Abstract
Nonribosomal peptides (NRPs) are produced by NRP synthetase (NRPS) enzymes that function as molecular assembly lines. The modular architecture of NRPSs suggests that a domain responsible for activating a building block could be replaced with a domain from a foreign NRPS to create a chimeric assembly line that produces a new variant of a natural NRP. However, such chimeric NRPS modules are often heavily impaired, impeding efforts to create novel NRP variants by swapping domains from different modules or organisms. Here we show that impaired chimeric NRPSs can be functionally restored by directed evolution. Using rounds of mutagenesis coupled with in vivo screens for NRP production, we rapidly isolated variants of two different chimeric NRPSs with ≈10-fold improvements in enzyme activity and product yield, including one that produces new derivatives of the potent NRP/polyketide antibiotic andrimid. Because functional restoration in these examples required only modest library sizes (103 to 104 clones) and three or fewer rounds of screening, our approach may be widely applicable even for NRPSs from genetically challenging hosts.
Keywords: nonribosomal peptide, polyketide
Many small molecules with important biological activities are synthesized by nonribosomal peptide synthetases (NRPSs), a class of assembly-line enzymes (1). NRPSs consist of modular sets of protein domains in which each module incorporates an amino acid monomer into a growing chain. Within each module, the adenylation domain (A domain) determines which monomer is incorporated by binding, activating, and covalently tethering the amino acid to the assembly line (2). The modular organization of protein domains in NRPSs has stimulated efforts to swap genes encoding heterologous domains into NRPSs with the goal of producing nonnatural variants of the original small-molecule product (3, 4). Although limited success has been achieved with the production of nonribosomal peptide (NRP) variants (5–14), the resultant chimeric NRPSs are usually nonfunctional or heavily impaired, often yielding too little of the new product to make production feasible. It is not yet known why chimeric NRPSs suffer large reductions in activity, although it is likely that domain swapping disrupts quaternary interactions between protein domains in the assembly line (15).
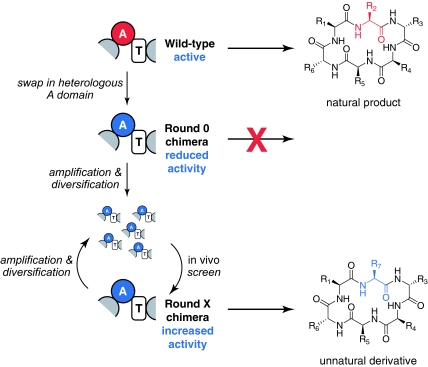
In contrast, nature has succeeded in producing families of related NRPs in which each member differs at specific positions by monomer substitutions. It is possible that these NRP variants could have arisen by divergent evolution of an ancestral synthetase to acquire mutations altering the monomer incorporated at various positions. However, phylogenetic evidence from at least one family of NRPs (16) suggests that their producing synthetases arose from an ancestral NRPS by A domain rearrangement. Based on the possibility that some natural NRPSs arose through domain swapping to generate chimeric NRPSs followed by evolutionary optimization, we set out to investigate whether we could recapitulate this process using directed evolution (Fig. 1).
Fig. 1.
Using directed evolution to improve the activity of chimeric NRPSs. A heterologous A domain is swapped into an NRPS, typically resulting in a significant loss of synthetase activity. A library of chimeric synthetase mutants is constructed in which the heterologous A domain has been diversified (for example, by mutagenic PCR). The library is subjected to an in vivo screen for production of the NRP variant. Clones showing improved production are characterized and subjected to further rounds of diversification and screening.
Results and Discussion
We began with the two-module Escherichia coli NRPS (17) that produces enterobactin (1) (Fig. 2a), an iron-scavenging small molecule excreted by E. coli in response to iron starvation (18). E. coli require enterobactin for growth on iron-deficient media, enabling us to develop a screen for enterobactin synthetase activity in which cell growth rate was highly correlated with the level of enterobactin production (19–22).
