Abstract
The immune reconstitution syndrome caused by bacillus Calmette-Guérin (BCG) was found in 4 HIV-infected children who were immunized with BCG at birth. The localized, suppurative, BCG-related complications developed within 10 weeks after initiation of antiretroviral therapy. The incidence rate was 2.7 cases per 100 persons (95% confidence interval, 0.7–6.7). Patients responded well to treatment with isoniazid and rifampicin.
Immune reconstitution syndrome is an untoward manifestation of vigorous immune recovery that develops on receipt of potent antiretroviral therapy (ART). This inflammatory reaction is directed against pathogens causing latent or subclinical infection. It has been associated with a variety of opportunistic pathogens, including mycobacterial organisms [1–3]. Many countries with a high prevalence of tuberculosis, including Thailand, routinely immunize children with bacillus Calmette-Guérin (BCG). In Thailand, all neonates receive BCG via intradermal injection of the World Health Organization WHO reference strain (Tokyo 172), which is produced by the Thai Red Cross Society. In addition, there is also a program to revaccinate children at the age of 6–7 years, at the time that they enter school, if they have no vaccination scar. The policy allows administration of BCG vaccine to newborns of HIV-infected mothers [4], because BCG is effective in preventing tuberculosis, particularly the disseminated form [5]. Although BCG is a live vaccine, it causes a relatively low rate of complications because HIV-infected newborns are not yet immunosuppressed at birth. Nevertheless, because of the latency of viable BCG bacilli, HIV-infected children are at risk for clinical manifestations of BCG infection at any later time, including after restoration of cellular immunity against bacillus organisms has been achieved as a result of ART.
Before antiretroviral drugs were available, there were several reports of BCG-related complications in HIV-infected children. These were usually cases of localized disease (e.g., regional lymphadenitis and/or ulceration of the vaccination site) [6–9]. Disseminated disease was a rare event. The only prospective study that used blood culture to assess the rate of disseminated BCG disease in hospitalized HIV-infected children in Zambia found a low prevalence rate of 0.26% [10]. In a review of the reported disseminated cases from 1980 through 1995, only 9 of 28 cases were found to have occurred in HIV-infected patients [11]. The incidence rates of local complication varied from <2% to 24%, depending on the vaccine strain, dose of the vaccine, and degree of immunosuppression [4]. To date, only 1 case of regional BCG lymphadenitis following initiation of ART has been reported in the English-language medical literature [12]. More cases may not have been reported because of the lack of facilities to culture and identify the organism. We describe 4 children with perinatally acquired HIV infection who received BCG vaccine at birth and who subsequently developed localized suppurative BCG-related disease within 10 weeks after initiation of ART.
Patients and methods
From May 2002 to October 2004, we prospectively observed all 150 HIV-infected children who started receiving ART in a national program providing access to ART at the Chiang Mai University hospital (Chiang Mai, Thailand) and Lamphun provincial hospital (Lamphun, Thailand). The inclusion criteria were symptomatic HIV infection with severe immunosuppression, defined as a baseline CD4 lymphocyte percentage of ≤15%. All patients were observed for at least 6 months after initiation of ART. Four patients were identified as having BCG-related complications due to immune reconstitution syndrome. The incidence rate was 2.7 cases per 100 persons (95% CI, 0.7–6.7 cases per 100 persons). Pus samples aspirated from the abscesses or lymph nodes were cultured on Löwenstein-Jensen media. The Mycobacterium bovis BCG strain was identified using a multiplex PCR method to detect the RD1 deletion [13]. Clinical characteristics of individual patients are shown in table 1.
Table 1.
