Abstract
Complementary medication is en vogue and an increasing number of patients consume herbal medicine without reporting their use to physicians. We report a case of iodine-induced hyperthyroidism due to the ingestion of a kelp-containing tea. A 39-year-old woman with multinodular goiter presented with typical signs of hyperthyroidism, which was confirmed by endocrine tests. She was not exposed to iodinated radiocontrast media and did not take medications containing iodine, such as amiodarone. However, a detailed medical history revealed that she had been treated for a period of 4 weeks by a Chinese alternative practitioner with a herbal tea containing kelp because of her enlarged thyroid. The consumption of the tea was discontinued and an antithyroid drug therapy was initiated. Physicians should advise patients with underlying thyroid disease to avoid all complementary or alternative medications containing iodine.
Keywords: iodine-induced hyperthyroidism, thyrotoxicosis, iodine, kelp
In healthy individuals, the thyroid gland has intrinsic autoregulatory mechanisms to adapt to excess iodine (for review, see Braverman 1). The oxidation of iodine, which is an essential first reaction in the synthesis of the thyroid hormones, is inhibited in the presence of large amounts of iodine. This acute inhibitory effect of excessive iodine on hormone production, known as the acute Wolff–Chaikoff effect, is only transient. 2 After approximately 48 hours, an escape from the acute Wolff–Chaikoff effect occurs leading to a normalization of thyroid hormone synthesis. 3 Animal studies suggest that the adaptation to this effect may be caused by the diminished active transport of increased plasma iodide into the thyroid leading to a protection of the thyroid from the adverse effects of excessive iodide on thyroid hormone production. 4, 5 In contrast, patients with underlying thyroid disease may fail to adapt to excessive iodine resulting in hypothyroidism or hyperthyroidism. The occurrence of these 2 disparate responses to iodide excess may be due to differences in the sensitivity to iodide-induced turn-off in hormone biosynthesis. 6 Subjects with an elevated sensitivity, such as patients previously treated for Graves' disease with radioactive iodine, subtotal thyroidectomy, antithyroid drugs, or those with chronic autoimmune thyroiditis, have an increased risk to develop hypothyroidism after administration of excessive iodide because of a failure to escape from the acute Wolff–Chaikoff effect. 1 In contrast, in subjects with lowered sensitivity, i.e., patients with endemic goiter and iodide deficiency or patients with nodular goiter containing autonomous nodules, iodide excess may result in hyperthyroidism. 6
Commonly used iodine-containing drugs, such as amiodarone, topical antiseptics, expectorants, and radiology contrast agents are sources of excessive iodine. 7 Furthermore, various foods and dietary supplements, including kelp, may contain high quantities of iodide. 8 Kelp are large seaweeds, belonging to the brown algae and classified in the order Laminariales, and are an important food source in many Asian cultures. Herbal medicine, including kelp and kelp-containing dietary supplements, are also used by an increasing number of patients. 9 In 1997, approximately one third of the adult population in the United States reported use of herbs to treat medical illnesses. 10 The growing consumer interest is also reflected by the herbal product annual retail sales increasing from $1 billion in 1994 to $4 billion in 1998. 11 However, 70% of patients do not inform their physicians of concomitant use of herbal medicines. 12 According to the prevailing belief that herbal products are safe owing to their natural origin, they are not subjected to the same restrictions as pharmaceutical drugs. Herbs are considered dietary supplements and do not have to receive approval from the Food and Drug Administration (FDA) before sale and marketing. However, many serious and even life-threatening adverse reactions have recently been reported. 13 Side effects include toxicity, allergic reactions, effects because of contamination, and interaction with drugs and other herbs. 14
Here, we present a case of iodine-induced thyrotoxicosis in a patient with multinodular goiter with autonomously functioning tissue. Initially, the source of iodine was not obvious, especially as iodinated contrast agents, amiodarone, or topical antiseptics were not used. Finally, after intensive questioning it was revealed that the consumption of a kelp-containing tea was the sought-after source of excessive iodine.
CASE REPORT
A 39-year-old woman (157 cm, 75 kg, body mass index [BMI] 30.4 kg/m2) was referred to our outpatient clinic for evaluation of an enlarged thyroid. The patient's medical history and family history were unremarkable. Physical examination confirmed a palpably enlarged thyroid. Normal values of free tri-iodothyronine (fT3), free thyroxine (fT4), and thyroid-stimulating hormone (TSH) were congruent with the lack of clinical signs of hyper- or hypothyroidism; antithyroid peroxidase (TPO), antithyroglobuline (TG), and anti-TSH-receptor (TSH-R) antibodies were negative (Table 1).
