Abstract
In perceptual experiments, within-individual fluctuations in perception are observed across multiple presentations of the same stimuli, a phenomenon that remains only partially understood. Here, by means of thulium–yttrium/aluminum–garnet laser and event-related functional MRI, we tested whether variability in perception of identical stimuli relates to differences in prestimulus, baseline brain activity. Results indicate a positive relationship between conscious perception of low-intensity somatosensory stimuli and immediately preceding levels of baseline activity in medial thalamus and the lateral frontoparietal network, respectively, which are thought to relate to vigilance and “external monitoring.” Conversely, there was a negative correlation between subsequent reporting of conscious perception and baseline activity in a set of regions encompassing posterior cingulate/precuneus and temporoparietal cortices, possibly relating to introspection and self-oriented processes. At nociceptive levels of stimulation, pain-intensity ratings positively correlated with baseline fluctuations in anterior cingulate cortex in an area known to be involved in the affective dimension of pain. These results suggest that baseline brain-activity fluctuations may profoundly modify our conscious perception of the external world.
Keywords: consciousness, functional MRI, pain
In perceptual experiments, within-individual fluctuations in perception are observed across multiple presentations of the same stimuli (1). In recent years, trial-to-trial variability in the magnitude of event-related blood oxygenation level-dependent (BOLD) responses has also been shown to be relevant to human perception and behavior (2). For example, the magnitude of the evoked BOLD response in the frontoparietal network relates to conscious (visual) perception (3) and to pain intensity perception in the posterior part of anterior cingulate cortex (pACC) (4). In many cases, this intertrial variability cannot be attributed to the variability in stimuli (5–7). Despite its demonstrated relevance for human behavior, the sources of these event-related BOLD responses and the related perception variability are only partially understood (2, 8).
The aim of our study was to test whether spatially specific differences in prestimulus baseline brain activity could predict subsequent differences in subjective perception of external stimuli. In the present experiment, we investigated somatosensory and pain perception. It is now increasingly accepted that perceptual awareness seems to be the result of the interaction between specialized sensory cortices and a higher-order frontoparietal network (9). However, the relative role of specialized sensory cortices (10, 11) vs. higher level areas (9, 12) in the genesis of conscious perception remains controversial. On the other hand, a set of particular brain areas, the so-called “pain neuromatrix,” has been involved in pain intensity perception (4, 13). These areas were thus candidates for the possible location of prestimulus baseline modulations. Our study aims to determine where in the brain, if at all, prestimulus BOLD activity relate to somatosensory awareness and pain intensity perception.
We tested our hypotheses by using an event-related thulium–yttrium/aluminum–garnet (YAG) laser functionial MRI (fMRI) paradigm (13) assessing somatosensory perception (contrasting perceived vs. unperceived stimuli) and pain-intensity perception for intensity-matched laser stimuli. Subjects received laser stimuli on the dorsum of their left hand. After each stimulus, subjects rated their sensory perception on a five-point scale: P0, no stimulus perceived; P1, warm but not painful sensation; and P2, P3, and P4, increasingly painful sensations. P2 was defined as mild pain and P4 as very painful. P3 was defined as an intermediate pain between P2 and P4. Afterward, stimuli were matched for their intensity in unperceived (P0 score) vs. perceived (P1 score) conditions and in comparison with two pain levels (P2 and P3 scores). We performed two distinct analyses by using a voxel-by-voxel statistical approach. A first analysis identified differences in brain activity in response to perceived-vs.-unperceived intensity-matched stimuli and to similar stimuli eliciting different levels of pain intensity. The second analysis looked for areas in which differences in brain activity shortly before the presentation of the stimuli (i.e., 3 sec) could predict changes in subsequent stimulus perception (i.e., in stimulus awareness for the sensory range or in pain intensity level for the painful range of intensities).
Results
Neural Correlates of Somatosensory Stimuli Awareness and Pain Intensity Perception.
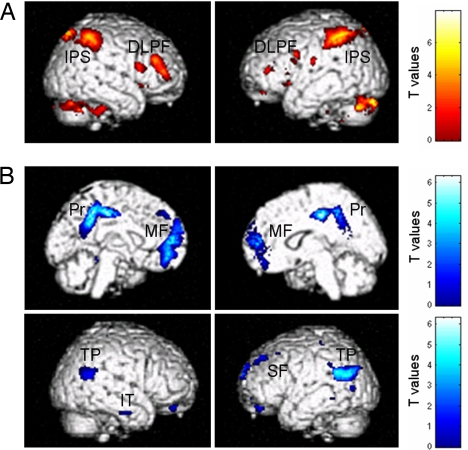
Consciously perceived laser stimuli elicited higher activation in bilateral middle and inferior frontal gyri, intraparietal sulci and adjacent posterior parietal cortices, and the dorsal anterior part of anterior cingulate cortex (aACC) as compared with unperceived, energy-matched stimuli (i.e., P1 > P0 contrast) (Fig. 1A and Table 1). Mean intensities of selected P1 events were not significantly different from mean intensities in selected P0 events, within subjects and at the group level (mean ± SD, 379 ± 39 mJ vs. 384 ± 37 mJ for P1 and P0, respectively). The inverse comparison (P0 > P1) identified bilateral posterior cingulate/precuneus and mesiofrontal cortices, bilateral temporoparietal junctions, right inferior temporal cortex, and left superior frontal and parahippocampal gyri (Fig. 1B and Table 1).
Fig. 1.
Neural correlates of somatosensory stimuli awareness. Consciously perceived stimuli compared with unperceived intensity-matched stimuli were associated with greater activity in bilateral dorsolateral prefrontal (DLPF) and intraparietal sulcus/posterior parietal cortex (IPS) activity (yellow-red sections) (A) and less activity in a network encompassing bilateral posterior cingulate precuneas (Pr), mesiofrontal cortices (MF), temporoparietal junctions (TP), right inferior temporal (IT), and left superior frontal gyri (SF) (blue sections) (B). Results are of a 24-subject group analysis displayed at uncorrected P < 0.001.
