Abstract
The virion host shutoff (vhs) protein encoded by the UL41 gene of herpes simplex virus 1 is an endoribonuclease. The enzyme is introduced into the cell during unpackaging of the virion upon entry and selectively degrades mRNA for several hours. The RNase activity ceases after the onset of synthesis of late (γ) viral proteins. Here we report that vhs protein does not accumulate in cells transiently transfected with only a plasmid encoding the UL41 gene. However, vhs does accumulate in cells cotransfected with plasmids expressing two other tegument proteins, VP16 and VP22. vhs does not directly interact with VP22 but, instead, binds VP22 only in the presence of VP16. In contrast to these findings, the amounts of vhs mRNA accumulating in the cells transfected solely with vhs are not significantly different from those detected in cells coexpressing vhs, VP16, and VP22. We conclude from these studies that the steady state of vhs mRNA, reflecting synthesis and turnover of mRNA, is not affected by the interaction of vhs protein with VP16 with VP22. A model is proposed in which the vhs protein may function to sequester mRNAs in compartments inaccessible to the cellular translational machinery and that VP16 and VP22 rescue the mRNAs by interacting with the vhs protein.
Keywords: herpesviruses, untranslated mRNA
Virion host shutoff (vhs) protein is the product of the UL41 ORF of herpes simplex virus (HSV)-1. During productive infection, copies of vhs protein enter the cell as components of the virion tegument and shut off host protein synthesis by mediating the degradation of preexisting and newly transcribed mRNAs during the first few hours after infection (1–3) (reviewed in ref. 4). The hypothesis that vhs is an endoribonuclease proposed by several investigators (5–9) was recently unambiguously demonstrated with purified protein (10). Indeed, the substrate specificity of the vhs is similar to that of RNase A in that it cleaves at C or U residues in single-stranded stretches of RNA (11).
vhs exists in three states. In chronological order, vhs is packaged in virions in a structure known as the tegument. This structure surrounds the capsid and consists of ≈12 proteins (12) and a selection of mRNAs recruited during virus assembly (13). vhs is not active in the virion inasmuch as the mRNAs are readily recovered either because it is not in the proximity of the mRNAs or because it is maintained in an inactive form. The second stage takes place after the entry of the virus into cells. In this instance, vhs is released in the cytoplasm, accumulates in punctuate structures in the cytoplasm (10), and selectively degrades mRNAs for several hours (14, 15). In the last stage, after the onset of synthesis of late viral proteins, the RNase activity ceases, although newly synthesized vhs begins to accumulate in significant amounts. The conclusion of studies reported in the past decade is that, late in infection, the nuclease activity of the de novo synthesized vhs is dampened by its interaction with the tegument protein designated VP16 or α-transinducing factor (16–18).
The studies we report stemmed from the observations that, although transfection of plasmids encoding vhs has been reported to degrade the mRNAs of cotransfected reporter genes, the synthesis and accumulation of vhs itself has not been unambiguously demonstrated (5, 6). In this report, we present three fundamental findings. First, vhs protein does not accumulate in cells transfected only with a plasmid encoding vhs. Optimal accumulation of vhs requires the cotransfection of plasmids encoding vhs, VP16, and VP22. Second, the vhs protein interacts with both VP16 and VP22 but not with VP22 alone. Third, relatively equal amounts of vhs mRNA accumulate in cells transfected with plasmids encoding vhs alone, vhs plus VP16, or all three ORFs. We conclude that the functions of VP16 and VP22 are to enable the translation of the mRNA rather than to block the degradation of the mRNAs by vhs.
Relevant to this report are some of the properties of VP22. Briefly, VP22 is an abundant tegument phosphoprotein (12, 19) that has been reported to be exported from infected to uninfected adjacent cells (20, 21). VP22, along with the products of UL47 and US11 ORFs, bind mRNA in vitro and may be responsible for the recruitment of mRNA in virions (22). VP22 expressed in transfected cells exports mRNA to adjacent, untransformed cells. Finally, it is noteworthy that VP22 has been reported to interact with VP16 (23).
Results
vhs Pulls Down the Tegument Protein VP22 from Infected Cells.
