Abstract
Otopetrin1 (Otop1) is a multitransmembrane domain protein required for the formation of otoconia in the vertebrate inner ear. Otoconia are complex calcium carbonate (CaCO3) biominerals that are required for the sensation of gravity. Examination of the phenotypes of animals with mutations or deficiencies in Otop1 suggests a direct role for Otop1 in the initiation of extracellular biomineralization, possibly through the regulation of intracellular Ca2+. Here, we demonstrate that Otop1 overexpression can modulate purinergic-mediated Ca2+ homeostasis in transfected cell lines. These experiments define a unique set of biochemical activities of Otop1, including depletion of endoplasmic reticulum Ca2+ stores, specific inhibition of the purinergic receptor P2Y, and regulation of the influx of extracellular Ca2+ in response to ATP, ADP, and UDP. These activities can be inhibited by the polyanion suramin in a rapidly reversible manner. This first characterization of the consequences of Otop1 overexpression indicates a profound effect on cellular Ca2+ regulation. In a physiologic setting, these activities could direct the formation and growth of otoconia and regulate other biomineralization processes.
Keywords: biomineralization, otoconia, purinergic receptor
In mammals, CaPO4 is the inorganic component of bones and teeth, whereas CaCO3 is the inorganic component of otoconia. Otoconia are complex biominerals in the mammalian inner ear required for normal balance and the sensation of gravity. Otoconia contain a proteinaceous core of Ca2+ binding and matrix proteins surrounded by a coating of minute CaCO3 crystals (1–5). Human otoconia are subject to demineralization and to alterations in structure because of aging, disease, and exposure to commonly used pharmaceuticals (6), which can result in fragmentation and displacement of otoconia into the semicircular canals, where they may cause abnormal sensations of motion and loss of balance, a condition referred to as benign positional vertigo (7, 8). Disorders of balance afflict ≈9% of people over age 65 (9).
Tilted (tlt) mice lack otoconia and are used as a model for vestibular function (10–13). Positional cloning identified tlt as a mutant allele (Ala151Glu) of the novel gene Otopetrin 1 (Otop1) that is required for normal otoconial development in mice (14–16) and zebrafish (17, 18). Otop1 encodes a highly conserved protein predicted to contain 10–12 transmembrane (TM) domains, with no homology to known channels, transporters or receptors. Otop1 is a member of a new family of proteins containing the domain of unknown function 270 motif (DUF270, pfam03189) identified in Caenorhabditis elegans and Drosophila melanogaster. The DUF270 motif consists of a highly conserved multi-TM domain structure with no known or proposed activities or homologies to other protein families (19, 20). The multi-TM domain structure of Otop1 suggests a function in membrane transport or signaling. The requirement for Otop1 in CaCO3 mineralization suggests a role in regulating Ca2+ in the developing inner ear.
During development, Otop1 is present in the otoconial gelatinous membrane (16), an extracellular matrix superstructure made up of numerous structural proteins. This extracellular location is surprising for a large multi TM domain protein and suggests that Otop1 may be associated with extruded membrane vesicles called “globular substance” that may serve as a site for the protected nucleation of otoconial CaCO3 crystals (2, 21, 22). Suzuki et al. (23) showed that globular substance vesicles in isolated otoconial membranes had a dose-dependent response to ATP characterized by a slow (1–2 min) 5- to 6-fold increase in intravesicular Ca2+. This suggested that ATP might serve as a trigger in vivo to increase Ca2+ ion concentration and permit the nucleation of CaCO3 crystals in a protected environment. The kinetics and agonist sensitivity of the ATP-induced intravesicular Ca2+ accumulation was similar in activity to known purinergic P2 receptors (P2Y and P2X families), which regulate intracellular Ca2+ in response to ATP.
P2Y receptors have seven TM domains and are coupled to G proteins. Several P2Y receptors activate the Gαq subunits of G proteins leading to subsequent activation of phospholipase C and the generation of inositol 1,4,5-triphosphate (IP3) in response to nucleotide binding. Activation of the IP3 receptor on the ER membrane releases ER Ca2+ stores, resulting in a rapid peak in cytoplasmic Ca2+ concentrations ([Ca2+]i). The P2X receptors have two TM domains. Binding of nucleotides to their extracellular domain l opens a nonselective cation channel formed by homo- or heterotrimers of P2X family members resulting in an influx of extracellular Ca2+ into the cytoplasm. The P2 family members are functionally distinguished by the source of the Ca2+ (intracellular versus extracellular), nucleotide or nucleotide-analogue sensitivity, the use or absence of second messengers, and sensitivity to a variety of pharmacologic agents (24, 25). Although the response of globular substance vesicles to ATP was similar to the activity of known P2 receptors (23), Suzuki et al. were not able to directly identify the protein target of ATP as a P2 receptor.
Given the apparent localization of Otop1 within extracellular otoconial membranes and genetic studies suggesting a prominent role for this protein in the initiation of Ca2+ mineralization, we hypothesized that Otop1 may have a role in modulating the Ca2+ flux observed by Suzuki et al. (23) and may therefore be responsive to purinergic stimuli. Using single cell ratiometric Ca2+ imaging, we show that Otop1 expression leads to a nonspecific depletion of ER Ca2+ stores, a specific inhibition of P2Y receptor signaling, and initiation of a novel influx of extracellular Ca2+ in response to certain purinergic nucleotides. These activities are inhibited by the polyanion suramin in a rapidly reversible manner similar to the globular substance response to ATP (23). These studies identify a biochemical activity for the Otopetrin family and suggest a role for Otop1 in regulating Ca2+ flux in response to purinergic stimuli. This combination of activities is unique to the Otopetrin family, and we suggest that one or all of these activities may be required for otoconial development.
Results
Otop1-Expressing Cells Lack a P2Y-Mediated Peak in [Ca2+] in Response to ATP and UTP.
