD and HA. In fact, if HA and AUD were independently transmitted within each family, concordance for HA would have been similar regardless of the AUD concordance or discordance. Pairs of concordant siblings might be more similar in HA levels than discordant pairs because they share an excess of genetic factors modulating HA which in turn have made them more or less likely to develop AUD. On the other hand, if one assumes that prolonged alcohol exposure is a major determinant of HA levels in alcoholics, genetic influences acting on HA might be obscured in the pairs discordant for diagnosis.
The lack of significant sib-pair correlations for NS and RD might be explained by the fact that almost all of the genetic influences acting on these traits have been reported to be non-additive, whereas for HA additive genetic influences appeared to be more important (Keller et al., 2005; Young et al., 2000). If interaction between several genes is required for expression of a trait, correlations between relatives rapidly decrease as the degree of relatedness decreases and might be detectable only in very large samples (Goldman et al., 2005). In line with this idea, for NS the correlation between DZ twins (who share on average half of their genes as well as non-twin siblings) has been reported to be only 0.02, while correlation between MZ was much higher (0.40) (Young et al., 2000).
Results from the present study should be interpreted in the context of several important limitations. First, none of the populations included can be considered an epidemiological sample. Therefore, some of the observed associations between temperament and AUD might reflect the ways in which these samples were selected rather than associations that exist in the general population. In the Finnish population, the high level of HA we detected among alcoholics, including alcoholics with ASPD, might be partially due to the persistent exposure to a stressful environment such as prison. In the COGA population, similarly to other populations of alcoholics mainly ascertained from treatment facilities, internalizing psychopathology has been described as largely drug-induced and to a lesser extent, primary (Schuckit et al., 1997). Finally, differences in mode of ascertainment, diagnostic instrument used, severity of AUD, cultural background etc. make these three samples hardly comparable one to the other. However, the multi-samples design we used might help in discriminating between associations that are consistent and probably related to AUD in general, from those that are more contextual and related to particular population of alcoholics. Thus, higher NS seems to be a feature of certain populations of alcoholics but not all; for example, not the Native American sample we studied. However, increase in trait anxiety might be a consistent feature of AUD despite the diversity of cultures and modes of ascertainment represented by the populations we, and others previously, have studied. Within populations of alcoholics, there is substantial variation in anxiety, but levels of anxiety are at least as high in alcoholics with earlier age at onset and antisocial behavior. Thus, such alcoholics frequently have more than one “strike against them” – early exposure, behavioral dyscontrol and negative mood. HA shows very similar levels of familiality in pairs of alcoholic siblings as well as in pairs of non-alcoholic siblings from the same families. The significant correlation for HA between alcoholic siblings suggest that, even after long-term exposure to alcohol, genetic influences might moderate anxiety.
Acknowledgments