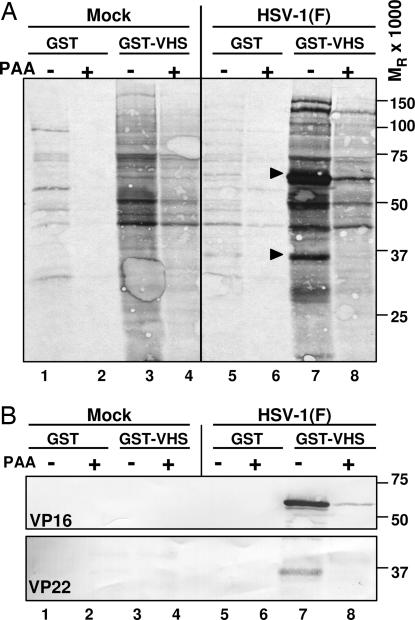
To investigate potential protein–protein interaction of vhs with other viral proteins, GST or GST–VHS fusion protein generated in Escherichia coli, as previously described (10), and captured on glutathione–agarose beads was reacted with [35S]methionine-labeled extracts prepared from HeLa cells that were either mock-infected or infected with HSV-1(F) (10 pfu per cell) and incubated in the presence or absence of inhibitory concentrations of phosphonoacetate (PAA), an inhibitor of viral DNA synthesis. After extensive rinsing, proteins bound to beads were solubilized, subjected to electrophoresis on a denaturing gel, transferred to a nitrocellulose sheet, and exposed to a film. The results (Fig. 1A) were as follows: two predominant bands of 65 and 37 kDa, respectively, were captured by GST–VHS from untreated, infected cell lysates (lane 7, arrows) but not from uninfected cell lysates (lanes 3 and 4) or lysates of infected cells treated with PAA (lane 8). GST failed to pull down these proteins from infected (lanes 5 and 6) or uninfected (lanes 1 and 2) cell lysates. Because the two proteins did not accumulate in cultures exposed to PAA, we concluded that they contained γ2 proteins that required viral DNA synthesis to accumulate.
Fig. 1.
vhs pulls down VP22 from infected cell lysates. One hour after mock infection or exposure to 10 pfu of HSV-1(F) per cell, confluent HeLa cell monolayers were incubated in methionine-free DMEM containing [35S]methionine. A replicate set of cultures was maintained in medium containing 300 μg/ml PAA. The cells were harvested 18 h after infection and lysed in GST lysis buffer (see Materials and Methods). Precleared cell lysates were reacted with GST (lanes 1, 2, 5, and 6) or GST–VHS (lanes 3, 4, 7, and 8) for pull-down assays, resolved by PAGE, and transferred to nitrocellulose membrane. (A) Autoradiographic image of proteins pulled down by GST–VHS. (B) Immunoblotting with anti-VP16 and anti-VP22 antibodies.
On the basis of earlier reports (16, 24), we anticipated that the 65-kDa band contained the tegument protein VP16. On the basis of apparent molecular mass and γ2 phenotype, we predicted the 37-kDa species to be the tegument protein VP22, which is the product of the UL49 gene. Indeed, as shown in Fig. 1B (lane 7), the 65- and 37-kDa bands reacted with the anti-VP16 and anti-VP22 antibodies, respectively. Mass spectrometric analysis further confirmed that the 37-kDa species was in fact VP22 (data not shown). Lastly, the other two bands in the 100–150 kDa range pulled down only by GST–VHS in the absence of PAA (Fig. 1A, lane 7) have not been identified yet.
The Interaction Between vhs and VP22 Is Not Direct but Mediated by VP16.
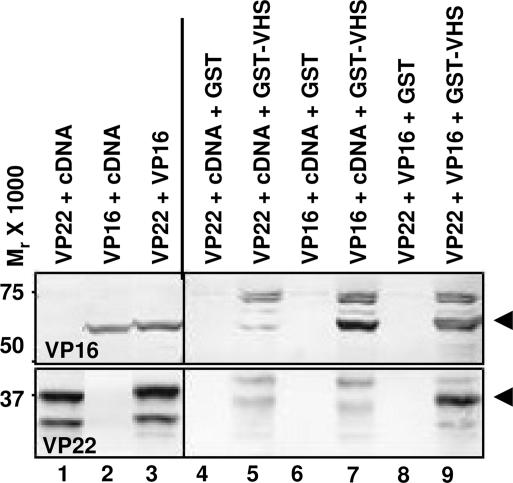
To determine whether vhs interacted directly with VP22, pcDNA 3.1(+) encoding full-length UL49 (pVP22) or full-length UL48 (pVP16) were transiently transfected in HEK 293T cells either alone or in combination. Soluble lysates of cells harvested 48 h after transfection were reacted with glutathione–agarose beads bound to GST or GST–vhs and processed as described in Materials and Methods. The proteins eluted from the beads were subjected to electrophoresis on denaturing polyacrylamide gel, transferred to a nitrocellulose membrane, and reacted with antibodies against either VP16 or VP22. The results shown in Fig. 2 were as follows. (i) VP16 was pulled down by GST–VHS from transfected cells either when expressed alone (Fig. 2, lane 7, upper blot) or in combination with VP22 (Fig. 2, lane 9, upper blot). The higher-molecular-mass bands were probably attributable to a cross-reaction of the anti-VP16 rabbit polyclonal antibody against the GST moiety in the GST–VHS fusion protein. (ii) VP22 was pulled down by GST–VHS from transfected cells only when coexpressed along with VP16 (Fig. 2, lane 9, lower blot) but not when expressed alone (Fig. 2, lane 5, lower blot). These data indicate that the VP22 does not interact directly with vhs but requires the presence of VP16.
