Abstract
The posterior ventral tegmental area (VTA) is a neuroanatomical substrate mediating the reinforcing effects of ethanol in rats. Repeated alcohol deprivations produce robust ethanol intakes of alcohol-preferring (P) rats during relapse and increase the reinforcing effects of oral alcohol self-administration. The objective of this study was to test the hypothesis that alcohol drinking and repeated alcohol deprivations will increase the reinforcing effects of ethanol within the posterior VTA of P rats. Groups of female P rats were used (alcohol-naive, continuous access, and repeatedly deprived). Each rat was implanted with a guide cannula aimed at the posterior VTA. Depression of the active lever produced the infusion of 100 nl of artificial cerebrospinal fluid (CSF) or ethanol (25–300 mg%). Each rat was given only one ethanol concentration during the 4-h sessions conducted every other day. Compared with the infusions of artificial CSF, the alcohol-naive group reliably self-infused 75 and 150 mg% ethanol, but not the lower or higher concentrations. On the other hand, the continuous access group had significantly higher self-infusions of 50, 75, 150, and 300 mg% ethanol compared with artificial CSF infusions. The repeatedly deprived group also self-infused significantly more of 50, 75, 150, and 300 mg% ethanol than artificial CSF; moreover, the number of infusions for all four concentrations was higher in the repeatedly deprived versus the continuous access group. Chronic alcohol drinking by P rats increased the reinforcing effects of ethanol within the posterior VTA, and repeated alcohol deprivations produced a further increase in these reinforcing effects of ethanol.
The alcohol-preferring (P) line of rats demonstrates a robust alcohol deprivation effect (ADE) under continuous 24-h access (Rodd-Henricks et al., 2000c) and limited access operant (Rodd et al., 2003) conditions. The ADE is defined as a temporary increase in the intake of or preference for ethanol after a period of deprivation (Sinclair, 1972, 1973). With repeated deprivations, the magnitude and duration of the ADE increase in the P line of rats (Rodd-Henricks et al., 2001; Rodd et al., 2003). The neural mechanisms underlying the expression of the ADE are not known.
The ventral tegmental area (VTA) dopamine (DA) system seems to have an important role in mediating the actions of ethanol. Electrophysiological studies demonstrated that ethanol administration increased the firing rate of VTA DA neurons in vivo (Gessa et al., 1985) and in vitro after bath application (Brodie et al., 1990, 1995). Microdialysis experiments indicated that oral operant self-administration of ethanol increased DA release in the nucleus accumbens (Weiss et al., 1993, 1996; Melendez et al., 2002). In addition, micro-injection of a D2,3 agonist into the VTA-reduced ethanol intake of P (Nowak et al., 2000) and Long-Evans (Hodge et al., 1993) rats. Injection of a D2,3 agonist into the VTA has been shown to reduce DA neuronal activity, presumably through its action at D2 autoreceptors (Jeziorski and White, 1989; Congar et al., 2002). Using midbrain slice preparations and isolated neurons, Brodie (2002), Brodie and Appel (2000), and Brodie et al. (1999) demonstrated that chronic ethanol drinking by C57BL mice increased the sensitivity of the VTA DA neurons to the stimulating effects of ethanol, there were differences in sensitivity to ethanol-induced excitation of DA neurons between C57 and DBA mice, and ethanol could directly stimulate DA neurons. Taken together, these studies indicate that ethanol can have an excitatory action on VTA DA neurons and that increased VTA DA neuronal activity may be an important factor contributing to the reinforcing effects of ethanol and alcohol drinking.
The intracranial self-administration (ICSA) technique has been used to identify brain sites that are involved in mediating the reinforcing effects of drugs of abuse (for review, see McBride et al., 1999). Gatto et al. (1994) was the first to demonstrate that the VTA supported the self-infusion of ethanol. These investigators demonstrated that female P rats self-infused 75 to 200 mg% ethanol into the VTA, whereas female alcohol-nonpreferring rats did not reliably self-infuse ethanol at any of the concentrations tested. These results suggested that the VTA was a neuroanatomical substrate mediating the reinforcing effects of ethanol and that genetic factors might play a role in determining these reinforcing effects. In a subsequent study, Rodd-Henricks et al. (2000a) established that female Wistar rats self-infused 150 to 400 mg% ethanol into the posterior VTA (−5.6 to −6.04 mm bregma), whereas placements anterior to −5.3 mm bregma did not support the self-infusion of 200 or 400 mg% ethanol. Additional ICSA studies demonstrated that coadministration of the D2,3 agonist quinpirole significantly reduced ethanol self-infusions into the posterior VTA of Wistar (Rodd et al., 2004b) and P (Rodd et al., 2005a) rats, suggesting that activation of VTA DA neurons may be involved in promoting the reinforcing effects of ethanol within the posterior VTA.
In a recent ICSA study, Rodd et al. (2005b) demonstrated that chronic continuous alcohol drinking (2 months) by P rats increased the sensitivity of the posterior VTA to the reinforcing effects of ethanol. In addition, this study demonstrated that higher infusions of 50, 75, and 125 mg% were also found in the alcohol-drinking group, suggesting that prior alcohol drinking experience may have increased the reinforcing effects of ethanol within the posterior VTA. The increased sensitivity to the reinforcing effects of ethanol in the posterior VTA may be a result of higher basal DA neuronal activity in the nucleus accumbens of the chronic alcohol-drinking P rats (Thielen et al., 2004).
Repeated alcohol deprivations have been shown to progressively increase responding for ethanol during each relapse session (Rodd et al., 2003). Moreover, a progressive ratio experiment (Rodd et al., 2003) demonstrated that P rats, which had been taken through repeated 2-week cycles of alcohol access and deprivation, had a 2-fold higher breakpoint value than did P rats that had not been deprived. These results suggest that repeated deprivations increased the reinforcing effects of oral ethanol self-administration. The objective of the present study was to test the hypothesis that, compared with nondeprived alcohol-drinking P rats, repeated cycles of ethanol access and deprivation will further increase the reinforcing effects of ethanol in the posterior VTA.
Materials and Methods
Animals
Female P rats, weighing 250 to 320 g at the start of the experiment, were used. Rats were double-housed upon arrival and maintained on a 12-h reverse light/dark cycle (lights off at 9:00 AM). Female rats were used in the present study and in previous ICSA studies (Gatto et al., 1994; Rodd-Henricks et al., 2000a, 2003; Rodd et al. 2004a, b, 2005a, b), because female rats maintain their body and head size better than male rats for more accurate and reliable stereotaxic placements. Also, the time requirement for the initial ethanol consumption period (greater than 7 months) precluded from using male rats since P males would have weighed in excess of 650 to 700 g at the end of the drinking phase. Additionally, in Wistar (Rodd et al., 2004b) and P (Z. A. Rodd, R. L. Bell, and W. J. McBride, unpublished data) rats, there have not been significant gender differences in ethanol self-infusions into the VTA. Food and water were freely available except in the test chamber. Animals used in this study were maintained in facilities fully accredited by the Association for the Assessment and Accreditation of Laboratory Animal Care. All research protocols were approved by the institutional animal care and use committee and are in accordance with the guidelines of the Institutional Care and Use Committee of the National Institute on Drug Abuse, National Institutes of Health, and the Guide for the Care and Use of Laboratory Animals (National Research Council 1996).
