Abstract
Renal ischemia/reperfusion (I/R) injury often occurs as a result of vascular surgery, organ procurement, or transplantation. We previously showed that renal I/R results in ATP depletion, oxidant production, and manganese superoxide dismutase (MnSOD) inactivation. There have been several reports that overexpression of MnSOD protects tissues/organs from I/R related damage, thus a loss of MnSOD activity during I/R likely contributes to tissue injury. The present study examined the therapeutic benefit of a catalytic antioxidant Mn(III) meso-tetrakis(N-hexylpyridinium-2-yl)porphyrin, (MnTnHex-2-PyP5+) using the rat renal I/R model. This was the first study to examine the effects of MnTnHex-2-PyP5+ in an animal model of oxidative stress injury. Our results showed that porphyrin pretreatment of rats for 24 hr protected against ATP depletion, MnSOD inactivation, nitrotyrosine formation, and renal dysfunction. The dose (50 μg/kg) used in this study is lower than doses of various types of antioxidants commonly used in animal models of oxidative stress injuries. In addition, using novel proteomic techniques, we identified ATP synthase- beta subunit as a key protein induced by MnTnHex-2-PyP5+ treatment alone, and complex V (ATP synthase) as a target of injury during renal I/R. These results showed that MnTnHex-2-PyP5+ protected against renal I/R injury via induction of key mitochondrial proteins that may be capable of blunting oxidative injury.
Keywords/Abbreviations: kidney, ischemia/reperfusion, metalloporphyrin, proteomics, MnSOD, mitochondria, oxidants, nitrotyrosine, blue native polyacrylamide gel electrophoresis BN-PAGE, two dimensional fluorescence differential in gel electrophoresis (2D-DIGE)
Introduction
Kidneys were the first successfully transplanted organs, and are the most commonly performed transplants today. Approximately 65,000 patients are currently on waiting lists for renal transplantation; however, only 10,000 donor kidneys were available in 2005 [9]. Renal transplantation is one of the most successful solid-organ transplant procedures as measured by 1-year survival rates. However, significant graft loss occurs due to delayed (>1 year) graft function and processes of chronic allograft nephropathy (CAN). The precise cause of CAN following renal allografts remains unclear, but appears to be related to pathological changes due to organ preservation techniques and ischemia/reperfusion (I/R) injury prior to and after surgical implantation [6,7,15,17,20]. It is well established that excessive oxidant production occurs during renal I/R [11,25,31,33]. The increase in oxidants overwhelms endogenous antioxidants leading to increased cellular injury. Several studies using models of I/R have clearly demonstrated free radical or reactive oxygen species (ROS) damage as evidenced by increased lipid peroxidation and loss of function of cellular antioxidant proteins including catalase, glutathione peroxidase, and superoxide dismutases, Cu,Zn SOD (SOD1), and MnSOD (SOD2) [11,15]. Finally, I/R has been shown to result in mitochondrial dysfunction. Mitochondria are extremely sensitive to I/R injury, with subsequent changes in oxidative phosphorylation, ATP depletion, a rise in intracellular calcium, mitochondrial swelling, and loss of respiratory complex activity [18,19,34,38].
Previously, using a rat model of renal I/R [11] and transplantation [26,27] we reported that MnSOD, the major mitochondrial antioxidant, was inactivated before the onset of renal dysfunction, suggesting that loss of mitochondria antioxidant protection might play an important role in I/R related renal injury. MnSOD is the major antioxidant in the mitochondria. Its main function is to catalyze the dismutation of O2.−, which is continually generated via misfires (~1–2% of total oxygen consumption) in the electron transport chain and from other sources. MnSOD is a nuclear-encoded protein that is transported into the mitochondria via an amino-terminal targeting sequence, and is subsequently cleaved to form its native homotetrameric structure of 96 kDa. Studies with MnSOD knockout mice provide unequivocal evidence that MnSOD is essential for life [22,24]. This is not the case for the other two superoxide dismutase family members: cytosolic SOD1 and extracellular SOD (SOD3), in which no lethality is observed following gene deletion [5,36].
MnSOD heterozygous knockout mice (expressing 50% of the normal complement of MnSOD) appear to develop normally and, in the absence of oxidative stress, fail to show any overt phenotypic change compared to wild-type animals [22,24]. However, recent studies have demonstrated that mitochondria from MnSOD heterozygous knockout mice do show oxidative damage and alterations in mitochondrial function [39]. Thus, it seems apparent that any condition that results in loss of MnSOD activity (even only a 50% reduction) in mitochondria would have serious, potentially lethal consequences. Importantly, our laboratory demonstrated a 50% reduction in MnSOD activity in kidneys exposed to warm I/R [11]. In addition, there have been several reports that overexpression of MnSOD protects tissues/organs from I/R related damage [1,7,8,20]; thus a loss of MnSOD activity during I/R likely contributes to tissue injury.
Experiments were designed to evaluate the therapeutic potential of a newly designed metalloporphyrin-based antioxidant MnTnHex-2-PyP5+ in the rat renal I/R model. This porphyrin has drawn attention because it is significantly more lipophilic than widely used MnTE-2-PyP5+, while possessing the same ability to eliminate O2.− [2] and ONOO− [16]. Due to its lipophilicity MnTnHex-2-PyP5+ was around 10-fold more efficient in allowing SOD-deficient E. coli to grow aerobically than MnTE-2-PyP5+ [32]. The data provided thus far indicate that the most potent Mn porphyrins studied in vivo are very effective in decreasing levels of oxidant species [13,14,30,35]. But there is also growing evidence that porphyrins may do more than quench oxidant production [10,37]. For example, porphyrins have been shown to inactivate transcription factors AP-1, NF-κB, HIF-1, either through eliminating reactive species or through directly oxidizing them [37], therefore affecting expression of corresponding genes. The goal of the current study was to determine whether MnTnHex-2-PyP5+ could blunt MnSOD inactivation and renal injury in our rat renal I/R model. Our data show that MnTnHex-2-PyP5+ treatment for a short period (30 minutes) was ineffective, while longer treatment (24 hr) was renal protective and appeared to involve upregulation of important mitochondrial proteins.
