Abstract
Presynaptic ionotropic glutamate receptors are emerging as key players in the regulation of synaptic transmission. Here we identify GluR7, a kainate receptor (KAR) subunit with no known function in the brain, as an essential subunit of presynaptic autoreceptors that facilitate hippocampal mossy fiber synaptic transmission. GluR7−/− mice display markedly reduced short- and long-term synaptic potentiation. Our data suggest that presynaptic KARs are GluR6/GluR7 heteromers that coassemble and are localized within synapses. We show that recombinant GluR6/GluR7 KARs exhibit low sensitivity to glutamate, and we provide evidence that presynaptic KARs at mossy fiber synapses are likely activated by high concentrations of glutamate. Overall, from our data, we propose a model whereby presynaptic KARs are localized in the presynaptic active zone close to release sites, display low affinity for glutamate, are likely Ca2+-permeable, are activated by single release events, and operate within a short time window to facilitate the subsequent release of glutamate.
Keywords: kainate receptors, presynaptic glutamate receptors, short-term plasticity, synaptic plasticity
Ionotropic glutamate receptors can regulate axonal excitability or transmitter release by acting as autoreceptors or heteroreceptors (1). Among these, presynaptic kainate receptors (KARs) play a prominent role in the regulation of synaptic transmission. Their pharmacological activation can either facilitate or depress GABAergic or glutamatergic synaptic transmission in several brain regions (2, 3). In comparison, relatively few reports describe a physiological role for presynaptic KARs activated by synaptically released glutamate. The most compelling example is found at the synapses between mossy fibers (MF) and CA3 pyramidal cells in the hippocampus (MF-CA3 synapses), where KARs contribute to several forms of short- and long-term synaptic plasticity (4–8). A crucial step toward understanding the molecular and biophysical mechanisms by which presynaptic KARs modulate synaptic transmission is to determine the subunit composition of the receptors involved in these processes; at MF-CA3 synapses, this is still a matter of debate. Antagonists for GluR5-containing receptors block the presynaptic action of KARs (refs. 6 and 9, but see ref. 10), leaving postsynaptic KAR-mediated excitatory postsynaptic currents (EPSCs) unaffected. However, these data are at odds with the analysis of GluR5−/− and GluR6−/− mice, because only the latter show altered MF-CA3 synaptic transmission (5, 11). In addition, GluR5 mRNA is not detected in dentate granule cells (12, 13), where MFs originate. These cells, however, express GluR7 mRNA, making it a possible candidate for presynaptic KARs at MF-CA3 synapses. No physiological function is known for GluR7, although it is abundantly expressed in the brain (14). One explanation for the elusive function of GluR7 may reside in the fact that it responds only to high concentrations of glutamate (15), thus questioning the physiological conditions under which native receptors can be activated. Here, we show that GluR7 participates to presynaptic KARs at the MF-CA3 synapse. We propose that, assembled with GluR6, it forms presynaptic receptors that are likely Ca2+-permeable, exhibit low sensitivity to glutamate, and facilitate synaptic plasticity.
Results
GluR7-Containing Presynaptic KARs Facilitate MF Short-Term Synaptic Plasticity.
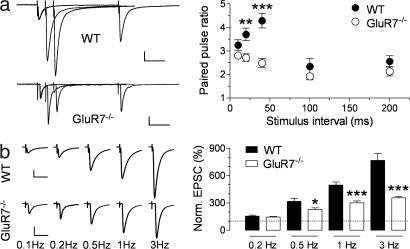
We examined the possible contribution of GluR7 to synaptic transmission at hippocampal MF synapses by the generation and analysis of GluR7−/− mice [supporting information (SI) Fig. 7]. MF EPSCs elicited in GluR7−/− mice displayed a large component mediated by AMPA receptors and a smaller component mediated by KARs that, as expected, did not differ from WT mice (SI Fig. 8), because CA3 pyramidal cells do not express GluR7 (12). Paired-pulse facilitation (PPF), measured at interstimulus intervals (ISIs) ranging from 10 to 200 ms, was markedly smaller in GluR7−/− mice at short ISIs (for 40-ms ISI, 4.3 ± 0.3, n = 14 for wild-type; 2.5 ± 0.2, n = 10 for GluR7−/−; P = 0.0002). Interestingly, at ISIs ≥100 ms, PPF was not altered in GluR7−/− mice (Fig. 1a). These results show that GluR7-containing KARs, in response to a single stimulus, facilitate subsequent MF-EPSCs within a restricted temporal frame. MF-CA3 synapses also display low-frequency facilitation (LFF), which develops over a slower time scale with repetitive stimulation. We tested LFF by increasing the stimulation frequency from a basal rate of 0.1 Hz to higher frequencies (0.2–3 Hz). LFF was reduced at rates of stimulation >0.2 Hz (e.g., 767 ± 75%, n = 8 for WT; 356 ± 15%, n = 8 for GluR7−/− at 3 Hz; P = 0.0001; Fig. 1b) (time courses are shown in SI Fig. 9). Therefore, presynaptic GluR7-containing KARs markedly facilitate PPF and LFF, but the expression of these forms of short-term plasticity (STP) does not absolutely require functional presynaptic KARs. STP was reduced in GluR6−/− mice to the same extent as in GluR7−/− mice [LFF at 1 Hz was 61 ± 4% of WT in GluR7−/− mice and 59 ± 8% in GluR6−/− (n = 10), P > 0.05; PPF at 40-ms ISI was 58 ± 4% of WT in GluR7−/− and 71 ± 6% in GluR6−/− (n = 8), P > 0.05] but was not affected in GluR5−/− mice (not shown), as reported (5). These results indicate that both GluR6 and GluR7 are necessary for the facilitatory action of presynaptic KARs at MF-CA3 synapses.
Fig. 1.