Table 1.
Peak voxels of brain areas associated to somatosensory stimuli awareness and pain intensity perception
| Brain regions | Side | x | y | z | Z value | P value |
|---|---|---|---|---|---|---|
| Sensory awareness | ||||||
| Activations | ||||||
| Inferior frontal gyrus | R | 46 | 44 | 12 | 4.26 | 0.001* |
| Middle frontal gyrus | R | 48 | 36 | 26 | 3.85 | 0.005* |
| L | −50 | 4 | 30 | 3.45 | 0.016* | |
| Intraparietal sulcus | R | 18 | −68 | 54 | 5.45 | <0.001* |
| L | −38 | −46 | 46 | 5.32 | <0.001* | |
| aACC | R | 6 | 24 | 44 | 3.68 | 0.008* |
| Cerebellar vermis | R | 6 | −54 | −32 | 4.81 | 0.035* |
| Deactivations | ||||||
| Posterior cingulated/precuneus | R | 6 | −24 | 42 | 4.76 | <0.001* |
| Mesiofrontal cortex | −8 | 48 | −6 | 4.66 | <0.001* | |
| Superior frontal gyrus | L | −16 | 42 | 44 | 3.55 | 0.012* |
| Temporoparietal junction | R | 58 | −54 | 28 | 3.23 | 0.030 |
| L | −52 | −66 | 22 | 3.97 | 0.003* | |
| Parahippocampal gyrus | L | −28 | −40 | −16 | 3.60 | 0.011* |
| Inferior temporal cortex | R | 64 | −16 | −24 | 3.58 | 0.036 |
| Pain intensity processing | ||||||
| pACC* | 0 | 14 | 34 | 3.40 | 0.018 | |
| Insula | R | 42 | 12 | −12 | 4.32 | 0.001 |
| L | −42 | 8 | −14 | 3.87 | 0.004 |
Results were thresholded at P < 0.05 corrected for 10-mm-radius small volumes of interest centered on a priori coordinates. Coordinates are defined in Montreal National Institute space. SVC, small-volume-corrected; R, right; L, left; ∗, false discovery rate (P < 0.05).
Pain-intensity-related activation (P3 > P2) was observed in bilateral insula and pACC (Table 1). Mean intensities of P3 events were not significantly different from mean intensities in selected P2 events, within subjects and at the group level (mean ± SD, 528 ± 34 mJ vs. 530 ± 36 mJ for P3 and P2, respectively). No area was found significantly less activated in P3 than in P2.
Predictive Effect of Baseline Brain Activity on Subsequent Stimuli Perception.
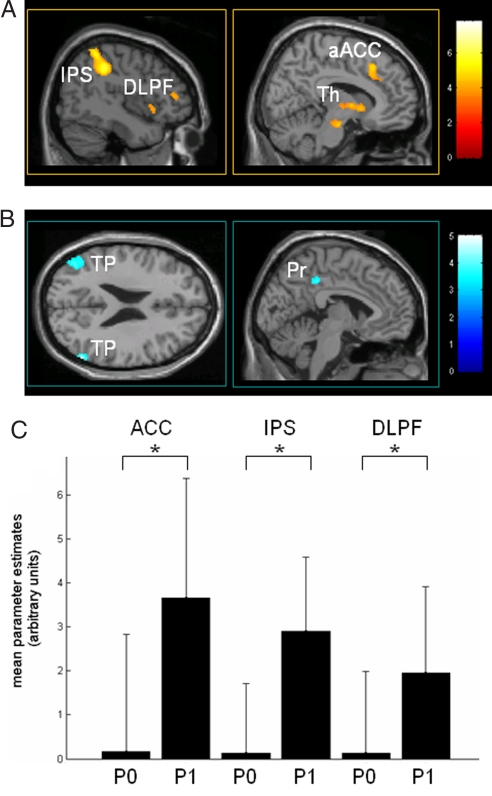
High activity in bilateral middle and inferior frontal gyri, intraparietal sulci, posterior parietal cortex, and aACC 3 sec before stimulus presentation predicted subsequent perception of the stimulus. Perceived stimuli were also associated with higher preceding baseline activity in medial thalamus (Fig. 2A and Table 2). Inversely, high activity in bilateral posterior cingulate/precuneus, temporoparietal and inferior temporal cortices, left superior frontal gyrus, and left parahippocampal cortices predicted stimuli to be subsequently rated as unperceived (Fig. 2B and Table 2).
Fig. 2.
Baseline brain activity predicting conscious perception of subsequent somatosensory stimuli. (A) Increased baseline brain activity in the medial thalamus (Th), dorsolateral prefrontal cortex (DLPF), intraparietal sulcus/posterior parietal cortex (IPS), and aACC 3 sec before stimulus presentation predicts perception of low-intensity sensory stimuli. (B) Decreased baseline activity in the default brain network encompassing posterior cingulate/precuneus (Pr) and bilateral temporoparietal junctions (TP) exerts a facilitatory effect upon perception of subsequent somatosensory stimuli. Contrasts are thresholded at an uncorrected P value of <0.001 on a canonical MR template. Color scales refer to T values of individual voxels. (C) Effect size (i.e., mean and standard deviation of the parameters estimates for baseline effect) in peak voxels of aACC, intraparietal sulcus (IPS), and middle frontal gyrus (DLPF) before stimuli subsequently rated as perceived (P1) or unperceived (P0). ∗, Significant difference between baseline effect sizes thresholded at a P value corrected for multiple comparisons of <0.05.
Table 2.
