Abstract
The chromatin-associated protein ATRX was originally identified because mutations in the ATRX gene cause a severe form of syndromal X-linked mental retardation associated with α-thalassemia. Half of all of the disease-associated missense mutations cluster in a cysteine-rich region in the N terminus of ATRX. This region was named the ATRX-DNMT3-DNMT3L (ADD) domain, based on sequence homology with a family of DNA methyltransferases. Here, we report the solution structure of the ADD domain of ATRX, which consists of an N-terminal GATA-like zinc finger, a plant homeodomain finger, and a long C-terminal α-helix that pack together to form a single globular domain. Interestingly, the α-helix of the GATA-like finger is exposed and highly basic, suggesting a DNA-binding function for ATRX. The disease-causing mutations fall into two groups: the majority affect buried residues and hence affect the structural integrity of the ADD domain; another group affects a cluster of surface residues, and these are likely to perturb a potential protein interaction site. The effects of individual point mutations on the folding state and stability of the ADD domain correlate well with the levels of mutant ATRX protein in patients, providing insights into the molecular pathophysiology of ATR-X syndrome.
Keywords: ATR-X syndrome, NMR structure, zinc finger
ATRX was identified when the gene encoding this protein was shown to be mutated in a form of X-linked mental retardation (ATR-X syndrome) in young males (1, 2). Furthermore, null mutations in mice are lethal at the embryonic stage of development (3). Because ATRX mutations reduce α-globin synthesis, causing α-thalassemia, it seems likely that ATRX normally plays a role in the regulation of globin gene expression (2, 4). The complexity of the disease also suggests that ATRX could be involved in the regulation of other as yet unidentified genes. ATRX is a large (2,492 residue; ≈280 kDa) nuclear protein predominantly localized to heterochromatin and nuclear PML bodies (5, 6). It contains two highly conserved domains, and missense mutations that give rise to ATR-X syndrome fall within these. At the C terminus is a helicase/ATPase domain, which characterizes ATRX as a member of the SNF2 (SWI/SNF) family of chromatin-associated proteins. Experimental evidence shows that ATRX acts as a DNA-dependent ATPase and as a DNA translocase, and it confers modest chromatin-remodeling activity in vitro (6). Thus, it seems likely that ATRX exerts its function by targeting chromatin.
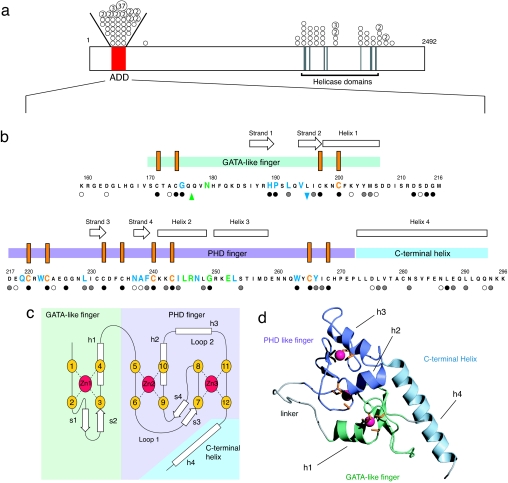
Of the missense mutations identified in the ATRX gene, 50% are located in the N terminus of the ATRX protein, which represents just 4% of the coding sequence (Fig. 1a) (7). This region is highly cysteine-rich and contains two different types of zinc-finger motif. It was first noticed that a region of the sequence shares homology with the plant homeodomain (PHD)-type zinc fingers (8). Mutations in other PHD-containing proteins (WSTF and AIRE) are also associated with human disease (9, 10). PHD fingers are found in nuclear proteins, and accumulating evidence suggests that the role of PHD fingers is to tether proteins (directly or indirectly) to chromatin (11).
Fig. 1.