Materials and Methods
Rat Ischemia/Reperfusion Model
Animals were treated according to UAMS IACUC guidelines. Male inbred Fisher 344 rats weighing 250–300 grams were used in this study. Ethrane was used to anesthetize the rats, followed by shaving and prepping with betadine. A 2-ml bolus of 0.9% (w/v) NaCl was administered intravenously and an incision made 1 cm superior to the symphysis pubis to the tip of the xyphoid process. The abdominal organs were retracted to the left to allow access to the retroperitoneum. A right nephrectomy was performed so that renal function reflects the function of the left kidney alone. The left renal artery and vein were clamped for 40 min, resulting in a color change of the left kidney, during which time moist gauze was placed in the abdominal cavity. After 40 min, the clamp was released and the kidney turned red (~3 min), indicating proper reperfusion. Postoperatively, the animals were given 2 ml of 0.9% (w/v) NaCl in the abdominal cavity and placed under a heating lamp to recover from the anesthesia. Immediately following the onset of reperfusion and 18 hr prior to sacrifice, rats were placed in metabolic cages to collect and measure urine. Urine was analyzed for creatinine concentration (uCr) using a Roche Mira Classic analyzer. At the end of the reperfusion (18 hr), the animals were anesthetized (Ethrane), the left kidney removed, and blood collected via intracardiac puncture.
Experimental Groups
I/R group
Rats were subjected to a right nephrectomy and renal artery occlusion (40 min) followed by 18 hr reperfusion (n = 5).
I/R + MnTnHex-2-PyP5+ group (24 hr)
Rats received an i.v. (penile vein) bolus of MnTnHex-2-PyP5+ (50 μg/kg in 0.5 ml saline) 24 hr prior to right nephrectomy and renal I/R (n = 5).
I/R + MnTnHex-2-PyP5+ group (30 min)
Rats received an i.v. (penile vein) bolus of MnTnHex-2-PyP5+ (50 μg/kg in 0.5 ml saline) 30 min prior to right nephrectomy and renal I/R (n = 5).
Sham-operated group
Rats underwent identical surgery (nephrectomy), but without the I/R episode (n = 5).
Sham + MnTnHex-2-PyP5+ group
Rats received an i.v. bolus (penile vein) of MnTnHex-2-PyP5+ (50 μg/kg in 0.5 ml saline) 24 hr prior to right nephrectomy (n = 5). MnTnHex-2-PyP5+ was synthesized as previously described [2].
Assessment of renal function
Creatinine clearance
At the time of kidney harvesting, rats were prepared for determination of creatinine clearance (CrCl) [40]. Maximum blood volumes (via intracardiac puncture) were recovered followed by determination of serum creatinine (sCr) using a Roche Mira Classic serum analyzer calibrated for rat samples: CrCl = (uCr × uVolume)/(sCr × 1080 min).
Histological Assessment
Renal tissue was formalin-fixed, paraffin-embedded, and processed as described [21]. Nitrotyrosine staining was performed using the polyclonal anti-nitrotyrosine antibody (1:700) as described [26]. Renal tissue injury was assessed in tissue sections stained using the Periodic Acid-Schiff (PAS) reaction, which permits assessment of tubular integrity.
Renal extract preparation
Renal extracts were made from frozen tissue by homogenizing (0.1 g/ml), using a Polytron homogenizer in buffer containing 50 mM potassium phosphate, pH 7.4 and 1mM phenylmethylsulfonylfluoride. Solubilized extracts were sonicated and centrifuged at 10,000 rpm (5 min, 4°C) to remove tissue debris. Protein concentrations were determined by Coomassie Plus Protein Assay Reagent (Pierce).
Western blot analysis
MnSOD Western analysis was performed using the polyclonal anti-MnSOD antibody (Upstate Biotechnology; 1:1000) or the polyclonal anti ATP synthase-beta subunit antibody (Invitrogen; 1:10,000). Probed membranes were washed three times and immunoreactive proteins were detected using horseradish peroxidase conjugated secondary antibodies and enhanced chemiluminescence.
MnSOD activity assay
Enzymatic activity of MnSOD was determined in renal extracts by the cytochrome c reduction method using 1mM KCN as described by Fridovich et al [28].
Blue-native polyacrylamide gel electrophoresis (BN-PAGE)
Mitochondrial isolation
Renal mitochondria were isolated by differential centrifugation in a sucrose-containing buffer. BN-PAGE was performed with minor modifications as described by Brookes et al. [4]. Briefly, mitochondrial pellets (200 μg protein) were resuspended in 50 μl of extraction buffer containing aminocaproic acid (0.75 M) and BisTris (50 mM, pH 7). 6.25 μl of L n-dodecyl-D-maltoside (lauryl-maltoside, 10% w/v) was added to this suspension and incubated on ice (20 min). Samples were centrifuged (14,000 × g for 10 min), and 6.3 μl of Coomassie brilliant blue G (5%, in aminocaproic acid, 0.5 M) was added to 50 μl of the supernatant. Subsequently, samples (80 μg) were quickly loaded into a native polyacrylamide gel (6%). Electrophoresis (40 V) used an anode buffer comprised of BisTris (50 mM pH 7) and cathode buffer comprised of Tricine (50 mM), BisTris (15 mM pH 7), and Coomassie Brilliant blue G (0.02% w/v). After 1 hr, the cathode buffer was replaced with one containing less Coomassie blue (0.002%), and electrophoresis resumed at 110 V, until the blue dye front reached the bottom. The gel was then stained (1 hr) with a Coomassie blue solution (Coomassie Brilliant blue G (0.05% w/v), Coomassie Brilliant blue R-250 (0.05% w/v) in 25% isopropanol, 10% acetic acid) followed by destaining (20% Isopropanol, 5% acetic acid).
Two Dimensional Fluorescence Differential in Gel Electrophoresis (2D-DIGE)
As a complementary approach to BN-PAGE, samples were also subjected to a highly quantitative, state-of-the-art two dimensional “differential in gel electrophoresis” (2D-DIGE) proteomics analysis. Renal homogenates were sent to Applied Biomics (Hayward, CA) and were pre-labeled with one of three CyDyes, which are matched in terms of size and charge and, therefore, do not alter protein migration in either dimension (IEF or SDS-PAGE) (see also www.appliedbiomics.com website). The excitation and emission wavelengths of the CyDyes are resolvable with filters such that each protein spot can be quantified. Equal protein amounts of all three fluorescently labeled renal samples were mixed and loaded onto the same electrophoresis gel. Proteins were resolved using 2-D electrophoresis (Isoelectric focusing followed by SDS-PAGE). The gel was then scanned with a sensitive imager and DeCyder software was used to locate and analyze the protein spots based on their specific fluorescence.
