Abstract
BACKGROUND
Orthostatic hypotension (OH) is a common finding among older patients. The impact of OH on mortality is unknown.
OBJECTIVE
To study the long-term effect of OH on total and cardiovascular mortality.
PATIENTS AND METHODS
A total of 471 inpatients (227 males and 244 females), with a mean age of 81.5 years who were hospitalized in an acute geriatric ward between the years 1999 and 2000 were included in the study. Orthostatic tests were performed 3 times during the day on all patients near the time of discharge. Orthostatic hypotension was defined as a fall of at least 20 mmHg in systolic blood pressure (BP) and/or 10 mmHg in diastolic BP upon assuming an upright posture at least twice during the day. Patients were followed until August 31, 2004. Mortality data were taken from death certificates.
RESULTS
One hundred and sixty-one patients (34.2%) experienced OH at least twice. Orthostatic hypotension had no effect on all cause and cause specific mortality. Over a follow-up of 3.47±1.87 years 249 patients (52.8%) had died 83 of whom (33.3%) had OH. Age-adjusted mortality rates in those with and without OH were 13.4 and 15.7 per 100 person-years, respectively. Cox proportional hazards model analysis demonstrated that male gender, age, diabetes mellitus, and congestive heart failure increased and high body mass index decreased total mortality.
CONCLUSIONS
Orthostatic hypotension is relatively common in elderly patients discharged from acute geriatric wards, but has no impact on vascular and nonvascular mortality.
Keywords: orthostatic hypotension, elderly, mortality
Orthostatic hypotension (OH) is a common clinical finding in old people. Its prevalence ranges from 5% to 50% 1, 2 and may even encompass more then 67% among hospitalized old patients. 3 The elderly are especially prone to various kinds of postural blood pressure (BP) drops because of both physiological and pathophysiological processes. 4 A low body mass index, 5, 6 high BP, Parkinson disease, stroke, transient ischemic attacks, and diabetes mellitus are all associated with OH. 7 Medications, volume depletion, immobility, and autonomic insufficiency may also contribute to OH. 8 Its clinical consequences vary from lack of symptoms through lightheadedness, dizziness, falls, syncope, or even coronary and cerebrovascular events. 9– 12 A few aspects of the clinical relevance of OH are under debate. One such issue relates to the effect of OH on the cognitive status of the elderly. 13– 16
The impact of OH on mortality is unclear; some claim that in certain circumstances OH even in lower than the consensus values might increase mortality, 7, 17, 18 while others have not found any cause and effect at all. 2, 19
We therefore decided to study the mortality rate among a cohort of patients who were evaluated for OH between the years 1999 and 2000 and were followed for 3.47±1.87 years.
PATIENTS AND METHODS
Study Population
Between the years 1999 and 2000 we performed orthostatic tests on 502 consecutive patients who were hospitalized in an acute 25-bed geriatric ward in a community hospital serving an area of 250,000 citizens. Patients were included in the study if they were at least 60 years old, able to get out of bed alone or with minor assistance, and able to stand up for at least 5 minutes. Most patients came from either private or sheltered homes where they led independent lives and had the standard health insurance applied by the law in Israel. Patients were studied during their convalescence, 3 days before their planned discharge from the hospital. The study was approved by the local Helsinki committee. As the study did not include any active intervention we obtained only an oral consent from the patients.
Patients were followed until August 31, 2004. Records on mortality were obtained from the day of the orthostatic test until the end of the follow-up period.
Data Collection
For each of the patients the following parameters were recorded; age, height, weight, body mass index (BMI), ethnicity or origin, reason for admission, diagnoses, and a list of the main drugs used.
Mortality data from the January 1, 1999 to August 31, 2004 were taken from the Bureau of Population Registry of the Ministry of Interior and the causes of death were taken from the medical records and death certificates.
Out of the 502 patients originally included in the study, 31 patients were lost to follow-up and therefore only 471 patients who had their entire database completed were enrolled in the present study. The baseline characteristics of those who were excluded were similar to the 471 patients who were included in the study.
BP Measurements and Orthostatic Test
Blood pressure and heart rate were measured with a device (Vital Signs Monitor 52 NTP model, Welch Allyn Protocol, Inc., Beaverton, OR) that was checked every day for accuracy against a mercury sphygmomanometer.