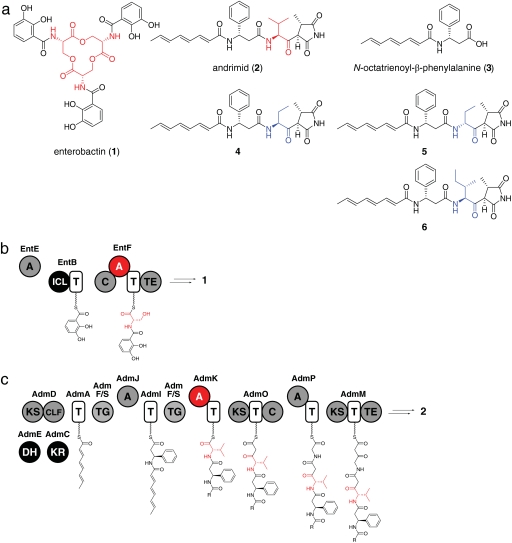
Fig. 2.
The enterobactin and andrimid assembly line enzymes. (a) Chemical structures of enterobactin, andrimid, N-octatrienoyl-β-phenylalanine, and andrimid derivatives. Building blocks incorporated by the wild-type NRPS are shown in red, and building blocks incorporated by evolved chimeric NRPSs are shown in blue. (b) The enterobactin NRPS. The EntF A domain and the serine it incorporates are highlighted in red. (c) The andrimid NRPS–polyketide synthase. The AdmK A domain and the valine it incorporates are shown in red.
We first targeted the serine-specific A domain in the second module of enterobactin synthetase, which is encoded by the four-domain protein EntF (Fig. 2b). Having previously shown that an entF-deficient E. coli strain (entF::cat) can be complemented by plasmid-borne entF (21), plasmid libraries encoding EntF derivatives could be introduced into entF::cat cells and the resulting transformants grown on minimal media in the presence of the iron chelator 2,2′-dipyridyl. Under these conditions, EntF activity is rate-limiting for cell growth as assayed by colony diameter (21) (Fig. 3a).
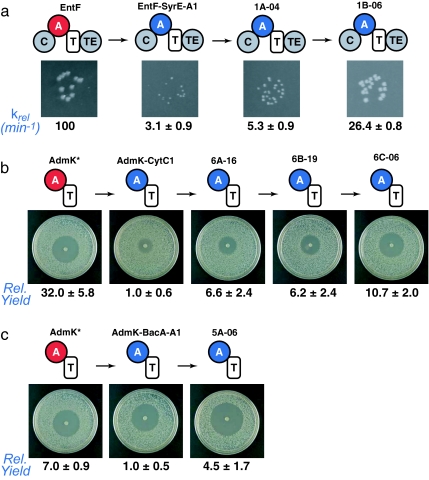
Fig. 3.
EntF-SyrE-A1, AdmK-CytC1, and AdmK-BacA-A1 are rapidly improved by directed evolution. (a) Summary of results from the EntF-SyrE-A1 screen showing the colony sizes of biochemical activity of each clone. The colony images come from strains grown on the same plate. (b) Summary of results from the AdmK-CytC1 screen showing the zone-of-inhibition size and relative yield of andrimid for each clone. (c) Summary of results from the AdmK-BacA-A1 screen showing the zone-of-inhibition size and relative yield of andrimid (AdmK) or the isoleucine derivative of andrimid (AdmK-BacA-A1 and 5A-06) for each clone. Relative yield is defined as the yield of andrimid or the isoleucine derivative of andrimid compared with the parental AdmK-CytC1 or AdmK-BacA-A1 chimera, with the yields of the parental chimeras normalized to a value of 1.
Swapping an A domain with an exogenous domain of different amino acid specificity could result in the production of an intermediate that is not an efficient substrate for subsequent biosynthetic steps, regardless of the ability of the exogenous A domain to function properly in its new context. To eliminate this possibility, we first replaced the A domain of EntF with SyrE-A1, an ≈50-kDa A domain from the Pseudomonas syringae syringomycin NRPS (23), which like the EntF A domain is specific for serine. The resulting chimera, EntF-SyrE-A1, exhibited a 30-fold loss of activity compared with wild-type EntF as measured by a previously described in vitro assay of enterobactin production. This loss of activity is consistent with previous reports of significantly impaired chimeric NRPS domains (9, 10).
We speculated that mutations in the exogenous SyrE-A1 domain may be sufficient to restore EntF function. To test this hypothesis we constructed a modest library (≈2 × 104 clones) of EntF-SyrE-A1 variants in which the exogenous 1.5-kb SyrE-A1 domain was diversified by mutagenic PCR (24) such that the average clone contained 1.6 mutations compared with EntF-SyrE-A1. After transforming this library into entF::cat and growing the transformants under screening conditions, the 28 largest colonies were isolated, and their phenotypic improvement was confirmed by rescreening at low cell density (Fig. 3a). The clone with the fastest growth rate (1A-04) as assayed by colony diameter was chosen as the template for a second round of diversification and screening, resulting in two round 2 clones (1B-02 and 1B-06) with especially promising phenotypes. The colony diameters of these clones were comparable to that of wild-type EntF in the in vivo assay. The hit rate based on the number of confirmed large colonies on the round 1 and 2 selection plates was 0.04% in each round.