Finally, protein spots of interest were excised from the gel using a ProPic (Genomic Solutions) imaging and spot picking robot. Protein digestion was performed with a ProGest (Genomic Solutions) in-gel enzymatic digestion robot using sequencing grade modified trypsin (Promega). The resulting peptide mixture was loaded using an autosampler onto a trapping column (Symmetry 300 C18 5 μm NanoEase, Waters) using a CapLC XE (Waters) system, a switching valve, and a flow rate of 20 μl/min. Peptides were separated by nanoflow capillary HPLC using a CapLC XE pump (Waters) operating at 12 μl/min; flow rate was controlled with a splitter in front of the switching valve. Peptides were eluted at 300 nl/min onto a self-packed PicoFrit (New Objective) 75 μm × 20 cm column (Jupiter 4u Proteo 90A, Phenomenex). The eluant was analyzed in-line by ESI-MS/MS using a Micromass Q-Tof Micro (Waters) mass spectrometer operating in the positive ion mode. Data acquisition was performed in a data dependent fashion, and the resulting data were processed to generate a peak list file using MassLynx (Waters) and searched against the NCBI non-redundant database using the Mascot search engine (www.matrixscience.com).
Cell Culture
A normal rat kidney proximal tubular cell line (NRK-52E; ATCC No. CRL-1571) was used as recently described [12]. Cells were maintained in a humidified incubator gassed with 5% CO2-95% air at 37°C in DMEM medium containing 5% fetal calf serum. Following treatment with MnTnHex-2-PyP5+ (2 μM; 24 hr), cells were trypsinized in 0.25% trypsin/EDTA and lysed in PBS plus 1% Triton at 4°C for 30 minutes. Following lysis, cells were centrifuged at 10,000 × g for 5 minutes and the supernatant was assayed for protein.
Statistical analysis
All data were expressed as means +/- SEM. Comparisons between groups were performed using one-way ANOVA. We used the Tukey’s test as the post-hoc ANOVA test; differences were considered statistically significant in the p value was less than 0.05 with a power of 0.80.
Results and Discussion
Experiments were designed to determine whether MnTnHex-2-PyP5+ could reduce renal injury using the rat renal I/R model
Male Fisher rats were pretreated (30 minutes) with MnTnHex-2-PyP5+ (50 μg/kg; iv) prior to renal I/R. Surprisingly, renal function, as measured by creatinine clearance, was not protected in the rats given the porphyrin (MnP) 30 min prior to ischemia compared to rats undergoing I/R without drug (I/R) (Figure 1). Due to the intense brown color of the porphyrin, we could visibly see that the compound was quickly delivered to the kidney during this 30 min incubation. Since the 30-minute treatment group was not protective we extended the time of porphyrin treatment to 24 hr. Rats receiving MnTnHex-2-PyP5+ 24 hr prior to ischemia (I/R + MnP, 24 hr), showed improved renal function compared to rats undergoing I/R without drug (I/R) (Figure 1). Rats not exposed to I/R (sham operated animals) treated with the same concentration of MnTnHex-2-PyP5+ (SH + MnP) showed no change in renal function, suggesting the compound had little renal toxicity at the dose given. The lack of toxicity was not surprising given the low dose (50 μg/kg) and route of administration (iv) used in these studies; other reports showing protective effects in vivo have used higher and often multiple doses of Mn-porphyrins or other antioxidants (from ~1–10 mg/kg with porphyrins and cyclic polyamines to as high as 600 mg/kg with nitroxides) and a different route of administration (intraperitoneal) [3,10,13,14,23,29,30,35,41]. It is also important to note that previous studies using higher doses of MnTnHex-2-PyP5+ did show cellular toxicity [32]. These data suggest that at the dose used in these studies, the longer incubation time (24 hr) was necessary for porphyrin-mediated protection against renal I/R injury.
Figure 1.
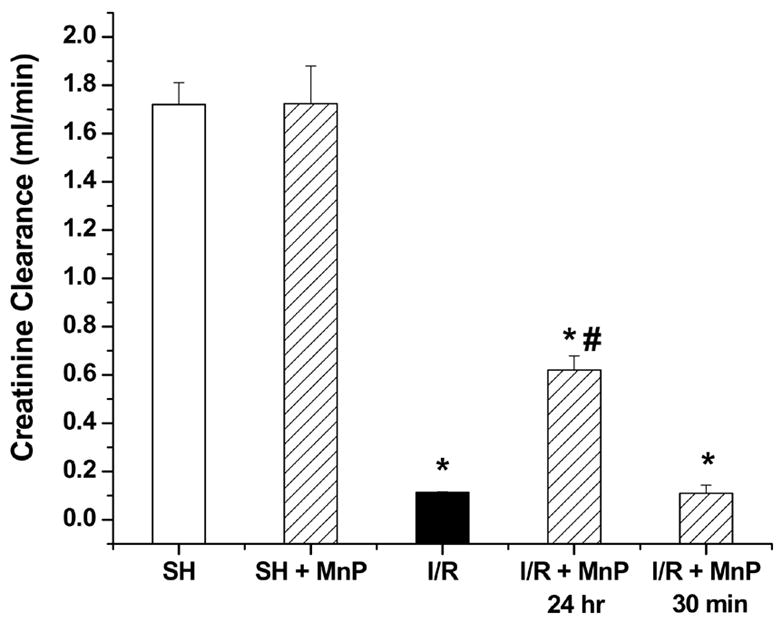
Effect of MnTnHex-2-PyP5+ on creatinine clearance following I/R (40 min ischemia/18 hr reperfusion). Creatinine clearance was determined in rats receiving MnTnHex-2-PyP5+ (50 μg/kg; 30 min or 24 hr prior to ischemia) (MnP). Values are expressed as Mean ± SEM (n = 5). * P < 0.05 compared with sham (SH); # P < 0.05 compared with I/R.
Additional studies examined the ability of MnTnHex-2-PyP5+ to blunt MnSOD inactivation in our rat model of renal I/R. MnTnHex-2-PyP5+ failed to increase MnSOD activity in sham treated rats (SH + MnP), suggesting that the dose and duration (30 min or 24 hr) of treatment did not increase basal antioxidant capacity (Figure 2A). This indicated that MnTnHex-2-PyP5+ was not acting as a classic ‘SOD mimetic’ in our system. However, rats receiving MnTnHex-2-PyP5+ 24 hr prior to I/R (I/R + MnP, 24 hr) showed improved MnSOD activity compared to rats exposed to I/R without drug. Consistent with the previous findings, no protection was observed in the 30-minute pretreatment group (I/R + MnP, 30 min). No alterations of MnSOD protein levels were noted in any of the rat groups (Figure 2B). These data suggest that 24 hr pretreatment with MnTnHex-2-PyP5+ protects MnSOD from inactivation in rats exposed to renal I/R. Thus, this compound may represent one therapeutic approach to preserving mitochondrial function and improving renal function during ischemia and reperfusion.