Measurements were taken in the supine and the standing positions 3 times a day, 30 minutes after meals beginning 3 days before planned discharge. The BP was measured in the supine position after at least 5 minutes of complete bed rest, and then after 2 minutes of standing. The BP was measured twice, 1 minute apart, in each position and the average of the 2 measurements was recorded.
Orthostatic hypotension was defined as a fall of at least 20 mmHg in systolic BP (SBP) and/or 10 mmHg in diastolic BP (DBP) upon assuming an upright posture at least twice during the day. 3
Statistical Analysis
The results are reported as mean±SD. Age-adjusted mortality rates, expressed per 100 person-years of follow-up, were calculated according to OH status. Kaplan-Meier's curves were used for 1-way comparison of survival for OH and non-OH patients (while using log-rank and Wilcoxon tests for group's comparison). Survival of OH and non-OH patients was also compared using Cox proportional hazard regression model, adjusting for additional factors such as age, gender, diabetes mellitus, hypertension, BMI, ischemic heart disease (IHD), congestive heart failure (CHF), smoking, Parkinsonism, and medications used. In addition, 2 indicator variables were built to identify patients with more than 1 chronic disease and patients who consumed more than 1 type of medication. The results are presented as hazard ratios (HR) and 95% confidence intervals (CI). All independent variables, including all 2-way interactions were entered/withdrawn from the model according to stepwise method. The variable was entered into the model if it made a significant contribution at the .15 level of significance, and it was removed, if after subsequent addition of other variables to the model, it no longer made a contribution at the .05 level of significance. Age, BMI, SBP, and DBP were defined as continuous variables, diseases presence/medications use were define as dichotomous variables.
The proportional hazards assumptions were tested using Baseline Empirical Cumulative Hazards Function Ĥ0t. The influence of the same factors was also tested by using logistic regression models.
Continuous variables were compared by using the Student's t-test/analysis of variance, and categorical variables by using χ2 test. Because of multiple comparisons, we applied Bonferoni correction for significance level. A P-value <.0023 indicated a significance level of .95.
RESULTS
Study Population
The study population included 471 patients (227 men) with a mean age of 81.5 years. Hypertension and IHD were the most common underlying diseases, and most patients used diuretics, angiotensin-converting enzyme inhibitors, and calcium antagonists. Of those 161 patients (34.2%) had OH at least twice during the initial evaluation. Patients who experienced and those who did not experience OH were similar in regard to age, BMI, history of IHD, diabetes mellitus, chronic lung disease, Parkinsonism, and medications (Table 1). Patients with OH had less CHF but were more likely to be hypertensive and had higher baseline supine BP levels than those without OH (Table 1). The most common reasons for admissions were stroke (24%), infections (18%), weakness (11%), CHF (5%), and syncope (3.6%). The prevalence of OH was unrelated to the reason for admission.
Table 1.