Free-Choice Alcohol Drinking Procedure
A total of 129 rats were used in the current experiment. The rats were randomly assigned to one of three groups. A group of rats were not exposed to ethanol and served as nonexposure controls (n = 43). The second group of rats were given continual (chronic) access to 15% ethanol for 8 months before ICSA testing (n = 46). The third group was given intermittent access to ethanol during the 7.5-month exposure period (repeatedly deprived; n = 40). The repeatedly deprived rats received continuous, concurrent free-choice access in the home cage to 15% (v/v) ethanol and water for 6 weeks. For the chronic and repeatedly deprived groups, the positions of the ethanol and water bottle randomly changed every day, and fluid intake was recorded to the nearest 0.1 g by weighing the water and ethanol tubes before and after each 24-h period. Fluid intake measures were converted into grams of ethanol per kilogram of body weight (grams per kilogram per day) and the ratio of intake for each solution (grams of solution per gram of total fluid intake per day). Body weights of the rats were recorded twice weekly, and fluid intake was measured daily. For rats in the repeatedly deprived group, at the end of the 6-week free-choice access period, ethanol was removed for a 2-week period. After the initial abstinence period, ethanol was reinstated for a 2-week period. The rats were then exposed to four more cycles of ethanol abstinence and access (2-week periods). Before ICSA testing, rats in the repeatedly deprived group were abstinent for at least 7 weeks before surgery.
For rats in the chronic drinking group, ethanol was removed for a specific duration each day (start time 1-h removal) at approximately 10:00 AM 2 weeks before surgery until gradually ethanol was removed for a 4-h period per day. Daily ethanol intakes were not affected by this brief period of ethanol abstinence. The chronic drinking group was never deprived of alcohol and consumed alcohol throughout ICSA testing. During ICSA testing, rats in the chronic drinking group were deprived of ethanol (EtOH) for 4 h before ICSA testing, did not have access to EtOH during ICSA testing, but access to EtOH was immediately returned after ICSA testing. Examining the drinking temporal data revealed that total 24-h EtOH consumption was not altered during ICSA testing in the chronic drinking group and that consumption of EtOH occurred in a similar pattern, albeit altered temporally, on non-ICSA testing days.
The number of animals indicated for each experiment represents 90% of the total number that underwent surgery; 9% of the animals were not included for analyses mainly due to the loss of the guide cannula before completion of all experimental sessions. The data for these animals were not used because their injection sites could not be verified.
Chemical Agents and Vehicle
The artificial CSF consisted of: 120.0 mM NaCl, 4.8 mM KCl, 1.2 mM KH2PO4, 1.2 mM Mg SO4, 25.0 mM NaHCO3, 2.5 mM CaCl2, and 10.0 mM D-glucose. Ethyl alcohol (190 proof; McCormick Distilling Co., Weston, MO) was dissolved in the aCSF solution and the pH adjusted to 7.4 ± 0.1.
Apparatus
The test chambers (30 × 30 × 26 cm; weight × height × diameter) were situated in sound-attenuating cubicles (64 × 60 × 50 cm; Coulbourn Instruments, Allentown, PA) and illuminated by a dim house light during testing. Two identical levers (3.5 × 1.8 cm) were mounted on a single wall of the test chamber, 15 cm above a grid floor, and were separated by 12 cm. Levers were raised to this level to avoid accidental brushing against the lever and to reduce responses as a result of general locomotor activation. Directly above each lever was a row of three different colored cue lights. The light (red) to the far right over the active bar was illuminated during resting conditions. A desktop computer equipped with an operant control system (L2T2 system; Coulbourn Instruments) recorded the data and controlled the delivery of infusate in relation to lever response.
An electrolytic microinfusion transducer system (Bozarth and Wise, 1980) was used to control infusions of drug or vehicle. Two platinum electrodes were placed in an infusate-filled gas-tight cylinder (28 mm in length × 6 mm in diameter) equipped with a 28-gauge injection cannula (Plastics One, Roanoke, VA). The electrodes were connected to a constant current generator (MNC, Shreveport, LA) by a spring-coated cable (Plastics One) and a swivel (model 205; Mercotac, Carlsbad, CA), which delivered 6 μA of quiescent current (during resting conditions) and 200 μA of infusion current (when the active lever was pressed) between the electrodes. Depression of the active lever delivered the infusion current for 5 s, which led to the rapid generation of H2 gas (increasing the pressure inside the gas-tight cylinder) and, in turn, forcing 100 nl of the infusate through the injection cannula. During the 5-s infusion and additional 5-s time-out period, the house and right cue lights (red) were extinguished and the left cue light (green) over the active lever flashed on and off at 0.5-s intervals.
Animal Preparation
While under isoflurane anesthesia, subjects were prepared for stereotaxically implanting a 22-gauge guide cannula (Plastics One) into the right hemisphere; the guide cannula was aimed 1.0 mm above the target region. Coordinates (Paxinos and Watson, 1986) for placements into the posterior VTA were 5.4 mm posterior to bregma, 2.1 mm lateral to the midline, and 8.5 mm ventral from the surface of the skull at a 10° angle to the vertical. In between experimental sessions, a 28-gauge stylet was placed into the guide cannula and extended 0.5 mm beyond the tip of the guide. After surgery, rats were individually housed and allowed to recover 7 to 10 days. Alcohol was returned to the chronic drinking group 1 h after surgery to prevent a prolong deprivation period occurring. Animals were handled for at least 5 min daily beginning on the fifth recovery day. Subjects were not acclimated to the experimental chambers before the commencement of data collection, nor did they receive any prior operant training.
General Test Condition
For the chronic drinking group, ethanol was removed at 10:00 AM, and testing did not begin until at least 2:00 PM (range, 1400–1500 h). This period of abstinence results in undetectable blood ethanol concentrations. For testing, subjects were brought to the testing room, the cannula stylet was removed, and the injection cannula was screwed into place. To avoid trapping air at the tip of the injection cannula, the infusion current was delivered for 5 s during insertion of the injector, which resulted in a single noncontingent administration of infusate at the beginning of the session. Injection cannulae extended 1.0 mm beyond the tip of the guide. The test chamber was equipped with two levers. Depression of the active lever (FR1 schedule of reinforcement) caused the delivery of a 100-nl bolus of infusate over 5 s followed by a 5-s time-out period. During both the 5-s infusion period and 5-s time-out period, responses on the active lever were recorded but did not produce further infusions. Thus, the number of responses on the active lever does not match 1:1 with the number of infusions received. Typically, rats respond approximately 2 to 2.5 times on the active lever for each infusion (Rodd-Henricks et al., 2000a). Responses on the inactive lever did not result in infusions. The number of infusions and responses on the active and inactive lever were recorded. The assignment of active and inactive lever with respect to the left or right position was counterbalanced among subjects and remained the same for each rat throughout the experiment. No shaping technique was used to facilitate the acquisition of lever responses. The duration of each test session was 4 h, and sessions occurred every other day. At the end of each test session, rats in the alcohol-drinking group were again given access to 15% ethanol.