MF short-term synaptic plasticity is impaired in GluR7−/− mice. (a) PPF was found to be significantly reduced in GluR7−/− mice for short ISIs. (Left) Representative traces of averaged MF-EPSCs evoked by paired stimuli delivered at intervals of 20, 40, and 200 ms for WT and GluR7−/− mice. [Scale, 60 pA for WT and 50 pA for GluR7−/− (50 ms).] (Right) Summary graph of paired-pulse ratios at intervals ranging from 10 to 200 ms. ∗∗, P < 0.01; ∗∗∗, P < 0.001. (b) LFF, recorded when increasing the stimulation frequency from a basal value of 0.1 Hz, was also much reduced in GluR7−/− mice. (Left) Representative traces of averaged MF-EPSCs at stimulation frequencies of 0.1–3 Hz for WT and GluR7−/− mice. [Scale, 100 pA for WT and 50 pA for GluR7−/− (25 ms).] (Right) Summary graph of MF-EPSC amplitudes for stimulation frequencies of 0.2–3 Hz normalized to the amplitude of the responses at 0.1 Hz. ∗, P < 0.05; ∗∗∗, P < 0.001.
Long-Term Potentiation (LTP) Is Impaired in GluR7−/− Mice.
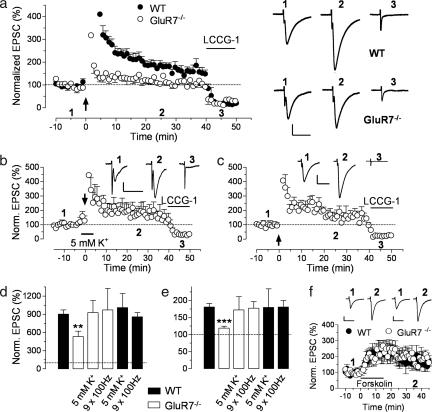
MF LTP is an NMDA receptor-independent presynaptic form of plasticity (16) that depends on Ca2+ entry at the MF terminal (but see ref. 17). KARs play an important role in MF LTP (5, 18), but the identity of the receptors involved also remains controversial. We examined the potential contribution of GluR7 to MF LTP induced by high-frequency stimulation (see SI Text). In the first minute after the last tetanic burst, a significant difference in the magnitude of post-tetanic potentiation (PTP) was observed between the two genotypes (902 ± 71%, n = 12 for WT and 534 ± 86%, n = 9 for GluR7−/−; P = 0.0036; Fig. 2 a and d). LTP was also markedly reduced but not completely abolished in GluR7−/− mice (181 ± 10%, n = 12 for WT and 119 ± 6%, n = 9 for GluR7−/−; P < 0.0001), supporting the notion that KARs play a facilitating rather than an inducting role in MF LTP (18). We thus examined whether LTP could be rescued in GluR7−/− mice under conditions that enhance presynaptic excitability. First, we increased the concentration of extracellular K+ to 5 mM before and during the induction protocol. This caused a slight increase in the amplitude of MF-EPSCs and the tetanus that previously induced partial LTP in GluR7−/− mice now induced a large enhancement of MF-EPSC amplitude (172 ± 39%, n = 7; Fig. 2 b and e). Second, a more robust induction protocol, consisting of nine bursts of 100 Hz instead of 3, also rescued LTP in GluR7−/− mice (177 ± 19%, n = 5; Fig. 2 c and e). Both protocols also restored PTP but had no effect when tested in WT mice (Fig. 2 d and e). Forskolin can directly activate adenylyl cyclases and induce a long-lasting enhancement of MF-EPSCs that occludes burst-induced LTP (19), bypassing the need for synaptic activity. Application of forskolin (10 μM, 15 min) increased MF-EPSCs to the same extent in both genotypes (177 ± 39%, n = 5 for WT and 184 ± 29%, n = 8 for GluR7−/−; P = 0.89; Fig. 2f), indicating that the impairment of MF LTP in GluR7−/− mice occurred upstream of PKA activation.
Fig. 2.
MF PTP and LTP are impaired in the absence of GluR7 but can be restored by increasing excitability. (a) Time course of MF LTP in WT and GluR7−/− mice. Sample traces from recordings in WT and GluR7−/− mice are also shown (Right). (Scale bars, 50 pA × 20 ms.) (b) Time course of MF LTP in slices of GluR7−/− mice, where 5 mM K+ was applied for 5 min before and during the normal induction protocol (arrow). Sample traces for the indicated time points in the graph are also shown. (Scale bars, 20 pA × 20 ms.) (c) Time course of MF LTP in slices of GluR7−/− mice, where the normal induction protocol was repeated three times at 10-s intervals (arrow). Sample traces for the indicated time points in the graph are shown. (Scale bars, 40 pA × 20 ms.) To confirm that only MFs were stimulated, we routinely checked that activation of group II mGluRs (that are present only on MF terminals) with 10 μM [2(s), 1′(S), 2′(s)]-2-)carboxycyclopropyl)glycine (LCCG-I) resulted in a large decrease in EPSC amplitude. In all time courses, the ordinate was limited to <500% for a better appreciation of the LTP and blockade by LCCG-1. (d and e) Summary graphs of MF PTP and LTP, respectively. Impaired PTP and LTP observed in GluR7−/− mice can be restored to the levels found in WT mice by increasing the extracellular K+ concentration or by providing additional stimuli for the induction protocol. ∗∗, P < 0.01; ∗∗∗, P < 0.001. These same protocols produced no change in PTP or LTP in WT mice. (f) The PKA-dependent enhancement of MF-EPSCs by forskolin is not changed in GluR7−/− mice. Sample traces for slices from WT and GluR7−/− mice are shown for the indicated time points. [Scale bars, 50 pA for WT and 25 pA for GluR7−/− (20 ms).]
Subcellular Localization, Coassembly, and Properties of GluR6/GluR7 Receptors.