Characteristics of Patients With and Without OH
| Characteristics | Without OH | With OH (n = 161) | P-Value |
|---|---|---|---|
| Mean±SD | Mean±SD | ||
| Age (y) | 81.4±6.9 | 81.7±6.7 | .69 |
| Gender (male/female) | 139/171 | 88/73 | .05 |
| BMI (kg/m2) | 25.3±4.5 | 24.8±4.5 | .26 |
| Supine morning SBP (mmHg) | 145±24 | 150±25 | .03 |
| Supine morning DBP (mmHg) | 71±14 | 75±16 | .008 |
| Supine morning HR (beats/min) | 77±14 | 76±14 | .23 |
| Comorbid diseases | |||
| Hypertension | 184 (59.4%) | 111 (68.9%) | .045 |
| Ischemic heart disease | 176 (56.7%) | 91 (56.5%) | 1.0 |
| Stroke | 91 (29.2%) | 59 (36.6%) | .12 |
| Congestive heart failure | 109 (35.3%) | 38 (23.3%) | .01 |
| Diabetes mellitus | 88 (28.4%) | 50 (31.1%) | .6 |
| Chronic lung disease | 69 (22.4%) | 37 (23%) | .9 |
| Parkinsonism | 36 (11.7%) | 24 (14.9%) | .4 |
| Patients with more than 1 disease | 203(65.5%) | 112 (69.6%) | .37 |
| Medications used | |||
| Diuretics | 114 (36.8%) | 47 (29.2%) | .10 |
| ACE inhibitors | 102 (32.9%) | 46 (28.6%) | .4 |
| Calcium antagonists | 90 (29%) | 49 (30.4%) | .75 |
| Nitrates | 89 (28.7%) | 42 (26.1%) | .59 |
| β-blockers | 52 (16.8%) | 33 (20.5%) | .32 |
| Sleeping pills | 89 (28.7%) | 39 (24.2%) | .33 |
| Patients with more than 1 medication | 167 (53.9%) | 78 (48.4%) | .26 |
BMI, body mass index; OH, orthostatic hypotension; ACE, angiotensin converting enzyme; SBP, systolic blood pressure; DBP, diastolic blood pressure; HR, heart rate
Mortality Rate
There were a total of 249 deaths in the entire cohort over a mean follow-up of 3.47±1.87 years (range 1.6 to 5.34 years). The mortality rate was 15.3 per 100 person-years. Those who died and those who remained alive were similar in regard to age, baseline supine morning BP and heart rate, hypertension, stroke, chronic lung disease, and medications (Table 2). Those who died had lower BMI and were more likely to be men and to have IHD, CHF, diabetes mellitus, Parkinsonism, and more than 1 disease (Table 2).
Table 2.
Characteristics of Survivors and Nonsurvivors
| Characteristics | Alive (n = 222) | Died (n = 249) | P-Value |
|---|---|---|---|
| Mean±SD | Mean±SD | ||
| Age (y) | 79.4 (6.5) | 83.4 (6.6) | .4 |
| Gender (male/female) | 94/128 | 133/116 | .02 |
| BMI (kg/m2) | 26.3 (4.55) | 24.2 (4.16) | .0001 |
| Supine morning SBP (mm Hg) | 148 (24.2) | 145 (24.4) | .98 |
| Supine morning DBP (mmHg) | 72.8 (13.7) | 71.5 (15.4) | .92 |
| Supine morning HR (beats/min) | 75.7 (13.7) | 77.5 (13.6) | .18 |
| Comorbid diseases | |||
| Orthostatic hypotension | 78 (35.1%) | 83 (33.3%) | .67 |
| Hypertension | 142 (64%) | 156 (63%) | .7 |
| Ischemic heart disease | 115 (52%) | 153 (61%) | .03 |
| Stroke | 69 (31%) | 83 (33%) | .6 |
| Congestive heart failure | 53 (24%) | 94 (38%) | .0015 |
| Diabetes mellitus | 56 (25%) | 84 (34%) | .04 |
| Chronic lung disease | 42 (19%) | 64 (26%) | .1 |
| Parkinsonism | 17 (8%) | 44 (18%) | .0014 |
| Patients with more than 1 disease | 130 (58.6%) | 185 (74%) | .0004 |
| Medications used | |||
| Diuretics | 69 (31%) | 94 (38%) | .15 |
| ACE inhibitors | 66 (30%) | 83 (33%) | .4 |
| Calcium antagonists | 68 (31%) | 75 (30%) | .9 |
| Nitrates | 53 (24%) | 80 (32%) | .05 |
| β-blockers | 48 (22%) | 37 (15%) | .07 |
| Sleeping pills | 61 (27%) | 70 (28%) | .9 |
| Patients with more than 1 medication | 115 (51.8%) | 130 (52.2%) | .93 |
BMI, body mass index; OH, orthostatic hypotension; ACE, angiotensin converting enzyme; SBP, systolic blood pressure; DBP, diastolic blood pressure; HR, heart rate
The main causes for mortality were cardiovascular diseases, sepsis, and malignancy. Women died less from cardiovascular reasons (Hazard ratio [HR] = 0.68; CI [0.47 to 0.98], P = .036]. As for some patients we identified more than 1 cause of death we have 291 causes for 249 deaths. In 127 deaths (51%) cardiovascular causes were recorded, in 111 deaths (44.6%) sepsis was recorded and in 34 deaths (13.6%) malignancy was recorded.
