Abstract
CD4+ T cells producing IL-17 [T helper (Th)17], as distinct from Th1 or Th2 cells, have recently been shown to be associated with autoimmunity, but it is not entirely clear how Th17 cells are generated from naïve T cells. We demonstrate here that IL-6, but not TNF-α or IL-1β, can, in combination with TGF-β, induce Th17 cell generation from naïve T cells and inhibit TGF-β-induced Foxp3 expression. Moreover, conditioned medium from lipopolysaccharide-stimulated bone marrow-derived dendritic cells (DCCM) can induce IL-17 production in naïve T cells. Interestingly, IL-17 was produced by DCCM even with the addition of anti-gp130 antibody or DCCM from IL-6 KO mice. The combination of IL-6 and TGF-β could maintain activation of signal transducer and activator of transcription (Stat)3, but not of Stat1. IL-27 or IFN-γ suppressed the induction of Th17 cells by TGF-β plus IL-6 and maintained Stat1 activation under these conditions. In contrast, both Stat1 and Stat3 remained to be activated in naïve T cells cultured with DCCM. These findings represent a different basis for Th17 differentiation from naïve T cells.
Keywords: STAT, TGF-β, retinoid-related orphan receptor γt, regulatory T cells
CD4+ T cells [T helper (Th)] are essential regulators of immune responses and inflammatory diseases. They can be divided into different subsets such as Th1, Th2, and regulatory T (Treg) cells, whose development is dictated by the transcription factors T-bet, GATA3, and Foxp3, respectively (1–3). Development of Th1 cells, which evolved to enhance the defense against intracellular pathogens, is linked to the sequential actions of IFN-γ and IL-12 (4, 5). Th2 cells, whose differentiation is driven by IL-4, are important for clearing extracellular organisms (6–8). Besides these effector subsets, CD4+ T cells can differentiate into distinct regulatory subsets (Treg), which express the forkhead/winged helix transcription factor Foxp3. It is well known that TGF-β1 is an important cytokine for promotion of the differentiation of Treg cells, which suppress adaptive T cell responses and prevent autoimmunity (9, 10).
Recently, a subset of IL-17-producing Th cells (Th17) has been identified and shown to play a crucial part in the induction of autoimmune diseases such as rheumatoid arthritis and experimental autoimmune encephalomyelitis (EAE) as well as allergen-specific responses (11–13). Although some reports claim that IL-23 is required for the generation of Th17 cells from naïve T cells (12, 14), others indicate that IL-23 is not required for Th17 commitment and that Th17 cell differentiation is driven by the combination of IL-6 and TGF-β (15–17). It has also been demonstrated that IFN-γ and IL-27 inhibit Th17 development, which depends on signal transducer and activator of transcription (Stat)1 (14, 18–20). Furthermore, the orphan nuclear receptor, retinoid-related orphan receptor γt (RORγt), has been identified as the key transcription factor that determines the differentiation of Th17 lineage (21). Although these findings all pertain to the differentiation mechanisms of Th17 cells, it is not entirely clear how Th17 cells are generated from naïve T cells. In this study, we demonstrate the existence of a previously uncharacterized mechanism in Th17 differentiation.
Results
IL-6 Combined with TGF-β Is Required for the Generation of Th17 Lineage from Naïve T Cells.
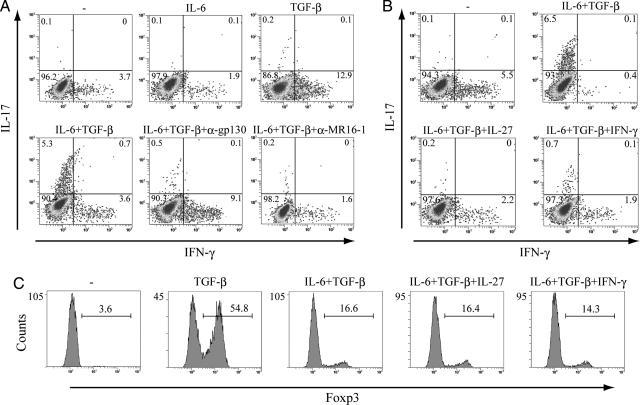
We used flow cytometry (FACS) to detect IL-17-producing T cells and that costimulation of IL-6 and TGF-β generated the Th17 lineage from naïve T cells, as previously reported (15–17) (Fig. 1A). The addition of anti-gp130 antibody or anti-IL-6 receptor antibody, MR16-1, to the combination of IL-6 and TGF-β to neutralize gp130 or IL-6R-mediated activity resulted in a drastic reduction in the number of IL-17-producing T cells (Fig. 1A). IL-27 is a heterodimeric cytokine, whose receptor consists of the IL-27Rα and gp130, a common receptor chain also used by several other cytokines, including IL-6. As reported elsewhere (18–20), IL-27 as well as IFN-γ suppress the generation of IL-17-producing T cells by IL-6 and TGF-β (Fig. 1B).