ATRX protein sequence, structure, and disease-associated mutations. (a) The locations of the highly conserved N-terminal cysteine-rich domain and the C-terminal helicase-like domain are shown. The positions of missense mutations are indicated with circles and the number of times (>1) the mutation has been identified in unrelated individuals is indicated within relevant circles. All of the circles drawn between the oblique lines above the bar refer to mutations within the ADD domain. (b) Locations of mutations and secondary structural elements in the ADD domain. The N-terminal GATA-like zinc finger is indicated by a light green bar, the PHD finger by a mauve bar, and the C-terminal extension by a light blue bar. The conserved cysteine residues are marked as orange vertical bars. Missense mutations are highlighted in green (surface), blue (buried), and orange (cysteines); the insertion mutation is highlighted by an upward green arrow and the deletion by a downward blue arrow. Residues where there is homology across the whole family of ADD domain sequences (ATRX, DNMT3A, DNMT3B, and DNMT3L) are marked with filled circles (absolute conservation), gray circles (strong conservation), and open circles (weak conservation); for the full alignment, see SI Fig. 5. (c) Schematic showing the zinc-binding topology and secondary structure elements of the ADD domain, color scheme as for b. β-Strands are labeled s1–s4 and helices h1–h4. The zinc binding within the PHD finger has the “cross-braced” topology characteristic of such domains, with each zinc coordinated by a noncontiguous set of ligands. (d) Ribbon representation of the NMR structure of the ADD domain (lowest energy structure from the accepted ensemble of 32) of ATRX. The GATA-like finger is shown in green, the PHD finger in mauve, and the C-terminal helix in blue. Linker and unstructured regions are shown in gray, zinc atoms in pink, and side chains of the zinc coordinating cysteines in orange.
Significantly, ATRX is unusual in that its PHD finger is not isolated but is flanked N-terminally by an additional C2C2 motif. Sequence database searches revealed that the only proteins that share this feature are DNMT3A, DNMT3B, and DNMT3L, three proteins involved in DNA methylation (12) [see also supporting information (SI) Fig. 5). This unique arrangement has been named the ATRX-DNMT3-DNMT3L (ADD) domain (13). Additionally, we note that the sequence homology extends C-terminally beyond the cysteine-rich region by ≈25 aa. The ADD domain is consistently found in orthologues of ATRX in vertebrates but is absent from the corresponding ATRX-like proteins in Caenorhabditis elegans and Drosophila, where DNA methylation is absent or rare. Consistent with this observation, the pattern of methylation is perturbed in the DNA of patients with ATR-X syndrome (14) and in mice in which ATRX has been inactivated (3). Together, these observations suggest that the ADD domain is present in chromatin-associated proteins that play a role in establishing and/or maintaining a normal pattern of DNA methylation. Thus, although the precise roles of ATRX in vivo are not known, mutations in the ADD domain clearly affect a number of biological pathways.
To understand the pathophysiology of mutations found in the ATRX-ADD domain, we determined the three-dimensional structure of this domain by NMR. Mapping 40 mutations to the structure reveals key functional regions and thereby provides an understanding of how mutations give rise to ATR-X syndrome.
Results
NMR Structure Determination of the ADD Domain of ATRX.
We report here the structure of the polypeptide K159–K296 (the ADD domain) from human ATRX protein, determined by heteronuclear multidimensional NMR. The N terminus of the domain was established by proteolysis experiments using a longer polypeptide. Expression trials of constructs with different C termini showed that the 25 residues C-terminal to the PHD motif were required for solubility. The first 10 (K159–I168) and final 4 (Q293–K296) residues of the ADD domain are unstructured in solution, whereas residues S210–D217 form a somewhat disordered linker between subdomains (see below). Unexpectedly, this linker showed 15N relaxation properties essentially identical to those of the ordered part of the protein, suggesting that any internal motions within it take place on a time scale slower than overall tumbling of the molecule (data not shown). For the ensemble of accepted structures, the backbone rmsd over residues 168–209 and 218–293 is 0.48 ± 0.12 Å.
Structure of the ADD Domain.
The ADD domain is composed of three clearly distinguishable modules that pack together through extensive hydrophobic interactions to form a single globular domain (Fig. 1 c and d). Starting at the N terminus, there is first a subdomain (residues 168–209) that binds a single zinc ion through four cysteines and is structurally very similar to the zinc fingers of the erythroid transcription factor GATA-1 (15). Packed against this GATA-like finger is a second subdomain (residues 218–272), which binds two zinc ions and closely resembles the structure reported for several PHD fingers (11, 16). Finally, there is a long C-terminal α-helix (residues 273–293) that runs out from the PHD finger and makes extensive hydrophobic contacts with the N-terminal GATA finger, bringing the N and C termini of the ADD domain close together. This combination of fused GATA-like and PHD fingers within a single domain is thus far unique.