Effect of OH on Mortality
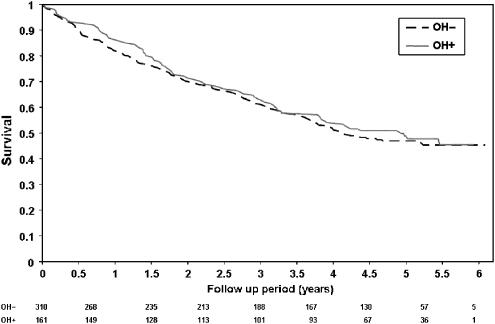
Total mortality rate was the same in those who experienced and those who did not experience OH. Of those who had OH, 83 patients (52%) died, and of those who did not have OH 166 (54%) died during the follow-up period (P = .7). The age-adjusted mortality rate was 13.4 and 15.7 per 100 person-years in those with and those without OH, respectively. Of those who died, 83 (33.3%) had OH and of those who remained alive 78 (35.1%) had OH (Table 2; P = .67). Kaplan-Meier survival analysis according to OH status showed a similar mortality in those with and without OH (Fig. 1; P = .44). Patients without OH died more than those with OH from CHF (P = .009). A multivariate Cox proportional hazards model analysis demonstrated that male gender, age and diabetes mellitus increased, and high BMI decreased total mortality (Table 3), whereas OH, hypertension, and other diseases or drug used had no effect on mortality (Table 3).
FIGURE 1.
Probability of mortality by orthostatic hypotension (OH) status. (OH−, without OH; OH+, with OH). Kaplan-Meier curves for 3.47 years total mortality rate in association with OH. (Wilcoxon test, P = .44, Log-rank test, P = .56). Rows indicate the number of persons under observation at each time period.
Table 3.
Factors Affecting Survival (Cox Proportional Hazards Model)
| Variable | Hazard Ratio | 95% Confidence Interval |
|---|---|---|
| Male gender* | 1.54 | 1.14 to 2.05* |
| Diabetes mellitus* | 1.58 | 1.15 to 2.15* |
| Age (1 y) | 1.08 | 1.05 to 1.10† |
| BMI (1 kg/m2) | 0.90 | 0.88 to 0.94† |
| CHF | 1.40 | 0.98 to 2.02 |
| OH | 0.96 | 0.72 to 1.28 |
| Hypertension | 1.22 | 0.84 to 1.79 |
| IHD | 0.98 | 0.67 to 1.40 |
| Smoking | 0.83 | 0.55 to 1.20 |
| Stroke | 0.97 | 0.72 to 1.30 |
| Chronic lung disease | 1.34 | 0.95 to 1.89 |
| Parkinsonism | 1.36 | 0.94 to 1.93 |
| More than 1 disease | 0.99 | 0.61 to 1.62 |
| Medications used | ||
| Diuretics | 1.19 | 0.81 to 1.75 |
| ACE inhibitors | 0.92 | 0.60 to 1.35 |
| Calcium antagonists | 0.99 | 0.70 to 1.39 |
| Nitrates | 1.32 | 0.90 to 1.88 |
| β-blockers | 0.82 | 0.54 to 1.23 |
| Sleeping pills | 1.03 | 0.74 to 1.42 |
| More than 1 medication | 0.94 | 0.58 to 1.55 |
BMI, body mass index; CHF, congestive heart failure; OH, orthostatic hypotension; IHD, ischemic heart disease
P ≤ .005
P <.0001
DISCUSSION
In our present study we did not find an increase in the 3.47-year risk of all cause and cause-specific mortality in patients who experienced OH while hospitalized in an acute geriatric ward. Orthostatic hypotension was diagnosed in 34.2% of our patients and was the same in those who died and in those who remained alive. This is a relatively high rate of OH reflecting a high-risk population with a high rate of concomitant diseases. Very few studies evaluated the impact of OH on mortality in elderly subjects. 7, 17, 18 Masaki et al. 17 found OH in 10.2% of the subjects older than 85 years. Luukinen et al. 7 found OH in 30% of the subjects, but they examined the subjects on 2 separate days at an average interval of 3 months. Sasaki et al. 18 found OH in 42% of the patients, but they examined patients with chronic renal failure. It is well known that the reproducibility of OH is low and the rate may vary during the day. 3, 20 Therefore more than 1 examination should be performed before a diagnosis of OH can be established. We performed orthostatic tests 3 times during the day and we included in the present analysis only those who exhibited OH at least twice. Some other studies that evaluated the effect of OH on mortality defined OH according to only 1 observation. 7, 17
In our cohort, OH did not predict all-cause and cause-specific mortality. Several studies found that OH is an independent predictor of mortality. 7, 17, 18 In one study, diastolic OH after 1 minute and systolic OH after 3 minutes were associated with excess vascular death but not with nonvascular mortality. 7 Masaki et al. 17 found a significant relationship between OH and total mortality among 3,741 elderly Japanese men, but they did not evaluate cause-specific mortality. Sasaki et al. 18 found that OH at the introductory phase of hemodialysis was an independent predictor of all-cause mortality.