Table 1.
Evaluation of the Thyroid Parameters in our Patient
| Date | Parameters | |||||
|---|---|---|---|---|---|---|
| fT4 (ng/dL) | fT3 (pg/dL) | TSH (mU/L) | TPO-AB (kU/L) | TG-AB (kU/L) | TSH-R-AB (U/L) | |
| Normal | 0.8–1.7 | 210–420 | 0.4–2.5 | <1.9 | <0.9 | <12 |
| Initially | 1.3 | 360 | 0.72 | Neg. | Neg. | <4.0 |
| After thyroxine | 2.5 | 380 | 0.05 | – | – | – |
| After 2 mo | 1.5 | 325 | 0.49 | – | – | – |
| After 4 mo | 3.2 | 781 | <0.01 | Neg. | Neg. | <4.0 |
| After 11 mo | 1.1 | 280 | 0.14 | Neg. | Neg. | <4.0 |
fT4, free thyroxin; fT3, free tri-iodothyronine; TSH, thyroid-stimulating hormone; TPO-AB, antithyroid peroxidase antibody; TG-AB, anti-thyroglobuline antibody; TSH-R-AB, anti-TSH-receptor antibody, Neg., negligible.
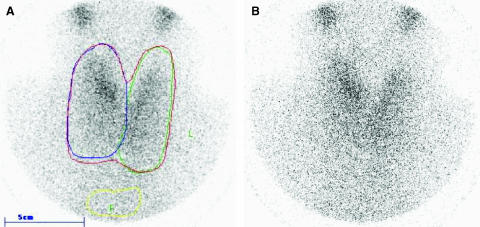
Ultrasonography revealed a multinodular goiter with a total volume of 62 mL. A thyroid 99mTC-pertechnetate scintigraphy showed a homogenous uptake with a moderate autonomy in the right upper lobe, confirmed by a thyroid scan under exogenous TSH suppression with levothyroxine (Fig. 1).
FIGURE 1.
Thyroid suppression 99mTC-pertechnetate scintigraphy, before (A) and after taking 100 μg levothyroxine daily for 4 weeks (B), showing a homogenous uptake with a moderate autonomy in the right upper lobe.
The patient was informed of the therapeutic options that included thyroid resection versus routine follow-up clinical visits. She decided against surgery, and a short-term control visit was arranged. Furthermore, it was pointed out to the patient to avoid the intake of large amounts of iodine, such as iodinated radiocontrast agents or iodine-containing drugs or food. Two months later, the patient was in good general health and endocrine tests revealed thyroid hormones and TSH plasma levels within the normal range (Table 1).
Four months after the initial visit, the patient presented with typical signs of hyperthyroidism, including tachycardia (100 beats/min), palpitations, tremor, nervousness, insomnia, fatigue, increased sweating, diarrhea, secondary amenorrhoea, and weight loss. Laboratory analysis revealed increased levels of fT3 and fT4 as well as a suppressed TSH concentration, anti-thyroid antibodies remained negative (Table 1). Ultrasonography showed a multinodular goiter with a total volume of 67 mL. The patient did not report any exposure to medications containing iodine, such as iodinated radiocontrast agents or amiodarone. However, on further questioning, the patient reported that for the last 4 weeks she had been consuming a herbal tea, prescribed by a Chinese alternative practitioner to treat her enlarged thyroid. The prescription of the tea, which is shown in Figure 2, revealed large amounts of kelp, Sargassum weed, and kombu.
FIGURE 2.
Prescription of the medicinal tea containing several species of seaweed (marked by arrows). Sargassum pallidum, a brown algae found in great abundance along the coasts of Japan and China, is, along with Sargassum fusiforme, referred to as sargassum seaweed or gulfweed. Laminaria japonica, belonging to the brown algae, is the most popular variety of kombu widely eaten in Northeast Asia. Seaweed (German translation: Seetang), any of a large number of marine benthic algae that are multicellular, macothallic (large-bodied), and thus differentiated from most algae that tend toward microscopic size.