As for both of the previously reported zinc fingers of GATA-1 (15, 17), the GATA-like finger of the ADD domain comprises an irregular hairpin loop carrying the first two zinc ligands, followed by a short antiparallel β-sheet (residues 186–189, s1, paired with residues 194–197, s2) and a short α-helix (residues 198–206, h1), the latter starting between the second pair of zinc ligands. The correspondence between the GATA-like finger of the ADD domain and the fingers from GATA-1 itself is very close (Fig. 2 a and b). The main differences are a four-residue insertion at positions 181–185 and a single-residue insertion at position 191 in the ADD domain structure. Discounting these, the backbone rmsd between this region of the ADD domain and the C-terminal finger of GATA-1 is 2.2 Å. Residue I196 of the ADD domain (analogous to V184 in GATA-1) forms the basis of a small hydrophobic core (contacting T172, A173, H189, P190, L192, F201, and Y204). For the ADD and both the GATA-1 fingers, the arrangement of the ligands around the zinc has S absolute chirality (following Berg's convention in ref. 18).
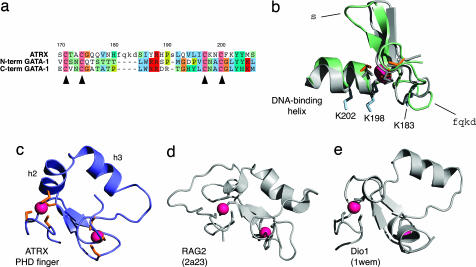
Fig. 2.
Sequence alignment and structural comparisons of the ADD domain with other GATA and PHD fingers. (a) Structure-based sequence alignment of the GATA-like zinc finger of ATRX with the N- and C-terminal zinc fingers of GATA-1. Residues considered as structurally equivalent are shown in uppercase, structurally dissimilar residues are shown in lowercase, and background colors follow the ClustalX scheme. Numbering is based on the ATRX sequence, and the positions of the metal-binding cysteines are indicated with triangles below the alignment. (b) Structural superposition of the GATA-like finger of the ADD domain of ATRX (color scheme as in Fig. 1 b–d) with the C-terminal zinc finger of GATA-1 (shown in gray, except for the zinc, which is red). The position in the structure of the single-residue insertion in the ADD domain relative to GATA-1 is indicated as “s” and that of the four-residue insertion is indicated as “fqkd.” The helix used for DNA binding by the C-terminal finger of GATA-1 and its analogue in the ADD domain are indicated, and the basic residues that the ADD domain might use for DNA binding (see text) are shown as side chains in blue and labeled with their sequence positions. The superposition was made by fitting the N, Cα, and C′ atoms of residues 167–180, 185–190, and 192–207 of ATRX to the corresponding residues of the C-terminal finger of GATA-1, based on the alignment in a. (c) PHD finger from the ADD domain of ATRX (color scheme as in Fig. 1 b–d). (d) Structure of the PHD finger from V(D)J recombination activating protein 2 (RAG2, Protein Data Bank ID code 2a23) (28), shown in the same orientation as in c. (e) Structure of the PHD finger from death-inducer obliterator-1 (Dio1, Protein Data Bank ID code 1wem), shown in the same orientation as in c. These PHD fingers were chosen for comparison, because they have the closest structural similarity to the PHD finger of ATRX in terms of the position and orientation of helical elements. The correct orientation in c–e was achieved by superposing the positions of the corresponding zinc-binding atoms of the eight zinc-binding residues in each protein.
The PHD finger in the ADD domain structure shares the cross-braced topology of zinc-binding interactions (Fig. 1c), but it differs from others in that all eight zinc-binding residues are cysteines (SI Fig. 5b). A more significant difference is the presence of two well formed α-helices in the ADD domain PHD finger spanning residues 241–248 (h2) and residues 250–258 (h3); this region of the sequence, which corresponds to “loop 2” of other PHD domains (11), is consequently expanded relative to other PHD fingers (SI Fig. 5b). As in other PHD fingers, the PHD finger of the ADD domain also contains a short antiparallel β-sheet (residues 230–232, s3, paired with residues 237–239, s4), and both the zinc sites have the S absolute chirality (18). There is considerable divergence among published structures of PHD fingers; some contain helices or approximately helical single turns at similar locations to the helices in the ADD domain (particularly h2), but none contains these two helices simultaneously and positioned as they are in ATRX (Fig. 2). The distinction between PHD and RING fingers can be difficult, but the presence of a largely buried tryptophan (W263) two residues N-terminal to the seventh zinc-binding ligand indicates that the structure can be classified as a PHD finger (19).