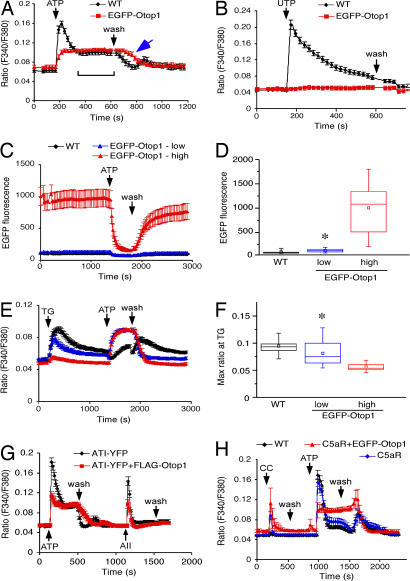
To directly test whether Otop1 could respond to purinergic stimuli, COS7 cells were transfected with Otop1 and assayed for their response to exogenous purines, using time lapse fluorescence microscopy to monitor cytosolic [Ca2+] with Fura-2 acetoxymethyl ester. Untransfected COS7 cells (WT) and those transfected with cytosolic EGFP (data not shown) respond to ATP (Fig. 1A) with a rapid peak in [Ca2+]i. We observed a dramatic absence of this [Ca2+]i peak in the response to ATP in cells expressing EGFP-Otop1. Similar responses to ATP were seen in cells coexpressing untagged Otop1 and cytosolic EGFP or Flag-tagged Otop1 [supporting information (SI) Fig. 5A and data not shown]. We hypothesized that this early peak in [Ca2+]i in WT COS7 cells was likely due to Gαq-coupled P2Y receptor signaling. To test this, we treated WT and EGFP-Otop1 transfected cells with UTP, a P2Y specific stimulus that does not activate P2X receptors. WT COS7 cells responded to UTP, whereas cells expressing EGFP-Otop1 showed no response to UTP (Fig. 1B). Lack of a peak in [Ca2+]i in EGFP-Otop1-expressing cells in response to ATP or UTP suggests that overexpression of Otop1 inhibited the activity of endogenous P2Y receptors.
Fig. 1.
Overexpression of EGFP-Otop1 in COS7 cells alters the purinergic response. (A) WT COS7 cells (n = 23) show a biphasic response to 200 μM ATP characterized by a sharp peak in [Ca2+]i and a rapid reduction in [Ca2+]i to an elevated plateau (bracket). After removal of purinergic stimulus (wash), [Ca2+]i returns to the prestimulation baseline. Cells transfected with EGFP-Otop1 (n = 17) respond to ATP with an increase in [Ca2+]i to an elevated plateau and a delay in the return of the [Ca2+]i to baseline after removal of ATP (arrow). (B) WT COS7 cells (n = 16) respond to 200 μM UTP with a sharp increase in [Ca2+]i. Cells transfected with EGFP-Otop1 (n = 11) did not respond to UTP. (C) EGFP-Otop1-expressing COS7 cells with an initial fluorescence of <200 EGFP fluorescence units were classified as “low expressers” (blue, n = 23). Cells with EGFP fluorescence units >200 units were grouped as “high expressers” (red, n = 19) and compared with untransfected cells (black, n = 35). (D) Bar plots of the experiment shown in C. Mean and median are indicated by a point and bar, respectively. Range by the box and standard deviation by the error bars [WT (∗) versus EGFP-Otop1-low expressers, P < 0.03]. (E) Treatment of the cells in C with 10 μM TG revealed a significant (P < 0.001) difference in IP3 releasable ER Ca2+ stores in low versus high EGFP-Otop1-expressing cells. Despite the low fluorescence/expression for EGFP-Otop1 in the low-expressing cells, the response to 200 μM ATP was identical to that of high-expressing cells. (F) Bar plot of the experiment shown in E. The maximum ratio value (maximum amount of ER Ca2+ released after TG stimulation) differs among WT and EGFP-Otop1 low and high expressers [WT (∗) versus EGFP-Otop1 low expressers, P < 0.05]. (G) COS7 cells transfected with both AT1-YFP and FLAG-Otop1 (n = 12) shows a significantly (P < 0.001) reduced P2Y response to 200 μM ATP compared with AT1-YFP transfected cells (n = 20), but retains a response to 20 nM AII. (H) COS7 cells transfected with C5aR (n = 7) respond to 2 μM CC peptide with a rapid and transient increase in [Ca2+]i without disrupting the P2Y-mediated increase in [Ca2+]i in response to 200 μM ATP. Cotransfection of C5aR and EGFP-Otop1 (n = 4) does not alter the 2 μM CC induced increase in [Ca2+]i, but eliminates the P2Y peak in response to 200 μM ATP. The data in A, G, and H are single experiments that were reproduced 3 times. The data in B–F are the combined results of three independent experiments.
The Level of Otop1 Expression Affects IP3 Sensitive ER Ca2+ Stores.
Inhibition of P2Y activity could result from depletion of ER Ca2+ stores, inhibition of the G protein cascade, or inhibition of receptor activation. To examine IP3 sensitive ER Ca2+ stores, COS7 cells expressing EGFP-Otop1 were treated with the sarcoplasmic/ER Ca2+ ATPase inhibitor thapsigargin (TG) (10 μM). This agent inhibits refilling of IP3 sensitive ER stores and triggers a slow leak of ER Ca2+ into the cytoplasm, thereby raising [Ca2+]i and allowing a direct evaluation of IP3 releasable stores (26, 27). Using the EGFP fluorescence intensity to compare EGFP-Otop1 levels in transfected cells, we determined that cells with high EGFP fluorescence have almost no IP3 releasable ER Ca2+ stores (Fig. 1 C–F), whereas low-expressing cells have reduced ER Ca2+ when compared with WT cells. WT cells showed a minimal response to ATP after pretreatment with TG, supporting the idea that the majority of the increase in [Ca2+]i after stimulation with ATP comes from intracellular IP3 releasable stores and thus is likely due to signaling through members of the P2Y receptor family.
Depletion of ER Ca2+ stores has been suggested to be a nonspecific effect of the overexpression of a variety of proteins (28, 29). To test this, we overexpressed another large multi-TM domain protein, the angiotensin (AII)-1 receptor [AT1-yellow fluorescent protein (YFP)], in COS7 cells. The presence of AT1-YFP conferred responsiveness to AII (Fig. 1G and SI Fig. 6A). Overexpression of AT1-YFP significantly (P < 0.05) reduced the amount of ER Ca2+ released in response to TG (SI Fig. 6B) but did not appreciably alter the characteristics of the P2Y peak in response to ATP (Fig. 1G and SI Fig. 6A). Thus, depletion of ER Ca2+ stores in cells expressing high levels of Otop1 may represent a nonspecific effect of protein overexpression. In high EGFP-Otop1-expressing cells, depletion of ER Ca2+ can contribute to the loss of P2Y activity in response to UTP and ATP, but low-expressing cells should have sufficient ER Ca2+ stores to allow an increase in [Ca2+]i in response to purinergic stimuli.