Baseline brain activity predicting differences in perception of subsequent intensity-matched laser stimuli
| Brain regions | Side | x | y | z | Z value | P value |
|---|---|---|---|---|---|---|
| Facilitatory baseline activity effect on low-intensity stimuli perception | ||||||
| Middle frontal gyrus | R | 42 | 38 | 28 | 3.30 | 0.038* |
| L | −54 | 6 | 30 | 4.48 | 0.001* | |
| Inferior frontal gyrus | R | 44 | 42 | 10 | 3.48 | 0.011* |
| Intraparietal sulcus/PPC | R | 26 | −50 | 38 | 5.42 | <0.001* |
| L | −44 | −36 | 46 | 5.05 | <0.001* | |
| aACC | 6 | 24 | 44 | 4.13 | 0.002* | |
| Medial thalamus | 6 | −12 | 2 | 3.76 | 0.008* | |
| Preventive baseline activity effect on low-intensity stimuli perception | ||||||
| Precuneus/posterior cingulated | −4 | −38 | 42 | 3.44 | 0.020 | |
| Superior frontal gyrus | L | −26 | 28 | 54 | 3.12 | 0.047 |
| Temporoparietal junctions | R | 50 | −74 | 32 | 3.38 | 0.024 |
| L | −46 | −70 | 26 | 3.51 | 0.016 | |
| Parahippocampal gyrus | L | −32 | −34 | −20 | 3.11 | 0.048 |
| Inferior temporal cortex | R | 64 | −16 | −24 | 3.11 | 0.047 |
| L | −56 | −34 | −20 | 3.10 | 0.04 | |
| Facilitatory baseline activity effect on high-intensity stimuli perception | ||||||
| pACC | 0 | 16 | 32 | 4.20 | 0.001* | |
| Insula | R | 36 | 10 | −10 | 3.33 | 0.021* |
| L | −50 | 8 | 0 | 3.11 | 0.038* |
Results were thresholded at P values of <0.05 corrected for 10-mm-radius small volumes of interest centered on a priori coordinates. Coordinates are defined in Montreal National Institute space. PPC, posterior parietal cortex; ∗, false discovery rate (P < 0.05).
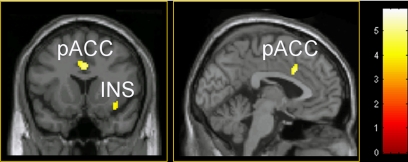
In the painful range of stimulation, increased baseline activity in bilateral insula and pACC predicted subsequent laser stimuli to be rated as more painful (Fig. 3 and Table 2).
Fig. 3.
Baseline brain activity predicting subsequent pain-intensity ratings. High baseline activity in the pain-related pACC and insula (Ins) predicts a sensation of higher pain intensity in response to painful-range laser stimuli. Results reflect a 24-subject group analysis masked by pain-intensity-related areas contrasted with the first analysis, thresholded at an uncorrected P value of <0.001, and displayed on a canonical MR template. Color scale refers to T values of individual voxels.
Finally, we tested whether these differences in baseline brain activity could be related to differences in intensity of the immediately preceding stimuli. For P0 and P1 stimuli, preceding stimuli mean intensities were not significantly different from one another (438 ± 21 mJ vs. 433 ± 23 mJ, respectively). Similarly, stimuli preceding painful P3 and P2 stimuli were not significantly different (453 ± 29 mJ vs. 471 ± 37 mJ, respectively).
Discussion
Do Spatially Specific Baseline (Prestimulus) Fluctuations Influence Sensory Perception?
Our results show that differences in perception of external stimuli can be predicted by using baseline brain activity in areas involved in the genesis of this perception. According to Sapir et al. (14), the presence of endogenous predictive signals is the strongest evidence that can be obtained by using correlational methods for a causal link between neural activity and perception or behavior. An alternative explanation that predictive signals are a consequence of perception is unlikely because the perceptual event had yet to take place at the time these signals were recorded. Our results provide a system-wide understanding of the relationships between prestimulus BOLD activity and perception, suggesting that ongoing changes of baseline brain activity could deeply modulate our awareness of the external world.
Two important recent studies also showed that prestimulus BOLD activity influences human behavior. Sapir et al. (14) demonstrated that the variability in accuracy in a visual motion discrimination task was predicted by preceding changes in BOLD signal, induced by a cue, in spatial attention cortices. Weissman et al. (15) showed that attentional lapses, defined as slow reaction times in a visual attention task, begin with reduced prestimulus activity in cingulate and prefrontal attentional regions. The important difference with our study is that we focused on somatosensory awareness and pain intensity perception, whereas the earlier studies focused on reaction times (15) or motion discrimination accuracy (14).
In line with previous studies, our data show that conscious perception of external sensory stimuli relates to activation of frontoparietal and anterior cingulate cortices (4, 9). Furthermore, we show that conscious perception of near-threshold sensory stimuli is predicted by increased baseline neural activity in this frontoparietal network 3 sec before stimulus occurrence. Our results emphasize the importance of frontoparietal cortices activity in sensory awareness. These results can also be linked to the preferential metabolic impairment of higher-order frontoparietal regions, compared with the relative preservation of primary sensory cortices in various states of altered consciousness (16). On the other hand, we also show a facilitatory effect of high baseline activity in medial thalamus on external stimuli perception. Medial thalamus has repeatedly been reported to be the interface between alertness and cognition in humans (17, 18). Our data corroborate a recently proposed model of access consciousness (8), predicting a facilitatory effect of a vigilance-related increase in regional spontaneous activity on external stimuli processing.