Figure 2.
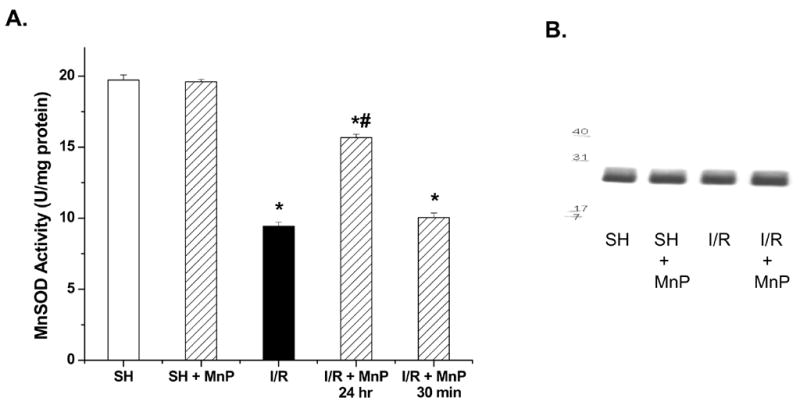
Effect of MnTnHex-2-PyP5+ on MnSOD inactivation following I/R (40 min ischemia/18 hr reperfusion). A. MnSOD activity of renal extracts were determined using the cytochrome c assay in rats receiving MnTnHex-2-PyP5+ (50 μg/kg; 24 hr or 30 min prior to ischemia) (MnP). Values are expressed as Mean ± SEM (n = 5). * P < 0.05 compared with sham (SH); # P < 0.05 compared with I/R. B. MnSOD Western blot showing equal protein levels in the 4 rat groups (MnP was 24 hr treatment).
Periodic acid-Schiff (PAS) staining was used to examine tubular histopathological changes associated with loss of membrane integrity, and also revealed improved renal histology in rats receiving MnTnHex-2-PyP5+ 24 hr prior to I/R. Specifically, rats undergoing I/R without drug showed extensive tubular damage (dilation, loss of brush border, extensive tubular casts and debris), whereas rats receiving pre-treatment with MnTnHex-2-PyP5+ (I/R + MnP, 24 hr) showed less severe tubular damage (Figure 3). Likewise, MnTnHex-2-PyP5+ (24 hr) also decreased the levels of nitrotyrosine within the kidney during I/R. Again, the 30 min pretreatment group (I/R + MnP, 30 min) did not protect against tubular damage or nitrotyrosine staining.
Figure 3.
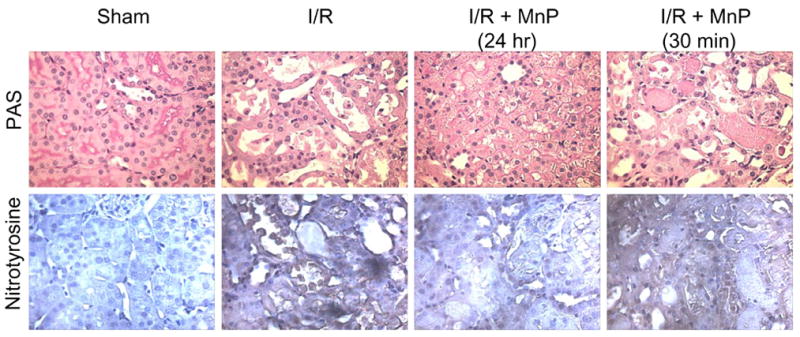
Periodic acid-Schiff (PAS) and Nitrotyrosine (1:500) staining of renal tissue following I/R with and without pretreatment of MnTnHex-2-PyP5+ (MnP); 400X. These sections are representative of the rats used in each group (n = 5).
We used a relatively new proteomic approach called two dimensional fluorescence differential in gel electrophoresis (2D-DIGE) to begin to dissect the mechanism by which the porphyrin protects against tyrosine nitration and I/R-mediated renal injury. This is a highly quantitative, state-of-the-art proteomics analysis. In 2-D DIGE, three separate protein samples (rat kidney homogenates) were pre-labeled with three distinct size and charge-matched, spectrally resolvable CyDye™ fluorophores. Thus, three samples can be compared to each other simultaneously, i.e., A compared to B, A to C, and B to C. This quantitative “crosschecking” provides an internal control for the accuracy and precision of each protein spot, making 2D-DIGE far superior to conventional 2-D gel overlay methods.
Kidneys from rats receiving MnTnHex-2-PyP5+ alone (50 μg/kg; 24 hr or 30 minutes prior to kidney harvest) were compared to control rats without drug to screen for alterations in renal proteins by drug alone. The scale and scope of the 2D-DIGE approach is very broad. For example, this analysis revealed 1840 distinct protein spots that could be rigorously quantified. Of these spots, 6.1% were increased by ≥ 1.5-fold and 5.8% were decreased by ≥ 1.5-fold. Only 15 spots increased or decreased by ≥ 2-fold. Since only the 24 hr drug treatment proved to be protective in vivo, the comparison of 24 hr drug to control is the most relevant; spots showing ≥ 1.5-fold increases/decreases in 24 hr drug (relative to control) were chosen for identification. Figure 4 shows the results when the control kidney is compared to 24 hr MnTnHex-2-PyP5+ (Panel A); as well as the 24 hr versus the 30-minute comparison (Panel B). Based on the labeling done with these samples, protein spots that are increased (relative to control in Panel A; and 30-minute drug treatment in Panel B) show up as red, and spots that are decreased show up green; equal alignment and intensity of red and green yield yellow, meaning that these proteins are unchanged.
Figure 4.