Depletion of ER Ca2+ can also result from the unfolded protein response when proteins are overexpressed (30–32). However, immunoreactivity for the glucose-binding and ER stress related protein BiP/GrP78 was not increased in cells overexpressing EGFP-Otop1 (SI Fig. 6 C–H). Therefore, the ER Ca2+ loss is unlikely to result from an unfolded-protein response initiated by overexpressing Otop1.
G Protein Coupled Receptor (GPCR)-Mediated Signaling Is Intact in Otop1-Expressing Cells.
If Otop1 blocked P2Y activity at the level of the G protein signaling cascade, Otop1 expression should alter the activity of other GPCRs that used the same G protein signaling cascade as P2Y. The isolate of COS7 cells used did not have other Gαq based GPCRs that could be readily assayed. Therefore, we transfected these cells with plasmids containing either the AT1-YFP or the human complement C5a (C5aR, containing an IRES Gα16) GPCRs. Transfected cells responded to AII or the Cha-Cha (CC) peptide, respectively, with activation of the downstream components of Gαq signaling and release of intracellular IP3 sensitive Ca2+ stores. Cells expressing AT1-YFP responded to ATP with an increase in [Ca2+]i similar to that seen in WT cells (Fig. 1G and SI Fig. 6A) and responded to a subsequent challenge with 20 nM AII with a rapid peak in [Ca2+]i released from IP3 sensitive ER stores (Fig. 1G). Cells cotransfected with FLAG-Otop1 and AT1-YFP exhibited the characteristic Otop1 inhibition of the P2Y dependent [Ca2+]i peak in response to ATP but retained their response to 20 nM AII (Fig. 1G). Reversing the order of ATP and AII stimulus did not alter these responses (data not shown). Similarly, COS7 cells overexpressing the C5aR responded to the CC peptide agonist and to ATP (Fig. 1H). Overexpression of both C5aR and EGFP-Otop1 eliminated the characteristic P2Y response to ATP but did not block the response to CC. Thus, Otop1 depletion of IP3 sensitive ER Ca2+ stores is not sufficient to prevent increases in [Ca2+]i in response to the G protein signaling cascade from other Gαq requiring GPCRs. These studies also indicate that the Gαq signaling cascade is intact in Otop1-expressing cells, suggesting that Otop1 interferes with P2Y upstream of Gαq activation.
Otop1 Activity Requires Extracellular Ca2+ and Has Unique Agonist Sensitivity.
Although EGFP-Otop1-expressing cells did not exhibit a P2Y-mediated peak in [Ca2+]i in response to ATP, these cells showed a slow (30–60 sec to plateau) rise and a sustained elevation in [Ca2+]i (Fig. 1A). This altered response to ATP was similar to the plateau in [Ca2+]i in WT cells exposed to ATP (Fig. 1A, bracket) and was further characterized. After removal of ATP (wash), cells overexpressing EGFP-Otop1 took longer than WT cells to return to baseline [Ca2+]i (WT t1/2 = 95 ± 7 sec, EGFP-Otop1 t1/2 = 186 ± 3 sec, P < 0.01) (Fig. 1A, arrow). Thus, expression of Otop1 in COS7 cells eliminated the P2Y-mediated rapid rise in [Ca2+]i and altered the elevated plateau in [Ca2+]i in response to ATP.
In addition to altering [Ca2+]i in response to ATP, EGFP fluorescence was greatly reduced in EGFP-Otop1-expressing cells after stimulation with ATP (average decrease in fluorescence intensity 50 ± 19%, Fig. 1C). This was particularly apparent in high-expressing cells, but was also noted in low-expressing cells. The significance of these observations is unknown (see Discussion).
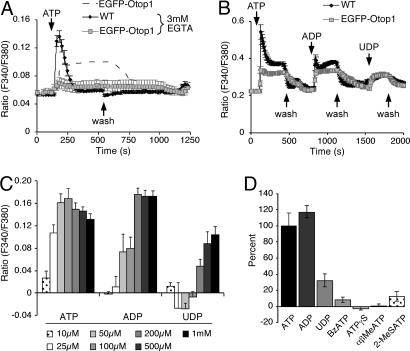
After exposure to TG, EGFP-Otop1 showed a significant increase in [Ca2+]i to an elevated plateau in response to ATP (Fig. 1E), which was identical in cells with either high or low fluorescence, suggesting that the Otop1-ATP-mediated increase in [Ca2+]i does not require IP3 releasable ER Ca2+ stores and is independent of Otop1 expression levels. As Otop1-expressing cells do not require the presence of ER IP3-releasible Ca2+, an alternative source of Ca2+ must be required for the observed plateau in [Ca2+]i in response to ATP. Chelation of extracellular Ca2+ with 3 mM EGTA did not reduce the P2Y-mediated rapid peak in [Ca2+]i in WT cells, but abolished the ATP-induced increase in [Ca2+]i in EGFP-Otop1-expressing cells (Fig. 2A). This indicated that Otop1 requires extracellular Ca2+ to respond to ATP. In this way, Otop1 activity resembles the activity of P2X receptors, which are distinguished, in part, by their ability to mediate extracellular Ca2+ entry in response to purinergic stimuli.
Fig. 2.
Unique characteristics of EGFP-Otop1 purinergic-induced increase in [Ca2+]i in COS7 cells. (A) In the absence of extracellular Ca2+ (3 mM EGTA), 200 μM ATP elicits a response in WT cells (n = 10) but not in EGFP-Otop1-expressing cells (n = 7). EGFP-Otop1-expressing COS7 cells in Ca2+-containing media are represented by the dashed line. (B) Two hundred micromolar ATP and ADP elicit similar increases in [Ca2+]i in EGFP-Otop1-expressing cells (n = 14), 200 μM UDP leads to an increase in [Ca2+]i of approximately one-half that seen with ATP or ADP. This is independent of the order of addition of nucleotides. Unlike in EGFP-Otop1-expressing cells, the P2Y component of WT cells (n = 27) remained in a refractory period after the initial treatment with ATP. (C) Treatment of EGFP-Otop1-expressing cells with varying concentrations of ATP (n = 11), ADP (n = 8), UDP (n = 5) leads to different levels of [Ca2+]i 200 sec after addition of agonist. All ratio values are subtracted from baseline values to indicate change in [Ca2+]i in response to stimulus. (D) EGFP-Otop1-expressing COS7 cells respond to 200 μM ATP (n = 11), ADP (n = 8), and UDP (n = 5) with increases in [Ca2+]i, but respond minimally to 200 μM BzATP (n = 18), 200 μM ATPγS (n = 18), 50 μM αβMeATP (n = 5), and 50 μM 2-(methylthio) adenosine 5′-triphosphate (2MeSATP) (n = 5). All increases in [Ca2+]i at 200 sec after addition of agonist were normalized to the response to 200 μM ATP. The results of a single experiment are shown and were reproduced at least 2 times.