Fig. 2.
The interaction between vhs and VP22 is mediated by VP16. HEK 293T cells were transiently transfected with plasmids encoding full-length VP22 or full-length VP16 either alone or in combination. Soluble cell extracts were prepared 48 h after transfection, reacted with glutathione–agarose beads bound to GST (lanes 4, 6, and 8) or GST–VHS (lanes 5, 7, and 9). The proteins bound to the beads were subjected to electrophoresis on a denaturing polyacrylamide gel, transferred to a nitrocellulose membrane, and reacted with antibodies against VP16 (upper blot) or VP22 (lower blot). Immunoreactivity of VP22 (lanes 1 and 3) and VP16 (lanes 2 and 3) from a one-tenth volume of whole-cell lysates also is shown. cDNA, pcDNA 3.1(+).
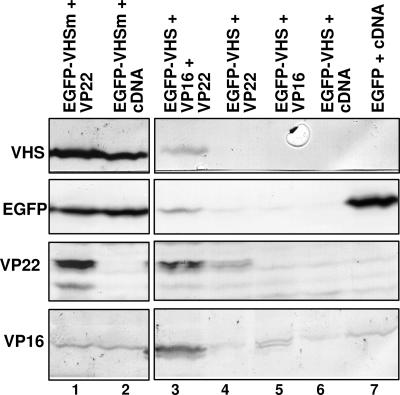
Both VP16 and VP22 Are Required for the Accumulation of vhs Protein in Transiently Transfected Cells.
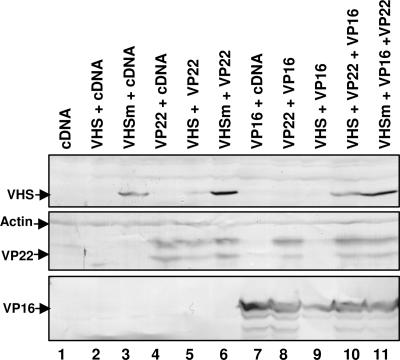
To investigate the role of VP22 in the regulation of vhs activity, HEK 293T cells were transiently transfected with pcDNA3.1(+)-expressing wild-type UL41 (pVHS) or the nuclease-defective vhs mutant (pVHSm) previously described (10) either alone or in combination with plasmids encoding VP22 and/or VP16. The cells were harvested 48 h after transfection, solubilized, and analyzed by immunoblotting as described in Materials and Methods. The results shown in Fig. 3 were as follows. (i) Cells transfected solely with plasmid encoding wild-type vhs (Fig. 3, lane 2) did not accumulate vhs. (ii) Cotransfection of pVHS with the plasmid expressing VP22 (Fig. 3, lane 5) partially rescued wild-type vhs accumulation, whereas the coexpression of VP16 was ineffective (Fig. 3, lane 9). (iii) Cells transfected with pVHSm accumulated the protein in the absence of either VP22 or VP16 (Fig. 3, lane 3), although the levels of the protein were higher in cells cotransfected with either VP22 (Fig. 3, lane 6) or both VP22 and VP16 (Fig. 3, lane 11). (iv) Lastly, cotransfection of pVHS with both VP22 and VP16 restored wild-type vhs accumulation at levels comparable with that of nuclease-defective vhs protein (Fig. 3, compare lane 10 with lane 3).
Fig. 3.
Both VP16 and VP22 are required for the accumulation of vhs protein in transiently transfected cells. HEK 293T cells were transiently transfected with the listed plasmids, harvested 48 h after transfection, solubilized as described in Materials and Methods, and analyzed by immunoblotting for the accumulation of vhs (upper blot), VP22 (middle blot), and VP16 (lower blot). Immunoreactivity to actin (arrow) is shown as a loading control. The results are representative of at least five independent transfection experiments.