The GATA-like and PHD fingers pack closely together through an extensive network of hydrophobic interactions. The key residues in this network are T172 on the GATA-like finger, which makes many hydrophobic contacts to side chains of residues on the PHD finger, and W222 on the PHD finger, which similarly makes many hydrophobic contacts to the GATA-like finger. Interestingly, the N-terminal finger of GATA-1 itself forms a complex with a partner protein, “friend of GATA” (FOG) (20), and the interface in this complex has a similar location to the “internal interface” between the GATA-like and PHD fingers of the ADD domain (SI Fig. 6).
The 25 residues C-terminal to the PHD motif form a long α-helix (residues 273–293, h4) that completes the structure by extending away from the PHD finger and across one surface of the GATA finger (Fig. 1d). Here, it makes a further network of hydrophobic interactions, extending significantly the structural core between the GATA-like and PHD fingers. Residues on the C-terminal helix that make hydrophobic contacts to the GATA-like finger include L276, V277, V283, F284, L287, and L290.
Surface Properties of the ADD Domain.
The discovery through the structural analysis that the ADD domain contains a GATA-like finger is intriguing and might shed light on the function of ATRX. GATA-1 itself is a transcription factor, and binding to its cognate DNA response element is mediated through the α-helix of its C-terminal finger (15). Interestingly, the corresponding helix (h1) is exposed in the ATRX-ADD domain structure, and basic residues on its surface (K198 and K202) combine with K183 on a neighboring loop to form a basic patch (Figs. 2 and 3), perhaps suggesting a DNA-binding function for the ATRX-ADD domain also.
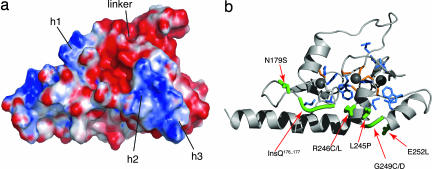
Fig. 3.
Electrostatic potential and location of mutations in the structure of the ADD domain. (a) Surface electrostatic potential of the ADD domain, shown in the same orientation as the ribbon view in b. The helix in the GATA-like finger (h1) is solvent-exposed and basic, and the two helices within loop 2 of the PHD finger (h2 and h3) form another basic patch. The linker between the GATA-like and PHD fingers is highly acidic. (b) Ribbon structure of the ADD domain showing the locations of mutations found in patients with ATR-X syndrome. Mutations are classified as surface (green), buried (blue), or cysteine (orange) and are represented by using their side chains, except for the glycine mutation G249C/D and the glutamine insertion, which are represented by thickening the backbone. The surface mutations are individually labeled.
Apart from the basic patch on the GATA-like finger, the only other significantly basic patch on the surface of the ATRX-ADD domain occurs on the PHD finger in the region involving helices 2 and 3, where residues K241, K242, R246, R251, and K252 combine to form the most prominent feature of the electrostatic surface (Fig. 3). The two basic patches lie on the same face of the ADD domain surface and are separated by ≈20 Å. Much of the remainder of the ADD domain surface is acidic, in particular the area immediately between the two basic patches, where several acidic side chains are contributed by the linker between the GATA-like and PHD fingers (D207, D208, D212, D214, and D217) and the PHD finger itself (E218 and E225).
Structure/Function Analysis of Amino Acid Point Mutations.
To relate the structural information to function, we mapped the 40 previously reported ATRX mutations of known functional relevance to the three-dimensional structure of the ADD domain (Fig. 3, see also SI Table 1). These occur as single point mutations in patients and change the identity of single amino acids at 28 positions in the structure, located in both the GATA-like and PHD fingers. Multiple mutations change the identity of 16 deeply buried amino acid residues, in the hydrophobic core of the structure, referred to as buried mutations. Additional mutations affect six zinc-coordinating cysteines (Fig. 3, C200, C220, C223, C240, C243, and C265). Interestingly, another set of point mutants map to six solvent-exposed amino acid residues located along a strip on one face of the ADD domain structure (Fig. 3), involving residues from both the GATA-like and PHD fingers. Within the GATA-like finger, two mutations (N179S and the glutamine insertion between Q176 and Q177) occur in a loop region, marginally increasing its polarity. Adjacent to these is a region that appears to be a mutational hotspot (L245–E252). These mutations fall within the loop 2 region that in other PHD fingers has been identified as a protein interaction site (11). In the PHD finger of ATRX, this loop 2 region is longer and more highly structured, containing two α-helices. Residues on the surface of the two α-helices give rise to the larger of the two basic patches mentioned (Fig. 3), and some of the mutations result in a reduction in basicity.