Additionally, deactivations were identified when comparing perceived with unperceived intensity-matched laser stimuli in bilateral posterior cingulate/precuneus and mesiofrontal cortices, bilateral temporoparietal junctions, right inferior temporal cortex, and left superior frontal and parahippocampal gyri. These areas, previously referred to as the “default network,” commonly exhibit greater activity at rest relative to attention-demanding cognitive tasks (19) and are thought to be involved in various aspects of self-referential processing (20) and task-unrelated thoughts (21). Furthermore, we show that sensory stimuli are predicted to be unperceived when preceding baseline activity is increased in this default system. Our results are likely to reflect a competition between conscious access to external stimuli and self-referential processes. In other words, if the activity 3 sec before stimulation in the lateral frontoparietal network is high, the subject is in a state of high receptivity to the external world, leading to higher chances of perception of the applied low intensity laser stimuli. Inversely, if the activity 3 sec before stimulation is high in the default network, the subject seems to be in a state of higher introspection or self-oriented thoughts and will have fewer chances to subsequently report the stimuli as consciously perceived. Our findings go against a widespread idea in theories of subjective awareness, posing a self-related observer function as an essential component without which awareness cannot emerge (22). In line with a recent study on sensorimotor processing (23), we show that self-related processes are not necessarily engaged in sensory awareness. Instead, they tend to be in competition with external stimuli perception.
Finally, with regard to pain, we show that enhanced activity in bilateral insula and pACC before high-intensity laser stimuli predicts these stimuli to be scored as more painful. In the painful range of stimulation, activity in pACC and insula correlated with pain intensity scores (4, 13). As expected, the location of pain-related pACC is more ventral and posterior than our identified anterior cingulate area related to awareness of stimuli (4). Our results could be related to a study showing that increased activity in pain-related areas (the so-called “pain matrix,” encompassing the pACC and insula) during expectation of painful stimuli correlates with higher pain scores during subsequent painful stimulation (24). In parallel, another article recently showed the possibility, by means of real-time fMRI feedback training, to teach subjects to gain control over activation in ACC (in coordinates close to our activation peak) (25). This voluntary control over activation in pACC reduced pain scores after training. Our results might also have potential implications for the understanding of chronic pain pathophysiology. Previous studies have shown increased baseline activity in ACC during experience of chronic neuropathic pain (26). In chronic pain patients, as in our healthy volunteers, baseline pain matrix activity might influence pain intensity perception. In other words, if the baseline cerebral activity in the pain-related pACC and insula is high before noxious stimulation, the subject is in a state of high receptivity to pain and is more likely to experience intensity-matched laser stimuli as highly painful. On the contrary, low baseline activity in the pain matrix decreases the likelihood to rate stimuli as painful. Our results also pertain to previous findings by members of our and other laboratories on cognitive modulation of pain during the hypnotic state (27, 28).
The aim of our study was to test whether the different BOLD responses (and related subjective perception) potentially elicited by identical stimuli could be linked to differences in prestimulus baseline brain activity. Studies of event-related mental imagery as well as binocular rivalry studies have shown that hemodynamic brain responses to instantaneous cognitive events can be modeled similarly as those evoked by external stimulation (29–31) and that conventional hemodynamic modeling could be used to predict the rapidly changing contents of the so-called “stream of consciousness” (31). In consequence, we modeled our baseline regressor as a cognitive event occurring 3 sec before the stimulus, convoluted with standard BOLD response function. We empirically chose to investigate baseline brain activity at a delay of 3 sec before the onset of the stimulus, for reasons of hemodynamic physiology (32) and according to the time scale of spontaneous slow brain fluctuations (<0.1 Hz) observed in previous resting-state fMRI studies (20, 33). The orthogonalization of design matrix regressors (34) allowed us to disentangle the effect due to baseline activity from the effect of the following stimulus presentation. It could be argued that baseline activity differences observed in our study could be related to differences in preceding stimulus intensities. However, intensities of stimuli preceding P0 vs. P1 and P2 vs. P3 events were shown to be not significantly different.
Potential Sources of Baseline Brain Activity Fluctuations.
Our results demonstrate that lateral frontoparietal activity predicts increased chances of perception of somatosensory stimuli. These findings are in line with a number of studies showing enhanced lateral frontoparietal activity during increased visual attention (e.g., refs. 15 and 35). Our results could thus possibly be interpreted to reflect attentional fluctuations in a stimulus-awareness-related network preceding stimulus presentation. On the other hand, several studies have shown that, as attentional demands in cognitive tasks are increased, activity in the default system is decreased (15, 36), suggesting that spontaneous modifications in attention could explain our current findings. Finally, attention to pain has also been shown to increase the BOLD signal in the pain neuromatrix encompassing ACC and insula (24).
Alternatively, fMRI studies recently identified spontaneous fluctuations in neural activity in the resting human brain. These slow BOLD fluctuations (in the range of 0.1 Hz) are not random but coherent within specific neuroanatomical systems (20, 37). In addition, it has been recently shown that coherent spontaneous brain activity is superimposed on task-related activity in fMRI measurements and accounts for a significant variability of measured event-related BOLD responses (2). Fox et al. (20) recently demonstrated that even in the absence of any task or behavior, in the so-called “conscious resting state” of the human brain, two networks very similar to that found in our study, encompassing on the one hand the lateral frontoparietal areas and on the other hand the posterior cingulate/precuneus and bilateral temporoparietal junctions, show a pattern of anticorrelated activity. Our results potentially extend the interpretation of these data, suggesting that this dichotomy is likely to directly influence the momentary contents of consciousness by causing a continuous modulation of the brain's responsiveness to the external world. Studies of human resting-state connectivity showed that anterior cingulate and insular cortices activity is spontaneously fluctuating in time (38, 39). Modifications in pain-related pACC and insula baseline activity observed in our study could thus also be due to ongoing fluctuations of local brain activity unrelated to external stimulus presentation. Interestingly, a recent study demonstrated the preservation of coherent BOLD fluctuations in deeply anesthetized macaque monkeys (40). These results suggest that spontaneous resting-state BOLD fluctuations are an aspect of brain functional organization that transcends levels of consciousness.