Fig. 1.
The combination of IL-6 and TGF-β induces the development of Th17 cells. Isolated naïve T cells were cultured with anti-CD3/CD28 beads and the indicated cytokines for 3 days. (A and B) Naïve T cells were stimulated with the indicated cytokines in the presence or absence of anti-mouse gp130 antibody or anti-mIL-6R monoclonal antibody MR16-1. After stimulation, cells were restimulated with PMA and ionomycin for 5 h and with GolgiStop (last 2 h) and were then subjected to intracellular cytokine staining. Dot plots show intracellular staining for IFN-γ and IL-17. (C) Foxp3 expression was determined by staining with anti-mouse Foxp3 antibody. These results are representative of three independent experiments.
We next used FACS to investigate the expression of Foxp3. Naïve T cells stimulated by TGF-β expressed Foxp3, and this expression was inhibited by IL-6 (Fig. 1C). As shown in Fig. 1B, IL-27 as well as IFN-γ inhibited the induction of Th17 cells by IL-6 and TGF-β, whereas neither IL-27 nor IFN-γ had any effect on the pattern of Foxp3 expression by IL-6 and TGF-β (Fig. 1C).
Neither TNF-α nor IL-1β Participates in Th17 Cell Differentiation in the Presence of TGF-β.
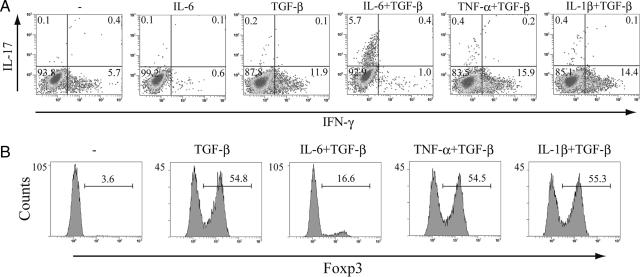
Because IL-17 itself has been implicated in the induction of TNF-α and IL-1β as well as IL-6 (12, 22), we next investigated whether TNF-α and IL-1β are involved in Th17 differentiation. We found that the combination of IL-6 and TGF-β controlled the differentiation of naïve T cells into IL-17-producing T cells, whereas TNF-α or IL-1β did not participate in Th17 commitment in the presence of TGF-β (Fig. 2A). Although IL-6 inhibited the expression of Foxp3 by TGF-β (Fig. 1C), TNF-α and IL-1β did not suppress TGF-β-mediated Foxp3 expression (Fig. 2B).
Fig. 2.
TNF-α or IL-1β in combination with TGF-β has no effect on the differentiation of Th17 cells. (A) MACS-sorted naïve T cells were cultured with anti-CD3/CD28 beads. Cells were stimulated with IL-6 or TGF-β alone and with IL-6, TNF-α, or IL-1β combined with TGF-β. After 3 days, cells were restimulated with PMA and ionomycin before intracellular cytokine staining for IFN-γ and IL-17 and analysis by flow cytometry. Dot plots show intracellular staining for IFN-γ and IL-17. (B) Isolated naïve T cells were activated with anti-CD3/CD28 beads and cultured with TGF-β alone and with IL-6, TNF-α, or IL-1β combined with TGF-β. Foxp3 expression was determined by staining with anti-mouse Foxp3 antibody. These results are representative of three independent experiments.
RORγt Is Induced by IL-6 and TGF-β also in the Presence of IL-27 or IFN-γ.
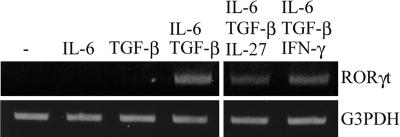
It has been reported recently that RORγt is required for the induction of IL-17 in Th cells (21). To understand how IL-27 or IFN-γ inhibits Th17 differentiation driven by IL-6 and TGF-β, we assessed the expression of RORγt during stimulation by IL-6 and TGF-β with the addition of IL-27 or IFN-γ. Consistent with the finding of a previous study, RORγt was induced by IL-6 and TGF-β. Although IL-27 and IFN-γ almost completely suppressed the generation of Th17 cells by IL-6 and TGF-β (Fig. 1), these cytokines only partially inhibited the expression of RORγt by IL-6 and TGF-β (Fig. 3). Therefore, RORγt is required for the differentiation of Th17 cells but may not be sufficient for the induction of IL-17 expression in naïve T cells.