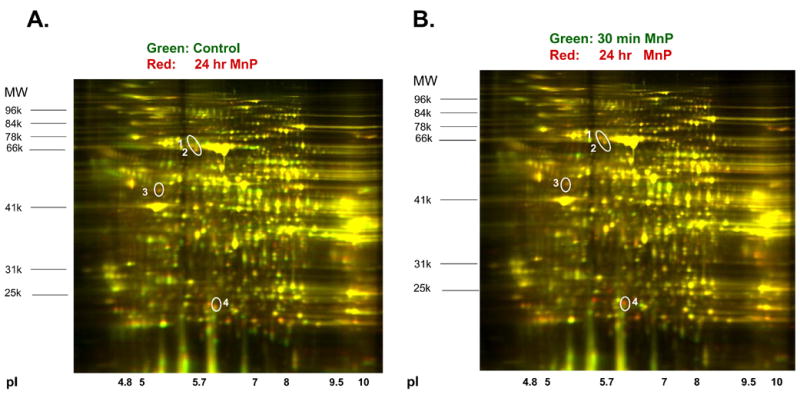
Two-Dimensional Differential in Gel Electrophoresis of renal proteins following treatment with Porphyrin (MnP). Rats were injected with MnTnHex-2-PyP5+ (50 μg/kg 24 hr prior to kidney harvest). Renal proteins (200 μg) were subjected to 2D-DIGE (Applied Biomics). Proteins were separated by isoelectric point (pI), from pH ~3–10 along the horizontal axis, and by molecular mass, from ~100 to 20 kD, vertically. The circle represents the spots which were increased by porphyrin with respect to control (Panel A) and the 30 min pretreatment group (Panel B).
Using the naked eye, numerous red and green spots are readily evident; however, the DeCyder software makes quantitative comparisons via calculations of 3D “volumes” (spot area × intensity). The circled numbers represent 4 spots that were increased ≥1.5–fold in the kidneys of rats treated for 24 hr with MnP compared either to control animals (Panel A), or compared to rats treated for 30 min with MnP (Panel B). Some additional red spots in the 24 hr vs. 30 min comparison were evident, but were not due to an induction of protein at 24 hr. Rather, these spots were due to the fact that 30 min of MnP treatment decreased protein expression. In this regard, the advantage of correlating functional and proteomic data is necessary and apparent. For example, we know that MnP treatment for 30 min did not confer protection from I/R; hence proteins that changed in control versus the 30 min treatment group would not be candidate targets to microsequence, even if these same proteins changed in the control versus 24 hr treatment group. We chose to identify the protein depicted in spot #3. A robotic system (ProPic from Genomic Solutions) was used to excise the spot. Following in gel trypsin digestion and mass spectroscopy, spot #3 was identified as ATP synthase beta subunit (Table 1). The enzyme appeared to be significantly upregulated in rats treated with MnTnHex-2-PyP5+ (24 hr) compared to control rats alone. Additional evidence that MnTnHex-2-PyP5+ induces the ATP synthase-beta subunit was seen in our in vitro model using normal rat renal proximal tubule cells (NRK cells) [12]. Treatment of these cells with MnTnHex-2-PyP5+ (2 μM; 24 hr) resulted in increased protein expression of ATP synthase-beta subunit (Figure 5).
Table 1.
Summary of mass spectroscopy findings on excised gel spot #3.
| Spot | Protein name | Mass | Total Peptides | Unique Peptides | Mascot Score | Coverage |
|---|---|---|---|---|---|---|
| 3 | ATP synthase beta subunit | 51171 | 10 | 10 | 456 | 28 % |
Figure 5.
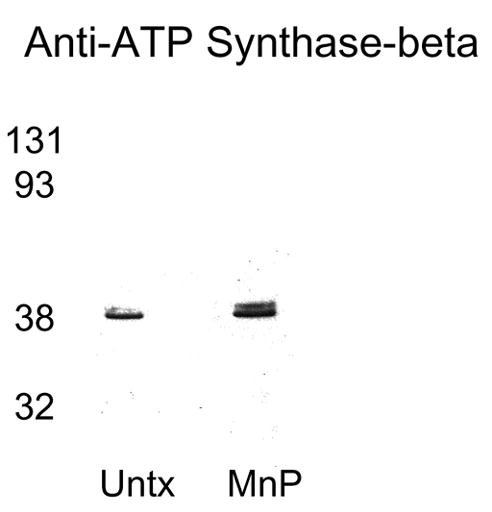
ATP Synthase-beta subunit Western blot showing porphyrin (MnP) mediated induction of ATP synthase-beta subunit in normal rat kidney proximal tubule cells treated with MnTnHex-2-PyP5+ (2 μM; 24 hr). Cellular proteins (25 μg) were separated on a 12% SDS-PAGE and blotted with a ATP synthase-beta subunit polyclonal antibody (Molecular Probes 1:10,000). Untx reflects untreated renal cells. Gel is representative of three separate experiments.
Finally, studies using blue native gel electrophoresis (BN-PAGE), a proteomic technique that separates and detects membrane-bound mitochondrial complexes, was used to pinpoint which respiratory complexes revealed the most intense changes in expression patterns or function during I/R. To our knowledge, this technique has not been applied to evaluate critical changes in mitochondrial proteins in renal I/R. Mitochondria were isolated from control and ischemic kidneys without or with pretreatment of MnTnHex-2-PyP5+ (24 hr) followed by I/R and analyzed using BN-PAGE, during which the complexes remain intact (shown by Roman numerals). Interestingly, the mitochondria from the ischemic kidney appeared to have an altered level of complex V (ATP synthase), which may indicate complex instability or inactivation (Figure 6A). In addition, mitochondria from rats pretreated with MnTnHex-2-PyP5+ were not only protected from renal I/R injury; but the porphyrin also appeared to restore the levels of complex V (ATP synthase) comparable to control levels. It is important to remember that complex V comprises 14 subunits, thus future studies could use the modified protocol of 2D-BN-PAGE [4] to determine precisely which subunits of ATP synthase are altered during renal I/R. The precise mechanism by which upregulation of ATP synthase might protect the kidney from I/R injury hasn’t been evaluated previously, but increased ATP production prior to ischemia would likely serve to protect mitochondria from I/R- mediated injury. Further studies were completed to determine ATP levels in the renal homogenates of control rats versus rats undergoing I/R, and I/R + MnTnHex-2-PyP5+ pretreatment (Figure 6B). These data support our prior report showing that I/R reduces renal ATP levels [11], but importantly that pretreatment with MnTnHex-2-PyP5+ (24 hr) significantly restored ATP levels. Thus, these new studies suggest that the induction of complex V using MnP also correlates with increased ATP levels.
Figure 6.