Our study was not designed to identify the origin of baseline brain activity fluctuations. We speculate that our results reflect spontaneous modifications in the attention state of the subjects throughout the experiment. However, the exact origin of baseline fluctuations needs to be assessed in future studies.
Conclusion
In conclusion, variability in prestimulus baseline or default brain activity in discrete brain areas predicted the quality of perception of subsequent intensity-matched somatosensory stimuli. Our findings nicely fit in a recently proposed neuronal model for consciousness (8), in which higher spontaneous activity in the vigilance system and in areas involved in external stimuli perception has a facilitatory effect on external stimuli perception. Moreover, activity in areas involved in spontaneous trains of thoughts, unrelated to external stimulation and instructions, exerts a preventing effect on external stimuli perception. Our results add to the previous model in subdividing the “global workspace” into two competing subsystems: one predominantly lateral and involved in external monitoring and one predominantly medial and preferentially involved in internal- or self-referential processes. Spontaneous anticorrelated BOLD signal fluctuations found between these two networks in the resting human brain (20) could possibly reflect ongoing modifications of the brain's receptiveness to external stimulation. However, the source of the spontaneous baseline brain-activity fluctuations observed in our study remains to be directly investigated in future work. In the same line, variations in baseline activity in the pain-related ACC predicted subsequent pain intensity perception in response to similar stimuli. Our data suggest that baseline brain activity profoundly modifies conscious perception of the external world. These results possibly provide a physiological basis of the decoupling of the organism's internal activity from its current inputs and may provide novel insights on the neural foundations of the autonomy of consciousness.
Methods
Subjects.
Twenty-six healthy volunteers (16 males; age, 26 ± 4 yr; mean ± SD) were recruited from Liège University and gave their written informed consent to participate in the study. Two subjects were excluded from further analysis because of reported discomfort during scanning, altering somatosensory and pain ratings. None of the subjects had any neurological or psychiatric disease history. Subjects were paid for their participation in the experiment. The study was approved by the Ethics Committee of the University of Liège and was conducted according to the Declaration of Helsinki (41) and to the International Association for the Study of Pain Ethical Guidelines for Pain Research in Humans (42).
Stimuli.
A thulium–YAG laser (Baasel Lasertech, Starnberg, Germany) was used to apply computer-controlled brief radiant pulses to the skin of the subjects. The thulium–YAG laser emits calibrated near-infrared radiation (wavelength, 1.96 μm; spot diameter, 5 mm; pulse duration, 1 msec) with a penetration depth of 360 μm into the human skin. At low intensities, laser stimuli elicit a sensation of light touch. At higher intensities, the sensation evoked is comparable to a pinprick.
Experimental Protocol and Sensory Rating.
The experiment was conducted in a single fMRI session. Somatosensory stimuli were delivered to the dorsum of the left hand. To decrease anticipation, interstimulus intervals were randomized between 8 and 12 sec. The stimulation site was changed slightly after each stimulus to avoid sensitization and habituation. Stimulation intensities randomly ranged from 250 to 650 mJ. After each stimulus, subjects rated their sensory perception on a five-point scale: P0, no stimulus perceived; P1, warm but not painful sensation; and P2, P3, and P4, increasingly painful sensations, comparable to a pinprick. P2 was defined as mild pain and P4 as very painful. P3 was defined as an intermediate pain between P2 and P4. The subjects indicated their ratings on the keyboard with their right hand. The total study duration was ≈60 min; the total number of applied laser stimuli ranged from 160 to 200 stimuli (one subject received only 128 stimuli for technical reasons). To better separate motor response from sensory- or pain-related activity, subjects were prompted by a tone (600 Hz sine wave, 250 msec) to respond at a randomized interval of 3–5 sec after the laser stimulation. Volunteers were familiarized with the laser and rating scale procedure before fMRI data acquisition.
fMRI Data Acquisition.
Data were acquired by using a 3-T head-only MRI scanner (Allegra; Siemens Medical Systems, Erlangen, Germany) with a T2*-sensitive gradient echo sequence [time to repetition (TR), 2,130 msec; echo time (TE), 40 msec; flip angle, 90°; matrix size, 64 × 64 × 32; voxel size, 3.4 × 3.4 × 3 mm3]. Thirty-two contiguous 3-mm-thick transverse slices were acquired, covering the whole brain. Anatomical images were obtained by using a T1-weighted 3D magnetization-prepared rapid gradient echo sequence (TR, 1,960 msec; TE, 4.43 msec; time after inversion pulse, 1,100 msec; field of view, 230 × 173 cm2; matrix size, 256 × 256 × 176; voxel size, 0.9 × 0.9 × 0.9 mm) for each subject. A standard head coil was used in the data acquisition. Subjects were lying down in the scanner in front of a mirror box that allowed them to see the display of the screen by using a liquid crystal display projector. Subjects kept their eyes closed during the experiment. A vacuum cushion minimized head movements. Before the fMRI session, the acoustic level of the tone was individually adjusted for optimal comfort during a sham fMRI acquisition.
fMRI Data Analysis.
For each subject, laser stimuli were retrospectively sorted in such a way that laser energy for perceived and unperceived stimuli (P0 and P1 scores) were automatically intensity-matched by using Matlab (version 6.1; Mathworks, Sherbom, MA). A similar procedure was applied for the pain-intensity study (P2 and P3 scores). Differences between samples were assessed by using two sample t tests and considered significant at P < 0.001. The number of trials for P0 and P1 conditions were 30 ± 16 and 31 ± 13, respectively (P value not significant); for P2 and P3 the number of trials were 28 ± 15 and 19 ± 7, respectively (P value not significant). Scored stimuli for P4 conditions were not taken into account because of their insufficient number (11 ± 11, mean ± SD).