Fig. 3.
RORγt mRNA expression by IL-6 and TGF-β with or without IL-27 or IFN-γ. Isolated naïve T cells were stimulated with anti-CD3/CD28 beads and the indicated cytokines, followed by total RNA and cDNA prepared as described in Materials and Methods. RORγt induction was examined by using RT-PCR.
Possibility of Participation of Factors Other Than IL-6 in Differentiation of IL-17-Producing T Cells.
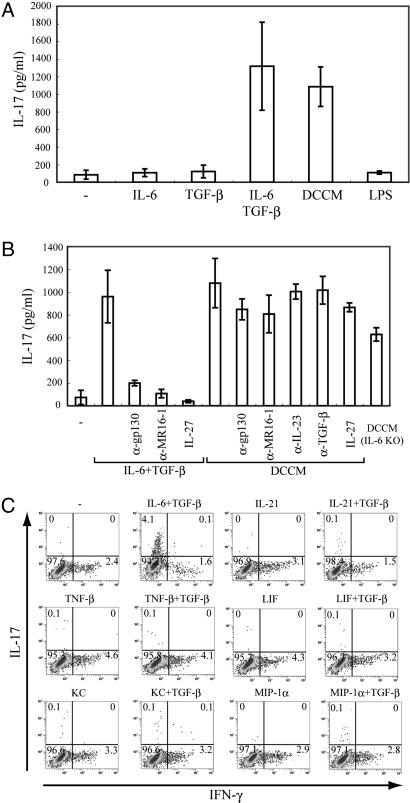
IL-6 performs a critical role in the commitment of Th17 cells from naïve T cells. To test whether factors other than IL-6 are involved in generating IL-17-producing T cells, we first generated conditioned medium from lipopolysaccharide (LPS)-stimulated bone marrow-derived dendritic cells (DCCM). Naïve T cells were stimulated by DCCM, and IL-17 production was measured by means of ELISA. Culturing of naïve T cells with DCCM, but not LPS, resulted in the production of IL-17 to the same extent as that generated by the combination of IL-6 and TGF-β (Fig. 4A).
Fig. 4.
IL-17 is produced by DCCM without IL-6-mediated activity in naïve T cells. (A) Purified naïve T cells were cultured with anti-CD3/CD28 beads and the indicated cytokines, LPS, or CM from DCs stimulated with LPS. (B) Naïve T cells were stimulated with TGF-β plus IL-6, DCCM, or DCCM from IL-6 KO mice in the presence or absence of IL-27 or neutralizing antibodies for IL-6. Supernatants were collected 2 days after stimulation, and IL-17 production was measured by means of ELISA. Data show means ± SE of three independent experiments. (C) MACS-sorted naïve T cells were activated with anti-CD3/CD28 beads. Cells were cultured with the indicated stimulators. After 3 days, cells were restimulated with PMA and ionomycin before intracellular cytokine staining for IFN-γ and IL-17 and analysis by flow cytometry. Dot plots show intracellular staining for IFN-γ and IL-17.
To examine whether IL-17 is produced by DCCM without IL-6-mediated activity, we added anti-gp130 or anti-MR16-1 antibody to DCCM. IL-17 production by IL-6, and TGF-β was substantially inhibited in the presence of either neutralizing antibody, which was consistent with the flow cytometry data (Fig. 1A). Interestingly, production of IL-17 by DCCM was not inhibited by the addition of anti-gp130 or MR16-1 antibody. In addition, secretion of IL-17 was also observed in DCCM from IL-6 KO mice (Fig. 4B). We further confirmed that DCCM could induce IL-17 production also in the presence of anti-IL23 or anti-TGF-β antibody (Fig. 4B). Next, we investigated whether IL-27 suppressed the production of IL-17 by DCCM. Consistent with the previously obtained result (Fig. 1B), IL-27 completely suppressed IL-17 production by TGF-β plus IL-6, whereas IL-17 was produced by DCCM in the presence of IL-27 (Fig. 4B). These findings indicate that other distinct factors regulate the differentiation of Th17 cells independently of IL-6, IL-23, or TGF-β.
To identify the factors other than IL-6 in the development of Th17 cells, we tried several cytokines or chemokines including IL-21, TNF-β, LIF, KC, or MIP-1α, which were nominated by a DNA microarray analyzed to examine the profile of genes induced by DCCM in naïve T cells (data not shown). However, these factors alone or together with TGF-β could not induce the differentiation of IL-17-producing T cells (Fig. 4C).