The patient was advised to discontinue the consumption of the tea and an antithyroid drug therapy with 40 mg thiamazole and 40 mg propranolol daily was initiated. Her follow-up visit, 7 months after the diagnosis of hyperthyroidism, revealed normal laboratory values of fT3, fT4, and just slightly decreased TSH levels (Table 1) that were congruent with the lack of clinical signs of hyper or hypothyroidism. Her thiamazole therapy was then reduced to 20 mg daily.
DISCUSSION
Iodine-induced thyrotoxicosis is not a single etiologic entity (Table 2). However, common causes are iodine supplementation in areas of iodine deficiency or administration of high doses of iodide, such as iodine-containing drugs or iodinated radiographic contrast agents, in patients with nodular goiters including areas of autonomy. 6, 15, 16 In countries with a higher iodine intake, such as the United States, iodine-induced thyrotoxicosis is not frequent. 7 However, the incidence of this disorder may increase with the aging of the population, as elderly persons with long standing multinodular goiters have an increased risk to develop iodine-induced hyperthyroidism. 17
Table 2.
Causes of Iodine-Induced Hyperthyroidism 1
| Iodine supplementation for endemic iodine-deficiency goiter |
| Iodine administration to patients with underlying thyroid disease, more common in areas of marginal iodine intake Nontoxic nodular goiter Autonomous nodule Nontoxic diffuse goiter |
| Iodine administration to euthyroid patients previously treated with antithyroid drugs |
| Iodine administration to patients with no recognized underlying disease, especially in areas of mild to moderate iodine deficiency |
Common sources of excessive iodine are iodinated radiocontrast material and the antiarrhythmic drug amiodarone. 18, 19 Iodine-induced thyrotoxicosis after consumption of kelp or kelp-containing dietary supplements is rare, as reflected by the small number of case reports that have been documented. 20– 24 However, they do require clinical attention in view of the increasing use of herbal products, including kelp. 14 Patients typically do not inform their physicians of ingestion of herbal medicine. 12 Similarly, on admission, our patient also did not report on her herbal use. Only after specific questioning for drug intake, including alternative medicine, revealed consumption of a herbal tea. Study of the tea's prescription (Fig. 2) showed large amounts of seaweed, a known rich source of iodine (Table 3).
Table 3.
Comparison of Iodine Content of Seaweed by Genus, Geographic Location, and Study
| Seaweed origin | Teas et al. 25 (μg/g) | Lee et al. 8 (μg/g) |
|---|---|---|
| U.S., Canada, Namibia, Tasmania, Japan | UK | |
| Arame (Eisenia bicyclis) | 586 | 714* |
| Dulse (Palmaria palmata) | 72 | 44 |
| Hijiki (sea grass) | 629 | 391 |
| Kelp granules (salt substitute) | 8165 | 67 |
| Kelp/kombu | 1542† | 2650 |
| Nori (Porphyra tenera) | 16 | 43 |
| Wakame | 66‡ | 161 |
Average of 2 reported values.
Average of 10 kinds of kelp analyzed.
Average of 3 sample sites.
Considering the average iodine content of kelp of 1,500 to 2,500 μg/g, 8, 25 our patient was taking an estimated 580 to 990 μg iodine daily, i.e., 3.8 to 6.6 times the recommended daily allowance of 150 μg. 26 For comparison, in the United States the estimated average iodine intake of men and women aged 25 to 30 years is 410 and 260 μg/day, respectively. 27 In the literature, several cases of iodine-induced hyperthyroidism after consumption of seaweed have been described, this occurred mainly while taking kelp tablets. 20– 23, 28 The consumption of kombu, a long dark brown to grayish-black seaweed, has also been reported to cause hyperthyroidism. 29 We report the first case of iodine-induced hyperthyroidism after ingestion of a medicinal tea containing kelp. Furthermore, several dietary supplements have been shown to contain significant amounts of iodine and, therefore, could endanger iodine-sensitive subjects (Table 4).
Table 4.