Ethanol Dose Response
For ethanol self-infusions, P rats with either chronic access to 15% ethanol and water (ethanol group, n = 6–9/concentration), repeated cycles of EtOH access and abstinence (n = 6–7/concentration), or water alone (n = 6–8/concentration) were assigned to one of six groups, which received artificial CSF alone for all seven sessions or 25, 50, 75, 150, or 300 mg% EtOH for the first four sessions (acquisition), artificial CSF alone during sessions 5 and 6, and their respective ethanol concentration again in session 7. Previous studies (Rodd-Henricks et al., 2000a; Rodd et al., 2004a, b) indicated that P and Wistar rats acquired stable responding on the active lever by the third or fourth session, extinguished responding on the active lever when artificial CSF was substituted for ethanol, and reinstated responding on the active lever when ethanol was returned.
Histology
At the termination of the experiment, animals were sacrificed by CO2 inhalation. Subsequently, 1% bromophenol blue (0.5 μl) was injected into the infusion site. Brains were removed and immediately frozen at −70°C. Frozen brains were subsequently equilibrated at −15°C in a cryostat microtome and then sliced into 40-μm sections. Sections were then stained with cresyl violet and examined under a light microscope for verification of the injection site using the rat brain atlas of Paxinos and Watson (1986).
Statistical Analyses
The initial analysis consisted of a two-factor (group × concentration) ANOVA performed on the average number of reinforcers infused during the initial four sessions. Further data analysis consisted of a group × concentration × session-mixed ANOVA, with a repeated measure of session, performed on the number of infusions and active lever responses. Additionally, for each individual group, lever discrimination was determined by type (active or inactive) × session-mixed ANOVA with a repeated measure of session. Lever discrimination is a key factor when a stimulant is self-administered to distinguish between response-contingent behavior and drug-stimulated locomotor activity.
Results
The posterior VTA was defined as the region of the VTA at the level of the interpeduncular nucleus, coronal sections at −5.3 to −6.0 mm bregma (Fig. 1). Cannula placements surrounding the VTA included injection sites located in the substantia nigra, interpeduncular nucleus, red nucleus, and caudal linear nucleus of the raphe (n = 4–6/pretreatment condition). Data for these rats were not included in the statistical analyses. Cannula placements surrounding the VTA did not support ethanol self-infusions (average number of active and inactive lever responses during acquisition were 17 ± 5 and 16 ± 4, respectively, for placements outside the posterior VTA), which is in agreement with findings previously reported (Rodd-Henricks et al., 2000a, 2003; Rodd et al., 2004a, b).
Fig. 1.
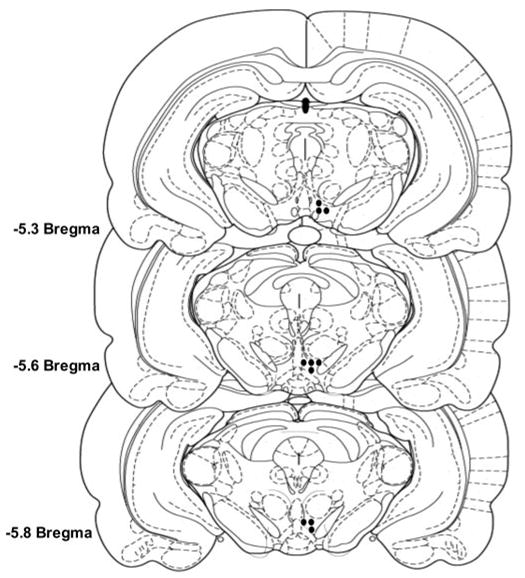
Representative injection sites within the posterior VTA of ethanol-naive, chronic alcohol-drinking, and repeatedly deprived P rats. Circles, placements within the posterior VTA.
Average daily intakes of the chronic alcohol-drinking group were 5–6 g ethanol/kg/day for the 1st week; this intake increased to 6 to 7 g ethanol/kg/day by the end of the experiment. During the 2 weeks of ICSA testing, ethanol intakes were maintained at 6 to 7 g/kg/day (daily intake averages ranged from 6.2 ± 0.9 to 7.1 ± 1.1 g/kg/day). Specifically, the average mean intake for the 3 days before surgery was 6.5 ± 0.4 g/kg/day. During the immediate 3 days after surgery, there was a modest decrease in ethanol intake (mean 5.2 ± 0.8 g/kg/day). However, intake levels returned to presurgery levels and were stable during the 3 days before ICSA testing (6.4 ± 0.5 g/kg/day).
Average daily intakes for the groups exposed to repeated cycles of ethanol access and deprivation were equal to intake observed in the chronic group before the first deprivation period (5.8 ± 0.7 g ethanol/kg/day). After the first deprivation period, rats consumed 12.0 ± 0.7 g ethanol/kg during the first 24 h re-exposure day (data not shown). This increase was a transient phenomenon and was not observed during the 3rd re-exposure day. With each subsequent deprivation period, the amount of ethanol consumed during the 1st re-exposure day increased (i.e., after the third deprivation period, 13.5 g/kg was consumed during the first re-exposure day), and elevated ethanol intakes were observed for longer durations (i.e., after the third deprivation period elevated ethanol intakes were observed for 5 consecutive days). The amount of ethanol consumption was increased after each deprivation cycle and mirrored that observed in past studies (Rodd-Henricks et al., 2000c, 2001; Rodd et al., 2003).
Ethanol concentrations between 0 and 300 mg% were tested to determine the response-contingent behaviors of female P rats with no prior alcohol drinking experience, chronic ethanol drinking experience, and exposure to repeated cycles of ethanol access and deprivation, with injections sites in the posterior VTA (Fig. 2). Reducing the analysis to the average number of infusions received during the four acquisition sessions revealed that there was a significant effect of pretreatment (F2,111 = 61.4; p < 0.0001), concentration (F5,111 = 58.3; p < 0.0001), and a pretreatment × concentration interaction (F10,111 = 5.4; p < 0.0001).
Fig. 2.
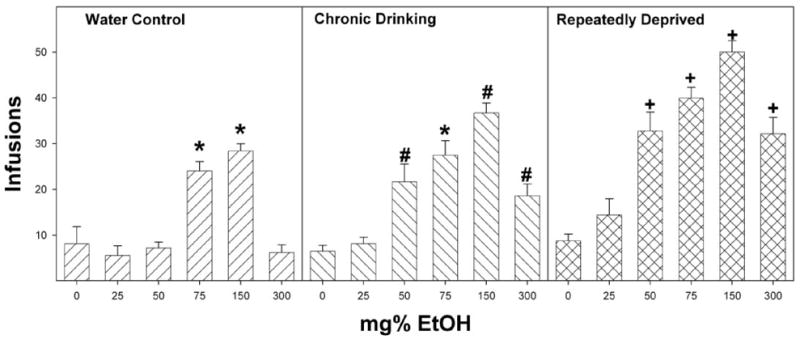
Dose-response effects for self-infusion of 0, 25, 50, 75, 150, and 300 mg% EtOH into the posterior VTA of P rats that are alcohol-naive (water control) have undergone chronic ethanol drinking (chronic drinking) or have been taken through repeated cycles of ethanol access and ethanol deprivation. The number of infusions was averaged over the four acquisition sessions for each animal. *, infusions were significantly greater than aCSF alone (p < 0.05, Tukey’s b). #, significantly greater infusions occurred at these ethanol concentrations compared with artificial CSF and compared with the infusions for the water control group at the same ethanol concentration. +, infusions were significantly greater than both the other groups at the same ethanol concentration and artificial CSF values. Data are the means ± S.E.M. (n = 6–8/dose).