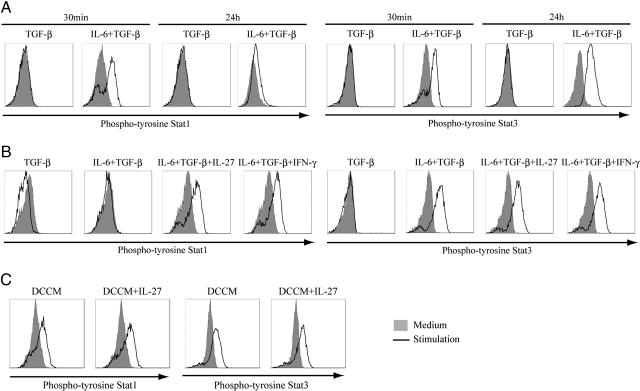
Distinct Patterns of Activation Between Stat1 and Stat3 in Generating Th17 Cells from Naïve T Cells.
Because it is well known that IL-6 activates Stat1 and Stat3 through gp130 (23), we examined the activation of Stat1 and Stat3 in the generation of Th17 cells by IL-6 and TGF-β. Both Stat1 and Stat3 were activated in naïve T cells 30 min after IL-6 and TGF-β stimulation (Fig. 5A). Stat3 remained activated at the same intensity 24 h after IL-6 and TGF-β stimulation, whereas Stat1 activation was not maintained 24 h after stimulation, compared with that at 30 min after stimulation (Fig. 5A).
Fig. 5.
Activation of Stat1 and Stat3 by stimulation to produce IL-17 in naïve T cells. MACS-sorted naïve T cells were cultured with anti-CD3/CD28 beads. (A) Naïve T cells were stimulated with TGF-β alone or TGF-β plus IL-6 for 30 min or 24 h. (B) Naïve T cells were stimulated with TGF-β alone or TGF-β plus IL-6 in the presence or absence of IL-27 or IFN-γ for 24 h. (C) Naïve T cells were cultured with DCCM with or without IL-27. Cells were fixed and permeabilized in 90% methanol, followed by staining with Alexa Fluor 488-conjugated phospho-Stat1 and phycoerythrin-conjugated phospho-Stat3. Intracellular phospho-Stat1 and -Stat3 were measured by flow cytometry. These results are representative of three independent experiments.
As already shown (Fig. 1B), IL-27 or IFN-γ suppresses the differentiation of Th17 cells by IL-6 and TGF-β. Previous reports have suggested that the inhibition of Th17 cell generation by IL-27 depends on Stat1 (19, 20). Next, we examined the activation of Stat1 and Stat3 24 h after IL-6 and TGF-β stimulation in the presence or absence of IL-27 or IFN-γ. Stat1 activation could be restored after this stimulation in the presence of IL-27 or IFN-γ (Fig. 5B). In addition, we confirmed that IL-17-producing T cells were not induced immediately after stimulation by IL-6 and TGF-β (data not shown). Taken together, these findings suggest that Th17 cell generation from naïve T cells stimulated by TGF-β plus IL-6 requires different patterns of activation between Stat1 and Stat3.
In contrast to the IL-6-dependent induction, DCCM maintained the activation of both Stat1 and Stat3 with or without IL-27 (Fig. 5C), again suggesting that the mechanism of Th17 differentiation by DCCM is different from that of TGF-β and IL-6.
Discussion
The IL-17 cytokine family is a recently discovered group of cytokines. Now, this family has six members and appears to have a very distinct biological role (11). IL-17 (also known as IL-17A) has the ability to induce other cytokines, such as IL-6, IL-8, and GM-CSF as well as chemokines from epithelial and vascular endothelial cells (24, 25).
Recently, IL-17-producing T cells have been identified as a new subset of T helper cells. It was previously suggested that IL-23 is the differentiation factor for Th17 cells (12, 26). However, recent data suggests that IL-23 is not the differentiation factor but TGF-β plus IL-6 induce Th17 cell development from naïve T cells (15–17). IL-23 may expand or stabilize previously differentiated Th17 cells. The orphan nuclear receptor RORγt has been recently identified as the key transcription factor in the regulation of Th17 cell differentiation. The involvement of IL-6 in Th17 differentiation has been clarified, but it is still not completely clear how IL-17 induction is regulated in naïve T cells.