To obtain a measure of the effect of the mutations on the ADD domain structure, we expressed many of the point mutants in Escherichia coli and analyzed their ability to fold by using one-dimensional NMR (SI Table 1 and SI Fig. 7). ADD domain proteins containing a mutation of buried amino acid residues expressed at low levels and those affecting zinc-coordinating cysteines expressed only in trace amounts. Surprisingly, when sufficient protein was available for one-dimensional NMR analysis, most of these mutant proteins appeared to be correctly folded. By contrast, protein constructs containing mutations of surface residues were expressed at relatively high levels and were correctly folded as judged by one-dimensional NMR. Therefore, these data reinforce the conclusions drawn from the structural analysis and suggest that the destabilization of the structural core results in lower recovery of soluble protein from E. coli.
Relating Structure to Function in Vivo.
Potentially, the most interesting and significant insight might come from investigating the effects of known mutations in the ADD structure on ATRX expression in vivo. Because ATRX is ubiquitously expressed throughout differentiation and development, we analyzed endogenously expressed ATRX by using EBV-transformed cell lines from normal individuals and from patients with ATR-X syndrome (SI Table 1). We first analyzed the ATRX mRNA expression levels and found, as expected, that the missense mutations had no significant effect on mRNA expression (Fig. 4a). We next analyzed the levels of ATRX protein by quantitative immunoblotting (Fig. 4b and SI Table 1). Patients with point mutations of the zinc-coordinating cysteines had very low, but readily detectable, levels of full-length protein (7–12% of normal levels). Mutations in buried residues also gave rise to lower levels of full-length ATRX protein (6–29% of normal levels). Mutations of surface residues (as defined in SI Table 1) had less effect on the amounts of ATRX (32–55% of normal levels). It is striking that the levels of ATRX protein found in patients correlate with the amount of ADD domain recovered from E. coli (SI Table 1). Given that the ATRX mRNA levels are not affected by missense mutations, the most likely explanation for the low levels of ATRX found in patients is that destabilization of the structural fold of the ADD domain causes loss of full-length ATRX protein in the cellular environment.
Fig. 4.
ATRX in vivo expression in EBV-transformed patient lymphocytes. (a) ATRX mRNA levels of patient mutations and normal controls as determined by quantitative RT-PCR. Patients are grouped according to the nature of their underlying mutation: cysteine mutations are orange, buried mutations are blue, and surface mutations are green. Values for normal individuals are represented by black circles. For each case, the ATRX mRNA level is expressed as the percentage of the average for 18 normal control individuals. (b) ATRX protein levels of patients and normal controls. Cases are grouped as in a. ATRX protein levels are expressed as a percentage of the average value for seven normal control individuals. (c) Representative Western blots showing ATRX protein levels (including loading control). Lane 1 represents the ATRX protein level for a cysteine mutation, lanes 2–4 are buried mutations, lanes 5–7 are surface mutations, and lane 8 is a normal control.
Thus, these structure/function studies demonstrate that there are at least two molecular mechanisms underlying ATR-X syndrome. Mutations that affect the structural core of the ADD domain destabilize the protein fold, which in turn leads to lower ATRX protein levels in vivo. The lower levels of protein as well as the instability of the structure are likely to affect function, including interactions with ATRX partners. The second mechanism involves the class of mutations that fall on the surface of the ADD domain (Fig. 4). Although these mutations also affect protein stability to some extent, they are most likely to affect interactions with specific ATRX ligands directly.
Discussion
The structure of the ADD domain of ATRX presented here represents a unique combination of zinc-binding modules, comprising a GATA-like zinc finger, a PHD zinc finger, and C-terminal α-helix that pack together to fold as a single globular domain. Given the sequence similarity across the members of the ADD domain family (12) (SI Fig. 5), it is likely that the essential features of this structure are also shared by the DNMT3 proteins.
The ADD domain of ATRX harbors 50% of all naturally occurring missense mutations of the ATRX gene. Mapping of the mutations to the structure provides insights into key functional regions of the ADD domain and hence an understanding of how these mutations lead to the genetic disease ATR-X syndrome. In general, ATR-X syndrome is thought to be caused by a loss of function arising from mutations in the ATRX protein and, where studied, is most commonly associated with low levels of ATRX protein in patients (Fig. 4 and unpublished data). With few exceptions (7), the ATRX phenotype is uniformly severe regardless of the type of mutation. The structure/function analysis presented here shows that most of the ADD point mutations associated with ATR-X syndrome affect buried amino acid residues that are important for structural integrity. In general, such mutations are likely to destabilize the structure, providing an explanation for the lower levels of ATRX protein found in patients. There is a close relationship between the location and function of mutated residues in the core of the ADD domain and the varying amount ATRX protein found in patients: the more essential the residue is for the structural integrity, the more severe is its effect on ATRX protein levels.