Functional data were preprocessed and analyzed by using Statistical Parametric Mapping software SPM2 (www.fil.ion.ucl.ac.uk/spm/software/spm2; Wellcome Department of Imaging Neuroscience, London, U.K.). The first four fMRI volumes were removed to allow for signal equilibration. Preprocessing steps included realignment and adjustment for movement-related effects (43), spatial normalization into standard stereotactic Montreal Neurological Institute space (43), and spatial smoothing with a Gaussian kernel of 8 mm full width at half maximum.
Data were analyzed with a mixed-effects model aimed at showing stereotypical effect in the population from which the subjects are drawn (44). The mixed-effects model was implemented in two processing steps accounting for fixed and random effects, respectively.
For each subject, a first-level intraindividual analysis aimed at modeling the data to partition the observed neurophysiological responses into components of interest, confounds, and errors by using a general linear model (45). An event-related analysis estimated BOLD responses evoked by the laser stimulation. Data analysis was performed by modeling the different trials as delta functions convolved with the canonical hemodynamic response function as implemented in SPM2.
A first analysis identified stimulus-induced brain activation in perceived vs. nonperceived (scores P0 and P1) and in mildly painful vs. moderately painful (P2 vs. P3) intensity-matched stimuli. These stimuli were incorporated as regressors of interest in the design matrix. The unmatched stimuli and motor responses were modeled in two supplementary regressors. Movement parameters derived from realignment of the functional volumes (translations in the x, y, and z directions and rotations around the x, y, and z axes) were included as covariates of no interest in the design matrix. High-pass filtering using a cut-off period of 128 sec was implemented in the design matrix to remove low-frequency drift from the time series. Serial correlations were estimated by using a restricted maximum likelihood algorithm with an intrinsic autoregressive model during parameter estimation. The effects of interest were tested by using linear contrasts, generating statistical parametric maps (SPM t) in each subject. Contrasts images were computed, identifying differences in brain activity in response to perceived stimuli compared with nonperceived stimuli (both P1 > P0 and P0 > P1 contrasts) and mildly vs. moderately painful intensity-matched laser stimuli (P3 > P2 and P2 > P3 contrasts). Because no inference was made at this (fixed effect) level of analysis, summary statistic images were thresholded at P < 0.95 (uncorrected) as described (46–48).
Individual summary statistics images were further spatially smoothed (a 6-mm full width at half maximum Gaussian kernel) and entered in a second-level analysis, corresponding to a random effects model in which subjects are considered random variables. The resulting set of voxel values for each contrast constituted a map of the t statistic (SPM T) thresholded at P < 0.001 (uncorrected for multiple comparisons) (46). Statistical inferences were then obtained after correction at the voxel level using Gaussian random field theory for small volume of interest (PSVC < 0.05) (49) computed on a sphere (radius of 10 mm) (46) around coordinates previously reported to be involved in sensory awareness (50–52), default-brain network (20, 36, 53, 54), and pain-intensity processing (4, 13, 55).
A second analysis searched for differences in baseline brain activity before the applied intensity-matched stimuli, depending on subsequent subjectively rated sensory perception (P0 or P1) and pain intensity (P2 or P3). Two distinct regressors therefore modeled each event type. A first regressor modeled the brain activity (i.e., a cognitive event) 3 sec before the occurrence of the stimuli. A second regressor, orthogonalized with respect to the first one, explicitly modeled the brain response to the presentation of the laser stimuli (i.e., externally induced sensory event) (34). As a result, the first time-course variable of the design matrix modeled the effect of prestimulus baseline activity and of the brain activity in the response to the stimulus correlated to preceding baseline changes. The second variable modeled the effect of stimulation, uncorrelated to prestimulus baseline activity.
Generation of individual statistical T-maps was then performed by using the same procedure as described above. Contrasts images were computed, identifying differences in baseline brain activity correlated to differences in perception of subsequent sensory stimuli. Subtraction analyses searched for brain areas in which increased baseline activity was associated to subsequent stimulus perception (baseline regressor, P1 > P0), and inversely, for brain areas in which increased baseline activity prevented subsequent stimulus perception (baseline regressor, P0 > P1). Similar subtraction analyses were performed for prestimulus baseline activity before intensity-matched stimuli, perceived as mildly or moderately painful (baseline regressors, P3 > P2 and P2 > P3). Second-level analyses were performed as described above. Resulting statistical maps were thresholded as described above at corrected PSVC < 0.05 (49). Small-volume correction was performed as reported above on the same areas as in the first analysis and on previously reported vigilance-related medial thalamus coordinates (17, 56, 57).
Acknowledgments
This study was supported by Belgian Fonds National de la Recherche Scientifique Grant 3.4517.04 and by grants from the Fondation Médicale Reine Elisabeth, the Centre Hospitalier Universitaire Sart Tilman, the University of Liège, and the Mind Science Foundation. M.B., E.B., C.P., P.M., and S.L. were supported by Fonds National de la Recherche Scientifique.