To compare Otop1 activity with the agonist sensitivities of described P2X receptors, Otop1-expressing cells were treated with a variety of purine analogs and were examined for alterations in [Ca2+]i. EGFP-Otop1-expressing cells responded to ATP, ADP, and UDP with an increase in [Ca2+]i (Fig. 2B). Cells were exposed to varying doses of ATP, ADP or UDP and examined at ≈200 sec after agonist addition for levels of [Ca2+]i. The dose dependence of Otop1 activity showed a clear rank order of potency of ATP ≈ ADP > UDP (Fig. 2C). Initial maximum [Ca2+]i was also similar for ATP and ADP (SI Fig. 7 A and B). Cells expressing EGFP-Otop1 were insensitive to varying concentrations of 2′(3′)-O-(4-Benzoylbenzoyl)adenosine 5′-triphosphate (BzATP), adenosine 5′-[γ-thio]triphosphate (ATPγS), α-β-methylene ATP (αβMeATP), and 2-(methylthio) adenosine 5′-triphosphate (Fig. 2D), or UTP (Fig. 1B). This response profile to extracellular nucleotides did not correspond to the agonist sensitivity of any described P2X family homo- or hetero-trimer analyzed in vivo or in vitro (24, 25, 33, 34), suggesting that Otop1 either has unique activity or modifies the activity of another ionotropic purinergic receptor.
Expression of Otop1 Induces ATP-Mediated [Ca2+]i Increases in Rat Osteosarcoma 17/2.8 (ROS) and HEK293 Cells.
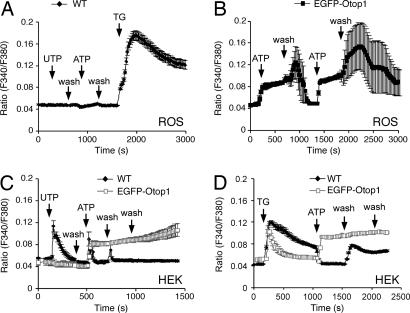
Otop1 expression in COS7 cells suppressed the P2Y response and activated an influx of extracellular Ca2+ ions. This could be due to intrinsic Otop1 activity or modulation of activity of endogenous P2 receptors. To test whether Otop1 expression could initiate a purinergic response in cells lacking endogenous P2 receptor activity, we examined ROS cells that lack response to purinergic stimuli (35, 36). In untransfected ROS cells, there was no alteration in [Ca2+]i in response to UTP or ATP, although IP3 sensitive ER Ca2+ stores were plentiful and responded to TG with increased [Ca2+]i (Fig. 3A). In contrast, ROS cells expressing EGFP-Otop1 responded to ATP with a sustained increase in [Ca2+]i (Fig. 3B). The [Ca2+]i remained elevated in the presence of agonist, as seen in COS7 cells. With removal of agonist, [Ca2+]i rose above the plateau level in some cells and then slowly returned to baseline. Thus, overexpression of Otop1 is sufficient to induce sensitivity to purinergic stimuli in ROS cells which lack active P2 receptors.
Fig. 3.
EGFP-Otop1 activity in ROS and HEK293 cells. (A) UTP (200 μM) and ATP (200 μM) do not elicit increases in [Ca2+]i in untransfected ROS cells (n = 4). Treatment with TG increases [Ca2+]I revealing abundant ER Ca2+ stores. (B) In cells transfected with EGFP-Otop1 (n = 4), 200 μM ATP elicits a slow increase in [Ca2+]i. Removal of stimulus results in an increase in [Ca2+]i before a return to baseline. Subsequent stimulation with ATP leads to an identical response. (C) WT HEK293 cells (n = 11) respond to 400 μM UTP with a brief increase in [Ca2+]i and a rapid return to baseline. Subsequent treatment with 200 μM ATP elicits a similar response. EGFP-Otop1-expressing HEK293 cells (n = 6) do not respond to UTP, and 200 μM ATP elicits a sustained increase in [Ca2+]i that does not return to baseline after removal of stimulus. (D) Treatment of WT (n = 19) and EGFP-Otop1-expressing (n = 16) HEK293 cells with 10 μM TG leads to an increase in [Ca2+]i. WT cells do not respond to 200 μM ATP after TG treatment, whereas EGFP-Otop1 cells show a sustained elevation of [Ca2+]i. Data show the result of single experiments that were reproduced at least 3 times.
HEK293 express P2Y receptors (data not shown); however, they failed to show inward current in response to ATP, suggesting that they do not express functional cell-surface P2X receptors (T. Egan, personal communication). EGFP-Otop1 expression in HEK293 cells inhibited the P2Y response to 400 μM UTP (Fig. 3C) and responded to ATP with an increase in [Ca2+]i similar to that seen in COS7 and ROS cells. However, in response to ATP, EGFP-Otop1-expressing cells sustained elevated [Ca2+]i, even after removal of the purinergic stimulus. Expression of Otop1 reduced ER Ca2+ stores releasable by TG (Fig. 3D), but the presence of TG did not affect the sustained increase in [Ca2+]i in response to ATP observed in Otop1-expressing cells. The sustained high [Ca2+]i in EGFP-Otop1-expressing HEK293 cells after removal of agonist was considerably different from that seen in other cell types expressing EGFP-Otop1. Although return of [Ca2+]i to baseline was slow in both COS7 and ROS cells after removal of ATP (wash), most cells initiated a return to baseline within ≈150 sec and attained baseline within ≈350 sec of agonist removal (Figs. 1A and 3B). In contrast, HEK293 cells expressing Otop1 maintained elevated [Ca2+]i for >1,800 sec after ATP removal, suggesting that HEK293 cells lack one or more components of the Otop1 signaling pathway that allow Otop1 to be inactivated after ATP is removed. These findings suggest that overexpression of Otop1 is sufficient for the initiation and maintenance of increased [Ca2+]i in response to ATP, but that inactivation of Otop1 after removal of agonist depends on cell type-specific mechanisms.