Iodine Content of Dietary Supplements 8
| Supplement type | n | Iodine content (μg/daily dose)* | Declared content %† | ||||
|---|---|---|---|---|---|---|---|
| Range | Mean | Median | Range | Mean | Median | ||
| Kelp supplements | 22 | 210–3,840 | 1,244 | 1,005 | 45–914 | 217 | 137 |
| Multinutrient supplements (total) | 42 | 11–171 | 94 | 104 | 8–197 | 82 | 79 |
| In detail | |||||||
| Children's supplements | 4 | 12–122 | 74 | 81 | – | – | – |
| Women's supplements | 3 | 11–124 | 47 | 16 | – | – | – |
| Multimineral supplements | 6 | 30–124 | 90 | 100 | – | – | – |
| Dolomite supplement | 1 | – | <1 | – | – | – | – |
| Multivitamin supplements | 1 | – | <1 | – | – | – | – |
| Iodine preparation | 1 | – | 185,000 | – | – | – | – |
Daily dose refers to manufacturers' maximum recommended daily dose of supplement.
Where given.
In our patient, hyperthyroidism did not resolve spontaneously following discontinuation of the kelp-containing tea, as described previously, 20, 23 but required antithyroid drug therapy. Our patient suffered from multinodular goiter in an endemic area of moderate iodine deficiency. This nontoxic multinodular goiter, however, with scintigraphic proof of autonomous tissue, turned toxic after ingestion of the iodine-rich kelp.
This case demonstrates the importance of careful and detailed investigations that may lead to the discovery of unusual sources of iodine. Successful diagnosis often requires specific questioning by the physician. Dietary supplements and herbal remedies are used by an increasing number of patients without prior medical consultation. Therefore, patients with known thyroid disease should be advised to avoid all complementary and alternative medications that contain iodine. Before starting any such supplements, patients with underlying thyroid disorder should check with their physician or pharmacist.
Acknowledgments
The authors are indebted to Prof. Dr. Fritz J. Seif for carefully reviewing the manuscript and Ms. Mary-Ann Schneider for proofreading the manuscript.
REFERENCES
- 1.Braverman LE. Iodine and the thyroid: 33 years of study. Thyroid. 1994;4:351–6. doi: 10.1089/thy.1994.4.351. [DOI] [PubMed] [Google Scholar]
- 2.Wolff J, Chaikoff IL. Plasma inorganic iodide as homeostatic regulator of thyroid function. J Biol Chem. 1948;174:555–64. [PubMed] [Google Scholar]
- 3.Wolff J, Chaikoff IL, Goldberg, Meier JR. The temporary nature of the inhibitory action of excess iodine on organic iodine synthesis in the normal thyroid. Endocrinology. 1949;45:504–13. doi: 10.1210/endo-45-5-504. [DOI] [PubMed] [Google Scholar]
- 4.Galton VA, Pitt-Rivers R. The effect of excessive iodine on the thyroid of the rat. Endocrinology. 1959;64:835–9. doi: 10.1210/endo-64-5-835. [DOI] [PubMed] [Google Scholar]
- 5.Braverman LE, Ingbar SH. Changes in thyroidal function during adaptation to large doses of iodide. J Clin Invest. 1963;42:1216–31. doi: 10.1172/JCI104807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fradkin JE, Wolff J. Iodide-induced thyrotoxicosis. Medicine (Baltimore) 1983;62:1–20. doi: 10.1097/00005792-198301000-00001. [DOI] [PubMed] [Google Scholar]
- 7.Roti E, Uberti ED. Iodine excess and hyperthyroidism. Thyroid. 2001;11:493–500. doi: 10.1089/105072501300176453. [DOI] [PubMed] [Google Scholar]
- 8.Lee SM, Lewis J, Buss DH, Holcombe GD, Lawrance PR. Iodine in British foods and diets. Br J Nutr. 1994;72:435–46. doi: 10.1079/bjn19940045. [DOI] [PubMed] [Google Scholar]
- 9.Miller LG. Herbal medicinals: selected clinical considerations focusing on known or potential drug-herb interactions. Arch Intern Med. 1998;158:2200–11. doi: 10.1001/archinte.158.20.2200. [DOI] [PubMed] [Google Scholar]