In the P rats that were ethanol naive before ICSA testing, there was a significant effect of ethanol concentration on the number of self-infusions (F5,37 = 23.5; p < 0.0001). Post hoc comparisons (Tukey’s b) indicated that rats allowed to self-infuse 75 and 150 mg% ethanol received significantly more self-infusions than rats self-administering aCSF, 25, 50, or 300 mg% ethanol (Fig. 2). In P rats that had continual access to ethanol, there was a significant effect of ethanol concentration on the number of self-infusions (F5,40 = 19.3; p < 0.0001). Post hoc comparisons (Tukey’s b) indicated that rats given 50, 75, 150, and 300 mg% ethanol received significantly more self-infusions than rats given artificial CSF or 25 mg% ethanol. Additionally, rats given 150 mg% ethanol received significantly more self-infusions than all other concentrations of ethanol. In P rats that experienced repeated cycles of ethanol access and abstinence, there was also a significant effect of ethanol concentration on the number of self-infusions (F5,34 = 25.4; p < 0.0001). Post hoc comparisons (Tukey’s b) indicated that rats self-infusing 50, 75, 150, and 300 mg% ethanol received significantly more self-infusions than rats given artificial CSF or 25 mg% ethanol (Fig. 2). Additionally, rats given 150 mg% ethanol had more self-infusions than all the other groups.
Holding EtOH concentration constant allowed for a direct comparison among the three groups at each concentration tested. There were no differences between the three groups for the self-infusion of aCSF (F2,18 = 0.3; p = 0.73). For the self-infusion of 25 mg% ethanol, there was a significant effect of pre-exposure condition (F2,18 = 3.6; p = 0.048); the post hoc test (Tukey’s b) indicated that P rats exposed to repeated cycles of ethanol access, and deprivation had more self-infusions than P rats that were naive to ethanol before ICSA testing. For P rats self-infusing 50, 150, and 300 mg% ethanol, there was also a significant effect of group at each concentration (F2,18 > 15.4, p < 0.0001); post hoc tests indicated that repeatedly deprived rats had significantly more self-infusions than the nondeprived alcohol-consuming rats. In addition, post hoc tests indicated that the nondeprived alcohol-drinking group had more self-infusions of 50, 150, and 300 mg% ethanol than did the water-drinking control group (Fig. 2). For P rats self-administering 75 mg% EtOH, there was a significant effect of group (F2,18 = 6.9; p = 0.006), with the repeatedly deprived rats receiving more self-infusions than continuous ethanol-drinking or naive rats but no difference between the nondeprived and ethanol-naive groups.
Examining the number of active lever responses across all seven sessions (Figs. 3–6) revealed a significant effect of ethanol drinking experience (F2,111 = 55.5; p < 0.0001), ethanol concentration (F5,111 = 75.8; p < 0.0001), session (F6,106 = 69.2; p < 0.0001), and an ethanol drinking experience × concentration × session interaction (F60,666 = 1.8; p < 0.0001). Figures 3 to 6 show responses on the active and inactive lever for all three treatment groups given 50, 75, 150, and 300 mg% ethanol to self-infuse. Data for the groups given artificial CSF and 25 mg% ethanol are not shown. Regardless of prior ethanol drinking experience, the groups given artificial CSF or 25 mg% ethanol had low levels of responding on the active lever that did not differ across sessions (data for these groups were similar to responses on the active and inactive lever for the water control group given 50 mg% ethanol; see Fig. 3).
Fig. 3.
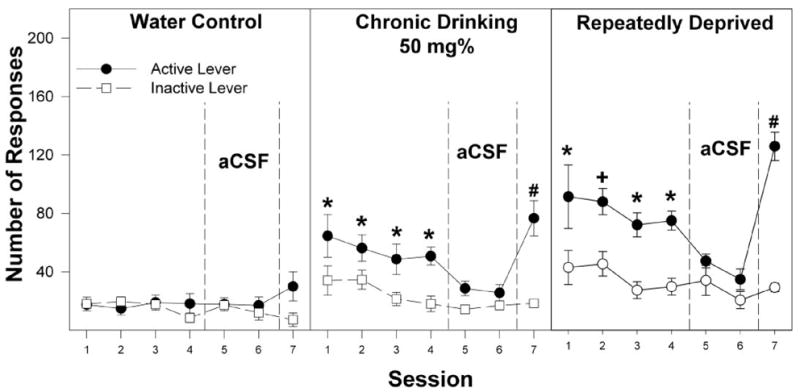
Responses on the active and inactive levers for the self-infusion of 50 mg% ethanol into the posterior VTA of P rats that are alcohol-naive (water control) have undergone chronic ethanol drinking (chronic drinking) or have been taken through repeated cycles of ethanol access and ethanol deprivation during the first four acquisition sessions (sessions 1–4), two extinction sessions (sessions 5 and 6), and the reinstatement session (session 7). *, responses on the active lever are significantly higher (p < 0.05; Tukey’s b) than responses on the inactive lever for that session. +, responses on the active lever are greater (p < 0.05) than responses on the inactive lever and higher than all other groups. #, responses on the active lever are greater (p < 0.05) than responses on the inactive lever for session 7 and higher than responses on the active lever for session 4. Data are the means ± S.E.M. (n = 7–8/group).
Fig. 6.
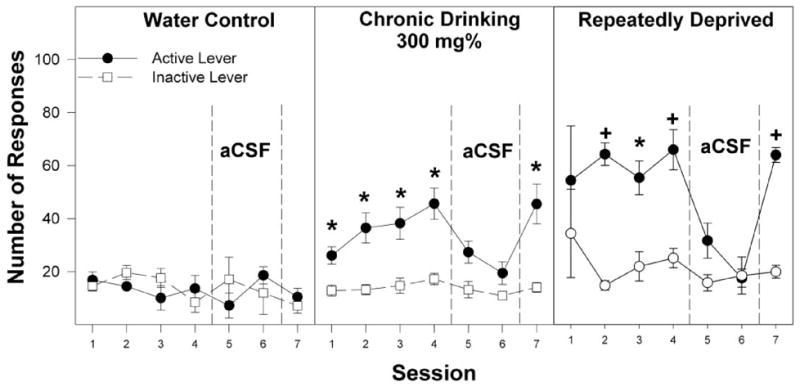
Responses on the active and inactive levers for the self-infusion of 300 mg% EtOH into the posterior VTA of P rats that are alcohol-naive (water control), have undergone chronic ethanol drinking (chronic drinking), or have been taken through repeated cycles of ethanol access and ethanol deprivation during the first four acquisition sessions (sessions 1–4), two extinction sessions (sessions 5 and 6), and the reinstatement session (session 7). *, responses on the active lever are significantly higher (p < 0.05; Tukey’s b) than responses on the inactive lever for that session. +, responses on the active lever are greater (p < 0.05) than responses on the inactive lever and higher than active lever responses for the other two groups for the same session. Data are the means ± S.E.M. (n = 7–8/group).