EntF, EntF-SyrE-A1, and evolved clones 1A-04, 1B-02, and 1B-06 were expressed as C-terminal His6 fusions and purified by nickel-chelate affinity chromatography. Overexpression of EntF-SyrE-A1 yielded less soluble protein than overexpression of wild-type EntF or either of the round 1 and 2 clones [supporting information (SI) Fig. 5], suggesting that domain replacement resulted in poor protein solubility and that mutations in the most active round 1 clone rescued this solubility defect.
Next, the activities of wild-type EntF and domain-swapped variants were determined by using a previously described (22) in vitro assay of enterobactin synthesis under conditions in which EntF activity is rate-limiting. In vitro assay of the purified proteins revealed that whereas the activity of 1A-04 had improved <2-fold relative to EntF-SyrE-A1 and the activity of 1B-02 had improved ≈3-fold (3.3 ± 0.7-fold), the activity of 1B-06 had improved ≈8-fold and was within 4-fold of wild-type EntF activity (Fig. 3a). Both 1B-02 and 1B-06 have only four amino acid substitutions (SI Table 1), indicating that the number of mutations required for functional rescue may be quite small. Taken together, these results support the hypothesis that directed evolution can rapidly restore the activity of chimeric NRPS modules containing heterologous A domains.
To further investigate the generality of this approach, we pursued A domain swapping in an unrelated and more challenging system. A recently described hybrid, NRPS–polyketide synthase from Pantoea agglomerans (25), produces the acetyl-CoA carboxylase inhibitor andrimid (2) (Fig. 2a), a broad-spectrum antibiotic (26). A cosmid harboring the andrimid gene cluster enables E. coli XL1-Blue MR cells to produce andrimid (25).
We developed an in vivo screen for production of andrimid in which colonies of andrimid-producing E. coli were oversprayed with the andrimid-sensitive indicator strain E. coli imp. Those colonies expressing the wild-type andrimid gene cluster gave rise to a zone of inhibited indicator strain growth (SI Fig. 6), whereas control colonies lacking the gene cluster did not inhibit indicator strain growth.
Based on our previously reported proposal for the biosynthesis of andrimid (25), we targeted the valine-specific A domain of the 64-kDa A-T di-domain protein AdmK for replacement (Fig. 2c) because substitution of the valine in andrimid with other aliphatic amino acids does not disrupt its antibacterial activity (27), whereas replacement of its β-phenylalanine or its glycine-derived succinimide abrogates activity (26). We constructed a knockout of the admK gene (admK::cat) in a cosmid expressing the andrimid gene cluster and confirmed that a plasmid-borne admK gene complements this knockout (SI Fig. 6).
The gene fragment encoding AdmK-A was replaced with CytC1, a 2-aminobutyrate-incorporating A domain from the Streptomyces sp. RK95-74 cytotrienin NRPS–polyketide synthase (28). CytC1 was chosen for its known substrate promiscuity (28) as a strategy for incorporating nonproteinogenic amino acids into the valine position of andrimid. Complementation of the admK::cat strain with plasmid-encoded AdmK-CytC1 generated andrimid, but at a 32-fold reduced level compared with the level of andrimid production arising from plasmid-based wild-type AdmK (Fig. 3b). Like the admK knockout strain, the AdmK-CytC1-complemented strain excreted the andrimid biosynthetic intermediate N-octatrienoyl-β-phenylalanine (3) (Fig. 2 and SI Fig. 7), suggesting that the A-swapped synthetase suffers from a blockade at the stage of condensing 3-S-AdmI with Val-S-AdmK.