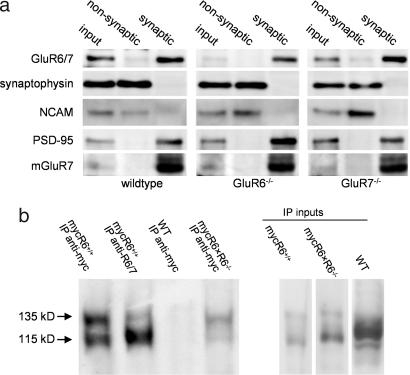
Both GluR6 and GluR7 appear necessary for the facilitatory effects of KARs on MF synaptic transmission, suggesting they may coassemble to form functional presynaptic KARs. We examined whether GluR6 and GluR7 colocalize in synapses using a biochemical fractionation procedure (20). Several synaptic proteins were enriched in fractions corresponding to synaptic junctions isolated from the hippocampi of WT, GluR6−/− and GluR7−/− mice (Fig. 3a; details in figure legend). In the absence of subunit-selective antibodies, we used an anti-GluR6/7 antibody in GluR6−/− and GluR7−/− mice to label GluR7 and GluR6, respectively. These experiments indicate that both subunits are present in synaptic junctions (Fig. 3a). Second, we investigated the coassembly of the two subunits in vivo by coimmunoprecipitation. It is already known that GluR6 and GluR7 can coassemble in recombinant systems (22, 23). In the absence of a suitable anti-GluR7 antibody, we used transgenic mice expressing myc-GluR6a under the control of the α-Ca2+/calmodulin-dependent kinase II (CAMKII) promoter on a GluR6−/− background [myc-GluR6×GluR6−/− mice (24)]. Immunoprecipitation with an anti-myc antibody yielded two bands corresponding to myc-GluR6 (135 kDa) and GluR7 (115 kDa; Fig. 3b), indicating that myc-GluR6 and GluR7 were associated within a heteromeric complex in vivo.
Fig. 3.
Subcellular localization and association of GluR6 and GluR7 subunits. (a) Immunoblots of hippocampal synaptosomal membranes (input) and from nonsynaptic and synaptic fractions prepared from WT, GluR6−/−, and GluR7−/− mice. PSD95, a characteristic protein of the postsynaptic density, was present in the synaptic junctions but excluded from the nonsynaptic fraction. The same separation occurred with the metabotropic receptor mGluR7, which is known to be present almost exclusively within the presynaptic active zone (21). On the contrary, the adhesion molecule NCAM and the synaptic vesicle protein synaptophysin were detected only in the nonsynaptic fraction. Analysis of the protein fractions with an anti-GluR6/7 antibody revealed that, in the absence of the GluR6 subunit, GluR7 is found at synapses, and vice versa. (b) KARs were purified by immunoprecipitation from mouse brains of different genotypes (WT, mycGluR6, and mycGluR6×GluR6−/−), with an anti-myc antibody. Western blots were probed with an anti-GluR6/7 antibody. In brains from mycGluR6×GluR6−/− mice, the upper band (135 kDa) corresponds to the immunoprecipitated transgene product (myc-GluR6a) and the lower band (115 kDa) to GluR7.
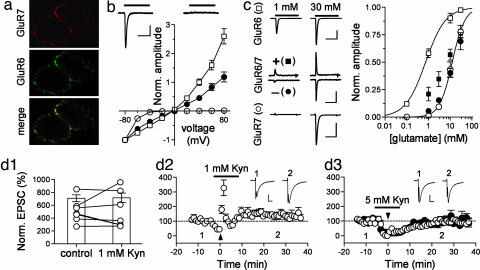
We next examined some properties of recombinant GluR6/GluR7 heteromers that putatively correspond to the presynaptic KARs on MF terminals. When transfected, alone or in combination, in HEK 293 cells, the receptors colocalized at the cell surface (Fig. 4a; see also ref. 23). Homomeric GluR7 receptors desensitized rapidly and completely, as shown (15). Interestingly, current—voltage (I–V) curves showed a very marked inward rectification; at −40 mV, the chord conductance was only 6.9 ± 0.8% of the one at −80 mV (n = 6), and no currents were detected at higher voltages (Fig. 4b). To study GluR6/7 receptors, we cotransfected GluR7a and the edited (R) form of GluR6a that, once incorporated into a receptor, yields an approximately linear I–V curve (25). I–V curves recorded from GluR6/7 transfected cells displayed an almost linear relationship, intermediate between those of GluR6R and GluR7 receptors (Fig. 4b). GluR6 and GluR7 also show very different sensitivities to glutamate (15, 26). We measured an EC50 of 740 ± 50 μM for GluR6 [n = 3, Hill coefficient (h) = 0.95] and 12.4 ± 0.4 mM for GluR7 (n = 6, h = 1.49; Fig. 4c). For GluR6/7 transfected cells, the sensitivity to glutamate, measured at −60 mV, was almost identical to that of GluR7 (Fig. 4c). To eliminate the contribution of homomeric GluR7 receptors we held the cells at +80 mV, at which these receptors do not generate any currents (Fig. 4b). In these conditions, the sensitivity to glutamate was still markedly different from that of GluR6 and closer to GluR7 (Fig. 4c). This shows that the glutamate-activated currents arise mainly from heteromeric GluR6/GluR7 receptors that display low sensitivity to glutamate. Consequently, native receptors composed of GluR6 and GluR7 may be expected to require relatively high concentrations of glutamate for effective activation.
Fig. 4.
Properties of recombinant and native GluR7-containing KARs. (a) When cotransfected in HEK 293 cells, GFP-GluR6 and myc-GluR7 colocalize at the cell surface, as revealed after staining with anti-GFP and anti-myc antibodies. (b) I–V relationships recorded from HEK 293 cells transfected with GluR6R alone (white squares, n = 7), GluR7 alone (white circles, n = 6), or both (black circles, n = 8). Current amplitudes are normalized to values at −80 mV. Traces show currents evoked in a GluR7-transfected cell at −80 mV (Left) and +80 mV (Right). (Scale bars, 50 ms × 200 pA.) (c) Examples of currents evoked by 1 or 30 mM glutamate in cells transfected with one or both subunits (Left). Holding potentials were −40 mV for GluR6 transfected cells, −60 mV (−) and +80 mV (+) for GluR6/7 transfected cells, and −80 mV for GluR7-transfected cells. [Scale bars, 2 nA, GluR6; 100 pA, GluR6/GluR7; and 400 pA, GluR7 (50 ms).] (Right) Concentration-response curves for three to six cells in each group. Symbols are the same as in Left. Black squares correspond to GluR6/GluR7-transfected cells recorded at +80 mV. Data fitting was done with the Hill equation. (d) MF LFF, when shifting stimulation from 0.1 to 1 Hz, is not changed by 1 mM kynurenate (d1). At this concentration, kynurenate does not completely block MF LTP (d2) but is effective in blocking LTP at a higher concentration (5 mM; d3). [Scale bars, 35 pA, d2; 46 pA, d3 (10 ms).]