Unlike these findings, 2 Finnish studies which also investigated community dwelling elders, found no correlation between OH and mortality. 2, 19 Similarly, Hossain et al. 21 failed to show a higher mortality rate among nursing home residents with OH. 21 The inconsistent results may be related to the type of subjects included in the various studies. Our cohort included very high-risk patients as the mortality rate was 15.3 per 100 person-years in comparison with only 3.86 to 11.2 per 100 person-years in some other studies. 7, 17, 18, 21, 22 The patients in our cohort were very old, relatively sick, and probably less mobile than the subjects studied by others. 7, 17 As a result they were less exposed to the deleterious effects of OH like falls and their consequences. This may explain, at least in part, why OH did not affect mortality in our cohort.
Alternatively, the setting in which the orthostatic test was performed was unique, as our patients were recovering from an acute disease. This may affect the occurrence of OH and may explain the high frequency of OH found among our population. 3 We cannot exclude the fact that some of our patients did not have OH after their discharge. The effect of hospitalization on OH was described in a small study in a rehabilitation unit. In this study OH was found upon admission in 37 old patients, but resolved in 23 on discharge. 23 If this is true, then some of our patients did not have OH in the long-run and therefore OH during hospitalization had no effect on long-term mortality. It may be worthwhile to divide patients with OH into 2 subgroups; those with episodic OH during acute illness and those with established OH who exhibit sustain OH on repeated examinations in the community.
It is possible that only established OH has a worse impact on mortality, 24, 25 whereas episodic OH, as in our cohort, has no effect on long-term mortality.
Unlike some other studies that assessed total mortality, 17– 19, 21 we assessed also cause specific mortality and we did not observe any effect of OH on cardiovascular or noncardiovascular mortality. We did, however, observe the effect of age, gender and diabetes mellitus on survival. These findings are in accordance with data regarding survival in elderly patients. 26– 30 Therefore, we believe that our cohort of elderly patients, admitted to an acute medical ward, was a typical and a representative one.
Our study had several limitations because some patients were lost to follow-up and we do not have information regarding the influence of OH on nonfatal outcomes and whether the OH resolved after discharge. However, these limitations have no effect on the major observation that OH found in elderly patients discharged from acute geriatric wards has no impact on vascular and nonvascular mortality.
Summary and Conclusions
Orthostatic hypotension, found in elderly patients admitted to an acute geriatric ward, has no effect on all cause and cause specific mortality. Further large-scale studies are needed to find out whether OH observed during hospitalization persists beyond discharge.