A small library of plasmids encoding AdmK-CytC1 derivatives (5,760 clones) was constructed in which cytC1 was diversified by mutagenic PCR (24), resulting in an average of 3.2 amino acid substitutions per clone. This library was subjected to the zone-of-inhibition screen described above, and 22 primary hits were isolated and further characterized by fermentation. Of these, the best-performing clone (6A-16) produced 6.6-fold more andrimid per unit culture volume than AdmK-CytC1 (Fig. 3b). After two more rounds of mutagenesis and screening, a clone (6C-06) was isolated that produced 10.7-fold more andrimid per unit culture volume than AdmK-CytC1 and just 3-fold less than AdmK, consistent with the activity improvement we observed in the EntF-SyrE-A1 derivative 1B-06. Biochemical analysis of AdmK-CytC1 and its derivatives will necessarily await biochemical characterization of the early stages of andrimid assembly, a scheme for which was recently proposed (25).
The known amino acid substrate promiscuity of CytC1 raises the possibility that a functionally restored AdmK-CytC1 chimera could generate new andrimid derivatives. Indeed, by adding l-2-aminobutyrate or d-2-aminobutyrate to the culture medium of cells harboring the admK::cat cosmid and plasmid-encoded 6C-06 we were able to generate compounds that have masses consistent with the corresponding andrimid derivatives 4 and 5 (Fig. 2a and SI Fig. 8) in a ratio to andrimid as high as ≈1:1 for 4. These results validate the use of our approach to restore the activity of a chimeric NRPS domain containing a promiscuous A domain, enabling nonproteinogenic amino acids to be incorporated into the andrimid scaffold.
To explore the capabilities of our andrimid screen, we next investigated whether we could screen for production of an andrimid derivative rather than andrimid itself. Because previous work had shown that substituting isoleucine for valine confers increased potency on a synthetic derivative of andrimid (27), we constructed a second AdmK chimera by replacing AdmK-A with the isoleucine-specific BacA-A1 domain from the Bacillus licheniformis bacitracin NRPS (29). Complementation of the admK::cat strain with plasmid-encoded AdmK-BacA-A1 generated the corresponding isoleucine-containing derivative of andrimid (6) with a 7-fold reduction in product yield compared with that of the wild-type enzyme (Fig. 2a, Fig. 3c, and SI Fig. 8). AdmK-BacA-A1 had no discernible phenotypic impairment (Fig. 3c), likely because of increased antibacterial activity of 8 as compared with andrimid against the indicator strain (27). After a single round of mutagenesis and screening, we isolated a clone (5A-06) from 21 retested hits with a 4.5-fold improvement in product yield (Fig. 3c). These results not only indicate that chimeric NRPSs producing unnatural derivatives of the original product can be functionally improved, but they suggest that, in some circumstances, the activity level of the chimeric NRPS can reach a level that approaches the activity of its wild-type counterpart.
Our results indicate that the activity losses due to domain swapping may be restored by directed evolution. The surprisingly high hit rate in both systems (0.02–0.2%) indicates that small libraries (103 to 104 clones) can yield sufficient coverage of sequence space necessary for detectable gains in activity. Moreover, the rapid activity improvement (≈10-fold in no more than three rounds) we observed suggests that only a small number of directed evolution rounds can significantly restore NRP production levels. Importantly, these characteristics should make this approach applicable to bacterial strains for which transformation is challenging and generating large libraries is impractical, such as those from the order Actinomycetales, the most prolific known producers of natural products among soil microorganisms (30).
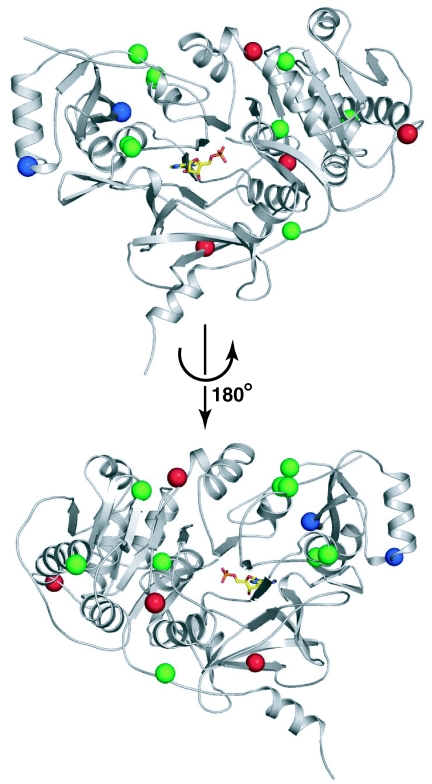
Evolved clones 1B-06, 5A-06, and 6C-06 (SI Table 1 and SI Fig. 9) have four, two, and nine amino acid substitutions, respectively, relative to their parental chimeric NRPSs. The mutations in these optimized clones do not map to one region of the A domain (Fig. 4). Rather, they are distributed throughout the domain, indicating that distinct regions of the A domain may be important in each of the clones. The unpredictable distribution of mutations that collectively confer improved activity suggests that a directed evolution approach is well suited for restoring the function of impaired chimeric NRPSs. Further genetic and biochemical analysis will be required to characterize the molecular basis for the improved function of the chimeric NRPS derivatives and to explore their implications for the protein–protein interactions that govern quaternary structure in NRPSs.