In our study reported here, we showed that IL-6 combined with TGF-β directs the differentiation of IL-17-producing T cells from naïve T cells, but not other inflammatory cytokines, such as TNF-α or IL-1β. Although IL-6 suppressed the expression of Foxp3 by TGF-β, TNF-α or IL-1β had no effect. Recently, it has been reported that the number of Th17 cells induced by IL-6 and TGF-β increased in the presence of TNF-α and IL-1β (17). Taken together, these findings indicate that IL-6 participates in the generation of Th17 cells from naïve T cells and that TNF-α or IL-1β expands the Th17 cell pool in combination with TGF-β. Anti-IL-6 receptor antibody therapy has been found to have a significant therapeutic effect in the treatment of systemic-onset juvenile idiopathic arthritis (sJIA) (27), whereas anti-TNF therapy is not so effective as anti-IL-6 receptor antibody therapy. Our data seem to suggest one of the reasons for the difference between anti-IL-6 receptor antibody therapy and anti-TNF therapy in the treatment of sJIA.
It has been reported that the participation of RORγt is crucial for the development of IL-17-producing T cells (21). We found that RORγt was induced by IL-6 and TGF-β even with the addition of IL-27 or IFN-γ, with partial reduction. This suggests that RORγt is necessary, but it alone may not be sufficient for the regulation of IL-17 transcription in naïve T cells because the partial reduction of its expression by IL-27 or IFN-γ cannot explain drastic suppression of Th17 cell differentiation by IL-6 and TGF-β. RORγt may be required for some ligand binding to it or for forming a complex with other transcriptional factors such as Stats to generate Th17 cells from naïve T cells.
To assess whether factors other than IL-6 participate in Th17 differentiation from naïve T cells, we cultured them with DCCM in the presence or absence of anti-gp130 or anti-MR16-1 antibody. Although naïve T cells secreted IL-17 when stimulated by TGF-β plus IL-6 or DCCM, IL-17 production by TGF-β plus IL-6 was almost completely inhibited in the presence of anti-gp130 or anti-MR16-1 antibody. In contrast, DCCM with anti-gp130 or anti-MR16-1 antibody permit the secretion of IL-17 in naïve T cells. Congruent with this finding, DCCM from IL-6 KO mice also induced IL-17 production from naïve T cells. Furthermore, we were able to show that IL-23 or TGF-β is not required for the development of Th17 cells by DCCM and that Th17 differentiation by DCCM is not repressed by IL-27. This makes it likely that there are pathways as yet unknown for the generation of Th17 cells. In this study, we could not identify other factors that are involved in the differentiation of Th17 cells. We found that Th17 development was induced by conditioned medium from LPS or CpG-DNA-stimulated bone marrow-derived dendritic cells, but not conditioned medium from Poly(I:C)-stimulated bone marrow-derived dendritic cells (data not shown). The comparison of factors among these conditioned media is required to define precisely which factors participate in Th17 differentiation.
We also analyzed the activation of Stat1 and Stat3 with the stimulation already described here. As expected, IL-6 and TGF-β activated Stat1 and Stat3 in 30 min. Interestingly, although Stat3 remained activated after 24 h, Stat1 activation was barely maintained. In addition, Stat1 activation by IL-6 and TGF-β after 24 h was restored in the presence of IL-27 or IFN-γ. These data are consistent with the previously reported findings that IL-27-mediated suppression of Th17 cell development depends on Stat1 activation and that Stat3 is required for its differentiation (19, 20, 28). Although IL-6 and IL-27 are closely related cytokines and share gp130-mediated signaling (29), it was shown that there is a difference in Stat1 activation between IL-6 and IL-27 when generating Th17 cells. Although this difference may have disparate effects on Th17 differentiation between IL-6 and IL-27 combined with TGF-β, it is not yet known how Stat1 activation is regulated between IL-6 and IL-27 signalings with TGF-β. Stat5, like Stat1 but in contrast to Stat3, functions as a suppressor of Th17 generation (30). Furthermore, it was found that these Stat family transcription factors bound IL-17 promoter (30). Because the actions of these Stats may take place via RORγt, it will be important to clarify the relation between RORγt and Stat family members. Interestingly, both Stat1 and Stat3 activation have been maintained in naïve T cells stimulated by DCCM. These data suggest that there is a different pathway for Th17 development that is not interfered with by Stat1 activation.
In summary, our findings indicate that mechanisms other than IL-6-dependent pathways are involved in the induction of IL-17 in naïve T cells in which Stat1 activation is maintained. Further analysis is expected to disclose more details of the mechanism for Th17 cell differentiation and to provide information for a more efficient approach to the treatment of several autoimmune diseases.
Materials and Methods
Mice.
C57BL/6 wild-type mice were obtained from CLEA (Tokyo, Japan), and IL-6 KO mice were provided by Dr. Manfred Kopf (Institute of Integrative Biology, Eidgenössische Technische Hochschule, Zurich, Switzerland). All animal experiments were performed in accordance with protocols approved by the Institutional Animal Care and Use Committees of the Graduate School of Frontier Bioscience, Osaka University.