REFERENCES
- 1.Puisieux F, Boumbar Y, Bulckaen H, Bonnin E, Houssin F, Dewailly P. Intraindividual variability in orthostatic blood pressure changes among older adults: the influence of meals. J Am Geriatr Soc. 1999;47:1332–6. doi: 10.1111/j.1532-5415.1999.tb07434.x. [DOI] [PubMed] [Google Scholar]
- 2.Raiha I, Luutonen S, Piha J, Seppanen A, Toikka T, Sourander L. Prevalence, predisposing factors, and prognostic importance of postural hypotension. Arch Intern Med. 1995;155:930–5. doi: 10.1001/archinte.1995.00430090067008. [DOI] [PubMed] [Google Scholar]
- 3.Weiss A, Grossman E, Beloosesky Y, Grinblat J. Orthostatic hypotension in acute geriatric ward: is it a consistent finding? Arch Intern Med. 2002;162:2369–74. doi: 10.1001/archinte.162.20.2369. [DOI] [PubMed] [Google Scholar]
- 4.Lipsitz LA. Orthostatic hypotension in the elderly. N Engl J Med. 1989;321:952–7. doi: 10.1056/NEJM198910053211407. [DOI] [PubMed] [Google Scholar]
- 5.Applegate WB, Davis BR, Black HR, Smith WM, Miller ST, Burlando AJ. Prevalence of postural hypotension at baseline in the Systolic Hypertension in the Elderly Program (SHEP) cohort. J Am Geriatr Soc. 1991;39:1057–64. doi: 10.1111/j.1532-5415.1991.tb02869.x. [DOI] [PubMed] [Google Scholar]
- 6.Rutan GH, Hermanson B, Bild DE, Kittner SJ, LaBaw F, Tell GS. Orthostatic hypotension in older adults. The Cardiovascular Health Study. CHS Collaborative Research Group. Hypertension. 1992;19(Part 1):508–19. doi: 10.1161/01.hyp.19.6.508. [DOI] [PubMed] [Google Scholar]
- 7.Luukinen H, Koski K, Laippala P, Kivela SL. Prognosis of diastolic and systolic orthostatic hypotension in older persons. Arch Intern Med. 1999;159:273–80. doi: 10.1001/archinte.159.3.273. [DOI] [PubMed] [Google Scholar]
- 8.Carlson JE. Assessment of orthostatic blood pressure: measurement technique and clinical applications. South Med J. 1999;92:167–73. doi: 10.1097/00007611-199902000-00002. [DOI] [PubMed] [Google Scholar]
- 9.Eigenbrodt ML, Rose KM, Couper DJ, Arnett DK, Smith R, Jones D. Orthostatic hypotension as a risk factor for stroke: the atherosclerosis risk in communities (ARIC) study, 1987–1996. Stroke. 2000;31:2307–13. doi: 10.1161/01.str.31.10.2307. [DOI] [PubMed] [Google Scholar]
- 10.Ensrud KE, Nevitt MC, Yunis C, Hulley SB, Grimm RH, Cummings SR. Postural hypotension and postural dizziness in elderly women. The study of osteoporotic fractures. The Study of Osteoporotic Fractures Research Group. Arch Intern Med. 1992;152:1058–64. [PubMed] [Google Scholar]
- 11.Rose KM, Tyroler HA, Nardo CJ, et al. Orthostatic hypotension and the incidence of coronary heart disease: the Atherosclerosis Risk in Communities study. Am J Hypertens. 2000;13(Part 1):571–8. doi: 10.1016/s0895-7061(99)00257-5. [DOI] [PubMed] [Google Scholar]
- 12.Low PA. Update on the evaluation, pathogenesis, and management of neurogenic orthostatic hypotension: introduction. Neurology. 1995;45(suppl 5):S4–S5. [PubMed] [Google Scholar]
- 13.Matsubayashi K, Okumiya K, Wada T, Osaki Y, Doi Y, Ozawa T. Cognitive and functional status of the Japanese oldest old. J Am Geriatr Soc. 1997;45:385–6. doi: 10.1111/j.1532-5415.1997.tb00963.x. [DOI] [PubMed] [Google Scholar]
- 14.Perlmuter LC, Greenberg JJ. Do you mind standing? Cognitive changes in orthostasis. Exp Aging Res. 1996;22:325–41. doi: 10.1080/03610739608254015. [DOI] [PubMed] [Google Scholar]