Fig. 4.
Structural mapping of the mutations in evolved clones 1B-06, 5A-06, and 6C-06. Two views of the A domain PheA (Protein Data Bank ID code 1AMU) are shown in which a molecule of adenosine-5′-monophosphate (yellow) partially occupies the enzyme's active site. Mutations were mapped to the structure of PheA by amino acid sequence alignment. 1B-06 mutation sites are shown as red spheres, 5A-06 mutation sites are shown as blue spheres, and 6C-06 mutation sites are shown as green spheres.
Materials and Methods
See SI Tables 2–4 for primers, plasmids, and stains used in this work. See SI Methods for details on plasmid and strain construction as well as protein expression and purification.
Library Construction.
Gene fragments encoding syrE-A1, cytC1, and bacA-A1 were amplified from pER410 (MAF088/MAF089), pMF212 (MAF407/MAF408), and pMF184 (MAF454/MAF455), respectively, by using the primer pairs indicated in parentheses and a previously described mutagenic PCR method (24) with mutagenic dNTP concentrations adjusted to result in one to five amino acid substitutions per clone. A typical 50-μl reaction contained 1× Taq buffer (Promega, Madison, WI), 5 mM MgCl2, 25 ng of template DNA, each primer at 4 μM, 800 μM dATP, 800 μM PTP, 800 μM dGTP, and 800 μM dCTP, 80 μM dPTP, and 80 μM 8-oxo-dGTP, and 5 units of Promega Taq polymerase. Mutagenic PCRs to amplify cytC1 were supplemented with betaine at 1 M. A typical PCR program comprised 25 cycles of denaturation at 94°C, annealing at 60°C, and extension at 72°C. The product of the PCR was digested with SacI and SpeI (syrE-A1) or KpnI and NheI (cytC1-A and bacA-A1) and ligated into the SacI and SpeI sites in pMF116 (syrE-A1) or the KpnI and SpeI sites in pMF183 (cytC1-A and bacA-A1). The resulting DNA was extracted with phenol/chloroform and buffer-exchanged into pure water.
In Vivo Screen for Enterobactin Production.
Typically, 1–2 μg of library DNA was transformed into electrocompetent E. coli ER1100 cells by electroporation. The transformed cells were washed three times with M9/dipyridyl medium [M9 salts, 2 mM MgSO4, 100 μM CaCl2, 0.4% glucose (wt/vol), and 100 μM 2,2′-dipyridyl], plated on M9 screening medium [M9 salts, 2 mM MgSO4, 100 μM CaCl2, 0.4% glucose (wt/vol), 0.3% casamino acids (wt/vol), 0.0002% thiamine (wt/vol), 100 μM 2,2′-dipyridyl, 50 μg/ml kanamycin, and 1.5% Bacto-Agar (wt/vol)], and incubated at 37°C for 24–48 h. Colonies with the largest diameter as judged by visual inspection were picked and restreaked on the same medium to ensure colony purity. Plasmid inserts isolated from putative hits were recloned into pMF116 and assayed as described below.
In Vivo Assay for Enterobactin Production.
E. coli ER1100 cells carrying plasmids encoding EntF or EntF-SyrE-A1 chimeras were grown to saturation in 2× YT medium containing 50 μg/ml kanamycin, washed once with M9/dipyridyl medium, and diluted 1:107 in M9/dipyridyl medium, and 40 μl were spotted on M9 screening medium. Plates were incubated at 37°C, typically for 24–48 h.
Biochemical Assay of EntF Activity.