Suramin Reversibly Inhibits Otop1 Activity.
Suramin is a sulfated polyanion that can inhibit the activity of a variety of proteins and was found to inhibit the ATP induced rise in [Ca2+]i in globular substance vesicles (23). To determine whether the Otop1-associated increase in [Ca2+]i in response to ATP was similarly sensitive to suramin, COS7 cells expressing high levels of EGFP-Otop1 were treated with 100 μM suramin. After prolonged (>10 min) exposure to suramin, EGFP-Otop1-expressing cells responded to ATP in a manner identical to WT cells (Fig. 4A), suggesting that this agent blocked the ability of Otop1 to inhibit the P2Y peak in [Ca2+]i and eliminated the Otop1 induced plateau in [Ca2+]i in response to ATP. Removal of suramin from the media before treatment with ATP resulted in a rapid return to the characteristic Otop1-ATP-induced Ca2+ entry and reduction in P2Y response (Fig. 4B). Thus, suramin reversibly inhibits both the Otop1-dependent inhibition of the P2Y response and prevents the Otop1-mediated influx of extracellular Ca2+. These data further support the similarities between Otop1 activity and the ATP-mediated increase in intravesicular [Ca2+] identified by Suzuki and colleagues (23).
Fig. 4.
Suramin inhibits Otop1 activity in COS7 cells. (A) EGFP-Otop1-expressing cells (n = 17) treated with 100 μM suramin for 20 min have an identical increase in [Ca2+]i in response to 200 μM ATP as WT cells (n = 22). After removal of suramin and ATP (wash), EGFP-Otop1-expressing cells respond to 200 μM ATP similar to that seen in cells never treated with suramin (Fig. 1A). Note that in WT cells the P2Y response to ATP was in the refractory period for the second dose of ATP. (B) COS7 cells expressing EGFP-Otop1 (n = 4) were pretreated with suramin for 10 min and then washed. Subsequent challenge with 200 μM ATP resulted in an increase in [Ca2+]i to an elevated plateau similar to that seen in untreated cells. WT cells (n = 11) are not affected by 100 μM suramin. Data show the result of single experiments that were reproduced at least 3 times.
Discussion
Otop1 is the first functionally described member of the DUF270 domain protein family. Here, we show that Otop1 can modulate Ca2+ homeostasis and Ca2+ flux in response to purinergic stimuli in several ways. (i) Otop1 expression depletes ER stores in a dose dependent and, most likely, nonspecific manner. (ii) Otop1 appears to selectively inhibit the activity of P2Y receptors without altering the activity of other overexpressed GPCRs. Such inhibition may result from altered expression or localization of the P2Y proteins or to alterations in P2Y conformation that prevents activation by ATP or interaction with Gαq in the presence of Otop1. (iii) In cells that do not have P2 receptor activity, Otop1 overexpression leads directly or indirectly to an influx of extracellular Ca2+ in response to exogenous ATP.
We propose that Otop1 may act as a key regulator of intracellular or intravesicular Ca2+ in response to ATP in the inner ear during otoconial development. To accomplish this, Otop1 may function similarly to P2 receptors, in that expression of Otop1 leads to an ATP induced increase in [Ca2+]i, as is seen in cells expressing P2 family subtypes (34, 35, 37). Both P2Y and P2X receptors increase [Ca2+]i, but have distinctly different primary structures and different mechanisms of action. The Otop1 primary sequence has no homology to either P2Y or P2X protein families or to other types of channels, transporters, exchangers, or receptors. Otop1 also lacks functional domains that are conserved in such proteins, for example ATP-binding domains, selectivity pores, or G protein binding consensus sequences. Thus, Otop1 either interacts with other proteins to form or activate sites for ATP-binding and Ca2+ flux, or contains atypical motifs, most likely within the highly conserved DUF270 domain, that bind purines and elicit Ca2+ influx.
Otop1 Function in Vitro.
The work of many labs has demonstrated ATP-induced Ca2+ currents in cells expressing cloned or native P2X receptors, using whole cell patch clamp experiments (24, 34, 38–41). However, we saw no ATP-gated membrane current in cells expressing Otop1 despite multiple attempts to patch clamp COS7, ROS, or HEK293, using techniques commonly used to characterize P2X receptors (D. Samway and T. Egan, personal communications). Thus, although Otop1 requires extracellular Ca2+ and responds to ATP, it does not behave like a classical P2X channel. The inability to identify membrane currents in response to ATP may suggest that Otop1 activity is electrically neutral.
It is possible that Otop1 functions as a novel ATPase or ATP-requiring transporter (along with other proteins that neutralize the entry of extracellular Ca2+), or as a Ca2+ pump or an ion exchanger to increase [Ca2+]i in response to purinergic stimuli. It is unclear whether ATP hydrolysis is required for Otop1 activity. Otop1-expressing COS7 cells lack an alteration in [Ca2+]i in response to ATPγS and rapidly inactivate when ATP concentrations are limiting (SI Fig. 7A), but in Otop1-expressing HEK293 cells, [Ca2+]i remains high even after removal of ATP from the media. In addition, inactivation or desensitization of Otop1 activity following repeated stimulation with agonist was not observed (data not shown), unlike that seen with P2Y receptors or with some P2X family members (24, 25, 34).