Kynurenate, a low-affinity competitive antagonist of glutamate receptors whose binding can be displaced by synaptically released glutamate (27) can, at high concentrations, block MF LTP (4) (but see refs. 17 and 28). We readdressed this issue by first testing its effects on LFF. Kynurenate (1 mM) blocked MF-EPSCs by ≈80% (not shown) but did not reduce LFF at 1 Hz (513 ± 69% for control and 519 ± 107% after kynurenate, n = 7; P = 0.93; Fig. 4d1). This same concentration of antagonist reduced but did not completely block LTP (140 ± 11%, n = 7; Fig. 4d2). A higher concentration of kynurenate (5 mM) completely blocked MF LTP (Fig. 4d3), consistent with the hypothesis that the high concentration of glutamate being sensed by presynaptic KARs receptors is able to displace bound kynurenate. Recombinant GluR6/GluR7 receptors were blocked by kynurenate (not shown), ruling out the possibility that the lack of effect of low concentrations of kynurenate on synaptic plasticity was due to a poor block of these receptors.
Insights into the Mechanisms of Action of Presynaptic KARs.
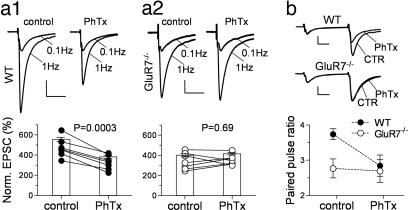
Presynaptic KARs on MF-CA3 synapses may act by depolarizing the nerve terminals (6, 8). It has also been proposed that they are Ca2+-permeable, because their action is blocked by philanthotoxin-433 [PhTx (29)] that selectively targets unedited Ca2+-permeable glutamate receptors (30). We therefore tested whether PhTx affected synaptic plasticity in GluR7−/− mice. To achieve a consistent block of the receptors, we delivered brief trains of stimulation [30 pulses at 50 Hz repeated five times at 2-min intervals (29)] in the presence of PhTx after recording the protocols in control conditions. These trains did not by themselves cause significant changes in synaptic plasticity (not shown). When PhTx (3 μM) was added, it significantly reduced LFF in WT mice (from 474 ± 36% to 327 ± 27%, n = 7; P = 0.0003; Fig. 5a1) without affecting EPSC amplitudes at basal frequency, as reported (31). In contrast, it did not cause any change in the already lower LFF in GluR7−/− mice (from 343 ± 31% to 354 ± 19%, n = 7; P = 0.69; Fig. 5a2). In a similar manner, PPF was significantly reduced by 3 μM PhTx in WT but not GluR7−/− mice (at 40-ms ISI, from 3.7 ± 0.2 to 2.6 ± 0.2 in WT, n = 7; P = 0.02; and from 2.8 ± 0.3 to 2.7 ± 0.3 in GluR7−/−, n = 8; P = 0.81; Fig. 5b). A similar lack of effect of PhTx was found in GluR6−/− mice (SI Fig. 10). These experiments show a selective action of PhTx on GluR6/GluR7-containing presynaptic KARs at MF-CA3 synapses, which are likely Ca2+-permeable. The AMPA receptor/KAR antagonist 6-cyano-7-nitroquinoxaline-2,3-dione (CNQX; 50 μM) was also able to reduce LFF in WT mice but not in GluR7−/− mice when monitoring the NMDA component of MF-EPSCs [LFF at 1 Hz, from 498 ± 37% to 406 ± 33% for WT, n = 7; P = 0.004; and from 402 ± 34% to 423 ± 61% for GluR7−/−, n = 7; P = 0.89 (see SI Fig. 11)].
Fig. 5.
KAR-dependent MF synaptic plasticity is blocked by PhTx. (a) MF LFF, when shifting stimulation from 0.1 to 1 Hz, is significantly reduced by 3 μM PhTx in slices from WT (a1) but not from GluR7−/− mice (a2). [Scale bars, WT, 100 pA × 20 ms; GluR7−/−, 50 pA for control, 36 pA for PhTx (20 ms).] (b) MF PPF, for an ISI of 40 ms, is also significantly reduced by 3 μM PhTx in WT but not in GluR7−/− mice. [Scale bars, 53 pA, WT; 170 pA, GluR7−/− (10 ms).]
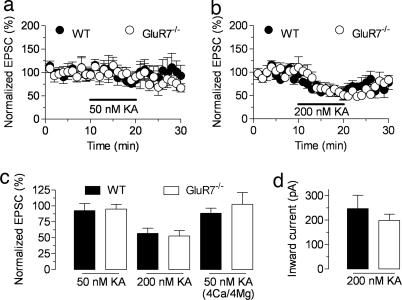
It has been shown that MF synaptic transmission is facilitated by bath application of kainate at “low” concentration (≤50 nM) and depressed by higher doses (8, 32, 33). These effects are lost in GluR6−/− mice, which also display impaired MF synaptic plasticity, suggesting that the same KARs act in a bimodal manner depending on the concentration of agonist (8). Given that GluR7−/− mice display a selective impairment of presynaptic KAR function, we repeated these experiments to further clarify the nature of the receptors involved in the effects of exogenous agonists on MF synaptic transmission. However, and at odds with previous reports, we were unable to observe any consistent facilitatory effect of bath-applied kainate (50 nM) on MF synaptic transmission, even when raising the extracellular Ca2+ concentration (29) (Fig. 6 a and c). This phenomenon was nevertheless apparent in a subset of the recordings (WT, three of 10 cells). However, this was also the case for GluR7−/− mice (three of eight cells). Bath application of 200 nM kainate caused a large decrease in MF-EPSC amplitudes (Fig. 6 b and c) and the activation of an inward current on the recorded CA3 neurons (Fig. 6d) that were not different between genotypes. Thus, presynaptic KARs activated by endogenous glutamate (those involved in homosynaptic plasticity) are likely distinct from the KARs that mediate the effects of bath-applied kainate.