Isolation of Naïve T Cells and Culture Conditions.
Naïve T cells were purified from spleens of C57BL/6 female mice by using the CD4+ T cell Isolation kit, and CD62L MicroBeads (Miltenyi Biotec, Bergisch-Gladbach, Germany). Purified naïve T cells were cultured in RPMI medium 1640 with 10% FCS, 100 μg/ml streptomycin, 100 units/ml penicillin G, 1× nonessential amino acids, 1 μM sodium pyruvate, and 2.5 μM β-mercaptoethanol at 37°C and in 5% CO2. Naïve T cells were stimulated with the Dynabeads Mouse CD3/CD28 T Cell Expander (Invitrogen, Carlsbad, CA) for 3 days. As indicated, cultures were supplemented with recombinant mouse IL-6 (20 ng/ml; R & D Systems, Minneapolis, MN) or recombinant human TGF-β1 (2 ng/ml; R & D Systems), alone or combined. Additionally, recombinant mouse IL-27 (20 ng/ml), recombinant mouse IFN-γ (100 ng/ml), recombinant mouse TNF-α (100 ng/ml), or recombinant mouse IL-1β (20 ng/ml; all from R & D Systems) was added to some samples. IL-6 was neutralized by anti-mouse gp130 antibody (4 μg/ml; R & D Systems) and anti-mIL-6R monoclonal antibody MR16-1 (1 μg/ml; kindly donated by Yoshiyuki Ohsugi, Chugai Pharmaceutical Co., Shizuoka, Japan).
Intracellular Cytokines and Foxp3 Staining.
T cells were stimulated with 50 ng/ml phorbol 12-myristate 13-acetate (PMA) (Calbiochem, Darmstadt, Germany), 800 ng/ml ionomycin (Calbiochem) for 5 h and GolgiStop (BD Pharmingen, San Diego, CA) for the last 2 h, followed by fixation and permeabilization with Cytofix/Cytoperb (BD PharMingen). Cells were stained intracellularly with PE-conjugated anti-IL-17 (TC11–18H10.1) (BD PharMingen) and FITC-labeled anti-IFN-γ (XMG1.2) (eBioscience, San Diego, CA). For Foxp3 staining, T cells were fixed and permeabilized with the Fixation/Permeabilization buffer (eBioscience) for 30 min at 4°C before intracellular staining with FITC-conjugated anti-Foxp3 (eBioscience). Flow cytometric analysis was performed with a Cytomics FC500 (Beckman Coulter, Fullerton, CA).
RT-PCR.
Total RNA was prepared by using RNeasy (Qiagen, Germantown, MD), and cDNA was prepared as described (31). Reaction conditions consisted of a 45-s denaturation step at 94°C, a 30-s annealing step at 58°C, and a 30-s extension step at 72°C for 25 cycles (G3PDH) or 35 cycles (RORγt). The specific primers were as follows: RORγt, sense 5′-GCGGAGCAGACACACTTACA-3′ and antisense 5′-TTGGCAAACTCCACCACATA-3′; G3PDH, sense 5′-TCCACCACCCTGTTGCTGTA-3′ and antisense 5′-ACCACAGTCCATGCCATCAC-3′.
Preparation of DCCM.
Bone marrow dendritic cells (DCs) (BMDCs) were generated from WT mice or IL-6 KO mice as follows. Briefly, bone marrow cells were flushed from tibiae and femurs of 7- to 10-week-old female C57BL/6 mice or IL-6 KO mice and seeded at 1 × 107 cells in cell culture dishes (150-mm diameter; Corning, Corning, NY) in a volume of 30 ml of RPMI medium 1640 containing 10 ng/ml GM-CSF (R & D Systems). On day 3, 30 ml of this medium was added to the dishes, and on day 6, 30 ml of the cultures was removed and centrifuged. The pellet was resuspended in 30 ml of fresh RPMI medium 1640 containing 10 ng/ml GM-CSF and returned to the same dish. On day 9, DCs were isolated from nonadherent and loosely adherent cells by using the CD11c MicroBeads (Miltenyi Biotec). 3 × 107 BMDCs were seeded in 40 ml of RPMI medium 1640 and stimulated with 1 μg/ml Escherichia coli LPS (Sigma, St. Louis, MO). After 12 h, supernatants were collected and used as DCCM.
IL-17 ELISA.
The cells were stimulated with indicated cytokines or with DCCM in the presence or absence of neutralizing antibodies for IL-6. After 48 h, mouse IL-17 from the supernatants was measured by means of ELISA according to the manufacture's instructions (R & D Systems).