If Otop1 behaves as, or interacts with, a pump or exchanger, a counterion would be required to balance Ca2+ influx. In support of this hypothesis, EGFP, when either coexpressed or as a fusion protein with Otop1, bleaches in response to ATP, ADP, and to a lesser extent UDP, but is unaffected by UTP (Fig. 1C, SI Fig. 5 A and B, and data not shown). EGFP fluorescence quenching in response to ATP also occurred in cells expressing only cytosolic EGFP, but was significantly less (P < 0.04) than in cells expressing either the EGFP-Otop1 fusion protein or coexpressing cytosolic EGFP and native Otop1 (SI Fig. 5 A and B). This change in EGFP fluorescence mimics the pattern of Ca2+ influx in response to these nucleotides. However, in the presence of EGTA-containing media, where little increase in [Ca2+]i was noted, EGFP bleaching was less (average decrease in fluorescence intensity 11 ± 13%) (SI Fig. 5 C and D). EGFP fluorescence is sensitive to alterations in intracellular anion concentrations (42); however, ion replacement and subsequent treatment with a purinergic stimulus has not identified a counter ion for Otop1-mediated Ca2+ influx or EGFP quenching. Replacement of Na+ ions with N-methyl d-glutamine did not affect the initiation or the level of [Ca2+]i influx in response to ATP in COS7 cells, but after removal of agonist, [Ca2+]i remained high for >400 sec (SI Fig. 8A), similar to the response seen in HEK293 cells (Fig. 3C). Replacement of Na+ ions to the media restored Otop1-mediated Ca2+ regulation, suggesting that Na+ is required to inactivate Otop1 activity or that a Na+/Ca2+ exchanger or other Na+ requiring protein is needed to reduce [Ca2+]i after Otop1 activity terminates. Similarly, Cl− depleted media, or pH6.8 media, also prevented Otop1 inactivation but did not alter other characteristics of the Otop1 ATP-induced [Ca2+]i increase (SI Fig. 8 B and C). Otop1 activity was also not affected by removal of extracellular glucose or increasing extracellular Ca2+ (data not shown). However, increasing extracellular K+ caused a small reduction in the influx of Ca2+ in response to ATP (data not shown).
In addition to examining ion sensitivities, we examined the activity of a variety of inhibitors and modulators of channel and transporter function on cells overexpressing Otop1. The Ca2+ channel blocker SKF96365 (10 mM) (43) had no effect on Otop1 activity in COS7 cells. Modulation of PLC-mediated pathways with the PLC activator m-3M3FBS (25 μM) (44, 45) or inhibitor U73122 (10 μM) (26) had no effect on Otop1 activity, but disrupted P2Y signaling in WT COS7 cells. Pretreatment with the polyanion suramin, however, was able to reversibly block Otop1 inhibition of ATP-induced P2Y activation and Otop1-mediated influx of extracellular Ca2+. This blockade was time dependent, requiring >10 min of suramin pretreatment, but was almost immediately reversible (Fig. 4 A and B). In cells treated for >10 min with suramin, TG-induced release of IP3 sensitive stores revealed near-normal levels of ER Ca2+ in high-expressing EGFP-Otop1 cells (data not shown). Suramin was also able to significantly reduce EGFP quenching in response to ATP in EGFP-Otop1-expressing cells (SI Fig. 5 E–H), suggesting that suramin exposure can inhibit the alterations in intracellular conditions that occur with Otop1 activation. It is also possible that suramin may disrupt interactions between Otop1 and other proteins that mediate P2Y inhibition, Ca2+, or counterion influx, or may act directly on Otop1 to reversibly inhibit its function.
Otop1 Function in Vivo.
Cells transfected with EGFP-Otop1 or Otop1 and EGFP responded to ATP with a novel increase in [Ca2+]i to an elevated plateau. The characteristics of this response were very similar to the atypical purinergic response studied by Suzuki et al. (23) in isolated globular substance vesicles, with a slow ATP-dependent increase in intravesicular [Ca2+] (taking 1–2 min to achieve maximal Ca2+ entry) and sustained intravesicular [Ca2+] after removal of ATP from the media. This similarity suggests that Otop1 is responsible for the ATP-mediated increase in intravesicular [Ca2+] seen in these studies. Suzuki et al. (23) found that the intravesicular Ca2+ increase in globular substance vesicles could not be elicited by application of αβMeATP, BzATP, ADP, or UTP but could be stimulated by 2-(methylthio) adenosine 5′-triphosphate and ATP (23). This profile of nucleotide sensitivity is somewhat different from that seen with Otop1 overexpression (ATP ≈ ADP ≫ UDP and αβMeATP, BzATP, ATPγS, UTP, and 2-(methylthio) adenosine 5′-triphosphate were not effective). This could be due to intrinsic differences in guinea pig and mouse Otop1, because similar differences have been noted across mouse, human, and rat for P2 receptors (24). Alternatively, these differences may be attributable to alternative splice forms of Otop1 (15, 16), differing accessory proteins, or differences in the ionic composition of the endolymph, which could alter the conformation or agonist sensitivity of Otop1 in globular substance vesicles.
Based on Otop1 localization to the extracellular space during otoconial formation (16) and its role in modulation of [Ca2+]i, we propose that Otop1 regulates increases in intravesicular [Ca2+] within globular substance vesicles which may be needed to initiate nucleation of CaCO3 crystals in the ATP-rich endolymph (46, 47). Although the complete biochemical mechanism of action of Otop1 remains unclear, this founding member of a novel gene family displays several important effects on cellular Ca2+ regulation in cell culture. Otop1 is expressed in a variety of tissues outside the inner ear, including the thymus, heart, kidney, skin, stomach, adrenal gland, and lactating mammary gland (16), where it and two other members of this gene family may play an important role in the movement of Ca2+ ions.
Materials and Methods
Reagents.
TG was from Calbiochem (San Diego, CA). EGTA, ATP, ADP, ATPγS, αβMeATP, 2-(Methylthio) adenosine 5′-triphosphate, BzATP, UTP, UDP, 1-[6-[((17β)-3-Methoxyestra-1,3,5[10]-trien-17-yl)amino]hexyl]-1H-pyrrole-2,5-dione, and N-methyl d-glutamine were from Sigma (St. Louis, MO). CC peptide, AII, ATI-YFP (48), and C5aR (49, 50) were from T. Baranski (Washington University School of Medicine). ROS 17/2.8 and HEK293 cells were from T. Steinberg (Washington University School of Medicine) (35, 36). pCS2-EGFP, pCS2-FLAG plasmids were from K. Kroll. BiP antibody was from P. Hanson.
Cell Culture and Transfection.
The full-length Otopetrin1 cDNA (A530025J20) was obtained from RIKEN Laboratories (Yokohama, Japan) (51, 52). Plasmid constructions and cell transfection are described in the SI Materials and Methods.
Calcium Signaling.
[Ca2+]i was detected with the Ca2+ sensitive dye Fura 2 acetoxymethyl ester (Invitrogen, Carlsbad, CA), using a Zeiss (Thornwood, CA) Axiovert microscope. Images were captured with an Intelligent Imaging Innovations (Denver, CO) 3-I digital camera system and analyzed with Slidebook software (Intelligent Imaging Innovations). Detailed methods are in the SI Materials and Methods. Transfected cells were stimulated with 200 μM purinergic agonist, unless otherwise stated.