Fig. 6.
GluR7-containing KARs are not involved in the depressing action of kainate on MF-EPSCs. (a and b) Time course of the normalized MF-EPSC amplitude during the application of 50 or 200 nM kainate in the extracellular medium, respectively. Basal stimulation was performed at 0.05 Hz. (c) Summary graph for the changes in MF-synaptic transmission upon application of 50 or 200 nM kainate. In either case, no differences between genotypes are observed, and an enhancing effect of kainate is not apparent even in the presence of higher concentrations of divalent cations (4 mM Ca2+ and 4 mM Mg2+). (d) Kainate (200 nM) activates a postsynaptic inward current, with similar average amplitude in neurons recorded from slices of WT and GluR7−/− mice.
Discussion
GluR7 forms functional recombinant receptors with very low sensitivity to glutamate (15), suggesting it may play a unique role in synaptic transmission. In this study, we show that GluR7 is a key subunit of presynaptic KARs involved in facilitating synaptic transmission at MF-CA3 synapses, and we provide insights into the mechanism of action of these autoreceptors.
Identity of Presynaptic KARs on MF Synapses.
The subunit composition of presynaptic KARs at MF synapses is still debated. It has been reported that the GluR5-selective antagonists LY382884 (4, 6, 29, 34) and UBP302 (9) affect MF-CA3 synaptic plasticity. However, and in substantiation of a previous report (10), we were unable to find any effect of these antagonists on MF LFF by using concentrations of LY382884 or UBP302 up to 50 μM, at which MF-EPSCs were already blocked by ≈50% (data not shown). We also found MF STP to be normal in GluR5−/− mice (data not shown; see also refs. 5 and 10). Instead, in GluR7−/− mice, the function of presynaptic KARs is selectively impaired, without any effects on postsynaptic KARs. In addition, in WT but not in GluR7−/− mice, STP was reduced by PhTx and 6-cyano-7-nitroquinoxaline-2,3-dione, (CNQX), lending further support to the participation of GluR7 to presynaptic KARs. GluR7−/− mice displayed a reduction in synaptic plasticity that resembles the one described for GluR6−/− mice, suggesting a functional interaction between the two subunits. Indeed, we show that GluR6 and GluR7 are associated in vivo. Therefore, our studies clearly favor a crucial role for GluR6/GluR7 receptors in MF synaptic plasticity and not for GluR5. The development of GluR7-selective antagonists, expected to target only presynaptic KARs and still be active in GluR5−/− mice, could help in solving this apparent paradox. The link between KARs involved in homosynaptic plasticity and KARs activated by bath application of kainate is also unclear. It was reported that MF synaptic transmission is facilitated by low concentrations of kainate (≤50 nM) (8, 33) and depressed by higher concentrations (8, 32). Both actions of kainate are absent in GluR6−/− mice (5, 32), along with a reduction of homosynaptic facilitation (5). In contrast, in KA2−/− mice, the facilitation by kainate is lost, whereas homosynaptic facilitation is preserved (33). We found no significant facilitation of MF-EPSCs by kainate both in WT and GluR7−/− mice, despite considerable efforts to approach experimental conditions used by others. On the other hand, inhibition by kainate was preserved in GluR7−/− mice but absent in GluR6−/− mice, although both genotypes show reduced homosynaptic facilitation. Together, these results suggest that the KARs involved in homosynaptic facilitation are distinct from the KARs mediating the effects of bath applied kainate, which are likely composed of GluR6 and KA2.
On the Mechanisms of Action of Presynaptic KARs at MF Synapses.
GluR6−/− mice display both pre- and postsynaptic phenotypes, whereas only presynaptic KAR function is impaired in GluR7−/− mice. These mice thus constitute a valuable tool to study the mechanism of action of presynaptic KARs. PPF is severely reduced in GluR7−/− mice, suggesting that presynaptic KARs containing GluR7 can be activated by single-release events to facilitate subsequent ones. The contribution of KARs to PPF is relevant only at ISIs <100 ms (Fig. 1; see also ref. 5), a timing that tightly coincides with the reported peak presynaptic intraterminal Ca2+ changes (35). This implies that, for short ISIs (<100 ms), presynaptic KARs enhance the classical mechanisms operating for PPF, which are attributed to residual presynaptic Ca2+ (36). The fast time course of action of presynaptic KARs tends to rule out the involvement of a metabotropic process as the primary trigger for synaptic facilitation. In addition to a possible depolarizing action, which could alter resting Ca2+ levels and heavily impact transmitter release (37), presynaptic KARs may also contribute directly to Ca2+ influx through the receptor itself. Given the marked effects of PhTx on synaptic facilitation in WT but not in GluR7−/− mice, we conclude that this toxin acts selectively on GluR7-containing presynaptic KARs, which are, in all probability, Ca2+-permeable. This Ca2+ influx would enhance release by summating with Ca2+ entering the terminals through voltage-gated Ca2+ channels and/or triggering release from intracellular stores (29) (but see ref. 10). It seems likely that a similar mechanism of facilitation of Ca2+ entry by presynaptic KARs also operates in the induction of MF LTP, by lowering the levels of activity required to attain sufficient activation of the Ca2+-dependent adenylyl cyclases implicated in this form of plasticity (38, 39). This is supported by the fact that chemical LTP is normal in GluR7−/− mice and that LTP can be rescued by manipulations that boost Ca2+ entry into the terminals (see also ref. 18). In addition, we found that KAR function can be bypassed in the presence of high extracellular Ca2+ such that short-term facilitation is the same in WT, GluR6−/−, and GluR7−/− mice (SI Fig. 12; see also ref. 29). It should be noted that, although 100-Hz trains of stimulation are routinely used for the induction of MF-LTP, the activity of granule cells in vivo consists of short bursts of action potentials (40).