In P rats with no prior access to ethanol, there was a significant effect of ethanol concentration (F5,37 = 20.3; p < 0.0001) and a concentration × session interaction (F30,180 = 2.4; p < 0.0001) on the number of active lever responses. Using individual ANOVAs, the interaction term was examined for each session to determine the dose effect. For the ethanol-naive group, during the initial four sessions, there was a significant effect of ethanol concentration (F5,37 > 3.9; p < 0.006), and post hoc comparisons (Tukey’s b) indicated that the 75 and 150 mg% ethanol infusate groups responded more on the active lever than the artificial CSF and 25, 50, and 300 mg% ethanol infusate groups. The 75 and 150 mg% EtOH groups discriminated between the active and inactive lever (p < 0.001) for sessions 2 to 4 and during session 7. There was no significant difference between the ethanol concentration groups during session 6 (F5,37 = 0.2; p = 0.95). Extinction was examined by analyzing active lever responses in session 4 versus sessions 5 and 6 in each individual infusate group. In P rats with no prior access to ethanol given 75 and 150 mg% ethanol, there was a significant reduction in the number of active lever responses when artificial CSF was substituted for ethanol in sessions 5 and 6 (p < 0.001). In addition, in session 5 for the group given 150 mg% ethanol, responses on the inactive lever increased significantly. During session 7, when ethanol was returned, there were again significant differences between dose groups (F5,37 = 19.3; p < 0.0001), with post hoc comparisons indicating that the 75 and 150 mg% ethanol groups responded significantly more on the active lever than the groups given artificial CSF or 25, 50, and 300 mg% ethanol. Moreover, during session 7, the 75 and 150 mg% ethanol groups responded significantly more on the active lever than all other dose groups. In addition, responses on the active lever were significantly higher in session 7 compared with session 4.
For the chronic alcohol-drinking group, there was a significant effect of concentration (F5,40 = 34.0; p < 0.0001) and session (F6,35 = 26.2; p < 0.0001) and a significant concentration × session interaction (F30,195 = 2.4; p < 0.0001) for active lever responses (Figs. 3–6). P rats in the chronic ethanol-drinking group responded more on the active lever for 50, 75, 150, and 300 mg% ethanol than did the artificial CSF group. These subgroups also discriminated between the active and inactive levers (p < 0.002) during sessions 1 to 4 and 7. For the chronic alcohol-drinking group, active lever responses decreased when artificial CSF was substituted for ethanol in sessions 5 and 6, and responding on the active lever was reinstated when ethanol was returned in session 7. In session 7, responses on the active lever were significantly higher than in session 4 for the groups given 50, 75, and 150 mg% ethanol but not for 300 mg% ethanol.
In P rats with a history of repeated cycles of alcohol access and deprivation followed by an 8-week of abstinence, there was a significant effects of concentration (F5,34 = 32.7; p < 0.0001) and session (F6,29 = 33.4; p < 0.0001) and a significant concentration × session interaction (F30,165 = 2.5; p < 0.0001) for active lever responses. The repeatedly deprived rats responding significantly more on the active lever for 50, 75, 150, and 300 mg% ethanol than for artificial CSF during sessions 1 to 4 and 7; these rats also readily discriminated the active and inactive levers during these sessions. During sessions 5 and 6 when artificial CSF alone was given, responding on the active lever was reduced to the low levels usually observed for the inactive lever for the groups given 50, 75, 150, and 300 mg% ethanol; when ethanol was returned in session 7, responding on the active lever increased for these four subgroups. Moreover, responding on the active lever in session 7 was significantly higher than session 4 for the 50, 75, and 150 mg% ethanol subgroups but not for the 300 mg% subgroup.
Comparing the number of infusions in sessions 7 versus 4 (Fig. 7) revealed that there was a significant effect of alcohol drinking experience (F2,111 = 43.8; p < 0.0001), ethanol concentration (F5,111 = 69.3; p < 0.0001), and session (F1,111 = 70.0; p < 0.0001) and a significant drinking experience × concentration × session interaction (F10,111 = 10.6; p < 0.0001). Individual repeated measure ANOVAs were performed on the water control, chronic drinking, and repeatedly deprived groups. For the water control group, rats given 75 and 150 mg% EtOH received more self-infusions during session 7 compared with session 4 (all p < 0.001). For the chronic alcohol-drinking group, rats given 50, 75, and 150 mg% ethanol received significantly more self-infusions during session 7 compared with session 4 (all p < 0.001). For the repeatedly deprived group, rats given 25, 50, 75, and 150 mg% EtOH received significantly more infusions during session 7 compared with session 4 (all p < 0.0001). Individual ANOVAs performed on each concentration of EtOH indicated that P rats exposed to repeated cycles of ethanol access and deprivation received more infusions of 25, 50, 75, and 150 mg% ethanol during session 7 than during session 4. Moreover, rats in the repeatedly deprived group received significantly more infusions of 25, 50, and 150 mg% ethanol during session 7 compared with the chronic drinking group.
Fig. 7.
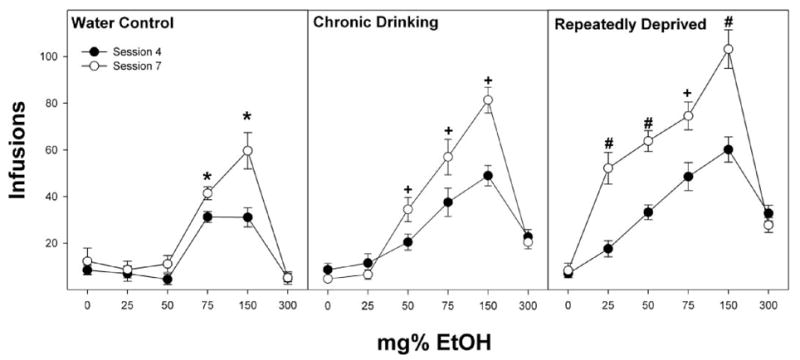
Comparison of infusions of 0 to 300 mg% EtOH into the posterior VTA between session 7 (open circles) and session 4 (closed circles) of P rats that are alcohol-naive (water control), have undergone chronic ethanol drinking (chronic drinking), or have been taken through repeated cycles of ethanol access and ethanol deprivation. *, significant (p < 0.05; Tukey’s b) differences between sessions 4 and 7 for that concentration. +, significant (p < 0.05; Tukey’s b) differences between sessions 4 and 7 for that concentration and higher number of infusions during session 7 than observed in water control rats. #, significant (p < 0.05; Tukey’s b) differences between sessions 4 and 7 for that concentration and higher number of infusions during session 7 than all other groups at that concentration.
Discussion
The major findings of this study support our hypothesis that alcohol drinking by P rats increases the reinforcing effects of ethanol in the posterior VTA of P rats and that repeated deprivations exacerbate these effects (Fig. 2). This interpretation is supported by the data indicating that lower concentrations of ethanol are self-administered into the posterior VTA of ethanol-drinking rats compared with the water control and that higher ethanol self-infusions were administered by the repeatedly deprived group than the chronic alcohol-drinking group (Fig. 2). Also critical to remember is that repeatedly deprived P rats were alcohol-abstinent for 7 to 8 weeks before ICSA testing, a prolonged duration in the life of a rat (approximately one-half-life expectancy). The reinforcing effects of ethanol in the posterior VTA are supported by the findings that the P rats readily discriminated the active from the inactive lever during the acquisition sessions, reduced responding on the active lever when artificial CSF alone was given in sessions 5 and 6 and resumed responding on the active lever when ethanol was restored in session 7 (Figs. 3–6). The 50 to 150 mg% ethanol concentrations that were self-infused into the posterior VTA are physiologically relevant and are within a range that have been attained by P rats under limited or 24-h access conditions (Murphy et al., 1986; Rodd-Henricks et al., 2001).