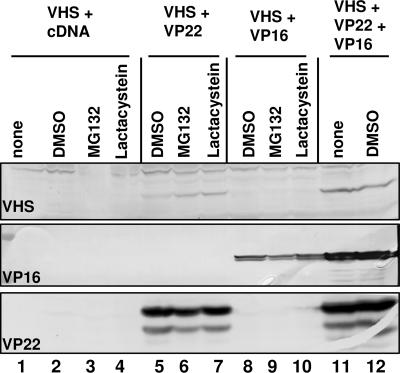
To exclude a trivial hypothesis (namely that in singly transfected cells, wild-type vhs is subject to proteasomal degradation, whereas mutant vhs is not), HEK 293T cells were transfected with pVHS either alone or in combination with pVP22 and/or pVP16 and incubated in medium containing DMSO or the proteasome inhibitors MG132 and lactacystin as described in Materials and Methods. The cells were harvested and analyzed as described above. The results shown in Fig. 4 were as follows. (i) The accumulation of wild-type vhs protein was not rescued by treatment of the cells with either MG132 (Fig. 4, lane 3) or lactacystin (Fig. 4, lane 4), even in the presence of VP16 (Fig. 4, lanes 8–10). (ii) As observed before, the accumulation of vhs absolutely required the coexpression of VP22 (Fig. 4, lanes 5–7), reaching an optimal level when VP16 was also present (Fig. 4, lanes 11 and 12). The slight increase in vhs protein level observed when the cells were cotransfected with VP22 and treated with the proteasome inhibitors (Fig. 4, compare lanes 6 and 7 with lane 5) is presently unclear. We conclude that vhs is not subject to proteasomal degradation in the absence of VP22 or VP16. Furthermore, it should be noted that although vhs interacts with VP22 via VP16, the accumulation of vhs protein in presence of only VP22 (Fig. 3, lane 5, and Fig. 4, lanes 5–7) suggests that VP22 alone can rescue vhs, albeit to a lesser extent than VP22 and VP16 together.
Fig. 4.
Wild-type vhs protein is not degraded by the proteasome pathway in the absence of VP22. HEK 293T cells were transiently transfected with the listed plasmids. Six hours before harvesting, the cells were incubated with mock medium (lanes 1 and 11) or medium containing DMSO (lanes 2, 5, 8, and 12) or 10 μM MG132 (lanes 3, 6, and 9) or 15 μM lactacystin (lanes 4, 7, and 10). The cells were solubilized as described in Materials and Methods and analyzed by immunoblotting for the accumulation of vhs (top blot), VP16 (middle blot), and VP22 (bottom blot).
VP22 and VP16 Can Rescue the Accumulation of a Reporter Gene Coexpressed with vhs.
It as been previously reported that vhs strongly inhibits the expression of a reporter gene in transiently cotransfected mammalian cells and that this effect is eliminated by the mutation that abolishes virion-inducing host shutoff during HSV-1 infection (5, 6). The objective of the next series of experiments was to determine whether, in cells transfected with a plasmid coexpressing wild-type vhs and EGFP, the accumulation of EGFP was inhibited by vhs and could be rescued by coexpression of VP22 and VP16. HEK 293T cells were transfected with pEGFP–VHS or pEGFP–VHSm alone or in combination with pVP22 and/or pVP16. The cells were harvested 48 h after transfection, solubilized, and analyzed for the accumulation of vhs, EGFP, VP22, and VP16, as described in Materials and Methods. The results shown in Fig. 5 were as follows. (i) EGFP accumulated at a very high level when transfected alone (Fig. 5, lane 7) or as a plasmid coexpressing the mutated form of vhs (Fig. 5, lanes 1 and 2). (ii) The expression of EGFP was strongly inhibited by wild-type vhs (Fig. 5, lane 6) and was not rescued by coexpression of solely VP22 (Fig. 5, lane 4) or VP16 (Fig. 5, lane 5). (iii) As for wild-type vhs protein accumulation, the accumulation of EGFP was restored, albeit partially, if both VP22 and VP16 were coexpressed (Fig. 5, lane 3). We conclude that in the absence of VP16 and VP22, vhs causes extensive degradation of mRNA and is probably lethal and that both VP16 and VP22 are required to counteract its toxic effects.
Fig. 5.
VP22 and VP16 rescue the accumulation of a reporter gene coexpressed with vhs. HEK 293T cells were transiently transfected with the listed plasmids, harvested 48 h after transfection, solubilized as described in Materials and Methods, and analyzed by immunoblotting for the accumulation of (from top to bottom) vhs, EGFP, VP22, and VP16.
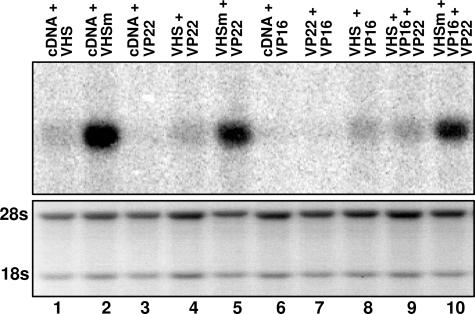
The Amounts of vhs mRNAs Are Not Augmented by the Presence of VP22 and/or VP16.