The mutations that result in the lowest ATRX protein levels in patients affect the structurally crucial zinc-coordinating cysteines. It is surprising that patients with such mutations have any ATRX protein at all (7–10% of normal levels), suggesting that the extensive hydrophobic core of such mutant proteins partly rescues their fold. This observation would also explain why mutations of other buried or partly buried residues have intermediate effects (6–29% of normal levels). These observations of the effects of mutations are reminiscent of the well documented case of the tumor suppressor p53 (21).
It was therefore of particular interest that there is a small subset of six mutated locations in the ADD domain of ATRX involving surface amino acid residues. Patients with these mutations are clinically indistinguishable from those with mutations in the structural core of the ADD domain. Because such patients have relatively normal levels of the protein, these surface mutations must cause the disease by affecting an essential ATRX function. Four of the mutated locations on the surface lie within or adjacent to a large basic patch (Fig. 3) that corresponds to the loop 2 region in other PHD fingers. In some cases, loop 2 has been shown to act as a protein interaction surface. For example, interaction of the PHD finger of KAP1 with Mi-2a depends on residues within loop 2 (22). Similarly, this region of the PHD domain is found in the Drosophila protein Pygopus (a nuclear component of the Wnt signaling pathway), which binds Legless/BCL9. These observations suggest that the exposed loop 2 region in the PHD finger of ATRX may similarly be involved in interacting with specific protein ligands and that point mutations in this region result in disease because they disrupt such interactions.
Although the specific ligand(s) for the ADD domain of ATRX is unknown, studies on other PHD-finger-containing proteins suggest that the function of such domains is to interact (directly or indirectly) with DNA or chromatin. Recent studies have shown that the PHD fingers of at least two chromatin-associated proteins (BPTF and ING2) specifically recognize trimethylated H3 histone tails (H3K4me3). In these cases, discrimination of the methylation state of the side chain is achieved by accommodating the trimethylated group of H3K4 into a hydrophobic aromatic cage on the surface of these PHD domains. No such aromatic cage is present in the ADD domain structure, suggesting that if the PHD finger of ATRX participates in histone tail recognition, it does so by recognizing unmodified histone tails or tails with other posttranscriptional modifications.
The presence of a basic GATA-1-like zinc finger in the ADD domain of ATRX suggests a function in DNA or chromatin binding. GATA-1 itself is a transcription factor; its C-terminal zinc finger binds DNA sequence specifically, and a structure of the complex has been determined (15). Such a binding site has not been identified for ATRX, but modeling the α-helix of the GATA-like finger of the ADD domain onto the recognition helix of GATA-1 itself in its complex with DNA suggests that an analogous interaction would probably be sterically accessible for the ADD domain (SI Fig. 8). Consistent with this view, previous studies demonstrated that in vitro, the ADD domain of ATRX is able to bind DNA homopolymers (23) and genomic DNA fragments (unpublished data). Notably, DNA binding was substantially decreased by mutations of the two basic lysine residues (K198A and K202A) on the DNA-recognition helix (unpublished data). Although the structural homology to GATA-1 and DNA-binding activity are tantalizing, it remains to be shown whether the interaction is sequence-specific or is relevant to ATRX function.
In conclusion, the mapping of the point mutations on the three-dimensional structure of the ADD domain of ATRX, together with in vivo analysis of the effects of mutations on ATRX protein levels, provides a molecular understanding of ATR-X syndrome. This structure/function analysis has led to the discovery of both putative DNA and protein-interaction regions (the GATA-like finger and the loops 1 and 2 regions of the PHD finger), which may act independently or in concert.
Materials and Methods
Preparation of the ADD Domain of ATRX.
The polypeptide-spanning residues 159–296 of ATRX (Fig. 1b) were chosen for NMR studies based on experiments to test different fragments for their expression, solubility, and monodisperse behavior. Proteins were expressed and purified as described in ref. 24 and set out in SI Methods.
Site-Directed Mutagenesis.
Site-directed mutagenesis was performed by using the QuikChange Site-Directed Mutagenesis kit (Stratagene, La Jolla, CA) according to manufacturer's instructions. The oligonucleotides designed to generate mutant proteins are set out in SI Table 2. All constructs were checked by DNA sequence analysis of both strands.