LFF is another form of STP that develops upon repetitive stimulation and involves CaMKII (41). That PPF was not impaired in GluR7−/− mice at ISIs >100 ms suggests LFF should be unchanged in these mice at such comparatively longer ISIs, which we found not to be the case. This implies that LFF probably relies more on the sheer amount of Ca2+ that enters the terminals at each stimulus (and to which KARs contribute) and on the repetitive nature of the protocol that will allow enough activated CaMKII to exert its action on glutamate release and disfavors a mechanism relying on Ca2+ buildup inside the terminals. The presynaptic action of KARs might also depend on specific interactions with other proteins that require both GluR6 and GluR7 subunits; for example, a close interaction with the protein complexes involved in synaptic release might be important.
Properties and Localization of GluR6/GluR7 Receptors: Possible Implications.
Characterizing the functional properties and subcellular localization of presynaptic KARs, which we identified as putative GluR6/GluR7 heteromers, is an important step toward understanding their mechanism of action. GluR6 and GluR7 are enriched in synaptic junctions and should, therefore, be localized within the active zone at the presynaptic level. Additionally, recombinant GluR6 and GluR7 coassemble and display low sensitivity to glutamate. This suggests that presynaptic KARs on MF synapses are localized close to glutamate release sites, where they would sense concentrations of glutamate high enough for their activation, because vesicular glutamate is estimated to be as high as 60–210 mM (42). This concurs with the need of high concentrations of the low-affinity competitive antagonist kynurenate to block MF LTP (see also ref. 4). However, previous studies reported that high kynurenate could not block the induction of MF-LTP (28) (see also ref. 17). A plausible explanation is that, in these studies, very low (or no) EGTA was used in the intracellular solution. In fact, in the study by Yeckel et al. (17), LTP induced by the same protocol as in our study is blocked by kynurenate when 1 mM 1,2-bis(2-aminophenoxy)ethane-N,N,N′,N′-tetraacetate (BAPTA) is included in the recording pipette, suggesting that another form of LTP was probably being recorded with low postsynaptic Ca2+ buffering.
The overall reduced facilitation of MF synaptic transmission in the absence of functional presynaptic KARs may cause a severe reduction in spike transmission at MF-CA3 pyramidal cell synapses. This may lead to impairment in the pattern of activity of place cells and, hence, in the definition of place fields in the CA3 region, that should impact behaviorally relevant tasks relying on hippocampal-dependent forms of learning. Genetic data suggest that GluR7 may be implicated in complex neurological conditions, such as recurrent major depressive disorder (43). This should fuel the study of the functions of GluR7 in a more widespread manner and possibly open new therapeutic opportunities.
Methods
Subcellular Fractionation.
Synaptic junctions were prepared as described (20) with slight modifications (44). Briefly, the hippocampi from six to eight mice were homogenized at 4°C in 3 ml of isolation solution (320 mM sucrose, 0.1 mM CaCl2, 1 mM MgCl2, and 0.1 mM phenylmethylsulfonyl fluoride). The concentration of sucrose was raised to 1.25 M, and the suspension was divided into ultracentrifuge tubes. The homogenate was overlaid with 1.0 M sucrose/0.1 mM CaCl2 and centrifuged (100,000 × gmax, 3 h at 4°C). Synaptosomes were collected at the 1.25/1.0 M sucrose interface, diluted 1:10 in cold 0.32 M sucrose with 0.1 mM CaCl2, and pelleted (15,000 × gmax, 30 min, 4°C). Synaptosomes were then diluted 1:10 in ice-cold 0.1 mM CaCl2 and added to an equal volume of solubilization buffer (2% Triton X-100/40 mM Tris, pH 6.0). Membranes were incubated for 30 min on ice with mild agitation, and synaptic junctions were pelleted by centrifugation (40,000 × gmax, 30 min at 4°C). Proteins in the supernatant (nonsynaptic proteins) were precipitated with acetone (6 volumes at −20°C) and recovered by centrifugation (18,000 × gmax, 30 min at −10°C). The proteins were resuspended in 5% SDS and quantified by the bicinchoninic acid method. Samples were further treated with SDS/PAGE sample buffer and analyzed by Western blotting (see the list of antibodies in SI Text). Immunoprecipitation experiments were performed by using a standard protocol (24) (details are in SI Text).
Electrophysiological Recordings from CA3 Pyramidal Cells.
Experiments were performed on parasaggital hippocampal slices from 14- to 21-day-old WT and GluR7−/− mice by using standard techniques (45). Whole-cell voltage–clamp recordings (3.5- to 4.5-MΩ electrodes, −70 mV holding potential) were made at room temperature (22–24°C) from pyramidal cells of the CA3 field visualized by infrared videomicroscopy. Slices were perfused with extracellular solution composed of 125 mM NaCl, 2.5 mM KCl, 1.25 mM NaH2PO4, 26 mM NaHCO3, 2.3 mM CaCl2, 1.3 mM MgCl2, and 25 mM glucose saturated with 95% O2/5% CO2. Bicuculline (10 μM) was added to the bath to block GABAA receptors. The intracellular solution was composed of: 122 mM CsCl, 10 mM Hepes, 10 mM EGTA, 2 mM MgCl2, 2 mM NaCl, and 4 mM Na2ATP, pH 7.3. EPSCs in CA3 pyramidal cells were evoked by stimulation of MFs by using a patch pipette filled with a Hepes-based extracellular solution placed in the hilus or in the stratum lucidum, in the vicinity of the recorded cell. Minimal intensities of stimulation were used to limit indirect activation of non-MFs (45).
Experiments on Recombinant Receptors.