This research was supported by the Intramural Research Program of the National Institute on Alcohol Abuse and Alcoholism, NIH, and in part by the Office of Research on Minority Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ando J, Suzuki A, Yamagata S, Kijima N, Maekawa H, Ono Y, Jang KL. Genetic and environmental structure of Cloninger’s temperament and character dimensions. J Personal Disord. 2004;18:379–393. doi: 10.1521/pedi.18.4.379.40345. [DOI] [PubMed] [Google Scholar]
- Babor TF, Hofmann M, DelBoca FK, Hesselbrock V, Meyer RE, Dolinsky ZS, Rounsaville B. Types of alcoholics, I. Evidence for an empirically derived typology based on indicators of vulnerability and severity. Arch Gen Psychiatry. 1992;49:599–608. doi: 10.1001/archpsyc.1992.01820080007002. [DOI] [PubMed] [Google Scholar]
- Brewer RD, Swahn MH. Binge drinking and violence. JAMA. 2005;294:616–618. doi: 10.1001/jama.294.5.616. [DOI] [PubMed] [Google Scholar]
- Bucholz KK, Cadoret R, Cloninger CR, Dinwiddie SH, Hesselbrock VM, Nurnberger JI, Jr, Reich T, Schmidt I, Schuckit MA. A new, semi-structured psychiatric interview for use in genetic linkage studies: a report on the reliability of the SSAGA. J Stud Alcohol. 1994;55:149–158. doi: 10.15288/jsa.1994.55.149. [DOI] [PubMed] [Google Scholar]
- Cleckley H. The mask of sanity: An attempt to clarify some issues about the so-called psychopathic personality. 5. St Luis, Mo: Mosby-Year Book Inc; 1976. [Google Scholar]
- Cloninger CR, Bohman M, Sigvardsson S. Inheritance of alcohol abuse. Cross-fostering analysis of adopted men. Arch Gen Psychiatry. 1981;38:861–868. doi: 10.1001/archpsyc.1981.01780330019001. [DOI] [PubMed] [Google Scholar]
- Cloninger CR. A systematic method for clinical description and classification of personality variants. A proposal, Arch Gen Psychiatry. 1987;44:573–588. doi: 10.1001/archpsyc.1987.01800180093014. [DOI] [PubMed] [Google Scholar]
- Cloninger CR, Sigvardsson S, Przybeck TR, Svrakic DM. Personality antecedents of alcoholism in a national area probability sample. Eur Arch Psychiatry Clin Neurosci. 1995;245:239–244. doi: 10.1007/BF02191803. [DOI] [PubMed] [Google Scholar]
- Copeland W, Landry K, Stanger C, Hudziak JJ. Multi-informant assessment of temperament in children with externalizing behavior problems. J Clin Child Adolesc Psychol. 2004;33:547–556. doi: 10.1207/s15374424jccp3303_12. [DOI] [PubMed] [Google Scholar]
- Del Boca FK, Hesselbrock MN. Gender and alcohol subtypes. Alcohol Health and Research World. 1996;20:56–62. [PMC free article] [PubMed] [Google Scholar]
- Dell’Osso L, Saettoni M, Papasogli A, Rucci P, Ciapparelli A, Di Poggio AB, Ducci F, Hardoy C, Cassano GB. Social anxiety spectrum: gender differences in Italian high school students. J Nerv Ment Dis. 2002;190:225–232. doi: 10.1097/00005053-200204000-00003. [DOI] [PubMed] [Google Scholar]
- Endicott J, Spitzer RL. A diagnostic interview: the Schedule for Affective Disorders and Schizophrenia. Arch Gent Psychiatry. 1978;35:837–844. doi: 10.1001/archpsyc.1978.01770310043002. [DOI] [PubMed] [Google Scholar]
- Enoch MA, Waheed JF, Harris CR, Albaugh B, Goldman D. Sex differences in the influence of COMT Val158Met on alcoholism and smoking in plains American Indians. Alcohol Clin Exp Res. 2006;30:399–406. doi: 10.1111/j.1530-0277.2006.00045.x. [DOI] [PubMed] [Google Scholar]
- Enoch MA, Schwartz L, Albaugh B, Virkkunen M, Goldman D. Dimensional Anxiety Mediates Linkage of GABRA2. Haplotypes with Alcoholism, AM J Med Genet (Neuropsychiatr Genet) 2006 doi: 10.1002/ajmg.b.30336. [Epub ahead of print] PMID: 16874763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu Q, Heath AC, Bucholz KK, Nelson E, Goldberg J, Lyons MJ, True WR, Jacob T, Tsuang MT, Eisen SA. Shared genetic risk of major depression, alcohol dependence, and marijuana dependence: contribution of antisocial personality disorder in men. Arch Gen Psychiatry. 2002;59:1125–1132. doi: 10.1001/archpsyc.59.12.1125. [DOI] [PubMed] [Google Scholar]