Abbreviations
- ACC
anterior cingulate cortex
- pACC
posterior part of the ACC
- aACC
anterior part of the ACC
- BOLD
blood oxygenation level-dependent
- fMRI
functional MRI
- YAG
yttrium/aluminum–garnet.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
References
- 1.Sergent C, Baillet S, Dehaene S. Nat Neurosci. 2005;8:1391–1400. doi: 10.1038/nn1549. [DOI] [PubMed] [Google Scholar]
- 2.Fox MD, Snyder AZ, Zacks JM, Raichle ME. Nat Neurosci. 2006;9:23–25. doi: 10.1038/nn1616. [DOI] [PubMed] [Google Scholar]
- 3.Dehaene S, Naccache L, Cohen L, Bihan DL, Mangin JF, Poline JB, Riviere D. Nat Neurosci. 2001;4:752–758. doi: 10.1038/89551. [DOI] [PubMed] [Google Scholar]
- 4.Buchel C, Bornhovd K, Quante M, Glauche V, Bromm B, Weiller C. J Neurosci. 2002;22:970–976. doi: 10.1523/JNEUROSCI.22-03-00970.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ress D, Heeger DJ. Nat Neurosci. 2003;6:414–420. doi: 10.1038/nn1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pessoa L, Padmala S. Proc Natl Acad Sci USA. 2005;102:5612–5617. doi: 10.1073/pnas.0500566102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pessoa L, Gutierrez E, Bandettini P, Ungerleider L. Neuron. 2002;35:975–987. doi: 10.1016/s0896-6273(02)00817-6. [DOI] [PubMed] [Google Scholar]
- 8.Dehaene S, Changeux JP. PLoS Biol. 2005;3:e141. doi: 10.1371/journal.pbio.0030141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rees G, Kreiman G, Koch C. Nat Rev Neurosci. 2002;3:261–270. doi: 10.1038/nrn783. [DOI] [PubMed] [Google Scholar]
- 10.Zeki S. Trends Cognit Sci. 2003;7:214–218. doi: 10.1016/s1364-6613(03)00081-0. [DOI] [PubMed] [Google Scholar]
- 11.Lamme VA. Trends Cognit Sci. 2003;7:12–18. doi: 10.1016/s1364-6613(02)00013-x. [DOI] [PubMed] [Google Scholar]
- 12.Dehaene S, Changeux JP, Naccache L, Sackur J, Sergent C. Trends Cognit Sci. 2006;10:204–211. doi: 10.1016/j.tics.2006.03.007. [DOI] [PubMed] [Google Scholar]
- 13.Bornhovd K, Quante M, Glauche V, Bromm B, Weiller C, Buchel C. Brain. 2002;125:1326–1336. doi: 10.1093/brain/awf137. [DOI] [PubMed] [Google Scholar]
- 14.Sapir A, d'Avossa G, McAvoy M, Shulman GL, Corbetta M. Proc Natl Acad Sci USA. 2005;102:17810–17815. doi: 10.1073/pnas.0504678102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Weissman DH, Roberts KC, Visscher KM, Woldorff MG. Nat Neurosci. 2006;9:971–978. doi: 10.1038/nn1727. [DOI] [PubMed] [Google Scholar]
- 16.Laureys S, Owen AM, Schiff ND. Lancet Neurol. 2004;3:537–546. doi: 10.1016/S1474-4422(04)00852-X. [DOI] [PubMed] [Google Scholar]
- 17.Kinomura S, Larsson J, Gulyas B, Roland PE. Science. 1996;271:512–515. doi: 10.1126/science.271.5248.512. [DOI] [PubMed] [Google Scholar]
- 18.Foucher JR, Otzenberger H, Gounot D. NeuroImage. 2004;22:688–697. doi: 10.1016/j.neuroimage.2004.01.048. [DOI] [PubMed] [Google Scholar]
- 19.Raichle ME, Mintun MA. Annu Rev Neurosci. 2006;29:449–476. doi: 10.1146/annurev.neuro.29.051605.112819. [DOI] [PubMed] [Google Scholar]
- 20.Fox MD, Snyder AZ, Vincent JL, Corbetta M, Van Essen DC, Raichle ME. Proc Natl Acad Sci USA. 2005;102:9673–9678. doi: 10.1073/pnas.0504136102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.McKiernan KA, D'Angelo BR, Kaufman JN, Binder JR. NeuroImage. 2006;29:1185–1191. doi: 10.1016/j.neuroimage.2005.09.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Baars BJ, Ramsoy TZ, Laureys S. Trends Neurosci. 2003;26:671–675. doi: 10.1016/j.tins.2003.09.015. [DOI] [PubMed] [Google Scholar]
- 23.Goldberg II, Harel M, Malach R. Neuron. 2006;50:329–339. doi: 10.1016/j.neuron.2006.03.015. [DOI] [PubMed] [Google Scholar]
- 24.Koyama T, McHaffie JG, Laurienti PJ, Coghill RC. Proc Natl Acad Sci USA. 2005;102:12950–12955. doi: 10.1073/pnas.0408576102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.deCharms RC, Maeda F, Glover GH, Ludlow D, Pauly JM, Soneji D, Gabrieli JD, Mackey SC. Proc Natl Acad Sci USA. 2005;102:18626–18631. doi: 10.1073/pnas.0505210102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hsieh JC, Belfrage M, Stone-Elander S, Hansson P, Ingvar M. Pain. 1995;63:225–236. doi: 10.1016/0304-3959(95)00048-W. [DOI] [PubMed] [Google Scholar]
- 27.Faymonville ME, Laureys S, Degueldre C, DelFiore G, Luxen A, Franck G, Lamy M, Maquet P. Anesthesiology. 2000;92:1257–1267. doi: 10.1097/00000542-200005000-00013. [DOI] [PubMed] [Google Scholar]
- 28.Kupers R, Faymonville ME, Laureys S. Prog Brain Res. 2005;150:251–269. doi: 10.1016/S0079-6123(05)50019-0. [DOI] [PubMed] [Google Scholar]
- 29.Formisano E, Linden DE, Di Salle F, Trojano L, Esposito F, Sack AT, Grossi D, Zanella FE, Goebel R. Neuron. 2002;35:185–194. doi: 10.1016/s0896-6273(02)00747-x. [DOI] [PubMed] [Google Scholar]