The activity of EntF and EntF-SyrE-A1 enzymes was determined by using a previously described assay of reconstituted enterobactin biosynthesis (17) under conditions in which EntF is rate-limiting. Reaction mixtures containing 75 mM Tris·HCl (pH 8.0), 10 mM MgCl2, 2.5 mM TCEP, 500 μM CoA, 300 nM Sfp, 15 μM EntB, and 100 nM EntF or EntF-SyrE-A1 chimera were preincubated at 37°C for 20 min to effect phosphopantetheinylation of EntB and EntF by Sfp. After preincubation, 50 mM l-serine, 500 μM 2,3-dihydroxybenzoate, and 300 nM EntE were added. Reactions were initiated by the addition of 10 mM ATP, incubated at 37°C, and terminated by quenching aliquots with 1.5 volumes of 1 N hydrochloric acid. Aliquots were removed at time points between 5 min and 30 min, quenched, incubated on ice for 10 min, and then centrifuged for 5 min at 16,000 × g to pellet precipitated protein. The supernatant was analyzed by RP-HPLC and monitored at 316 nm. The peak corresponding to enterobactin was integrated to calculate the rate of enterobactin production, and the average values were scaled such that wild-type EntF had a relative rate (krel) of 100 min−1. The values reported are the mean ± SD for three assays.
In Vivo Screen for Andrimid Production.
Typically, 1–2 μg of library DNA was transformed into electrocompetent E. coli Top10 cells by electroporation and plated on LB agar containing 50 μg/ml spectinomycin and 50 μg/ml streptomycin in two 24.5-cm2 plates. Cells were scraped from the surface of the plate and pooled, and plasmid DNA was isolated. A total of 100 ng of this AdmK chimera-encoding plasmid DNA was mixed with 100 ng of cosmid MF300, and the mixture was used to transform chemically competent E. coli XL1-Blue MR cells. Transformed cells were plated on LB agar containing 100 μg/ml carbenicillin, 50 μg/ml spectinomycin, and 50 μg/ml streptomycin. A total of 5,000–6,000 single colonies from a transformation were robotically picked and used to inoculate 125-μl cultures (LB medium containing 100 μg/ml carbenicillin and 50 μg/ml spectinomycin) in 96-well culture plates using a Genetix QBot (FAS Center for Systems Biology, Harvard University). After incubating stationary cultures at 37°C overnight, ≈2-μl spots of each culture were arrayed on LB agar containing 100 μg/ml carbenicillin and 50 μg/ml spectinomycin in 25-cm2 plates using a floating pin gridder (V & P Scientific). After incubation at 30°C overnight, plates were oversprayed with a 1:250 aqueous dilution of a saturated culture of the indicator strain E. coli imp ASR and incubated at room temperature (≈25°C) until enough indicator growth was observed to allow visual discrimination based on differential inhibition zone size. Plasmid inserts isolated from putative hits were recloned into pMF183 and assayed as described below.
Fermentation Assay for Andrimid Production.
Freshly transformed clones were prepared for each assay; chemically competent E. coli XL1-Blue MR cells were cotransformed with a plasmid encoding AdmK or an AdmK chimera and cosmid MF300 as described above. Single colonies were picked and used to inoculate 3-ml cultures of LB medium containing 100 μg/ml carbenicillin and 50 μg/ml spectinomycin, which were incubated at room temperature (≈25°C) on a drum roller for 24–30 h. After incubation, cultures were centrifuged and 1.8 ml of cell-free supernatant was evaporated to dryness by using a Speedvac (Thermo Savant, Waltham, MA). The dry residue was resuspended in 150 μl of 1:1 methanol:water, 115 μl of which was analyzed by RP-HPLC using a gradient of 30–60% acetonitrile in 0.1% trifluoroacetic acid/water over 8 min with monitoring at 305 nm. The peak corresponding to andrimid or the isoleucine-containing derivative of andrimid was integrated to determine the raw yield. To obtain the scaled yield, a background was subtracted from the raw yield; the background was determined by integrating the corresponding region of the HPLC spectrum from supernatant of the empty vector control strain. The scaled specific production was calculated by dividing the scaled yield by the optical density of the culture at the time of harvesting, which was determined spectrophotometrically by measuring absorbance at 600 nm. To obtain the normalized yield and normalized specific production, the scaled yield and scaled specific production were divided by the average scaled yield and the average scaled specific production values for the parental AdmK-CytC1 or AdmK-Bac-A1 clones. The values reported are the mean ± SD for three fermentation cultures inoculated with different colonies.
Production and Detection of Andrimid Derivatives Containing Nonproteinogenic Amino Acids.