Flow Cytometric Analysis of Phospho-Stat1 (Y701) and Phospho-Stat3 (Y705).
Naïve T cells were cultured with indicated cytokines or with DCCM for 30 min or 24 h. Cells were fixed with Fixation Buffer (BD PharMingen) for 10 min at 37°C and then permeabilized in 90% methanol for 30 min on ice. Cells were then washed twice in Stain Buffer (BD PharMingen), and stained with Alexa Fluor 488-conjugated phospho-Stat1 (Y701) antibody or PE-conjugated phospho-Stat3 (Y705) antibody for 1 h at room temperature (BD PharMingen). Flow cytometric analysis was performed with a Cytomics FC500 (Beckman Coulter).
Acknowledgments
We gratefully acknowledge the provision of MR16-1 by Dr. Yoshiyuki Ohsugi (Chugai Pharmaceutical Co. Ltd., Tokyo, Japan). This work was supported by Research Fellowships of the Japan Society for the Promotion of Science for Young Scientists and Chugai Pharmaceutical Co. Ltd.
Abbreviations
- DCCM
conditioned medium from LPS-stimulated bone marrow-derived dendritic cells
- Foxp3
forkhead box p3
- LPS
lipopolysaccharides
- RORγt
retinoid-related orphan receptor γt
- PMA
phorbol 12-myristate 13-acetate
- Stat
signal transducer and activator of transcription
- TGF-β
transforming growth factor-β1
- Th17
proinflammatory T helper cells producing IL-17.
Footnotes
The authors declare no conflict of interest.
References
- 1.Fontenot JD, Rasmussen JP, Williams LM, Dooley JL, Farr AG, Rudensky AY. Immunity. 2005;22:329–341. doi: 10.1016/j.immuni.2005.01.016. [DOI] [PubMed] [Google Scholar]
- 2.Szabo SJ, Kim ST, Costa GL, Zhang X, Fathman CG, Glimcher LH. Cell. 2000;100:655–669. doi: 10.1016/s0092-8674(00)80702-3. [DOI] [PubMed] [Google Scholar]
- 3.Zheng W, Flavell RA. Cell. 1997;89:587–596. [Google Scholar]
- 4.Hsieh CS, Macatonia SE, Tripp CS, Wolf SF, O'Garra A, Murphy KM. Science. 1993;260:547–549. doi: 10.1126/science.8097338. [DOI] [PubMed] [Google Scholar]
- 5.Scharton TM, Scott P. J Exp Med. 1993;178:567–577. doi: 10.1084/jem.178.2.567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Min B, Prout M, Hu-Li J, Zhu J, Jankovic D, Morgan ES, Urban JF, Jr, Dvorak AM, Finkelman FD, LeGros G, Paul WE. J Exp Med. 2004;200:507–517. doi: 10.1084/jem.20040590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shinkai K, Mohrs M, Locksley RM. Nature. 2002;420:825–829. doi: 10.1038/nature01202. [DOI] [PubMed] [Google Scholar]
- 8.Bottomly K. Imunol Today. 1988;9:268–274. doi: 10.1016/0167-5699(88)91308-4. [DOI] [PubMed] [Google Scholar]
- 9.Chen W, Jin W, Hardegen N, Lei KJ, Li L, Marinos N, McGrady G, Wahl SM. J Exp Med. 2003;198:1875–1886. doi: 10.1084/jem.20030152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sakaguchi S. Cell. 2000;101:455–458. doi: 10.1016/s0092-8674(00)80856-9. [DOI] [PubMed] [Google Scholar]
- 11.Kolls JK, Linden A. Immunity. 2004;21:467–476. doi: 10.1016/j.immuni.2004.08.018. [DOI] [PubMed] [Google Scholar]
- 12.Langrish CL, Chen Y, Blumenschein WM, Mattson J, Bashman B, Sedgwick JD, McClanahan T, Kastelein RA, Karlsson S. J Exp Med. 2005;201:233–240. doi: 10.1084/jem.20041257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nakae S, Komiyama Y, Nambu A, Sudo K, Iwase M, Homma I, Sekikawa K, Asano M, Iwakura Y. Imunity. 2002;17:375–387. doi: 10.1016/s1074-7613(02)00391-6. [DOI] [PubMed] [Google Scholar]