The objective of this series of experiments was to determine whether in cells transfected with a plasmid encoding wild-type vhs in the absence of plasmids encoding VP22 and VP16 the nascent vhs would degrade its own RNA resulting in lack of protein accumulation. The rationale underlying this experiment was based on the expectation that, at the very least, nascent vhs would degrade its own mRNA and possibly other mRNAs before decay. In these experiments, HEK 293T cells were transfected as described above and cytoplasmic RNA was isolated 48 h after transfection and probed for vhs RNA as described in Materials and Methods. The results are shown in Fig. 6. Cells transfected with the plasmid encoding wild-type vhs only accumulated very low levels of UL41 RNA (Fig. 6, lane 1), whereas the latter was expressed in much larger amounts in cells transfected with the plasmid encoding the nuclease-defective mutant (Fig. 6, lanes 2, 5 and 10). Surprisingly, the level of UL41 RNA was not increased by the concomitant expression of VP22 (Fig. 6, lane 4), VP16 (Fig. 6, lane 8), or both proteins (Fig. 6, lane 9). We conclude that (i) vhs mRNA is transported into the cytoplasm and (ii) the amounts of vhs mRNA are not augmented in cells coexpressing VP16, VP22, or both VP16 and VP22.
Fig. 6.
VP22 does not up-regulate the expression of vhs RNA. Cytoplasmic RNA was purified from HEK 293T cells harvested 48 h after transfection with the indicated plasmids. RNA (8 μg) was loaded onto denaturing formaldehyde gel, transferred onto a nylon membrane, and probed with 32P-labeled fragment containing the entire coding sequence of vhs. The ethidium-bromide-stained rRNAs served as loading controls.
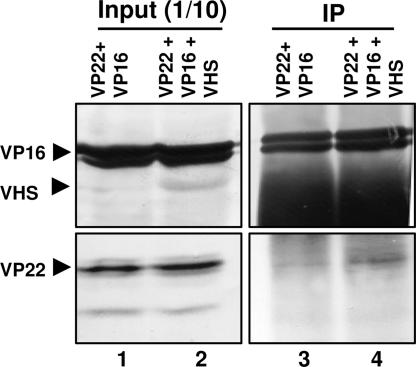
The Interaction Between VP16 and VP22 Is Stabilized by vhs.
To investigate the role, if any, of vhs in the interaction between VP16 and VP22, HEK 293T cells were transiently cotransfected with both VP16- and VP22-expressing plasmids with or without the plasmid expressing wild-type vhs. Soluble cell extracts were prepared 48 h after transfection, incubated with a mouse monoclonal antibody against VP16, and processed as described in Materials and Methods. The proteins bound to the antibody were subjected to electrophoresis on denaturing polyacrylamide gel, transferred to a nitrocellulose membrane, and reacted with antibodies against VP22, VP16, or vhs. As shown in Fig. 7, the amount of VP22 that coimmunoprecipitated with VP16 was greater in the presence of vhs (Fig. 7, lane 4) than in its absence (Fig. 7, lane 3). The results suggest that vhs stabilizes the interaction between VP22 and VP16 in the absence of other viral proteins.
Fig. 7.
vhs stabilizes the interaction between VP22 and VP16. Plasmids encoding VP22 and full-length VP16 were transiently transfected in HEK 293T cells with (lanes 1 and 3) or without (lanes 2 and 4) the plasmid expressing wild-type vhs. Soluble cell extracts were prepared 48 h after transfection and incubated with mouse monoclonal anti-VP16 antibody (lanes 3 and 4). The proteins bound to the antibody were subjected to electrophoresis on a denaturing polyacrylamide gel, transferred to a nitrocellulose membrane, and reacted with antibodies against VP16 and vhs (upper blot) or VP22 (lower blot). Immunoreactivity of VP22, VP16, and vhs (lanes 1 and 2) from a 1/10th volume of whole-cell lysates also is shown.
Discussion
The HSV RNase activity plays an important role in the biology of the infected cell. For several hours after infection, vhs degrades in a selective fashion both preexisting and newly synthesized mRNAs. These early events preclude host responses to infection and eliminate from the infected cells short-lived proteins by blocking the repopulation of their mRNAs (e.g., TNF-α receptor 1) (25). Another function attributed to vhs is synchronization of the transition from early to late protein synthesis because, in the absence of vhs, this transition is delayed.