Chemically competent E. coli XL1-Blue MR cells were cotransformed with a plasmid encoding AdmK or an AdmK chimera and cosmid MF300 as described above. Single colonies were picked and used to inoculate 3-ml cultures of LB medium containing 100 μg/ml carbenicillin and 50 μg/ml spectinomycin, which were incubated at room temperature (≈25°C) on a drum roller for 24 h. The cultures were centrifuged, the culture medium was discarded, and the cells were resuspended in a modified M63 medium comprising M63 salts [per liter: 2 g of (NH4)2SO4, 13.6 g of KH2PO4, and 0.5 mg of FeSO4·7H2O, adjusted to pH 7.0 with KOH], 0.4% glucose (wt/vol), 2 mM MgSO4, 1 μg/ml thiamine, 1 mM l-arginine, 1 mM l-proline, 100 μg/ml carbenicillin, 50 μg/ml spectinomycin, and 5 mM nonproteinogenic amino acid. After incubating at room temperature (≈25°C) on a drum roller for another 6–8 h, cultures were centrifuged and 1.8 ml of cell-free supernatant was evaporated to dryness by using a Speedvac. The dry residue was resuspended in 500 μl of water and extracted with 1 ml of ethyl acetate, and 750 μl of the organic layer was evaporated to dryness by using a Speedvac. The dry residue was resuspended in 105 μl of 1:1 methanol:water, 90 μl of which was analyzed by RP-HPLC MS using a gradient of 0–60% 0.1% formic acid/acetonitrile in 0.1% formic acid/water over 8 min with monitoring at 305 nm. Separately, RP-HPLC analysis was carried out by using a gradient of 35–50% acetonitrile in 0.1% trifluoroacetic acid/water over 30 min with monitoring at 305 nm.
Supplementary Material
Acknowledgments
We thank Christian Daly (FAS Center for Systems Biology, Harvard University) for help with the colony picker. This research was supported by the Howard Hughes Medical Institute (D.R.L.) and by National Institutes of Health/National Institute of General Medical Sciences Grants GM065400 (to D.R.L.) and GM20011 (to C.T.W.). M.A.F. is supported by a fellowship from the Hertz Foundation, and J.R.L. is supported by a fellowship from the Helen Hay Whitney Foundation.
Abbreviations
- NRP
nonribosomal peptide
- NRPS
nonribosomal peptide synthetase
- A domain
adenylation domain.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/cgi/content/full/0705348104/DC1.
References
- 1.Sieber SA, Marahiel MA. Chem Rev. 2005;105:715–738. doi: 10.1021/cr0301191. [DOI] [PubMed] [Google Scholar]
- 2.Fischbach MA, Walsh CT. Chem Rev. 2006;106:3468–3496. doi: 10.1021/cr0503097. [DOI] [PubMed] [Google Scholar]
- 3.Baltz RH. Nat Biotechnol. 2006;24:1533–1540. doi: 10.1038/nbt1265. [DOI] [PubMed] [Google Scholar]
- 4.Cane DE, Walsh CT, Khosla C. Science. 1998;282:63–68. doi: 10.1126/science.282.5386.63. [DOI] [PubMed] [Google Scholar]
- 5.Coeffet-Le Gal MF, Thurston L, Rich P, Miao V, Baltz RH. Microbiology. 2006;152:2993–3001. doi: 10.1099/mic.0.29022-0. [DOI] [PubMed] [Google Scholar]
- 6.Doekel S, Marahiel MA. Chem Biol. 2000;7:373–384. doi: 10.1016/s1074-5521(00)00118-6. [DOI] [PubMed] [Google Scholar]
- 7.Miao V, Coeffet-Le Gal MF, Nguyen K, Brian P, Penn J, Whiting A, Steele J, Kau D, Martin S, Ford R, et al. Chem Biol. 2006;13:269–276. doi: 10.1016/j.chembiol.2005.12.012. [DOI] [PubMed] [Google Scholar]