- Goldman D, Oroszi G, Ducci F. The genetics of addictions: uncovering the genes. Nat Rev Genet. 2005;6:521–532. doi: 10.1038/nrg1635. [DOI] [PubMed] [Google Scholar]
- Goodwin RD, Hamilton SP. Lifetime comorbidity of antisocial personality disorder and anxiety disorders among adults in the community. Psychiatry Res. 2003;117:159–166. doi: 10.1016/s0165-1781(02)00320-7. [DOI] [PubMed] [Google Scholar]
- Grant BF, Stinson FS, Dawson DA, Chou SP, Ruan WJ, Pickering RP. Co-occurrence of 12-month alcohol and drug use disorders and personality disorders in the United States: results from the National Epidemiologic Survey on Alcohol and Related Conditions. Arch Gen Psychiatry. 2004a;61:361–368. doi: 10.1001/archpsyc.61.4.361. [DOI] [PubMed] [Google Scholar]
- Grant BF, Stinson FS, Dawson DA, Chou SP, Dufour MC, Compton W. Pickering, R. P. & Kaplan, K. Prevalence and co-occurrence of substance use disorders and independent mood and anxiety disorders: results from the National Epidemiologic Survey on Alcohol and Related Conditions. Arch Gen Psychiatry. 2004b;61:807–816. doi: 10.1001/archpsyc.61.8.807. [DOI] [PubMed] [Google Scholar]
- Greene RL, Robin RW, Albaugh B, Caldwell A, Goldman D. Use of the MMPI-2 in American Indians: II. Empirical correlates. Psychol Assess. 2003;15:360–369. doi: 10.1037/1040-3590.15.3.360. [DOI] [PubMed] [Google Scholar]
- Heath AC, Cloninger CR, Martin NG. Testing a model for the genetic structure of personality: a comparison of the personality systems of Cloninger and Eysenck. J Pers Soc Psychol. 1994;66:762–775. doi: 10.1037//0022-3514.66.4.762. [DOI] [PubMed] [Google Scholar]
- Howard MO, Kivlahan D, Walker RD. Cloninger’s tridimensional theory of personality and psychopathology: applications to substance use disorders. J Stud Alcohol. 1997;58:48–66. doi: 10.15288/jsa.1997.58.48. [DOI] [PubMed] [Google Scholar]
- Keller MC, Coventry WL, Heath AC, Martin NG. Widespread Evidence for Non-Additive Genetic Variation in Cloninger’s and Eysenck’s Personality Dimensions using a Twin Plus Sibling Design. Behav Genet. 2005;35:707–721. doi: 10.1007/s10519-005-6041-7. [DOI] [PubMed] [Google Scholar]
- Kendler KS, Heath AC, Neale MC, Kessler RC, Eaves LJ. Alcoholism and major depression in women. A twin study of the causes of comorbidity. Arch Gen Psychiatry. 1993;50:690–698. doi: 10.1001/archpsyc.1993.01820210024003. [DOI] [PubMed] [Google Scholar]
- Kendler KS, Prescott CA, Myers J, Neale MC. The structure of genetic and environmental risk factors for common psychiatric and substance use disorders in men and women. Arch Gen Psychiatry. 2003;60:929–937. doi: 10.1001/archpsyc.60.9.929. [DOI] [PubMed] [Google Scholar]
- Kessler RC, Crum RM, Warner LA, Nelson CB, Schulenberg J, Anthony JC. Lifetime co-occurrence of DSM-III-R alcohol abuse and dependence with other psychiatric disorders in the National Comorbidity Survey. Arch Gen Psychiatry. 1997;54:313–321. doi: 10.1001/archpsyc.1997.01830160031005. [DOI] [PubMed] [Google Scholar]
- Krueger RF, Hicks BM, Patrick CJ, Carlson SR, Iacono WG, McGue M. Etiologic connections among substance dependence, antisocial behavior, and personality: modeling the externalizing spectrum. J Abnorm Psychol. 2002;111:411–424. [PubMed] [Google Scholar]
- Lappalainen J, Long JC, Eggert M, Ozaki N, Robin RW, Brown GL, Naukkarinen H, Virkkunen M, Linnoila M, Goldman D. Linkage of antisocial alcoholism to the serotonin 5-HT1B receptor gene in 2 populations. Arch Gen Psychiatry. 1998;55:989–994. doi: 10.1001/archpsyc.55.11.989. [DOI] [PubMed] [Google Scholar]