- 15.Elmstahl S, Rosen I. Postural hypotension and EEG variables predict cognitive decline: results from a 5-year follow-up of healthy elderly women. Dement Geriatr Cogn Disord. 1997;8:180–7. doi: 10.1159/000106629. [DOI] [PubMed] [Google Scholar]
- 16.Viramo P, Luukinen H, Koski K, Laippala P, Sulkava R, Kivela SL. Orthostatic hypotension and cognitive decline in older people. J Am Geriatr Soc. 1999;47:600–4. doi: 10.1111/j.1532-5415.1999.tb02576.x. [DOI] [PubMed] [Google Scholar]
- 17.Masaki KH, Schatz IJ, Burchfiel CM, et al. Orthostatic hypotension predicts mortality in elderly men: the Honolulu Heart Program. Circulation. 1998;98:2290–5. doi: 10.1161/01.cir.98.21.2290. [DOI] [PubMed] [Google Scholar]
- 18.Sasaki O, Nakahama H, Nakamura S, et al. Orthostatic hypotension at the introductory phase of haemodialysis predicts all-cause mortality. Nephrol Dial Transplant. 2005;20:377–81. doi: 10.1093/ndt/gfh614. [DOI] [PubMed] [Google Scholar]
- 19.Tilvis RS, Hakala SM, Valvanne J, Erkinjuntti T. Postural hypotension and dizziness in a general aged population: a four-year follow-up of the Helsinki Aging Study. J Am Geriatr Soc. 1996;44:809–14. doi: 10.1111/j.1532-5415.1996.tb03738.x. [DOI] [PubMed] [Google Scholar]
- 20.Ward C, Kenny RA. Reproducibility of orthostatic hypotension in symptomatic elderly. Am J Med. 1996;100:418–22. doi: 10.1016/S0002-9343(97)89517-4. [DOI] [PubMed] [Google Scholar]
- 21.Hossain M, Ooi WL, Lipsitz LA. Intra-individual postural blood pressure variability and stroke in elderly nursing home residents. J Clin Epidemiol. 2001;54:488–94. doi: 10.1016/s0895-4356(00)00322-x. [DOI] [PubMed] [Google Scholar]
- 22.Davis BR, Langford HG, Blaufox MD, Curb JD, Polk BF, Shulman NB. The association of postural changes in systolic blood pressure and mortality in persons with hypertension: the Hypertension Detection and Follow-up Program experience. Circulation. 1987;75:340–6. doi: 10.1161/01.cir.75.2.340. [DOI] [PubMed] [Google Scholar]
- 23.Kong KH, Chuo AM. Incidence and outcome of orthostatic hypotension in stroke patients undergoing rehabilitation. Arch Phys Med Rehabil. 2003;84:559–62. doi: 10.1053/apmr.2003.50040. [DOI] [PubMed] [Google Scholar]
- 24.Purewal TS, Watkins PJ. Postural hypotension in diabetic autonomic neuropathy: a review. Diabet Med. 1995;12:192–200. doi: 10.1111/j.1464-5491.1995.tb00458.x. [DOI] [PubMed] [Google Scholar]
- 25.Valensi P, Thi BN, Lormeau B, Paries J, Attali JR. Cardiac autonomic function in obese patients. Int J Obes Relat Metab Disord. 1995;19:113–8. [PubMed] [Google Scholar]
- 26.Perkins I. Diabetes mellitus epidemiology—classification, determinants, and public health impacts. J Miss State Med Assoc. 2004;45:355–62. [PubMed] [Google Scholar]
- 27.Muhammad S. Epidemiology of diabetes and obesity in the United States. Compend Contin Educ Dent. 2004;25:195–8. 200, 202; quiz 204. [PubMed] [Google Scholar]
- 28.Merlino JI, Yowler CJ, Malangoni MA. Nosocomial infections adversely affect the outcomes of patients with serious intraabdominal infections. Surg Infect (Larchmt) 2004;5:21–7. doi: 10.1089/109629604773860273. [DOI] [PubMed] [Google Scholar]
- 29.Peterson E, Bensimhon DR, et al. Coronary heart disease. In: Hazzard WR, Blass JP, Halter JB, editors. Principles of Geriatric Medicine & Gerontology. New York: McGraw-Hill; 2003. pp. 433–44. [Google Scholar]
- 30.High KP, Loeb M, et al. Infection in the elderly. In: Hazzard WR, Blass JP, Halter JB, editors. Principles of Geriatric Medicine & Gerontology. New York: McGraw-Hill; 2003. p. 1071. [Google Scholar]