The present results are in agreement with a previous study indicating that increased sensitivity to the reinforcing effects of ethanol occurs with chronic alcohol drinking experience by the P rat (Rodd et al., 2005b). In the present study, 50 mg% ethanol was reliably self-administered by the chronic alcohol-drinking group but not by the water control group (Fig. 2). Furthermore, for 50 mg% ethanol, the alcohol-experienced group discriminated the active from the inactive lever in all four acquisition sessions, whereas for the water control group, responses on the active and inactive lever were similar and low for all four sessions (Fig. 3). The increased sensitivity of the VTA to the reinforcing effects of ethanol are also in agreement with the findings of Brodie (2002), who reported that chronic alcohol drinking increased ethanol-induced excitation of DA neurons in the VTA. Chronic 24-h free-choice ethanol drinking by P rats was reported to reduce D2 autoreceptor function and increase basal DA neurotransmission in the nucleus accumbens (Thielen et al., 2004). Therefore, it is possible that the higher sensitivity and number of ethanol infusions in the chronic alcohol-drinking group might be a result of higher DA neuronal activity in the VTA.
Previous studies with alcohol-naive rats (Rodd-Henricks et al., 2000a; Rodd et al., 2004a, 2005a) indicated that the dose-response curve for ethanol self-infusions exhibited an inverted U-shape with concentrations of 300 mg% and higher not being reliably self-infused. In the present study, the water control group did not reliably self-infuse 300 mg% ethanol, whereas the chronic alcohol-drinking group did self-infuse this high concentration (Fig. 2). The inverted U-shaped dose-response plot and the findings that higher concentrations of ethanol are not reliably self-administered into the posterior VTA of alcohol-naive rats suggests that higher concentration of ethanol is having another action within the VTA, which inhibits intracranial self-infusion. The mechanisms underlying this secondary high-dose effect of ethanol are unknown. Previous findings indicate that ethanol self-infusion into the posterior VTA is dependent upon DA neuronal activity (Rodd et al., 2004b, 2005a). Therefore, one possible mechanism is that the higher concentrations of ethanol may be reducing VTA DA neuronal activity.
Future projects are planned to assess any alterations in neurotransmitter systems that occur after chronic EtOH consumption and/or after repeat exposures to cycles of EtOH access and deprivation. Already, some published research allows us to speculate about some possible neurotransmitter systems that may underlie the current findings. Repeated twice daily injections (21 days) of EtOH (3.5 g/kg) increased VTA DA neurons to the stimulating effects of EtOH, while reducing the inhibitory influence of GABA on VTA DA neurons (Brodie, 2002). Previously, we have reported that the efficacy of 5HT3 receptor antagonists to reduce EtOH drinking was decreased after a period of deprivation (Rodd-Henricks et al., 2000b). Similar to EtOH, 5HT3 receptor agonists are self-administered into the posterior, but not anterior, VTA (Z. A. Rodd, unpublished findings). Therefore, a valid experiment would be to ascertain the sensitivity of the posterior VTA to the reinforcing actions of 5HT3 receptor agonists after chronic EtOH consumption and exposure to repeated cycles of EtOH access and deprivation. However, it is possible that the neuroadaptations are independent of an alteration in any neurotransmitter systems but may be based upon alterations in the VTA DA neurons.
Chronic alcohol exposure may result in the development of tolerance to the secondary non-reinforcing effect of ethanol and allow expression of the reinforcing effects of ethanol. The development of tolerance to the non-reinforcing effects of ethanol may unmask the reinforcing effects of ethanol at other concentrations, resulting in increased sensitivity and higher self-infusions of ethanol at all concentrations. Alternatively, an explanation for a reduction in the non-reinforcing effects of ethanol with chronic ethanol exposure could be due to the higher basal level of DA neurotransmission observed in the nucleus accumbens of alcohol-drinking P rats (Thielen et al., 2004). The higher basal DA neuronal activity increases the sensitivity of the VTA to the reinforcing effects of ethanol and, at the same time, reduces the effects of the non-reinforcing high concentration of ethanol.
Another important finding is that in session 7, when ethanol is restored after two extinction sessions, higher responding on the active lever (Figs. 4 and 5), and higher number of infusions (Fig. 7) were attained for the water control group self-administering 75 and 150 mg% ethanol. This higher responding and infusions for ethanol after extinction have been previously reported for P rats (Rodd et al., 2004a, b, 2005a, b) but were not found for Wistar rats (Rodd et al., 2004a, b), suggesting that genetic factors may play a role in this phenomenon. The robust responding in session 7 after the two extinction sessions may be a significant factor contributing to the robust expression of an ADE observed for P rats during relapse drinking (Rodd-Henricks et al., 2000c, 2001; Rodd et al., 2003). The mechanisms underlying this effect are unknown but might be due to higher DA neuronal activity resulting from the incomplete re-establishment of the function of one or more receptors that have been down- or up-regulated within the posterior VTA. In the chronic alcohol-drinking group, the differences between sessions 7 and 4 for the self-infusions of 50, 75, and 150 mg% ethanol are significantly greater than were observed for the water control group (Fig. 7). These results suggest that the mechanisms underlying the higher responding and infusions in session 7 may be exacerbated by chronic alcohol exposure.
Fig. 4.
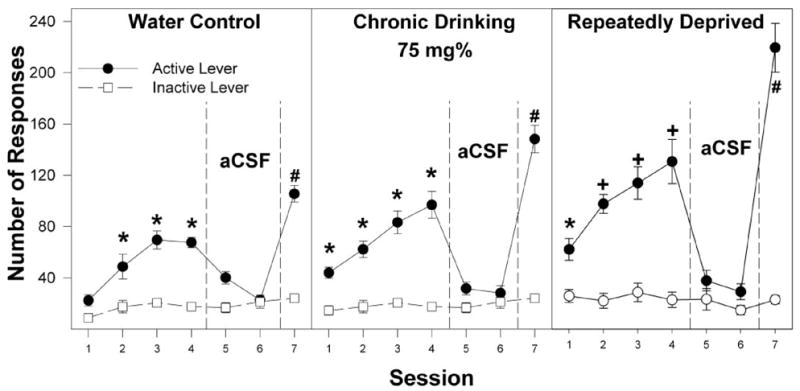
Responses on the active and inactive levers for the self-infusion of 75 mg% ethanol into the posterior VTA of P rats that are alcohol-naive (water control), have undergone chronic ethanol drinking (chronic drinking), or have been taken through repeated cycles of ethanol access and ethanol deprivation during the first four acquisition sessions (sessions 1–4), two extinction sessions (sessions 5 and 6), and the reinstatement session (session 7). *, responses on the active lever are significantly higher (p < 0.05; Tukey’s b) than responses on the inactive lever for that session. +, responses on the active lever are greater (p < 0.05) than responses on the inactive lever and higher than all other groups. #, responses on the active lever are greater (p < 0.05) than responses on the inactive lever for session 7 and higher than responses on the active lever for session 4. Data are the means ± S.E.M. (n = 7–8/group).