- 8.Mootz HD, Schwarzer D, Marahiel MA. Proc Natl Acad Sci USA. 2000;97:5848–5853. doi: 10.1073/pnas.100075897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nguyen KT, Ritz D, Gu JQ, Alexander D, Chu M, Miao V, Brian P, Baltz RH. Proc Natl Acad Sci USA. 2006;103:17462–17467. doi: 10.1073/pnas.0608589103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schneider A, Stachelhaus T, Marahiel MA. Mol Gen Genet. 1998;257:308–318. doi: 10.1007/s004380050652. [DOI] [PubMed] [Google Scholar]
- 11.Stachelhaus T, Schneider A, Marahiel MA. Science. 1995;269:69–72. doi: 10.1126/science.7604280. [DOI] [PubMed] [Google Scholar]
- 12.Yakimov MM, Giuliano L, Timmis KN, Golyshin PN. J Mol Microbiol Biotechnol. 2000;2:217–224. [PubMed] [Google Scholar]
- 13.Schauwecker F, Pfennig F, Grammel N, Keller U. Chem Biol. 2000;7:287–297. doi: 10.1016/s1074-5521(00)00103-4. [DOI] [PubMed] [Google Scholar]
- 14.Chiocchini C, Linne U, Stachelhaus T. Chem Biol. 2006;13:899–908. doi: 10.1016/j.chembiol.2006.06.015. [DOI] [PubMed] [Google Scholar]
- 15.Hans M, Hornung A, Dziarnowski A, Cane DE, Khosla C. J Am Chem Soc. 2003;125:5366–5374. doi: 10.1021/ja029539i. [DOI] [PubMed] [Google Scholar]
- 16.Moyne AL, Cleveland TE, Tuzun S. FEMS Microbiol Lett. 2004;234:43–49. doi: 10.1016/j.femsle.2004.03.011. [DOI] [PubMed] [Google Scholar]
- 17.Gehring AM, Mori I, Walsh CT. Biochemistry. 1998;37:2648–2659. doi: 10.1021/bi9726584. [DOI] [PubMed] [Google Scholar]
- 18.Fischbach MA, Lin H, Liu DR, Walsh CT. Nat Chem Biol. 2006;2:132–138. doi: 10.1038/nchembio771. [DOI] [PubMed] [Google Scholar]
- 19.Lai JR, Fischbach MA, Liu DR, Walsh CT. Proc Natl Acad Sci USA. 2006;103:5314–5319. doi: 10.1073/pnas.0601038103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lai JR, Fischbach MA, Liu DR, Walsh CT. J Am Chem Soc. 2006;128:11002–11003. doi: 10.1021/ja063238h. [DOI] [PubMed] [Google Scholar]
- 21.Roche ED, Walsh CT. Biochemistry. 2003;42:1334–1344. doi: 10.1021/bi026867m. [DOI] [PubMed] [Google Scholar]
- 22.Zhou Z, Lai JR, Walsh CT. Chem Biol. 2006;13:869–879. doi: 10.1016/j.chembiol.2006.06.011. [DOI] [PubMed] [Google Scholar]
- 23.Guenzi E, Galli G, Grgurina I, Gross DC, Grandi G. J Biol Chem. 1998;273:32857–32863. doi: 10.1074/jbc.273.49.32857. [DOI] [PubMed] [Google Scholar]
- 24.Zaccolo M, Williams DM, Brown DM, Gherardi E. J Mol Biol. 1996;255:589–603. doi: 10.1006/jmbi.1996.0049. [DOI] [PubMed] [Google Scholar]
- 25.Jin M, Fischbach MA, Clardy J. J Am Chem Soc. 2006;128:10660–10661. doi: 10.1021/ja063194c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pohlmann J, Lampe T, Shimada M, Nell PG, Pernerstorfer J, Svenstrup N, Brunner NA, Schiffer G, Freiberg C. Bioorg Med Chem Lett. 2005;15:1189–1192. doi: 10.1016/j.bmcl.2004.12.002. [DOI] [PubMed] [Google Scholar]
- 27.Freiberg C, Pohlmann J, Nell PG, Endermann R, Schuhmacher J, Newton B, Otteneder M, Lampe T, Habich D, Ziegelbauer K. Antimicrob Agents Chemother. 2006;50:2707–2712. doi: 10.1128/AAC.00012-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ueki M, Galonic DP, Vaillancourt FH, Garneau-Tsodikova S, Yeh E, Vosburg DA, Schroeder FC, Osada H, Walsh CT. Chem Biol. 2006;13:1183–1191. doi: 10.1016/j.chembiol.2006.09.012. [DOI] [PubMed] [Google Scholar]
- 29.Konz D, Klens A, Schorgendorfer K, Marahiel MA. Chem Biol. 1997;4:927–937. doi: 10.1016/s1074-5521(97)90301-x. [DOI] [PubMed] [Google Scholar]
- 30.Walsh C. Antibiotics: Actions, Origins, Resistance. Washington, DC: Am Soc Microbiol; 2003. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.