Immune reconstitution syndrome associated with bacillus Calmette-Guérin (BCG) vaccination in 4 children after initiation of antiretroviral therapy.
| CD4 T cell percentagea |
HIV RNA level, log10 copies/mL
|
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Patient | Sex, age | Time of BCG vaccination | Clinical findings and manifestations and findings of mycobacterial culture | Time to onset, weeks | Baseline | Nearest to onset of IRS | Baseline | Nearest to onset of IRS | Treatment | Outcome |
| 1 | F, 9 years | At birth | Right axillary lymphadenitis (diameter, 5 cm) (figure 1A); Kinyoun stain of pus showed acid-fast bacilli; culture of pus aspirate showed Mycobacterium BCG strain; blood culture showed no growth | 4 | 0 | 2 | >5.88 | 2.21 | Isoniazid (10 mg/kg/day) plus rifampin (15 mg/kg/day) for 9 months | Improved in 8 weeks and healed in 24 weeks |
| 2 | F, 8 years | At birth and 6 years of age | Abscess at vaccination (diameter, 5 cm) (figure 1B) with ipsilateral axillary lymphadenopathy; Kinyoun stain of pus showed acid-fast bacilli; culture of pus aspirate showed Mycobacterium BCG strain; blood culture was not done | 10 | 1 | 7 | >5.88 | 2.53 | Needle aspiration and isoniazid (10 mg/kg/day) plus rifampin (15 mg/kg/day) for 9 months | Improved in 8 weeks and healed in 16 weeks |
| 3 | F, 8 years | At birth and 7 years of age | Abscess at vaccination site with 3 cc of pus; Kinyoun stain of pus showed acid-fast bacilli; culture of the pus was not done; blood culture was not done | 4 | 3 | 6 | 4.38 | 1.87 | Needle aspiration and isoniazid (10 mg/kg/day) for 6 months | Improved in 4 weeks and healed in 15 weeks |
| 4 | F, 10 months | At birth | Abscess at vaccination site (diameter, 1 cm) with ipsilateral axillary lymphadenopathy; abscess aspiration was not done; blood culture was not done | 8 | 13 | 24 | >5.88 | 3.73 | None | Healed in 16 weeks |
NOTE. IRS, immune reconstitution syndrome.
In children, a CD4 lymphocyte percentage of <15% is considered to be severe immunosuppression, and a percentage between 15% and 24% is considered to be moderate immunosuppression.
All patients received BCG vaccine at birth. Two patients received an additional vaccination at 6 and 7 years of age. The intervals between revaccination and the development of symptoms in these 2 patients (patients 2 and 3) were 24 and 13 months, respectively. All patients had recently started receiving potent ART consisting of either nevirapine or efavirenz in combination with 2 nucleoside reverse-transcriptase inhibitors. The clinical manifestations included suppurative lymphadenitis ipsilateral to the BCG vaccination site (figure 1A) and/or abscess at the vaccination site (figure 1B). The symptoms developed within 10 weeks (range, 4–10 weeks) after the commencement of ART and occurred at the time when all patients were responding to ART with increased CD4 lymphocyte percentages and decreased plasma HIV RNA levels. Findings of chest radiographs obtained for all patients were normal. Mycobacterial blood culture was performed for only 1 patient (patient 1) who was admitted to the hospital because of high-grade fever. The culture did not reveal any organism. Aspiration of the abscess in patient 4 was not done because of a parent’s refusal.
Figure 1.
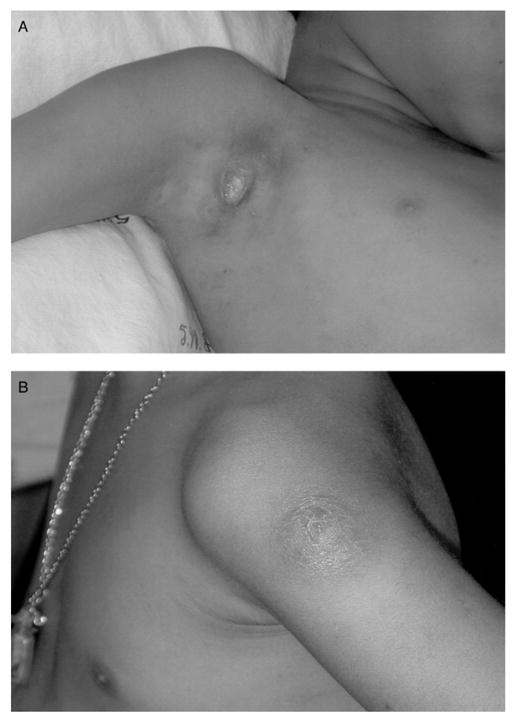
A, A photograph of severe suppurative axillary lymphadenitis 4 weeks after the commencement of combination antiretroviral therapy. B, A photograph of an abscess at a bacillus Calmette-Guérin (BCG) vaccination site with ipsilateral axillary lymphadenopathy 4 weeks after the commencement of combination antiretroviral therapy.