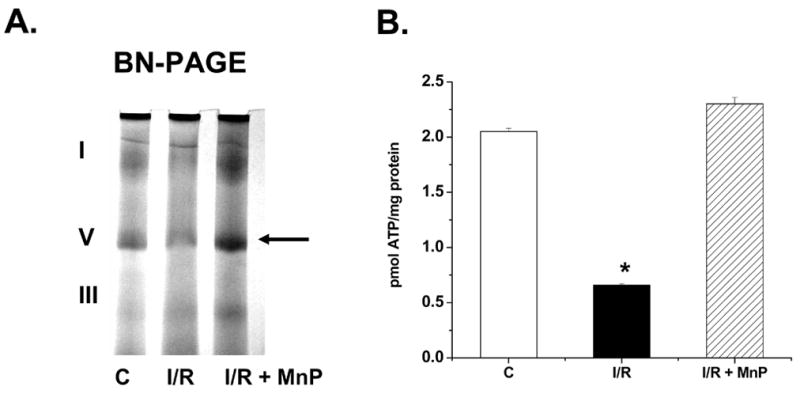
A. Blue Native Gel Electrophoresis (BN-PAGE) showing alterations in mitochondrial complex proteins during I/R, and restoration following porphyrin pretreatment.
Renal mitochondria (80 μg) isolated from rat kidney tissue following I/R were electrophoresed (6% native), stained, and destained as described in methods section. The arrow represents complex V (ATP Synthase) which differs in control (C), I/R, and rats pretreated with MnTnHex-2-PyP5+ (50 μg/kg; 24 hr prior to ischemia) (MnP) mitochondria. The roman numerals represent the migration of mitochondrial electron transport complexes (I-NADH Dehydrogenase; V-ATP synthase, III-Ubiquinol-Cytochrome c Oxidoreductase). The gel is representative of three separate experiments. B. ATP levels of renal extracts following I/R or with pretreatment with MnTnHex-2-PyP5+ (MnP). Values are expressed as Mean ± SEM (n = 5). * P < 0.05 compared with control (C).
To our knowledge, this is the first report showing induction of a key protein involved with mitochondrial respiration following treatment with a manganese porphyrin. Interestingly, the finding of altered ATP synthase complements the results of 2D-DIGE (Figure 5), which revealed an induction of ATP synthase-beta subunit in response to MnTnHex-2-PyP5+ pretreatment. Importantly, these findings also suggest that MnTnHex-2-PyP5+ may prevent renal injury via induction of protective factors. Although we haven’t identified the full complement of proteins that are altered in response to MnTnHex-2-PyP5+ treatment, our data demonstrate the feasibility of using proteomic techniques to detect specific proteins altered by drug treatment in this model of renal I/R. It is important to note that only 15 out of 1840 proteins were significantly altered by MnTnHex-2-PyP5+ and that this induction occurred independent of oxidant stress (i.e. in control rats). Future studies will be performed to better understand the molecular mechanism by which 24-hr pretreatment of MnTnHex-2-PyP5+ leads to protein induction, as well as identification of these proteins.
Interestingly, our studies showed that MnTnHex-2-PyP5+ (at the dose and time points studied) did not upregulate MnSOD protein or activity in the cell or rat model but did significantly blunt nitration of renal proteins during renal I/R (Figure 3). These data suggest that MnTnHex-2-PyP5+ is not acting as a classic ‘SOD mimetic’, but rather appears to indirectly block oxidant production during renal I/R. The precise mechanism of this protection remains unknown, but may relate to its ability to induce ATP synthase-beta subunit and prevention of ATP depletion (Figure 6), which would decrease mitochondrial superoxide production (and protein nitration), leading to less MnSOD inactivation. This in turn, would preserve mitochondrial and renal function during renal I/R.
In conclusion, a proteomic approach was used to identify abnormalities of specific mitochondrial proteins in renal I/R, and the reversal of these abnormalities by MnTnHex-2-PyP5+ pretreatment. Importantly, the porphyrin used here was protective at a single dose of 50 μg/kg, which is significantly lower than doses of various types of antioxidants commonly used in animal models of oxidative stress injuries. Prevention or rapid reversal of these mitochondrial modifications may preserve mitochondrial function and improve long-term success of renal transplants. Notably, renal damage clearly occurs during I/R, and is an unavoidable complication of cross-clamping the donor kidney for removal during transplant surgery. Therefore, strategies to limit the extent of renal damage during ischemic periods will increase the number of viable donor organs and improve graft function following renal transplantation.
Acknowledgments
The authors would like to thank Dr. John P. Crow for helpful discussions. The Arkansas Cancer Research Center Proteomics Core Facility is supported in part by NIH Grant Number P20 RR-16460 from the IDeA Networks of Biomedical Research Excellence (INBRE) Program of the National Center for Research Resources and by the Arkansas Biosciences Institute (funded by the Tobacco Settlement Proceeds Act).