Supplementary Material
Acknowledgments
We thank E. Kim, I. Boime, T. Steinberg, and T. Baranski (Washington University School of Medicine), and J. Hiken, T. Egan and D. Samways, (St. Louis University) for extensive discussion and for providing cell lines and plasmids. This research was supported by National Institute on Deafness and Other Communication Disorders Grants DC02236 and DC06974.
Abbreviations
- αβMeATP
α-β-methylene ATP
- ATPγS
adenosine 5′-[γ-thio]triphosphate
- AII
angiotensin
- BzATP
2′(3′)-O-(4-Benzoylbenzoyl)adenosine 5′-triphosphate
- [Ca2+]i
Ca2+ concentrations
- IP3
1,4,5-triphosphate
- CC
Cha-Cha
- GPCR
G protein coupled receptor
- ROS
rat osteosarcoma 17/2.8
- TM
transmembrane
- TG
thapsigargin
- YFP
yellow fluorescent protein.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/cgi/content/full/0705182104/DC1.
References
- 1.Ross MD, Pote KG, Perini F. In: Auditory Biochemistry. Drescher DG, editor. Springfield, IL: Charles C Thomas; 1985. pp. 500–514. [Google Scholar]
- 2.Suzuki H, Ikeda K, Takasaka T. Hear Res. 1995;90:212–218. doi: 10.1016/0378-5955(95)00168-7. [DOI] [PubMed] [Google Scholar]
- 3.Mann S, Parker SB, Ross MD, Skarnulis AJ, Williams RJ. Proc R Soc London B. 1983;218:415–424. doi: 10.1098/rspb.1983.0048. [DOI] [PubMed] [Google Scholar]
- 4.Thalmann I, Hughes I, Tong BD, Ornitz DM, Thalmann R. Electrophoresis. 2006;27:1598–1608. doi: 10.1002/elps.200500768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lins U, Farina M, Kurc M, Riordan G, Thalmann R, Thalmann I, Kachar B. J Struct Biol. 2000;131:67–78. doi: 10.1006/jsbi.2000.4260. [DOI] [PubMed] [Google Scholar]
- 6.Ross MD, Peacor D, Johnsson LG, Allard LF. Ann Otol Rhinol Laryngol. 1976;85:310–326. doi: 10.1177/000348947608500302. [DOI] [PubMed] [Google Scholar]
- 7.House MG, Honrubia V. Audiol Neurootol. 2003;8:91–99. doi: 10.1159/000068998. [DOI] [PubMed] [Google Scholar]
- 8.Welling DB, Parnes LS, O'Brien B, Bakaletz LO, Brackmann DE, Hinojosa R. Laryngoscope. 1997;107:90–94. doi: 10.1097/00005537-199701000-00018. [DOI] [PubMed] [Google Scholar]
- 9.Oghalai JS, Manolidis S, Barth JL, Stewart MG, Jenkins HA. Otolaryngol Head Neck Surg. 2000;122:630–634. doi: 10.1016/S0194-5998(00)70187-2. [DOI] [PubMed] [Google Scholar]
- 10.Ornitz DM, Bohne BA, Thalmann I, Harding GW, Thalmann R. Hearing Res. 1998;122:60–70. doi: 10.1016/s0378-5955(98)00080-x. [DOI] [PubMed] [Google Scholar]
- 11.Andreescu CE, De Ruiter MM, De Zeeuw CI, De Jeu MT. J Neurophysiol. 2005;94:3487–3496. doi: 10.1152/jn.00147.2005. [DOI] [PubMed] [Google Scholar]
- 12.de Caprona MD, Beisel KW, Nichols DH, Fritzsch B. Brain Res Bull. 2004;64:289–301. doi: 10.1016/j.brainresbull.2004.08.004. [DOI] [PubMed] [Google Scholar]
- 13.Jones SM, Erway LC, Johnson KR, Yu H, Jones TA. Hear Res. 2004;191:34–40. doi: 10.1016/j.heares.2004.01.008. [DOI] [PubMed] [Google Scholar]
- 14.Hurle B, Lane K, Kenney J, Tarantino LM, Bucan M, Brownstein BH, Ornitz DM. Genomics. 2001;77:189–199. doi: 10.1006/geno.2001.6632. [DOI] [PubMed] [Google Scholar]
- 15.Besson V, Nalesso V, Herpin A, Bizot JC, Messaddeq N, Romand R, Puech A, Blanquet V, Herault Y. Biol Cell. 2005;97:787–798. doi: 10.1042/BC20040525. [DOI] [PubMed] [Google Scholar]
- 16.Hurle B, Ignatova E, Massironi SM, Mashimo T, Rios X, Thalmann I, Thalmann R, Ornitz DM. Hum Mol Genet. 2003;12:777–789. doi: 10.1093/hmg/ddg087. [DOI] [PubMed] [Google Scholar]
- 17.Hughes I, Blasiole B, Huss D, Warchol ME, Rath NP, Hurle B, Ignatova E, Dickman JD, Thalmann R, Levenson R, Ornitz DM. Dev Biol. 2004;276:391–402. doi: 10.1016/j.ydbio.2004.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sollner C, Schwarz H, Geisler R, Nicolson T. Dev Genes Evol. 2004;214:582–590. doi: 10.1007/s00427-004-0440-2. [DOI] [PubMed] [Google Scholar]
- 19.Bateman A, Coin L, Durbin R, Finn RD, Hollich V, Griffiths-Jones S, Khanna A, Marshall M, Moxon S, Sonnhammer EL, et al. Nucleic Acids Res. 2004;32:D138–D141. doi: 10.1093/nar/gkh121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sonnhammer EL, Eddy SR, Birney E, Bateman A, Durbin R. Nucleic Acids Res. 1998;26:320–322. doi: 10.1093/nar/26.1.320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hughes I, Thalmann I, Thalmann R, Ornitz DM. Brain Res. 2006;1091:58–74. doi: 10.1016/j.brainres.2006.01.074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tateda M, Suzuki H, Ikeda K, Takasaka T. Hear Res. 1998;124:91–98. doi: 10.1016/s0378-5955(98)00115-4. [DOI] [PubMed] [Google Scholar]