Discussion
We described 4 HIV-infected children who developed localized suppurative complications due to BCG vaccination within 10 weeks after initiation of ART. All children had received BCG vaccine at birth, as recommended by a national immunization program, and 2 children received revaccination >1 year before the initiation of ART. The symptoms coincided with a marked reduction in HIV RNA levels and an increase in the CD4 lymphocyte percentage. This suggests that the suppuration may have occurred as a result of restoration of the immune response to a previously latent BCG infection.
The incidence rates of immune reconstitution syndrome due to BCG in this report are 2% for vaccine site abscess and 0.7% for lymphadenitis. These rates are similar to the incidence rates of BCG complications in a non–HIV-infected population: 2.5% for vaccine site abscess and 1.1% for lymphadenitis [14]. In HIV-infected children who were not receiving ART, the incidence rate of local BCG complications was reported to be between 2% and 24% [4].
It is unclear how best to manage HIV-infected children already immunized with BCG who are about to receive potent ART. At present, the authors recommend close observation of the patient during the first 3 months of ART for signs of regional or disseminated BCG infection. Differentiation from tuberculous lymphadenitis may be difficult, but cases of isolated axillary glandular tuberculosis are very rare [15].
There is no guideline for the treatment of patients with immune reconstitution syndrome due to BCG. The limited data reported for BCG adenitis in HIV-noninfected infants might be applicable. In a randomized, controlled trial, it was demonstrated that drainage of pus (e.g., by needle aspiration) resulted in more-rapid rates of healing [16]. M. bovis BCG is known to be susceptible to rifampin and isoniazid and inherently resistant to pyrazinamide [17]. In previous reports, treatment with isoniazid and rifampin with or without ethambutol for 6–9 months was used with variable efficacy [7, 9]. In this case series, 2 patients received treatment with isoniazid and rifampin for 9 months, 1 received treatment with isoniazid for 6 months, and 1 did not receive any intervention. In all cases, the lesion markedly improved after 8 weeks and healed within 16–24 weeks. However, without a control group, it is unclear whether these antimicrobial agents would shorten the duration of healing. In our report, viable M. bovis BCG was documented in 2 of 4 patients years after immunization, and in all patients, the reactivation caused vaccine site abscess and/or ipsilateral lymphadenitis. Although the incidence rate is quite low, clinicians should be aware of these conditions; otherwise, the events might cause unnecessary use of antistaphylococcal antibiotics or overuse of a 4-drug antituberculous regimen.
In conclusion, we described immune reconstitution syndrome due to BCG in 4 HIV-infected children. The events occurred within the first 10 weeks after the initiation of ART. Clinicians should be aware of this syndrome and should be able to differentiate it from other mycobacterial infections. Treatment guidelines for BCG immune reconstitution syndrome should be established in countries that have a high prevalence of HIV infection and in countries that employ routine BCG vaccination.
Acknowledgments
Financial support. Thailand Research Fund of the Royal Thai Government and Fogarty International Center (5R01TW006187-03).
Potential conflicts of interest. All authors: no conflicts.