As noted in the Introduction, vhs is inactive in virions and late in infection but highly active during the first several hours after virus entry into cells. The studies reported here focus on the neutralization of the functions of vhs presumed to take place late in infection at the time of the synthesis and accumulation of vhs. The salient features of the results are twofold. Foremost, in the absence of the full complement of viral genes, maximal accumulation of vhs requires the presence of both VP16 and VP22. The results also show that a reporter gene cotransfected with vhs is not expressed in the absence of VP16 and VP22. The second, and equally important, finding is that the amounts of vhs mRNA accumulating in cells transfected with a plasmid encoding wild-type vhs only are not significantly different from those accumulating in cells cotransfected with VP16 or VP22 or both VP16 and VP22. In all cases, the amounts of wild-type vhs mRNAs were significantly lower than those detected in cells transfected with the plasmid encoding a nuclease-defective form of vhs. To fully appreciate the potential role of VP16 and VP22, it is necessary to examine the implications of the results of the vhs mRNA analysis shown in Fig. 6.
Briefly, the conclusion of the results shown in Fig. 6 is that the steady state of vhs mRNA is not dependent on the presence or absence of VP16 or VP22. Rather, VP16 and VP22 are required for the accumulation of vhs protein. We have excluded the trivial hypothesis that VP16 and VP22 preclude the degradation of newly synthesized vhs. The alternative we propose is that VP16 and VP22 rescue the vhs mRNA and make it available to the machinery of the cell for translation. The role of VP22 in this process would be of particular interest because it is a RNA-binding protein. According to this model, in the absence of VP16 and VP22, the vhs mRNA is sequestered and unavailable for translation. The model also predicts that the main function of VP16 and VP22 is to enable the translation of the mRNA, rather than to block the RNase activity, which in turn may depend on the compartment in which vhs is confined. The observations reported earlier that vhs brought into the infected cells accumulates in punctuate structures immediately after viral infection (10) is in support of vhs localization in specific cellular compartments, the nature of which is still under investigation.
Irrespective of whether the model we propose is tenable, the observation that VP16 and VP22 affect the translation rather than accumulation of the vhs mRNA alters the landscape of our understanding of the functions of vhs and of the vhs–VP16–VP22 complex that is prevalent late in the course of the viral-replication cycle.
Materials and Methods
Cells and Virus.
HeLa and HEK 293T cells were obtained from the American Type Culture Collection (Manassas, VA) and propagated as described elsewhere (26). HSV-1(F) is the prototype HSV-1 wild-type strain used in our laboratory (27).
Plasmids.
The wild-type and nuclease-defective mutant UL41, as well as UL48 and UL49 ORFs, was subcloned into pCDNA3.1(+)-transfer vector from previously generated constructs (10, 22). The resulting plasmids were named pVHS, pVHSm, pVP16, and pVP22, respectively. Furthermore, the wild-type and nuclease-defective mutant UL41 ORF were subcloned in the multiple cloning site of pCMS–EGFP vector (Clontech, Palo Alto, CA), which also expresses EGFP under the control of simian virus 40 early promoter. The resulting plasmids were named pEGFP–VHS and pEGFP–VHSm, respectively.
Antibodies.
The rabbit polyclonal antibodies against VHS, VP22, or VP16 have been described elsewhere (10, 28, 29). Mouse monoclonal against VP16 (LP1) was a gift from A. Minson (Cambridge University, Cambridge, U.K.). Mouse monoclonal antibody against actin was purchased from Sigma (St. Louis, MO). Mouse monoclonal antibody against EGFP was purchased from Santa Cruz Biotechnology (Santa Cruz, CA).
HeLa Cell Infection and [35S] Labeling.
Replicate 25-cm2 cultures of HeLa cell monolayers were either mock-infected or exposed to 10 pfu of HSV-1(F) per cell. After 1 h, the inoculum was removed and replaced with methionine-free DMEM containing 100 μCi of [35S]methionine, and the cells were incubated for an additional 18 h. PAA (300 μg/ml) was present in one set of cultures throughout the infection to prevent viral DNA synthesis.
HEK 293T Cell Transient Transfection.
HEK 293T cells were seeded in 25-cm2 flasks the day before transfection in DMEM containing 10% FBS. The following day, a total of 3.6 μg of plasmid DNAs described above were mixed with 10 μl of Plus reagent (Invitrogen, Carlsbad, CA) in 300 μl of serum and antibiotic-free DMEM for 15 min. Then 300 μl of antibiotic-free DMEM containing 15 μl of Lipofectamine (Invitrogen) was added to the plasmid DNA mixture. The mixture was incubated for an additional 15 min and added to the cells in 2 ml of medium. After 4 h, 3 ml of DMEM containing 20% newborn calf serum and 2× antibiotics were added to the cells. The cells were harvested 48 h after transfection and lysed according to the protocol for the subsequent analyses. Where indicated, the transfected cells were exposed for 6 h before harvesting to 10 μM proteasome inhibitor MG132 (Biomol, Plymouth Meeting, PA) or to 15 μM clastolactacystin β-lactone (Biomol), a more specific inhibitor of the 20S proteasome.