HEK 293 cells were transfected and recorded as described (46). Cells were cotransfected with GluR6a or GluR7a subunits and GFP at a cDNA ratio of 2:1. To study heteromeric GluR6/GluR7 receptors, GluR7a, and the edited form of GluR6a (GluR6aR) were cotransfected with GFP at a ratio of 1:3:2. One day later, cells were bathed in a solution at room temperature containing 145 mM NaCl, 2 mM KCl, 2 mM MgCl2, 2 mM CaCl2, 10 mM glucose, and 10 mM Hepes (pH 7.4, 320 mOsm per liter). Whole-cell recordings were performed on fluorescent cells held at −80 to −40 mV. Recording pipettes (resistance 2–4 MΩ) were filled with a solution containing 122 mM CsCl, 2 mM NaCl, 2 mM MgCl2, 10 mM EGTA, 10 mM Hepes, 4 mM Na2ATP, and 0.06 mM spermine (pH 7.2, 310 mOsm per liter) (detailed in SI Text).
For immunocytochemical experiments, HEK 293 cells were transfected with GFP-tagged GluR6a and myc-tagged GluR7a. Detection of surface receptors was performed as described (23) (detailed in SI Text).
Statistics.
Results are presented as mean ± SEM of n cells from at least three to four different mice, where applicable. Comparison between groups was performed by using the paired or unpaired two-tailed t test, as adequate.
Supplementary Material
Acknowledgments
We thank Elisabeth Normand and Alice Vimeney for technical assistance and Frédéric Jaskolski for immunocytochemical experiments. This work was supported by grants from the Centre National de la Recherche Scientifique, the Ministère de la Recherche of France, the Conseil Régional d'Aquitaine, the European Commission (EUSynapse contract LSHM-CT-2005-019055, to C.M.), and the National Institutes of Health [National Institute of Neurological Disorders and Stroke (S.F.H.)]. P.S.P. was supported by Fundação para a Ciência e a Tecnologia (Grants FEDER SFRH/BD/5319/2001 and POCTI/FCB/46804/2002), a Federation of European Biochemical Societies (FEBS) Short-Term Fellowship, and a grant from Fundação Calouste Gulbenkian. GluR7-deficient mice were generated at The Salk Institute in the laboratory of S.F.H. by C.M., B.B., J.B., and J.R.M.
Abbreviations
- KAR
kainate receptor
- MF
mossy fiber
- EPSC
excitatory postsynaptic current
- PPF
paired-pulse facilitation
- ISI
interstimulus interval
- LFF
low-frequency facilitation
- STP
short-term plasticity
- LTP
long-term potentiation
- PTP
post-tetanic potentiation
- PhTx
philanthotoxin-433
- I–V
current–voltage.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0608891104/DC1.
References
- 1.Engelman HS, MacDermott AB. Nat Rev Neurosci. 2004;5:135–145. doi: 10.1038/nrn1297. [DOI] [PubMed] [Google Scholar]
- 2.Huettner JE. Prog Neurobiol. 2003;70:387–407. doi: 10.1016/s0301-0082(03)00122-9. [DOI] [PubMed] [Google Scholar]
- 3.Lerma J. Nat Rev Neurosci. 2003;4:481–495. doi: 10.1038/nrn1118. [DOI] [PubMed] [Google Scholar]
- 4.Bortolotto ZA, Clarke VR, Delany CM, Parry MC, Smolders I, Vignes M, Ho KH, Miu P, Brinton BT, Fantaske R, et al. Nature. 1999;402:297–301. doi: 10.1038/46290. [DOI] [PubMed] [Google Scholar]
- 5.Contractor A, Swanson G, Heinemann SF. Neuron. 2001;29:209–216. doi: 10.1016/s0896-6273(01)00191-x. [DOI] [PubMed] [Google Scholar]
- 6.Lauri SE, Bortolotto ZA, Bleakman D, Ornstein PL, Lodge D, Isaac JT, Collingridge GL. Neuron. 2001;32:697–709. doi: 10.1016/s0896-6273(01)00511-6. [DOI] [PubMed] [Google Scholar]
- 7.Schmitz D, Frerking M, Nicoll RA. Neuron. 2000;27:327–338. doi: 10.1016/s0896-6273(00)00040-4. [DOI] [PubMed] [Google Scholar]
- 8.Schmitz D, Mellor J, Nicoll RA. Science. 2001;291:1972–1976. doi: 10.1126/science.1057105. [DOI] [PubMed] [Google Scholar]
- 9.More JC, Nistico R, Dolman NP, Clarke VR, Alt AJ, Ogden AM, Buelens FP, Troop HM, Kelland EE, Pilato F, et al. Neuropharmacology. 2004;47:46–64. doi: 10.1016/j.neuropharm.2004.03.005. [DOI] [PubMed] [Google Scholar]
- 10.Breustedt J, Schmitz D. J Neurosci. 2004;24:10093–10098. doi: 10.1523/JNEUROSCI.3078-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mulle C, Sailer A, Perez-Otano I, Dickinson-Anson H, Castillo PE, Bureau I, Maron C, Gage FH, Mann JR, Bettler B, et al. Nature. 1998;392:601–605. doi: 10.1038/33408. [DOI] [PubMed] [Google Scholar]
- 12.Wisden W, Seeburg PH. J Neurosci. 1993;13:3582–3598. doi: 10.1523/JNEUROSCI.13-08-03582.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bureau I, Bischoff S, Heinemann SF, Mulle C. J Neurosci. 1999;19:653–663. doi: 10.1523/JNEUROSCI.19-02-00653.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bettler B, Egebjerg J, Sharma G, Pecht G, Hermans-Borgmeyer I, Moll C, Stevens CF, Heinemann S. Neuron. 1992;8:257–265. doi: 10.1016/0896-6273(92)90292-l. [DOI] [PubMed] [Google Scholar]
- 15.Schiffer HH, Swanson GT, Heinemann SF. Neuron. 1997;19:1141–1146. doi: 10.1016/s0896-6273(00)80404-3. [DOI] [PubMed] [Google Scholar]