References
- 1.Shelburne SA, Hamill RJ, Rodriguez-Barradas MC, et al. Immune reconstitution inflammatory syndrome, emergence of a unique syndrome during highly active antiretroviral therapy. Medicine. 2002;81:213–27. doi: 10.1097/00005792-200205000-00005. [DOI] [PubMed] [Google Scholar]
- 2.Hirsch HH, Kaufmann G, Sendi P, Battegay M. Immune reconstitution in HIV-infected patients. Clin Infect Dis. 2004;38:1159–66. doi: 10.1086/383034. [DOI] [PubMed] [Google Scholar]
- 3.French MA, Price P, Stone SF. Immune restoration disease after antiretroviral therapy. AIDS. 2004;18:1615–27. doi: 10.1097/01.aids.0000131375.21070.06. [DOI] [PubMed] [Google Scholar]
- 4.Moss WJ, Clements CJ, Halsey NA. Immunization of children at risk of infection with human immunodeficiency virus. Bull World Health Organ. 2003;81:61–70. [PMC free article] [PubMed] [Google Scholar]
- 5.Colditz GA, Berkey CS, Mosteller F, et al. The efficacy of bacillus Calmette-Guérin vaccination of newborns and infants in the prevention of tuberculosis: meta-analyses of the published literature. Pediatrics. 1995;96:29–35. [PubMed] [Google Scholar]
- 6.O’Brien KL, Ruff AJ, Louis MA, et al. Bacillus Calmette-Guérin complications in children born to HIV-1-infected women with a review of the literature. Pediatrics. 1995;95:414–8. [PubMed] [Google Scholar]
- 7.Sirisanthana V. Complication of bacillus Calmette Guérin (BCG) vaccine in HIV-infected children. J Infect Dis Antimicrobial Agents. 1995;12:63–7. [Google Scholar]
- 8.Hofstadler G, Schmitt K, Tulzer G, Binder L, Brandstetter B. BCG lymphadenitis in an HIV-infected child 9.5 years after vaccination AIDS. Patient Care STDs. 1998;12:677–80. doi: 10.1089/apc.1998.12.677. [DOI] [PubMed] [Google Scholar]
- 9.Hesseling AC, Schaaf HS, Hanekom WA, et al. Danish bacilli Calmette-Guerin vaccine-induced disease in human immunodeficiency virus–infected children. Clin Infect Dis. 2003;37:1226–33. doi: 10.1086/378298. [DOI] [PubMed] [Google Scholar]
- 10.Waddell RD, Lishimpi K, von Reyn CF, et al. Bacteremia due to Mycobacterium tuberculosis, or M.bovis, bacille Calmette-Guerin (BCG) among HIV-positive children and adults in Zambia. AIDS. 2001;15:55–60. doi: 10.1097/00002030-200101050-00009. [DOI] [PubMed] [Google Scholar]
- 11.Talbot EA, Perkins MD, Silva SF, Frothingham R. Disseminated bacilli Calmette-Guerin disease after vaccination: case report and review. Clin Infect Dis. 1997;24:1139–46. doi: 10.1086/513642. [DOI] [PubMed] [Google Scholar]
- 12.Sharp MJ, Mallon DF. Regional bacillus Calmette-Guerin lymphadenitis after initiating antiretroviral therapy in an infant with human immunodeficiency virus type 1 infection. Pediatr Infect Dis J. 1998;17:660–2. doi: 10.1097/00006454-199807000-00021. [DOI] [PubMed] [Google Scholar]
- 13.Talbot EA, Williams DL, Frothingham R. PCR identification of Mycobacterium bovis BCG. J Clin Microbiol. 1997;35:566–9. doi: 10.1128/jcm.35.3.566-569.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Turnbull FM, McIntyre PB, Achat HM, et al. National study of adverse reactions after vaccination with bacille Calmette-Guérin. Clin Infect Dis. 2002;34:447–53. doi: 10.1086/338462. [DOI] [PubMed] [Google Scholar]
- 15.Goraya JS, Virdi VS. Bacille Calmette-Guerin lymphadenitis. Postgrad Med J. 2002;78:327–9. doi: 10.1136/pmj.78.920.327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Banani SA, Alborzi A. Needle aspiration for suppurative post-BCG adenitis. Arch Dis Child. 1994;71:446–7. doi: 10.1136/adc.71.5.446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.American Thoracic Society. Diagnosis standards and classification of tuberculosis in adults and children. Am J Respir Crit Care Med. 2000;161:1376–95. doi: 10.1164/ajrccm.161.4.16141. [DOI] [PubMed] [Google Scholar]