Footnotes
This work was supported in part by a grant from NIH (RO1 DK59872 LAMC)
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- 1.Abunasra HJ, Smolenski RT, Morrison K, Yap J, Sheppard MN, O’Brien T, Suzuki K, Jayakumar J, Yacoub MH. Efficacy of adenoviral gene transfer with manganese superoxide dismutase and endothelial nitric oxide synthase in reducing ischemia and reperfusion injury. Eur J Cardiothorac Surg. 2001;20:153–158. doi: 10.1016/s1010-7940(01)00704-7. [DOI] [PubMed] [Google Scholar]
- 2.Batinic-Haberle I, Spasojevic I, Stevens RD, Hambright P, Fridovich I. Manganese(III) meso-tetrakis(ortho-N-alkylpyridyl)porphyrins. Synthesis, characterization, and catalysis of O2– dismutation. J Chem Soc, Dalton Trans. 2002:2689–2696. [Google Scholar]
- 3.Benov L, Batinic-Haberle I. A manganese porphyrin suppresses oxidative stress and extends the life span of streptozotocin-diabetic rats. Free Radic Res. 2005;39:81–88. doi: 10.1080/10715760400022368. [DOI] [PubMed] [Google Scholar]
- 4.Brookes PS, Pinner A, Ramachandran A, Coward L, Barnes S, Kim H, Darley-Usmar VM. High throughput two-dimensional blue-native electrophoresis: a tool for functional proteomics of mitochondria and signaling complexes. Proteomics. 2002;2:969–977. doi: 10.1002/1615-9861(200208)2:8<969::AID-PROT969>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 5.Carlsson LM, Jonsson J, Edlund T, Marklund SL. Mice lacking extracellular superoxide dismutase are more sensitive to hyperoxia. Proc Natl Acad Sci, USA. 1995;92:6264–6268. doi: 10.1073/pnas.92.14.6264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chan PH. Role of oxidants in ischemic brain damage. [Review] [56 refs] Stroke. 1996;27:1124–1129. doi: 10.1161/01.str.27.6.1124. [DOI] [PubMed] [Google Scholar]
- 7.Chen Z, Siu B, Ho YS, Vincent R, Chua CC, Hamdy RC, Chua BH. Overexpression of MnSOD protects against myocardial ischemia/reperfusion injury in transgenic mice. J Mol Cell Cardiol. 1998;30:2281–2289. doi: 10.1006/jmcc.1998.0789. [DOI] [PubMed] [Google Scholar]
- 8.Chen Z, Siu B, Ho YS, Vincent R, Chua CC, Hamdy RC, Chua BH. Overexpression of MnSOD protects against myocardial ischemia/reperfusion injury in transgenic mice. Journal of Molecular & Cellular Cardiology. 1998;30:2281–2289. doi: 10.1006/jmcc.1998.0789. [DOI] [PubMed] [Google Scholar]
- 9.Cooper JT, Chin LT, Krieger NR, Fernandez LA, Foley DP, Becker YT, Odorico JS, Knechtle SJ, Kalayoglu M, Sollinger HW, D’Alessandro AM. Donation after cardiac death: the university of wisconsin experience with renal transplantation. Am J Transplant. 2004;4:1490–1494. doi: 10.1111/j.1600-6143.2004.00531.x. [DOI] [PubMed] [Google Scholar]
- 10.Crow JP, Calingasan NY, Chen J, Hill JL, Beal MF. Manganese porphyrin given at symptom onset markedly extends survival of ALS mice. Ann Neurol. 2005;58:258–265. doi: 10.1002/ana.20552. [DOI] [PubMed] [Google Scholar]
- 11.Cruthirds DL, Novak L, Akhi KM, Sanders PW, Thompson JA, MacMillan-Crow LA. Mitochondrial targets of oxidative stress during renal ischemia/reperfusion. Arch Biochem Biophys. 2003;412:27–33. doi: 10.1016/s0003-9861(03)00039-0. [DOI] [PubMed] [Google Scholar]
- 12.Cruthirds DL, Saba H, MacMillan-Crow LA. Overexpression of manganese superoxide dismutase protects against ATP depletion-mediated cell death of proximal tubule cells. Arch Biochem Biophys. 2005;437:96–105. doi: 10.1016/j.abb.2005.02.023. [DOI] [PubMed] [Google Scholar]
- 13.Cuzzocrea S, Mazzon E, Dugo L, Caputi AP, Riley DP, Salvemini D. Protective effects of M40403, a superoxide dismutase mimetic, in a rodent model of colitis. Eur J Pharmacol. 2001;432:79–89. doi: 10.1016/s0014-2999(01)01427-3. [DOI] [PubMed] [Google Scholar]
- 14.Cuzzocrea S, Mazzon E, Dugo L, Di Paola R, Caputi AP, Salvemini D. Superoxide: a key player in hypertension. FASEB J. 2004;18:94–101. doi: 10.1096/fj.03-0428com. [DOI] [PubMed] [Google Scholar]
- 15.Dobashi K, Ghosh B, Orak JK, Singh I, Singh AK. Kidney ischemia-reperfusion: modulation of antioxidant defenses. Mol Cell Biochem. 2000;205:1–11. doi: 10.1023/a:1007047505107. [DOI] [PubMed] [Google Scholar]
- 16.Ferrer-Sueta G, Vitturi D, Batinic-Haberle I, Fridovich I, Goldstein S, Czapski G, Radi R. Reactions of manganese porphyrins with peroxynitrite and carbonate radical anion. J Biol Chem. 2003;278:27432–27438. doi: 10.1074/jbc.M213302200. [DOI] [PubMed] [Google Scholar]
- 17.Fujimura M, Morita-Fujimura Y, Kawase M, Copin JC, Calagui B, Epstein CJ, Chan PH. Manganese superoxide dismutase mediates the early release of mitochondrial cytochrome C and subsequent DNA fragmentation after permanent focal cerebral ischemia in mice. J Neurosci. 1999;19:3414–3422. doi: 10.1523/JNEUROSCI.19-09-03414.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gupta M, Dobashi K, Greene EL, Orak JK, Singh I. Studies on hepatic injury and antioxidant enzyme activities in rat subcellular organelles following in vivo ischemia and reperfusion. Mol Cell Biochem. 1997;176:337–347. [PubMed] [Google Scholar]
- 19.Henke W, Jung K, Polster F. Effects of preservation solutions on cortical and medullary mitochondria of rat kidney. Cell Mol Biol. 1995;41:319–326. [PubMed] [Google Scholar]
- 20.Keller JN, Kindy MS, Holtsberg FW, St Clair DK, Yen HC, Germeyer A, Steiner SM, Bruce-Keller AJ, Hutchins JB, Mattson MP. Mitochondrial manganese superoxide dismutase prevents neural apoptosis and reduces ischemic brain injury: suppression of peroxynitrite production, lipid peroxidation, and mitochondrial dysfunction. J Neurosci. 1998;18:687–697. doi: 10.1523/JNEUROSCI.18-02-00687.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kerby JD, Verran DJ, Luo KL, Ding Q, Tagouri Y, Herrera GA, Diethelm AG, Thompson JA. Immunolocalization of FGF-1 and receptors in glomerular lesions associated with chronic human renal allograft rejection. Transplantation. 1996;62:190–200. doi: 10.1097/00007890-199607270-00008. [DOI] [PubMed] [Google Scholar]