- 30.Klein I, Paradis AL, Poline JB, Kosslyn SM, Le Bihan D. J Cognit Neurosci. 2000;12(Suppl 2):15–23. doi: 10.1162/089892900564037. [DOI] [PubMed] [Google Scholar]
- 31.Haynes JD, Rees G. Curr Biol. 2005;15:1301–1307. doi: 10.1016/j.cub.2005.06.026. [DOI] [PubMed] [Google Scholar]
- 32.Henson RN. In: Human Brain Function. Frackowiak R, Friston K, Frith C, Dolan R, Price C, Zeki S, Ashburner J, Penny W, editors. London: Academic; 2003. pp. 793–822. [Google Scholar]
- 33.Cordes D, Haughton VM, Arfanakis K, Carew JD, Turski PA, Moritz CH, Quigley MA, Meyerand ME. Am J Neuroradiol. 2001;22:1326–1333. [PMC free article] [PubMed] [Google Scholar]
- 34.Andrade A, Paradis AL, Rouquette S, Poline JB. NeuroImage. 1999;10:483–486. doi: 10.1006/nimg.1999.0479. [DOI] [PubMed] [Google Scholar]
- 35.Buschman TJ, Miller EK. Science. 2007;315:1860–1862. doi: 10.1126/science.1138071. [DOI] [PubMed] [Google Scholar]
- 36.McKiernan KA, Kaufman JN, Kucera-Thompson J, Binder JR. J Cognit Neurosci. 2003;15:394–408. doi: 10.1162/089892903321593117. [DOI] [PubMed] [Google Scholar]
- 37.Damoiseaux JS, Rombouts SA, Barkhof F, Scheltens P, Stam CJ, Smith SM, Beckmann CF. Proc Natl Acad Sci USA. 2006;103:13848–13853. doi: 10.1073/pnas.0601417103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.De Luca M, Beckmann CF, De Stefano N, Matthews PM, Smith SM. NeuroImage. 2006;29:1359–1367. doi: 10.1016/j.neuroimage.2005.08.035. [DOI] [PubMed] [Google Scholar]
- 39.Beckmann CF, DeLuca M, Devlin JT, Smith SM. Philos Trans R Soc London B. 2005;360:1001–1013. doi: 10.1098/rstb.2005.1634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Vincent JL, Patel GH, Fox MD, Snyder AZ, Baker JT, Van Essen DC, Zempel JM, Snyder LH, Corbetta M, Raichle ME. Nature. 2007;447:83–86. doi: 10.1038/nature05758. [DOI] [PubMed] [Google Scholar]
- 41.World Medical Association. J Am Med Assoc. 1997;277:925–926. [Google Scholar]
- 42.Charlton E. Pain. 1995;63:277–278. doi: 10.1016/0304-3959(95)90040-3. [DOI] [PubMed] [Google Scholar]
- 43.Friston K, Ashburner J, Frith C, Poline JB, Heather J, Frackowiak RSJ. Hum Brain Mapp. 1995;2:165–189. [Google Scholar]
- 44.Penny W, Holmes A. In: Human Brain Function. Frackowiak R, Friston K, Frith C, Dolan R, Price C, Zeki S, Ashburner J, Penny W, editors. London: Academic; 2003. p. 1114. [Google Scholar]
- 45.Friston KJ. In: Human Brain Function. Frackowiak R, Friston K, Frith C, Dolan R, Price C, Zeki S, Ashburner J, Penny W, editors. London: Academic; 2003. pp. 599–601. [Google Scholar]
- 46.Peigneux P, Orban P, Balteau E, Degueldre C, Luxen A, Laureys S, Maquet P. PLoS Biol. 2006;4:e100. doi: 10.1371/journal.pbio.0040100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Orban P, Rauchs G, Balteau E, Degueldre C, Luxen A, Maquet P, Peigneux P. Proc Natl Acad Sci USA. 2006;103:7124–7129. doi: 10.1073/pnas.0510198103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Vandewalle G, Balteau E, Phillips C, Degueldre C, Moreau V, Sterpenich V, Albouy G, Darsaud A, Desseilles M, Dang-Vu TT, et al. Curr Biol. 2006;16:1616–1621. doi: 10.1016/j.cub.2006.06.031. [DOI] [PubMed] [Google Scholar]
- 49.Worsley KJ. Hum Brain Mapp. 1996;4:58–73. doi: 10.1002/(SICI)1097-0193(1996)4:1<58::AID-HBM4>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- 50.Lumer ED, Rees G. Proc Natl Acad Sci USA. 1999;96:1669–1673. doi: 10.1073/pnas.96.4.1669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Marois R, Yi DJ, Chun MM. Neuron. 2004;41:465–472. doi: 10.1016/s0896-6273(04)00012-1. [DOI] [PubMed] [Google Scholar]
- 52.Beck DM, Rees G, Frith CD, Lavie N. Nat Neurosci. 2001;4:645–650. doi: 10.1038/88477. [DOI] [PubMed] [Google Scholar]
- 53.Shulman GL, Fiez JA, Corbetta M, Buckner RL, Miezin FM, Raichle ME, Petersen SE. J Cognit Neurosci. 1997;9:648–663. doi: 10.1162/jocn.1997.9.5.648. [DOI] [PubMed] [Google Scholar]
- 54.Binder JR, Frost JA, Hammeke TA, Bellgowan PS, Rao SM, Cox RW. J Cognit Neurosci. 1999;11:80–95. doi: 10.1162/089892999563265. [DOI] [PubMed] [Google Scholar]
- 55.Rainville P, Duncan GH, Price DD, Carrier B, Bushnell MC. Science. 1997;277:968–971. doi: 10.1126/science.277.5328.968. [DOI] [PubMed] [Google Scholar]
- 56.Portas CM, Rees G, Howseman AM, Josephs O, Turner R, Frith CD. J Neurosci. 1998;18:8979–8989. doi: 10.1523/JNEUROSCI.18-21-08979.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Portas CM, Krakow K, Allen P, Josephs O, Armony JL, Frith CD. Neuron. 2000;28:991–999. doi: 10.1016/s0896-6273(00)00169-0. [DOI] [PubMed] [Google Scholar]