- 14.Harrington LE, Hatton RD, Mangan PR, Turner H, Murphy TL, Murphy KM, Weaver CT. Nat Immunol. 2005;6:1123–1132. doi: 10.1038/ni1254. [DOI] [PubMed] [Google Scholar]
- 15.Bettelli E, Carrier Y, Gao W, Korn T, Strom TB, Oukka M, Weiner HL, Kuchroo VK. Nature. 2006;441:235–238. doi: 10.1038/nature04753. [DOI] [PubMed] [Google Scholar]
- 16.Mangan PR, Harrington LE, O'Quinn DB, Helms WS, Bullard DC, Elson CO, Hatton RD, Wahl SM, Schoeb TR, Weaver CT. Nature. 2006;441:231–234. doi: 10.1038/nature04754. [DOI] [PubMed] [Google Scholar]
- 17.Veldhoen M, Hocking RJ, Atkins CL, Locksley RM, Stockinger B. Immunity. 2006;24:179–189. doi: 10.1016/j.immuni.2006.01.001. [DOI] [PubMed] [Google Scholar]
- 18.Cruz A, Khader SA, Torrado E, Frage A, Pearl JE, Pedrosa J, Cooper AM, Castro AG. J Immunol. 2006;177:1416–1420. doi: 10.4049/jimmunol.177.3.1416. [DOI] [PubMed] [Google Scholar]
- 19.Batten M, Li J, Yi S, Kljavin NM, Danilenko DM, Lucasa S, Lee J, de Sauvage FJ, Ghilardi N. Nat Immunol. 2006;7:929–936. doi: 10.1038/ni1375. [DOI] [PubMed] [Google Scholar]
- 20.Stumhofer JS, Laurence A, Wilson EH, Huang E, Tato CM, Johnson LM, Villarino AV, Huang Q, Yoshimura A, Sehy D, et al. Nat Immunol. 2006;7:937–945. doi: 10.1038/ni1376. [DOI] [PubMed] [Google Scholar]
- 21.Ivanov II, McKenzie BS, Zhou L, Tadokoro CE, Lepelley A, Lafaille JJ, Cua DJ, Littman DR. Cell. 2006;126:1121–1133. doi: 10.1016/j.cell.2006.07.035. [DOI] [PubMed] [Google Scholar]
- 22.Fossiez F, Banchereau J, Murray R, Van Kooten C, Garrone P, Lebecque S. Int Rev Immunol. 1998;16:541–551. doi: 10.3109/08830189809043008. [DOI] [PubMed] [Google Scholar]
- 23.Taga T, Kishimoto T. Annu Rev Immunol. 1997;15:797–819. doi: 10.1146/annurev.immunol.15.1.797. [DOI] [PubMed] [Google Scholar]
- 24.Prause O, Laan M, Lotvall J, Linden A. Eur J Pharmacol. 2003;462:193. doi: 10.1016/s0014-2999(03)01341-4. [DOI] [PubMed] [Google Scholar]
- 25.Laan M, Prause O, Miyamoto M, Sjostrand M, Hytonen AM, Kaneko T, Lotvall J, Linden A. Eur Respir J. 2003;21:387–393. doi: 10.1183/09031936.03.00303503. [DOI] [PubMed] [Google Scholar]
- 26.Murphy CA, Langrish CL, Chen Y, Blumenschein W, McClanahan T, Kastelein RA, Sedgwick JD, Cua DJ. J Exp Med. 2003;198:1951–1957. doi: 10.1084/jem.20030896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yokota S, Miyamae T, Imagawa T, Iwata N, Katakura S, Mori M, Woo P, Nishimoto N, Yoshizaki K, Kishimoto T. Arthritis Rheum. 2005;52:818–825. doi: 10.1002/art.20944. [DOI] [PubMed] [Google Scholar]
- 28.Yang XO, Panopoulos AD, Nurieva R, Chang SH, Wang D, Watowich SS, Dong C. J Biol Chem. 2007;282:9358–9363. doi: 10.1074/jbc.C600321200. [DOI] [PubMed] [Google Scholar]
- 29.Villarino AV, Huang E, Hunter CA. J Immunol. 2004;173:715–720. doi: 10.4049/jimmunol.173.2.715. [DOI] [PubMed] [Google Scholar]
- 30.Laurence A, Tato CM, Davidson TS, Kanno Y, Chen Z, Yao Z, Blank RB, Meylan F, Siegel R, Hennighausen L, et al. Immunity. 2007;26:371–381. doi: 10.1016/j.immuni.2007.02.009. [DOI] [PubMed] [Google Scholar]
- 31.Kimura A, Naka T, Muta T, Takeuchi O, Akira S, Kawase I, Kishimoto T. Proc Natl Acad Sci USA. 2005;102:17089–17094. doi: 10.1073/pnas.0508517102. [DOI] [PMC free article] [PubMed] [Google Scholar]