Expression and Purification of GST Fusion Proteins.
GST fusion proteins containing GST alone or GST fused to full-length vhs were expressed and purified as reported elsewhere (10).
GST Pull-Down Assay.
Infected HeLa cells or transfected HEK 293T cells were lysed in GST lysis buffer [20 mM Tris (pH 8.0)/1 mM EDTA/1% Nonidet P-40/200 mM NaCl/0.1 mM sodium orthovanadate/10 mM NaF/2 mM DTT/protease inhibitor mixture (Complete protease mixture; Roche Diagnostics, Indianapolis, IN)] on ice for 1 h. Insoluble material was pelleted by centrifugation at maximum speed in a 5415C centrifuge (Eppendorf, Boulder, CO) for 10 min at 4°C. The supernatant fluids precleared with 50 μl of a 50% slurry of glutathione beads for 3 h at 4°C were incubated overnight at 4°C with equal amounts of a 50% slurry of glutathione beads bound to GST alone or GST fused to the vhs protein. The beads were pelleted by centrifugation and rinsed five times with GST buffer. The proteins bound to the beads were solubilized in 50 μl of SDS gel-loading buffer [2% SDS/5% 2-mercaptoethanol/50 mM Tris (pH 6.8)/2.75% sucrose), heated to 95°C for 5 min, resolved by PAGE, transferred to nitrocellulose membrane, and immunoblotted with the appropriate antibodies.
Immunoblotting.
Cells were collected 48 h after transfection, rinsed once with cold PBS, and then solubilized in disruption buffer [50 mM Tris·HCl (pH 7)/2% SDS/710 mM 2-mercaptoethanol/3% sucrose] and sonicated to shred genomic DNA. One-third of lysates was boiled for 5 min, and the solubilized proteins were subjected to electrophoresis in a 10% denaturing polyacrylamide gel, transferred to a nitrocellulose sheet, blocked with 5% nonfat milk, reacted with primary antibody followed by appropriate secondary antibody conjugated to alkaline phosphatase (Bio-Rad, Hercules, CA), and visualized according to the instructions of the manufacturer.
Cytoplasmic RNA Isolation and Northern Blot Analysis.
Cytoplasmic RNA was isolated from transfected HEK 293T cells with the aid of an RNeasy mini kit according to the protocol suggested by the manufacturer (Qiagen, Valencia, CA). Eight micrograms of cytoplasmic RNA were loaded onto denaturing formaldehyde gel and probed with random hexanucleotide-primed 32P-labeled fragment encompassing the entire vhs ORF upon transfer onto a nylon membrane. Prehybridization and hybridization were performed with the ULTRAhyb buffer (Ambion, Austin, TX) supplemented with 200 μg of denatured salmon sperm DNA per milliliter (Stratagene, La Jolla, CA). The membranes were prehybridized for 2 h at 42°C and then overnight after the addition of the 32P-labeled probe. The membranes were rinsed as suggested by the manufacturer of the ULTRAhyb buffer and exposed to film for signal detection.
Immunoprecipitation.
HEK 293T cells were harvested 48 h after transfection and lysed in high-salt lysis buffer [20 mM Tris·HCl (pH 8)/1 mM EDTA/400 mM NaCl/1% Nonidet P-40/0.1 mM sodium orthovanadate/10 mM NaF/2 mM DTT/protease inhibitor mixture]. Samples were kept on ice for 1 h, and insoluble material was pelleted by centrifugation. The supernatant was brought up in an equal volume of lysis buffer without NaCl (final concentration of NaCl, 200 mM). Lysates were precleared with preimmune rabbit serum for 2 h at 4°C, then 50 μl of a 50% slurry of protein A conjugated to agarose was added. Samples were centrifuged, and the supernatant was transferred to new tubes and reacted with mouse monoclonal anti-VP16 antibody (LP1) overnight at 4°C. The immune complex was captured by protein A agarose and then washed five times by using low-salt lysis buffer. The immunoprecipitate was resuspended in 50 μl of disruption buffer, boiled for 5 min, subjected to electrophoresis in 12% denaturing polyacrylamide gel, transferred to a nitrocellulose sheet, and reacted with anti-VP22, anti-VP16, and anti-VHS antibodies.
Acknowledgments
These studies were supported by National Cancer Institute Grants CA115662, CA83939, CA71933, CA78766, and CA88860.
Abbreviations
- HSV
herpes simplex virus
- PAA
phosphonoacetate
- vhs
virion host shutoff.
Footnotes
The authors declare no conflict of interest.