Fig. 5.
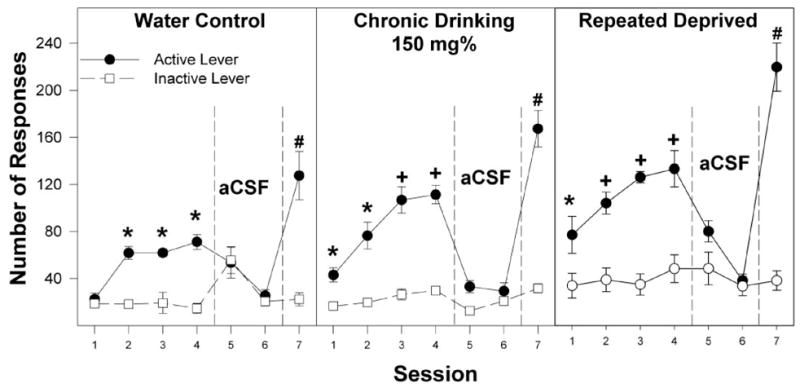
Responses on the active and inactive levers for the self-infusion of 150 mg% EtOH into the posterior VTA of P rats that are alcohol-naive (water control), have undergone chronic ethanol drinking (chronic drinking), or have been taken through repeated cycles of ethanol access and ethanol deprivation during the first four acquisition sessions (sessions 1–4), two extinction sessions (sessions 5 and 6), and the reinstatement session (session 7). *, responses on the active lever are significantly higher (p < 0.05; Tukey’s b) than responses on the inactive lever for that session. +, responses on the active lever are greater (p < 0.05) than responses on the inactive lever and higher than the water control values. #, responses on the active lever are greater (p < 0.05) than responses on the inactive lever for session 7 and higher than responses on the active lever for session 4. Data are the means ± S.E.M. (n = 7–8/group).
Another major finding of the present study is that the repeatedly deprived group responded more on the active lever (Figs. 3–6) and received more infusions (Fig. 2) of ethanol than the chronic alcohol-drinking group, suggesting that repeated deprivations have a greater impact on the reinforcing properties of ethanol in the posterior VTA than chronic alcohol drinking alone. These results are in agreement with several studies indicating that repeated cycles of alcohol access and deprivation increased ethanol intake (Rodd-Henricks et al., 2001), ethanol self-administration (Rodd et al., 2003; O’Dell et al., 2004), and withdrawal severity (Becker et al., 1997). The mechanisms underlying these robust effects on ethanol self-infusions into the posterior VTA are unknown. In addition, some of the neuronal alterations produced by repeated deprivations are long-lasting because the present study was conducted 8 weeks after the last ethanol drinking episode. These results indicate that the reinforcing effects of ethanol within the posterior VTA are more pronounced in the repeatedly deprived group than in the other two groups and that these effects are observed after protracted abstinence. These findings suggest the CNS of the P rat has undergone neuronal alterations within the mesolimbic DA system as a result of chronic alcohol drinking and repeated deprivations, which persists in the absence of alcohol and could contribute to alcohol craving and relapse in vulnerable individuals.
ABBREVIATIONS
- P
alcohol-preferring
- ADE
alcohol deprivation effect
- VTA
ventral tegmental area
- DA
dopamine
- ICSA
intracranial self-administration
- EtOH
ethanol
- CSF
cerebrospinal fluid
- aCSF
artificial CSF
- ANOVA
analysis of variance
Footnotes
This study was supported in part by National Institute on Alcohol Abuse and Alcoholism Grants AA11261, AA07611, AA12262, and AA13522.
Article, publication date, and citation information can be found at http://jpet.aspetjournals.org.
References
- Becker HC, Diaz-Granados JL, Weathersby RT. Repeated ethanol withdrawal experience increases the severity and duration of subsequent withdrawal seizures in mice. Alcohol. 1997;14:319–326. doi: 10.1016/s0741-8329(97)87949-9. [DOI] [PubMed] [Google Scholar]
- Bozarth MA, Wise RA. Electrolytic microinfusion transducer system: an alternative method of intracranial drug application. J Neurosci Methods. 1980;2:273–275. doi: 10.1016/0165-0270(80)90016-3. [DOI] [PubMed] [Google Scholar]
- Brodie MS. Increased ethanol excitation of dopaminergic neurons of the ventral tegmental area after chronic ethanol treatment. Alcohol Clin Exp Res. 2002;26:1024–1030. doi: 10.1097/01.ALC.0000021336.33310.6B. [DOI] [PubMed] [Google Scholar]
- Brodie MS, Appel SB. Dopaminergic neurons in the ventral tegmental area of C57BL/6J and DBA/2J mice differ in sensitivity to ethanol excitation. Alcohol Clin Exp Res. 2000;24:1120–1124. [PubMed] [Google Scholar]
- Brodie MS, Pesold C, Appel SB. Ethanol directly excites dopaminergic ventral tegmental area reward neurons. Alcohol Clin Exp Res. 1999;23:1848–1852. [PubMed] [Google Scholar]
- Brodie MS, Shefner SA, Dunwiddie TV. Ethanol increases the firing rate of dopamine neurons of the rat ventral tegmental area in vitro. Brain Res. 1990;508:65–69. doi: 10.1016/0006-8993(90)91118-z. [DOI] [PubMed] [Google Scholar]
- Brodie MS, Trifunovic RD, Shefner SA. Serotonin potentiates ethanol-induced excitation of ventral tegmental area neurons in brain slices from three different rat strains. J Pharmacol Exp Ther. 1995;273:1139–1146. [PubMed] [Google Scholar]
- Congar P, Bergevin A, Trudeau LE. D2 receptors inhibit the secretory process downstream from calcium influx in dopaminergic neurons: implication of K+ channels. J Neurophysiol. 2002;87:1046–1056. doi: 10.1152/jn.00459.2001. [DOI] [PubMed] [Google Scholar]
- Gatto GJ, McBride WJ, Murphy JM, Lumeng L, Li T-K. Ethanol self-infusion into the ventral tegmental area by alcohol-preferring rats. Alcohol. 1994;11:557–564. doi: 10.1016/0741-8329(94)90083-3. [DOI] [PubMed] [Google Scholar]
- Gessa GL, Muntoni F, Collu M, Vargui L, Mereu G. Low doses of ethanol activate dopaminergic neurons in the ventral tegmental area. Brain Res. 1985;348:201–293. doi: 10.1016/0006-8993(85)90381-6. [DOI] [PubMed] [Google Scholar]
- Hodge CW, Haraguchi M, Erickson H, Samson HH. Ventral tegmental microinjections of quinpirole decrease ethanol and sucrose-reinforced responding. Alcohol Clin Exp Res. 1993;17:370–375. doi: 10.1111/j.1530-0277.1993.tb00778.x. [DOI] [PubMed] [Google Scholar]
- Jeziorski M, White FJ. Dopamine agonists at repeated “autoreceptor-selective” doses: effects upon the sensitivity of A10 dopamine autoreceptors. Synapse. 1989;4:267–280. doi: 10.1002/syn.890040403. [DOI] [PubMed] [Google Scholar]