NMR Structure Determination.
Details of NMR sample preparation, data acquisition, assignment strategy, and structure calculations appear in SI Methods. For the PHD finger domain, metal–ligand connectivities were unambiguously established by analyzing preliminary structures calculated without metal-binding constraints in conjunction with sequence alignment against other PHD fingers; this analysis left no ambiguities for the GATA-like finger. Of 100 structures calculated in the final round, 32 correspond to a well defined plateau region in the energy and energy-ordered rmsd profiles (SI Fig. 9), indicating that they form a suitable set for reporting structural statistics (SI Table 3) (25).
Preparation and Quantitation of ATRX RNA.
RNA was prepared from EBV-transformed lymphoblastoid cell lines. Complementary DNA (cDNA) was prepared from 2 μg of RNA and analyzed by using quantitative real-time PCR (26). Details of primers, appropriate controls, and cycling conditions are given in SI Methods.
Western Blot Analysis and Quantification of ATRX Protein.
Nuclei from EBV-transformed cell lines from normal control individuals and those with ATR-X syndrome were prepared in duplicate. Proteins were isolated and analyzed by immunoblotting (27). Intensities of bands in the Western blots were quantified by using the Quantity One 1D ChemiDoc analysis software (Bio-Rad, Hemel Hempstead, U.K.).
Supplementary Material
Acknowledgments
We thank Alexey Murzin and Antonina Andreeva for assistance and helpful discussions concerning sequence alignment and structural homologies.
Abbreviations
- ADD
ATRX-DNMT3-DNMT3L
- PHD
plant homeodomain.
Footnotes
The authors declare no conflict of interest.
Data deposition: The NMR chemical shifts reported in this paper have been deposited in the BioMagResBank, www.bmrb.wisc.edu [accession no. 15001 (1H, 13C, and 15N NMR resonance assignments for ADD domain 159–296)]. The atomic coordinates of the 32 accepted structures have been deposited in the Protein Data Bank, www.pdb.org (PDB ID code 2jm1).
This article contains supporting information online at www.pnas.org/cgi/content/full/0704057104/DC1.
References
- 1.Weatherall DJ, Higgs DR, Bunch C, Old JM, Hunt DM, Pressley L, Clegg JB, Bethlenfalvay NC, Sjolin S, Koler RD, et al. N Engl J Med. 1981;305:607–612. doi: 10.1056/NEJM198109103051103. [DOI] [PubMed] [Google Scholar]
- 2.Gibbons RJ, Picketts DJ, Villard L, Higgs DR. Cell. 1995;80:837–845. doi: 10.1016/0092-8674(95)90287-2. [DOI] [PubMed] [Google Scholar]
- 3.Garrick D, Sharpe JA, Arkell R, Dobbie L, Smith AJH, Wood WG, Higgs DR, Gibbons RJ. PLoS Genet. 2006;2:438–450. doi: 10.1371/journal.pgen.0020058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gibbons RJ, Pellagatti A, Garrick D, Wood WG, Malik N, Ayyub H, Langford C, Boultwood J, Wainscoat JS, Higgs DR. Nat Genet. 2003;34:446–449. doi: 10.1038/ng1213. [DOI] [PubMed] [Google Scholar]
- 5.McDowell TL, Gibbons RJ, Sutherland H, O'Rourke DM, Bickmore WA, Pombo A, Turley H, Gatter K, Picketts DJ, Buckle VJ, et al. Proc Natl Acad Sci USA. 1999;96:13983–13988. doi: 10.1073/pnas.96.24.13983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Xue YT, Gibbons R, Yan ZJ, Yang DF, McDowell TL, Sechi S, Qin J, Zhou SL, Higgs D, Wang WD. Proc Natl Acad Sci USA. 2003;100:10635–10640. doi: 10.1073/pnas.1937626100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gibbons RJ, Wada T. In: Molecular Basis of Inborn Errors of Development. Epstein C, Erickson R, Wynshaw-Boris A, editors. London: Oxford Univ Press; 2004. pp. 747–757. [Google Scholar]