- 22.Lebovitz RM, Zhang H, Vogel H, Cartwright J, Dionne L, Lu N, Huang S, Matzuk MM. Neurodegeneration, myocardial injury, and perinatal death in mitochondrial superoxide dismutase-deficient mice. Proc Natl Acad Sci, USA. 1996;93:9782–9787. doi: 10.1073/pnas.93.18.9782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Leinenweber SB, Sheng H, Lynch JR, Wang H, Batinic-Haberle I, Laskowitz DT, Crapo JD, Pearlstein RD, Warner DS. Effects of a manganese (III) porphyrin catalytic antioxidant in a mouse closed head injury model. Eur J Pharmacol. 2006;531:126–132. doi: 10.1016/j.ejphar.2005.12.031. [DOI] [PubMed] [Google Scholar]
- 24.Li Y, Huang TT, Carlson EJ, Melov S, Ursell PC, Olson JL, Noble LJ, Yoshimura MP, Berger C, Chan PH, et al. Dilated cardiomyopathy and neonatal lethality in mutant mice lacking manganese superoxide dismutase. Nature Genetics. 1995;11:376–381. doi: 10.1038/ng1295-376. [DOI] [PubMed] [Google Scholar]
- 25.Linas SL, Whittenburg D, Parsons PE, Repine JE. Ischemia increases neutrophil retention and worsens acute renal failure: role of oxygen metabolites and ICAM 1. Kidney Int. 1995;48:1584–1591. doi: 10.1038/ki.1995.451. [DOI] [PubMed] [Google Scholar]
- 26.MacMillan-Crow LA, Crow JP, Kerby JD, Beckman JS, Thompson JA. Nitration and inactivation of manganese superoxide dismutase in chronic rejection of human renal allografts. Proc Natl Acad Sci, USA. 1996;93:11853–11858. doi: 10.1073/pnas.93.21.11853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.MacMillan-Crow LA, Cruthirds DL, Ahki KM, Sanders PW, Thompson JA. Mitochondrial tyrosine nitration precedes chronic allograft nephropathy. Free Radic Biol Med. 2001;31:1603–1608. doi: 10.1016/s0891-5849(01)00750-x. [DOI] [PubMed] [Google Scholar]
- 28.McCord JM, Fridovich I. Superoxide dismutase. An enzymic function for erythrocuprein (hemocuprein) J Biol Chem. 1969;244:6049–6055. [PubMed] [Google Scholar]
- 29.Nilakantan V, Zhou X, Hilton G, Shi Y, Baker JE, Khanna AK, Pieper GM. Antagonizing reactive oxygen by treatment with a manganese (III) metalloporphyrin-based superoxide dismutase mimetic in cardiac transplants. J Thorac Cardiovasc Surg. 2006;131:898–906. doi: 10.1016/j.jtcvs.2005.11.015. [DOI] [PubMed] [Google Scholar]
- 30.Nin N, Cassina A, Boggia J, Alfonso E, Botti H, Peluffo G, Trostchansky A, Batthyany C, Radi R, Rubbo H, Hurtado FJ. Septic diaphragmatic dysfunction is prevented by Mn(III)porphyrin therapy and inducible nitric oxide synthase inhibition. Intensive Care Med. 2004;30:2271–2278. doi: 10.1007/s00134-004-2427-x. [DOI] [PubMed] [Google Scholar]
- 31.Noiri E, Nakao A, Uchida K, Tsukahara H, Ohno M, Fujita T, Brodsky S, Goligorsky MS. Oxidative and nitrosative stress in acute renal ischemia. Am J Physiol Renal Physiol. 2001;281:F948–F957. doi: 10.1152/ajprenal.2001.281.5.F948. [DOI] [PubMed] [Google Scholar]
- 32.Okado-Matsumoto A, Batinic-Haberle I, Fridovich I. Complementation of SOD-deficient Escherichia coli by manganese porphyrin mimics of superoxide dismutase activity. Free Radic Biol Med. 2004;37:401–410. doi: 10.1016/j.freeradbiomed.2004.04.040. [DOI] [PubMed] [Google Scholar]
- 33.Paller MS, Hoidal JR, Ferris TF. Oxygen free radicals in ischemic acute renal failure in the rat. J Clin Invest. 1984;74:1156–1164. doi: 10.1172/JCI111524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Piantadosi CA, Zhang J. Mitochondrial generation of reactive oxygen species after brain ischemia in the rat. Stroke. 1996;27:327–331. doi: 10.1161/01.str.27.2.327. [DOI] [PubMed] [Google Scholar]
- 35.Piganelli JD, Flores SC, Cruz C, Koepp J, Batinic-Haberle I, Crapo J, Day B, Kachadourian R, Young R, Bradley B, Haskins K. A metalloporphyrin-based superoxide dismutase mimic inhibits adoptive transfer of autoimmune diabetes by a diabetogenic T-cell clone. Diabetes. 2002;51:347–355. doi: 10.2337/diabetes.51.2.347. [DOI] [PubMed] [Google Scholar]
- 36.Reaume AG, Elliott JL, Hoffman EK, Kowall NW, Ferrante RJ, Siwek DF, Wilcox HM, Flood DG, Beal MF, Brown RH, Jr, Scott RW, Snider WD. Motor neurons in Cu/Zn superoxide dismutase-deficient mice develop normally but exhibit enhanced cell death after axonal injury. Nature Genetics. 1996;13:43–47. doi: 10.1038/ng0596-43. [DOI] [PubMed] [Google Scholar]
- 37.Tse HM, Milton MJ, Piganelli JD. Mechanistic analysis of the immunomodulatory effects of a catalytic antioxidant on antigen-presenting cells: implication for their use in targeting oxidation-reduction reactions in innate immunity. Free Radic Biol Med. 2004;36:233–247. doi: 10.1016/j.freeradbiomed.2003.10.029. [DOI] [PubMed] [Google Scholar]
- 38.Weinberg JM, Venkatachalam MA, Roeser NF, Nissim I. Mitochondrial dysfunction during hypoxia/reoxygenation and its correction by anaerobic metabolism of citric acid cycle intermediates. Proc Natl Acad Sci U S A. 2000;97:2826–2831. doi: 10.1073/pnas.97.6.2826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Williams MD, Van Remmen H, Conrad CC, Huang TT, Epstein CJ, Richardson A. Increased oxidative damage is correlated to altered mitochondrial function in heterozygous manganese superoxide dismutase knockout mice. J Biol Chem. 1998;273:28510–28515. doi: 10.1074/jbc.273.43.28510. [DOI] [PubMed] [Google Scholar]
- 40.Wood PA, Hamm DA, Chen PY, Sanders PW. Studies of arginine metabolism and salt sensitivity in the Dahl/Rapp rat models of hypertension. Mol Genet Metab. 1998;64:80–83. doi: 10.1006/mgme.1998.2696. [DOI] [PubMed] [Google Scholar]
- 41.Zahmatkesh M, Kadkhodaee M, Moosavi SM, Jorjani M, Kajbafzadeh A, Golestani A, Ghaznavi R. Beneficial effects of MnTBAP, a broad-spectrum reactive species scavenger, in rat renal ischemia/reperfusion injury. Clin Exp Nephrol. 2005;9:212–218. doi: 10.1007/s10157-005-0359-6. [DOI] [PubMed] [Google Scholar]