- McBride WJ, Murphy JM, Ikemoto S. Localization of brain reinforcement mechanisms: intracranial self-administration and intracranial place-conditioning studies. Behav Brain Res. 1999;101:129–152. doi: 10.1016/s0166-4328(99)00022-4. [DOI] [PubMed] [Google Scholar]
- Melendez RI, Rodd-Henricks ZA, Engleman EA, Li T-K, McBride WJ, Murphy JM. Microdialysis of dopamine in the nucleus accumbens of alcohol-preferring (P) rats during anticipation and operant self-administration of ethanol. Alcohol Clin Exp Res. 2002;26:318–325. [PubMed] [Google Scholar]
- Murphy JM, Gatto GJ, Waller MB, McBride WJ, Lumeng L, Li T-K. Effects of scheduled access on ethanol intake by the alcohol-preferring P line of rats. Alcohol. 1986;3:331–336. doi: 10.1016/0741-8329(86)90010-8. [DOI] [PubMed] [Google Scholar]
- Nowak KL, McBride WJ, Lumeng L, Li T-K, Murphy JM. Involvement of dopamine D2 autoreceptors in the ventral tegmental area on alcohol and saccharin intake of the alcohol-preferring P rat. Alcohol Clin Exp Res. 2000;24:476–483. [PubMed] [Google Scholar]
- O’Dell LE, Roberts AJ, Smith RT, Koob GF. Enhanced alcohol self-administration after intermittent versus continuous alcohol vapor exposure. Alcohol Clin Exp Res. 2004;28:1676–1682. doi: 10.1097/01.alc.0000145781.11923.4e. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. Academic Press; New York: 1986. [Google Scholar]
- Rodd ZA, Bell RL, Kuc KA, Murphy JM, Lumeng L, Li T-K, McBride WJ. Effects of repeated alcohol deprivations on operant ethanol self-administration by alcohol-preferring (P) rats. Neuropsychopharmacology. 2003;28:1614–1621. doi: 10.1038/sj.npp.1300214. [DOI] [PubMed] [Google Scholar]
- Rodd ZA, Bell RL, McQueen VK, Davids MR, Hsu CC, Murphy JM, Li T-K, Lumeng L, McBride WJ. Chronic ethanol drinking by alcohol-preferring (P) rats increases the sensitivity of the posterior ventral tegmental area to the reinforcing effects of ethanol. Alcohol Clin Exp Res. 2005a;29:358–366. doi: 10.1097/01.alc.0000156127.30983.9d. [DOI] [PubMed] [Google Scholar]
- Rodd ZA, Bell RL, Melendez RI, Kuc KA, Lumeng L, Li T-K, Murphy JM, McBride WJ. Comparison of intracranial self-administration of ethanol within the posterior ventral tegmental area between alcohol-preferring (P) and Wistar rats. Alcohol Clin Exp Res. 2004a;28:1212–1219. doi: 10.1097/01.alc.0000134401.30394.7f. [DOI] [PubMed] [Google Scholar]
- Rodd ZA, Bell RL, Zhang Y, Murphy JM, Goldstein A, Zaffaroni A, Li T-K, McBride WJ. Regional heterogeneity for intracranial self-administration of ethanol and acetaldehyde within the ventral tegmental area of alcohol-preferring (P) rats: involvement of dopamine and serotonin. Neuropsychopharmacology. 2005b;30:330–338. doi: 10.1038/sj.npp.1300561. [DOI] [PubMed] [Google Scholar]
- Rodd ZA, Melendez RI, Bell RL, Kuc KA, Zhang Y, Murphy JM, McBride WJ. Intracranial self-administration of ethanol within the ventral tegmental area of male Wistar rats: evidence for regional heterogeneity and involvement of dopamine neurons. J Neurosci. 2004b;24:1050–1057. doi: 10.1523/JNEUROSCI.1319-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodd-Henricks ZA, Bell RL, Kuc KA, Murphy JM, McBride WJ, Lumeng L, Li T-K. Effects of concurrent access to multiple ethanol concentrations and repeated deprivations on alcohol intake of alcohol-preferring rats. Alcohol Clin Exp Res. 2001;25:1140–1150. [PubMed] [Google Scholar]
- Rodd-Henricks ZA, McKinzie DL, Crile RS, Murphy JM, McBride WJ. Regional heterogeneity for the intracranial self-administration of ethanol within the ventral tegmental area of female Wistar rats. Psychopharmacology. 2000a;149:217–224. doi: 10.1007/s002139900347. [DOI] [PubMed] [Google Scholar]
- Rodd-Henricks ZA, McKinzie DL, Edmundson VE, Dagon CL, Murphy JM, McBride WJ, Lumeng L, Li T-K. Effects of 5-HT3 receptor antagonists on daily alcohol intake under acquisition, maintenance and relapse conditions in alcohol-preferring (P) rats. Alcohol. 2000b;21:73–85. doi: 10.1016/s0741-8329(00)00083-5. [DOI] [PubMed] [Google Scholar]
- Rodd-Henricks ZA, McKinzie DL, Melendez RI, Berry N, Murphy JM, McBride WJ. The effects of serotonin-3 receptor antagonists on the intracranial self-administration of ethanol into the posterior VTA of Wistar rats. Psychopharmacology. 2003;165:252–259. doi: 10.1007/s00213-002-1300-2. [DOI] [PubMed] [Google Scholar]
- Rodd-Henricks ZA, McKinzie DL, Shaikh SR, Murphy JM, McBride WJ, Lumeng L, Li T-K. Alcohol deprivation effect is prolonged in the alcohol-preferring (P) rat after repeated deprivations. Alcohol Clin Exp Res. 2000c;24:8–16. [PubMed] [Google Scholar]
- Sinclair JD. The alcohol deprivation effect: influence of various factors. Q J Stud Alcohol. 1972;33:769–782. [PubMed] [Google Scholar]
- Sinclair JD, Walker S, Jordan W. Behavioral and physiological changes associated with various durations of alcohol deprivation in rats. Q J Stud Alcohol. 1973;34:744–757. [PubMed] [Google Scholar]
- Thielen RJ, Engleman EA, Rodd ZA, Murphy JM, Lumeng L, Li T-K, McBride WJ. Ethanol drinking and deprivation alter dopaminergic and serotonergic function in the nucleus accumbens of alcohol-preferring rats. J Pharmacol Exp Ther. 2004;309:216–225. doi: 10.1124/jpet.103.059790. [DOI] [PubMed] [Google Scholar]
- Weiss F, Lorang MT, Bloom FE, Koob GF. Oral alcohol self-administration stimulates dopamine release in the rat nucleus accumbens: genetic and motivational determinants. J Pharmacol Exp Ther. 1993;276:250–258. [PubMed] [Google Scholar]
- Weiss F, Parsons LH, Schutleis G, Hyytia P, Lorang MT, Bloom FE, Koob GF. Ethanol self-administration restores withdrawal-associated deficiencies in accumbal dopamine and 5-hydroxytryptamine release in dependent rats. J Neurosci. 1996;16:3474–3485. doi: 10.1523/JNEUROSCI.16-10-03474.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]