- McGue M, Slutske W, Taylor J, Iacono WG. Personality and substance use disorders: I. Effects of gender and alcoholism subtype. Alcohol Clin Exp Res. 1997;21:513–520. [PubMed] [Google Scholar]
- Mulder RT. Alcoholism and personality. Aust N Z J Psychiatry. 2002;36:44–52. doi: 10.1046/j.1440-1614.2002.00958.x. [DOI] [PubMed] [Google Scholar]
- Ono Y, Ando J, Onoda N, Yoshimura K, Momose T, Hirano M, Kanba S. Dimensions of temperament as vulnerability factors in depression. Mol Psychiatry. 2002;7:948–953. doi: 10.1038/sj.mp.4001122. [DOI] [PubMed] [Google Scholar]
- Prescott CA, Neale MC, Corey LA, Kendler KS. Predictors of problem drinking and alcohol dependence in a population-based sample of female twins. J Stud Alcohol. 1997;58:167–181. doi: 10.15288/jsa.1997.58.167. [DOI] [PubMed] [Google Scholar]
- Prescott CA, Aggen SH, Kendler KS. Sex-specific genetic influences on the comorbidity of alcoholism and major depression in a population-based sample of US twins. Arch Gen Psychiatry. 2000;57:803–811. doi: 10.1001/archpsyc.57.8.803. [DOI] [PubMed] [Google Scholar]
- Prescott CA, Caldwell CB, Carey G, Vogler GP, Trumbetta SL, Gottesman II. The Washington University Twin Study of alcoholism. Am J Med Genet B Neuropsychiatr Genet. 2005;134:48–55. doi: 10.1002/ajmg.b.30124. [DOI] [PubMed] [Google Scholar]
- Roberts AJ, Koob GF. The neurobiology of addiction: an overview. Alcohol Health Res World. 1997;21:101–106. [PMC free article] [PubMed] [Google Scholar]
- Sher KJ, Bartholow BD, Wood MD. Personality and substance use disorders: a prospective study. J Consult Clin Psychol. 2000;68:818–829. [PubMed] [Google Scholar]
- Schuckit MA, Tipp JE, Bucholz KK, Nurnberger JI, Jr, Hesselbrock VM, Crowe RR, Kramer J. The life-time rates of three major mood disorders and four major anxiety disorders in alcoholics and controls. Addiction. 1997;92:1289–1304. [PubMed] [Google Scholar]
- Spitzer RL, William JBW, Gibbon M, First MB. Structured Clinical Interview for DSM-III-R Patient Edition (with psychotic screen-W/Psychotic screen)-Version 1.0. Washington, DC: American Psychiatry Press; 1990a. [Google Scholar]
- Spitzer RL, William JBW, Gibbon M, First MB. Structured Clinical Interview for DSM-III-R -Non patient Edition (SCID-NP, version 1.0) Washington, DC: American Psychiatry Press; 1990b. [Google Scholar]
- Spitzer RL, William JBW, Gibbon M, First MB. Structured Clinical Interview for DSM-III-R Personality Disorder SCID-II (Version.01) Washington, DC: American Psychiatry Press; 1990c. [Google Scholar]
- Stallings MC, Hewitt JK, Cloninger CR, Heath AC, Eaves LJ. Genetic environmental structure of the Tridimensional Personality Questionnaire: three or four temperament dimensions? J Pers Soc Psychol. 1996;70:127–140. doi: 10.1037//0022-3514.70.1.127. [DOI] [PubMed] [Google Scholar]
- Windle M, Scheidt DM. Alcoholic subtypes: are two sufficient? Addiction. 2004;99:1508–1519. doi: 10.1111/j.1360-0443.2004.00878.x. [DOI] [PubMed] [Google Scholar]
- Young SE, Stallings MC, Corley RP, Krauter KS, Hewitt JK. Genetic and environmental influences on behavioral disinhibition. Am J Med Genet. 2000;96:684–695. [PubMed] [Google Scholar]
- Zimmermann P, Wittchen HU, Hofler M, Pfister H, Kessler RC, Lieb R. Primary anxiety disorders and the development of subsequent alcohol use disorders: a 4-year community study of adolescents and young adults. Psychol Med. 2003;33:1211–1222. doi: 10.1017/s0033291703008158. [DOI] [PubMed] [Google Scholar]
- Zucker RA. The four alcoholisms: a developmental account of the etiologic process. Nebr Symp Motiv. 1986;34:27–83. [PubMed] [Google Scholar]