- 16.Harris EW, Cotman CW. Neurosci Lett. 1986;70:132–137. doi: 10.1016/0304-3940(86)90451-9. [DOI] [PubMed] [Google Scholar]
- 17.Yeckel MF, Kapur A, Johnston D. Nat Neurosci. 1999;2:625–633. doi: 10.1038/10180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schmitz D, Mellor J, Breustedt J, Nicoll RA. Nat Neurosci. 2003;6:1058–1063. doi: 10.1038/nn1116. [DOI] [PubMed] [Google Scholar]
- 19.Weisskopf MG, Castillo PE, Zalutsky RA, Nicoll RA. Science. 1994;265:1878–1882. doi: 10.1126/science.7916482. [DOI] [PubMed] [Google Scholar]
- 20.Phillips GR, Huang JK, Wang Y, Tanaka H, Shapiro L, Zhang W, Shan WS, Arndt K, Frank M, Gordon RE, et al. Neuron. 2001;32:63–77. doi: 10.1016/s0896-6273(01)00450-0. [DOI] [PubMed] [Google Scholar]
- 21.Dalezios Y, Lujan R, Shigemoto R, Roberts JD, Somogyi P. Cereb Cortex. 2002;12:961–974. doi: 10.1093/cercor/12.9.961. [DOI] [PubMed] [Google Scholar]
- 22.Cui C, Mayer ML. J Neurosci. 1999;19:8281–8291. doi: 10.1523/JNEUROSCI.19-19-08281.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jaskolski F, Normand E, Mulle C, Coussen F. J Biol Chem. 2005;280:22968–22976. doi: 10.1074/jbc.M413166200. [DOI] [PubMed] [Google Scholar]
- 24.Coussen F, Normand E, Marchal C, Costet P, Choquet D, Lambert M, Mege RM, Mulle C. J Neurosci. 2002;22:6426–6436. doi: 10.1523/JNEUROSCI.22-15-06426.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kohler M, Burnashev N, Sakmann B, Seeburg PH. Neuron. 1993;10:491–500. doi: 10.1016/0896-6273(93)90336-p. [DOI] [PubMed] [Google Scholar]
- 26.Heckmann M, Bufler J, Franke C, Dudel J. Biophys J. 1996;71:1743–1750. doi: 10.1016/S0006-3495(96)79375-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Diamond JS, Jahr CE. J Neurosci. 1997;17:4672–4687. doi: 10.1523/JNEUROSCI.17-12-04672.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Castillo PE, Weisskopf MG, Nicoll RA. Neuron. 1994;12:261–269. doi: 10.1016/0896-6273(94)90269-0. [DOI] [PubMed] [Google Scholar]
- 29.Lauri SE, Bortolotto ZA, Nistico R, Bleakman D, Ornstein PL, Lodge D, Isaac JT, Collingridge GL. Neuron. 2003;39:327–341. doi: 10.1016/s0896-6273(03)00369-6. [DOI] [PubMed] [Google Scholar]
- 30.Fletcher EJ, Lodge D. Pharmacol Ther. 1996;70:65–89. doi: 10.1016/0163-7258(96)00014-9. [DOI] [PubMed] [Google Scholar]
- 31.Toth K, Suares G, Lawrence JJ, Philips-Tansey E, McBain CJ. J Neurosci. 2000;20:8279–8289. doi: 10.1523/JNEUROSCI.20-22-08279.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Contractor A, Swanson GT, Sailer A, O'Gorman S, Heinemann SF. J Neurosci. 2000;20:8269–8278. doi: 10.1523/JNEUROSCI.20-22-08269.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Contractor A, Sailer AW, Darstein M, Maron C, Xu J, Swanson GT, Heinemann SF. J Neurosci. 2003;23:422–429. doi: 10.1523/JNEUROSCI.23-02-00422.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lauri SE, Delany C, VR J. C., Bortolotto ZA, Ornstein PL, Isaac TR, Collingridge GL. Neuropharmacology. 2001;41:907–915. doi: 10.1016/s0028-3908(01)00152-6. [DOI] [PubMed] [Google Scholar]
- 35.Kamiya H, Ozawa S, Manabe T. J Neurosci. 2002;22:9237–9243. doi: 10.1523/JNEUROSCI.22-21-09237.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zucker RS, Regehr WG. Annu Rev Physiol. 2002;64:355–405. doi: 10.1146/annurev.physiol.64.092501.114547. [DOI] [PubMed] [Google Scholar]
- 37.Awatramani GB, Price GD, Trussell LO. Neuron. 2005;48:109–121. doi: 10.1016/j.neuron.2005.08.038. [DOI] [PubMed] [Google Scholar]
- 38.Villacres EC, Wong ST, Chavkin C, Storm DR. J Neurosci. 1998;18:3186–3194. doi: 10.1523/JNEUROSCI.18-09-03186.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wang H, Pineda VV, Chan GC, Wong ST, Muglia LJ, Storm DR. J Neurosci. 2003;23:9710–9718. doi: 10.1523/JNEUROSCI.23-30-09710.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Henze DA, Wittner L, Buzsaki G. Nat Neurosci. 2002;5:790–795. doi: 10.1038/nn887. [DOI] [PubMed] [Google Scholar]
- 41.Salin PA, Scanziani M, Malenka RC, Nicoll RA. Proc Natl Acad Sci USA. 1996;93:13304–13309. doi: 10.1073/pnas.93.23.13304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Clements JD. Trends Neurosci. 1996;19:163–171. doi: 10.1016/s0166-2236(96)10024-2. [DOI] [PubMed] [Google Scholar]
- 43.Schiffer HH, Heinemann SF. Am J Med Genet B Neuropsychiatr Genet. 2006;144:20–26. doi: 10.1002/ajmg.b.30374. [DOI] [PubMed] [Google Scholar]
- 44.Pinheiro PS, Rodrigues RJ, Silva AP, Cunha RA, Oliveira CR, Malva JO. Neurosci Lett. 2003;336:97–100. doi: 10.1016/s0304-3940(02)01217-x. [DOI] [PubMed] [Google Scholar]
- 45.Marchal C, Mulle C. J Physiol. 2004;561:27–37. doi: 10.1113/jphysiol.2004.069922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Coussen F, Perrais D, Jaskolski F, Sachidhanandam S, Normand E, Bockaert J, Marin P, Mulle C. Neuron. 2005;47:555–566. doi: 10.1016/j.neuron.2005.06.033. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.