References
- 1.Kwong AD, Frenkel N. Proc Natl Acad Sci USA. 1987;84:1926–1930. doi: 10.1073/pnas.84.7.1926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Strom T, Frenkel N. J Virol. 1987;61:2198–2207. doi: 10.1128/jvi.61.7.2198-2207.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Karr BM, Read GS. Virology. 1999;264:195–220. doi: 10.1006/viro.1999.9986. [DOI] [PubMed] [Google Scholar]
- 4.Smiley JR. J Virol. 2004;78:1063–1068. doi: 10.1128/JVI.78.3.1063-1068.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jones FE, Smibert CA, Smiley JR. J Virol. 1995;69:4863–4871. doi: 10.1128/jvi.69.8.4863-4871.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pak AS, Everly DN, Knight K, Read GS. Virology. 1995;211:491–506. doi: 10.1006/viro.1995.1431. [DOI] [PubMed] [Google Scholar]
- 7.Zelus BD, Stewart RS, Ross J. J Virol. 1996;70:2411–2419. doi: 10.1128/jvi.70.4.2411-2419.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Elgadi MM, Hayes CE, Smiley JR. J Virol. 1999;73:7153–7164. doi: 10.1128/jvi.73.9.7153-7164.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Elgadi MM, Smiley JR. J Virol. 1999;73:9222–9231. doi: 10.1128/jvi.73.11.9222-9231.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Taddeo B, Zhang W, Roizman B. Proc Natl Acad Sci USA. 2006;103:2827–2832. doi: 10.1073/pnas.0510712103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Taddeo B, Roizman B. J Virol. 2006;80:9341–9345. doi: 10.1128/JVI.01008-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Heine JW, Honess RW, Cassai E, Roizman B. J Virol. 1974;14:640–651. doi: 10.1128/jvi.14.3.640-651.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sciortino MT, Suzuki M, Taddeo B, Roizman B. J Virol. 2001;75:8105–8116. doi: 10.1128/JVI.75.17.8105-8116.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Esclatine A, Taddeo B, Evans L, Roizman B. Proc Natl Acad Sci USA. 2004;101:3603–3608. doi: 10.1073/pnas.0400354101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Esclatine A, Taddeo B, Roizman B. Proc Natl Acad Sci USA. 2004;101:18165–18170. doi: 10.1073/pnas.0408272102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Smibert CA, Popova B, Xiao P, Capone JP, Smiley JR. J Virol. 1994;68:2339–2346. doi: 10.1128/jvi.68.4.2339-2346.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lam Q, Smibert CA, Koop KE, Lavery C, Capone JP, Weinheimer SP, Smiley JR. EMBO J. 1996;15:2575–2581. [PMC free article] [PubMed] [Google Scholar]
- 18.Knez J, Bilan PT, Capone JP. J Virol. 2003;77:2892–2902. doi: 10.1128/JVI.77.5.2892-2902.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Leslie J, Rixon FJ, McLauchlan J. Virology. 1996;220:60–68. doi: 10.1006/viro.1996.0286. [DOI] [PubMed] [Google Scholar]
- 20.Elliott G, O'Hare P. Gene Ther. 1999;6:149–151. doi: 10.1038/sj.gt.3300850. [DOI] [PubMed] [Google Scholar]
- 21.Aints A, Dilber MS, Smith CI. J Gene Med. 1999;1:275–279. doi: 10.1002/(SICI)1521-2254(199907/08)1:4<275::AID-JGM44>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- 22.Sciortino MT, Taddeo B, Poon AP, Mastino A, Roizman B. Proc Natl Acad Sci USA. 2002;99:8318–8323. doi: 10.1073/pnas.122231699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Elliott G, Mouzakitis G, O'Hare P. J Virol. 1995;69:7932–7941. doi: 10.1128/jvi.69.12.7932-7941.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schmelter J, Knez J, Smiley JR, Capone JP. J Virol. 1996;70:2124–2131. doi: 10.1128/jvi.70.4.2124-2131.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liang L, Roizman B. J Virol. 2006;(15):7756–7759. doi: 10.1128/JVI.00587-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Esclatine A, Taddeo B, Roizman B. J Virol. 2004;78:8582–8592. doi: 10.1128/JVI.78.16.8582-8592.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ejercito P, Kieff ED, Roizman B. J Gen Virol. 1968;2:357–364. doi: 10.1099/0022-1317-2-3-357. [DOI] [PubMed] [Google Scholar]
- 28.Blaho JA, Mitchell C, Roizman B. J Biol Chem. 1994;269:17401–17410. [PubMed] [Google Scholar]
- 29.McKnight JLC, Kristie TM, Roizman B. Proc Natl Acad Sci USA. 1987;84:7061–7065. doi: 10.1073/pnas.84.20.7061. [DOI] [PMC free article] [PubMed] [Google Scholar]