- 23.Suzuki H, Ikeda K, Furukawa M, Takasaka T. Am J Physiol. 1997;273:C1533–C1540. doi: 10.1152/ajpcell.1997.273.5.C1533. [DOI] [PubMed] [Google Scholar]
- 24.North RA. Physiol Rev. 2002;82:1013–1067. doi: 10.1152/physrev.00015.2002. [DOI] [PubMed] [Google Scholar]
- 25.Ralevic V, Burnstock G. Pharmacol Rev. 1998;50:413–492. [PubMed] [Google Scholar]
- 26.Bleasdale JE, Thakur NR, Gremban RS, Bundy GL, Fitzpatrick FA, Smith RJ, Bunting S. J Pharmacol Exp Ther. 1990;255:756–768. [PubMed] [Google Scholar]
- 27.Treiman M, Caspersen C, Christensen SB. Trends Pharmacol Sci. 1998;19:131–135. doi: 10.1016/s0165-6147(98)01184-5. [DOI] [PubMed] [Google Scholar]
- 28.Brostrom MA, Brostrom CO. Cell Calcium. 2003;34:345–363. doi: 10.1016/s0143-4160(03)00127-1. [DOI] [PubMed] [Google Scholar]
- 29.Dellis O, Dedos SG, Tovey SC, Taufi Qur R, Dubel SJ, Taylor CW. Science. 2006;313:229–233. doi: 10.1126/science.1125203. [DOI] [PubMed] [Google Scholar]
- 30.Zhang K, Kaufman RJ. Neurology. 2006;66:S102–S109. doi: 10.1212/01.wnl.0000192306.98198.ec. [DOI] [PubMed] [Google Scholar]
- 31.Lievremont JP, Rizzuto R, Hendershot L, Meldolesi J. J Biol Chem. 1997;272:30873–30879. doi: 10.1074/jbc.272.49.30873. [DOI] [PubMed] [Google Scholar]
- 32.Morris JA, Dorner AJ, Edwards CA, Hendershot LM, Kaufman RJ. J Biol Chem. 1997;272:4327–4334. doi: 10.1074/jbc.272.7.4327. [DOI] [PubMed] [Google Scholar]
- 33.Arreola J, Melvin JE. J Physiol. 2003;547:197–208. doi: 10.1113/jphysiol.2002.028373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.North RA, Surprenant A. Annu Rev Pharmacol Toxicol. 2000;40:563–580. doi: 10.1146/annurev.pharmtox.40.1.563. [DOI] [PubMed] [Google Scholar]
- 35.Jorgensen NR, Geist ST, Civitelli R, Steinberg TH. J Cell Biol. 1997;139:497–506. doi: 10.1083/jcb.139.2.497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.You J, Jacobs CR, Steinberg TH, Donahue HJ. J Biol Chem. 2002;277:48724–48729. doi: 10.1074/jbc.M209245200. [DOI] [PubMed] [Google Scholar]
- 37.Jiang LH, Kim M, Spelta V, Bo X, Surprenant A, North RA. J Neurosci. 2003;23:8903–8910. doi: 10.1523/JNEUROSCI.23-26-08903.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jarlebark LE, Housley GD, Raybould NP, Vlajkovic S, Thorne PR. NeuroReport. 2002;13:1979–1984. doi: 10.1097/00001756-200210280-00030. [DOI] [PubMed] [Google Scholar]
- 39.Salih SG, Jagger DJ, Housley GD. Neuropharmacology. 2002;42:386–395. doi: 10.1016/s0028-3908(01)00184-8. [DOI] [PubMed] [Google Scholar]
- 40.Egan TM, Khakh BS. J Neurosci. 2004;24:3413–3420. doi: 10.1523/JNEUROSCI.5429-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Khakh BS, Egan TM. J Biol Chem. 2005;280:6118–6129. doi: 10.1074/jbc.M411324200. [DOI] [PubMed] [Google Scholar]
- 42.Griesbeck O, Baird GS, Campbell RE, Zacharias DA, Tsien RY. J Biol Chem. 2001;276:29188–29194. doi: 10.1074/jbc.M102815200. [DOI] [PubMed] [Google Scholar]
- 43.Merritt JE, Armstrong WP, Benham CD, Hallam TJ, Jacob R, Jaxa-Chamiec A, Leigh BK, McCarthy SA, Moores KE, Rink TJ. Biochem J. 1990;271:515–522. doi: 10.1042/bj2710515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bae YS, Lee TG, Park JC, Hur JH, Kim Y, Heo K, Kwak JY, Suh PG, Ryu SH. Mol Pharmacol. 2003;63:1043–1050. doi: 10.1124/mol.63.5.1043. [DOI] [PubMed] [Google Scholar]
- 45.Lee YN, Lee HY, Kim JS, Park C, Choi YH, Lee TG, Ryu SH, Kwak JY, Bae YS. Cancer Lett. 2005;222:227–235. doi: 10.1016/j.canlet.2004.09.017. [DOI] [PubMed] [Google Scholar]
- 46.Munoz DJ, Thorne PR, Housley GD, Billett TE. Hear Res. 1995;90:119–125. doi: 10.1016/0378-5955(95)00153-5. [DOI] [PubMed] [Google Scholar]
- 47.Munoz DJ, Thorne PR, Housley GD, Billett TE, Battersby JM. Hear Res. 1995;90:106–118. doi: 10.1016/0378-5955(95)00152-3. [DOI] [PubMed] [Google Scholar]
- 48.Hansen JL, Servant G, Baranski TJ, Fujita T, Iiri T, Sheikh SP. Circ Res. 2000;87:753–759. doi: 10.1161/01.res.87.9.753. [DOI] [PubMed] [Google Scholar]
- 49.Floyd DH, Geva A, Bruinsma SP, Overton MC, Blumer KJ, Baranski TJ. J Biol Chem. 2003;278:35354–35361. doi: 10.1074/jbc.M305607200. [DOI] [PubMed] [Google Scholar]
- 50.Klco JM, Lassere TB, Baranski TJ. J Biol Chem. 2003;278:35345–35353. doi: 10.1074/jbc.M305606200. [DOI] [PubMed] [Google Scholar]
- 51.Carninci P, Hayashizaki Y. Methods Enzymol. 1999;303:19–44. doi: 10.1016/s0076-6879(99)03004-9. [DOI] [PubMed] [Google Scholar]
- 52.Kawai J, Shinagawa A, Shibata K, Yoshino M, Itoh M, Ishii Y, Arakawa T, Hara A, Fukunishi Y, Konno H, et al. Nature. 2001;409:685–690. doi: 10.1038/35055500. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.