- 10.Johnston BA. One-third of nation's adults use herbal remedies. Herbalgram. 1997;40:49. [Google Scholar]
- 11.NBJ Herbal and Botanical U.S. Consumer Sales 1999. San Diego, CA: Nutrition Business Journal; 2000. pp. 1–3. [Google Scholar]
- 12.Eisenberg DM, Kessler RC, Foster C, Norlock FE, Calkins DR, Delbanco TL. Unconventional medicine in the United States. Prevalence, costs, and patterns of use. N Engl J Med. 1993;328:246–52. doi: 10.1056/NEJM199301283280406. [DOI] [PubMed] [Google Scholar]
- 13.Ernst E. Harmless herbs? A review of the recent literature. Am J Med. 1998;104:170–8. doi: 10.1016/s0002-9343(97)00397-5. [DOI] [PubMed] [Google Scholar]
- 14.Bent S, Ko R. Commonly used herbal medicines in the United States: a review. Am J Med. 2004;116:478–85. doi: 10.1016/j.amjmed.2003.10.036. [DOI] [PubMed] [Google Scholar]
- 15.Vagenakis AG, Wang CA, Burger A, Maloof F, Braverman LE, Ingbar SH. Iodide-induced thyrotoxicosis in Boston. N Engl J Med. 1972;287:523–7. doi: 10.1056/NEJM197209142871101. [DOI] [PubMed] [Google Scholar]
- 16.Rajatanavin R, Safran M, Stoller WA, Mordes JP, Braverman LE. Five patients with iodine-induced hyperthyroidism. Am J Med. 1984;77:378–84. doi: 10.1016/0002-9343(84)90726-5. [DOI] [PubMed] [Google Scholar]
- 17.Martin FI, Deam DR. Hyperthyroidism in elderly hospitalised patients. Clinical features and treatment outcomes. Med J Aust. 1996;164:200–3. [PubMed] [Google Scholar]
- 18.Harjai KJ, Licata AA. Effects of amiodarone on thyroid function. Ann Intern Med. 1997;126:63–73. doi: 10.7326/0003-4819-126-1-199701010-00009. [DOI] [PubMed] [Google Scholar]
- 19.Martin FI, Tress BW, Colman PG, Deam DR. Iodine-induced hyperthyroidism due to nonionic contrast radiography in the elderly. Am J Med. 1993;95:78–82. doi: 10.1016/0002-9343(93)90235-h. [DOI] [PubMed] [Google Scholar]
- 20.Shilo S, Hirsch HJ. Iodine-induced hyperthyroidism in a patient with a normal thyroid gland. Postgrad Med J. 1986;62:661–2. doi: 10.1136/pgmj.62.729.661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hartman AA. Hyperthyroidism during administration of kelp tablets. Ned Tijdschr Geneesk. 1990;134:1373. [PubMed] [Google Scholar]
- 22.de Smet PA, Stricker BH, Wilderink F, Wiersinga WM. Hyperthyroidism during treatment with kelp tablets. Ned Tijdschr Geneesk. 1990;134:1373. [PubMed] [Google Scholar]
- 23.Eliason BC. Transient hyperthyroidism in a patient taking dietary supplements containing kelp. J Am Board Fam Pract. 1998;11:478–80. doi: 10.3122/jabfm.11.6.478. [DOI] [PubMed] [Google Scholar]
- 24.Salas Coronas J, Cruz Caparros G, Laynez Bretones F, Diez Garcia F. Hyperthyroidism secondary to kelp tablets ingestias. Med Clin (Barc) 2002;118:797–8. doi: 10.1016/s0025-7753(02)72534-7. [DOI] [PubMed] [Google Scholar]
- 25.Teas J, Pino S, Critchley A, Braverman LE. Variability of iodine content in common commercially available edible seaweeds. Thyroid. 2004;14:836–41. doi: 10.1089/thy.2004.14.836. [DOI] [PubMed] [Google Scholar]
- 26.Dahl L, Johansson L, Julshamn K, Meltzer HM. The iodine content of Norwegian foods and diets. Public Health Nutr. 2004;7:569–76. doi: 10.1079/PHN2003554. [DOI] [PubMed] [Google Scholar]
- 27.Pennington JA, Young BE. Total diet study nutritional elements, 1982–1989. J Am Diet Assoc. 1991;91:179–83. [PubMed] [Google Scholar]
- 28.Henzen C, Buess M, Brander L. Iodine-induced hyperthyroidism (iodine-induced Basedow's disease): a current disease picture. Schweiz Med Wochenschr. 1999;129:658–64. [PubMed] [Google Scholar]
- 29.Ishizuki Y, Yamauchi K, Miura Y. Transient thyrotoxicosis induced by Japanese kombu. Nippon Naibunpi Gakkai Zasshi. 1989;65:91–8. doi: 10.1507/endocrine1927.65.2_91. [DOI] [PubMed] [Google Scholar]