- 8.Gibbons RJ, Bachoo S, Picketts DJ, Aftimos S, Asenbauer B, Bergoffen J, Berry SA, Dahl N, Fryer A, Keppler K, et al. Nat Genet. 1997;17:146–148. doi: 10.1038/ng1097-146. [DOI] [PubMed] [Google Scholar]
- 9.Lu X, Meng X, Morris CA, Keating MT. Genomics. 1998;54:241–249. doi: 10.1006/geno.1998.5578. [DOI] [PubMed] [Google Scholar]
- 10.Nagamine K, Peterson P, Scott HS, Kudoh J, Minoshima S, Heino M, Krohn KJ, Lalioti MD, Mullis PE, Antonarakis SE, et al. Nat Genet. 1997;17:393–398. doi: 10.1038/ng1297-393. [DOI] [PubMed] [Google Scholar]
- 11.Bienz M. Trends Biochem Sci. 2006;31:35–40. doi: 10.1016/j.tibs.2005.11.001. [DOI] [PubMed] [Google Scholar]
- 12.Xie S, Wang Z, Okano M, Nogami M, Li Y, He WW, Okumura K, Li E. Gene. 1999;236:87–95. doi: 10.1016/s0378-1119(99)00252-8. [DOI] [PubMed] [Google Scholar]
- 13.Aapola U, Shibuya K, Scott HS, Ollila J, Vihinen M, Heino M, Shintani A, Kawasaki K, Minoshima S, Krohn K, et al. Genomics. 2000;65:293–298. doi: 10.1006/geno.2000.6168. [DOI] [PubMed] [Google Scholar]
- 14.Gibbons RJ, McDowell TL, Raman S, O'Rourke DM, Garrick D, Ayyub H, Higgs DR. Nat Genet. 2000;24:368–371. doi: 10.1038/74191. [DOI] [PubMed] [Google Scholar]
- 15.Omichinski JG, Clore GM, Schaad O, Felsenfeld G, Trainor C, Appella E, Stahl SJ, Gronenborn AM. Science. 1993;261:438–446. doi: 10.1126/science.8332909. [DOI] [PubMed] [Google Scholar]
- 16.Pascual J, Martinez-Yamout M, Dyson HJ, Wright PE. J Mol Biol. 2000;304:723–729. doi: 10.1006/jmbi.2000.4308. [DOI] [PubMed] [Google Scholar]
- 17.Kowalski K, Czolij R, King GF, Crossley M, Mackay JP. J Biomol NMR. 1999;13:249–262. doi: 10.1023/a:1008309602929. [DOI] [PubMed] [Google Scholar]
- 18.Berg J. Proc Natl Acad Sci USA. 1988;85:99–102. doi: 10.1073/pnas.85.1.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dodd RB, Allen MD, Brown SE, Sanderson CM, Duncan LM, Lehner PJ, Bycroft M, Read RJ. J Biol Chem. 2004;279:53840–53847. doi: 10.1074/jbc.M409662200. [DOI] [PubMed] [Google Scholar]
- 20.Liew CK, Simpson RJY, Kwan AHY, Crofts LA, Loughlin FE, Matthews JM, Crossley M, Mackay JP. Proc Natl Acad Sci USA. 2005;102:583–588. doi: 10.1073/pnas.0407511102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Joerger AC, Ang HC, Fersht AR. Proc Natl Acad Sci USA. 2006;103:15056–15061. doi: 10.1073/pnas.0607286103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Capili AD, Schultz DC, Rauscher FJI, Borden KL. EMBO J. 2001;20:165–177. doi: 10.1093/emboj/20.1.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cardoso C, Lutz Y, Mignon C, Compe E, Depetris D, Mattei MG, Fontes M, Colleaux L. J Med Genet. 2000;37:746–751. doi: 10.1136/jmg.37.10.746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Court R, Chapman L, Fairall L, Rhodes D. EMBO Rep. 2005;6:39–45. doi: 10.1038/sj.embor.7400314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fletcher CM, Jones DNM, Diamond R, Neuhaus D. J Biomol NMR. 1996;8:292–310. doi: 10.1007/BF00410328. [DOI] [PubMed] [Google Scholar]
- 26.Heid CA, Stevens J, Livak KJ, Williams PM. Genome Res. 1996;6:986–994. doi: 10.1101/gr.6.10.986. [DOI] [PubMed] [Google Scholar]
- 27.Harlow E, Lane D. Antibodies: A Laboratory Manual. Cold Spring Harbor, NY: Cold Spring Harbor Lab Press; 1988. [Google Scholar]
- 28.Elkin SK, Ivanov D, Ewalt M, Ferguson CG, Hyberts SG, Sun Z-YJ, Prestwich GD, Yuan J, Wagner G, Oettinger MA, et al. J Biol Chem. 2005;280:28701–28710. doi: 10.1074/jbc.M504731200. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.