Abstract
Recent studies in both animal models and clinical trials have demonstrated that the avidity of T cells is a major determinant of anti-tumor and anti-viral immunity. Here, we evaluated several different vaccine strategies for their ability to enhance both the quantity and avidity of CTL responses. CD8+ T-cell quantity was measured by tetramer binding precursor frequency, and avidity was measured by both tetramer dissociation and quantitative cytolytic function. We have evaluated a peptide, a viral vector expressing the antigen transgene alone, with one costimulatory molecule (B7-1), and with three costimulatory molecules (B7-1, ICAM-1 and LFA-3), with anti-CTLA-4 mAb, with GM-CSF, and combinations of the above. We have evaluated these strategies in both a foreign antigen model employing β-gal as immunogen, and in a “self” antigen model, employing CEA as immunogen in CEA transgenic (Tg) mice. The combined use of several of these strategies was shown to enhance not only the quantity, but, to a greater magnitude, the avidity of T cells generated; a combination strategy is also shown to enhance anti-tumor effects. The studies reported here thus demonstrate multiple strategies that can be employed in both anti-tumor and anti-viral vaccine settings to generate higher avidity host T-cell responses.
Keywords: Vaccine, Poxvirus, Costimulation, Avidity, CTLA-4
Introduction
The use of vaccines as therapeutic interventions for infectious diseases and cancer is in an active state of investigation. Numerous vaccine modalities are being evaluated in both preclinical models and in clinical trials. A principal evaluation of the efficiency of a therapeutic vaccine is its ability to generate antigen-specific T-cell responses, with emphasis currently being placed on the level of generation of cytotoxic T lymphocytes (CTL). This has been evaluated for the most part by tetramer binding, ELISPOT or other assays to monitor cytokine secretion by CTL, and by the ability of CTL to lyse target cells. While the vast majority of studies have quantitatively evaluated these responses, few studies employing different vaccines and vaccine strategies have evaluated or defined the actual avidity of the T cells generated.
As previously defined,(1-4) CTL that can recognize peptide/MHC only at high antigen density are termed low avidity CTL, while those that can recognize peptide/MHC at low densities are termed high avidity CTL. Studies have demonstrated that high avidity CTL are more effective in the elimination of tumor cells and viral clearance.(1, 5–9) It has also been shown that when high avidity CTL are selected by tetramer positive sorting of high TCR density, these higher avidity CTL can lyse targets more efficiently than their lower avidity counterparts.(7, 10) Higher avidity CTL have also been selected from immunized mice by culturing heterogeneous splenic lymphocyte populations with low peptide densities.(1) While these above methods have been used to select for higher avidity CTL in heterogeneous T-cell populations, only one report exists on a methodology of enhancing costimulation to enhance T-cell avidity in-vivo. In that study, Oh et al.(11) utilized peptide-pulsed splenocytes that overexpressed costimulatory molecules to vaccinate mice. The use of anti-CTLA-4 antibody to block the inhibitory role of CTLA-4 has also been hypothesized to select for higher affinity T-cell clones.(12, 13) However, to our knowledge, no experimental data have thus far been reported to support this hypothesis.
In the study reported here, we have evaluated several different vaccines and vaccine strategies for their ability to enhance the quantity and avidity of CTL responses. CD8+ T-cell quantity was measured by tetramer binding precursor frequency, and avidity was measured by both tetramer dissociation and cytolytic function. These methods were utilized to monitor T-cell precursor frequency and avidity directly from fresh mouse splenocytes (tetramer binding and dissociation assay), as well as after a short in-vitro restimulation (CTL assay). We have evaluated a peptide, a vector expressing the antigen transgene alone, with one costimulatory molecule (B7-1), and with three costimulatory molecules (B7-1, ICAM-1 and LFA-3, designated TRICOM), the use of anti-CTLA-4 mAb, the use of GM-CSF, and combinations of the above. We have evaluated these vaccines in both a non-self model employing β-gal as the immunogen, and in a “self” model, employing CEA as the immunogen in CEA-transgenic (Tg) mice. Finally, employing this model, we provide evidence that several of these strategies are complimentary not only in enhancing the quantity and avidity of T cells generated, but also in mediating anti-tumor effects in the absence of autoimmunity.
Materials and Methods
Animals
Female C57BL/6 mice were obtained from the National Cancer Institute, Frederick Cancer Research Facility (Frederick, MD). Female C57BL/6 mice transgenic for human CEA have been previously described.(14) Mice were housed and maintained under pathogen-free conditions in microisolator cages until used for experiments at 6–8 weeks of age.
Tumor cells
For quantitative cytotoxicity assays, the target tumor cell line EL-4 (H-2b, thyoma, ATCC TIB-39) pulsed with different concentrations of peptide was used. For in-vivo studies, murine colon adenocarcinoma MC38 cells (H-2b) expressing human CEA (designated MC38-CEA+) were used.(15)
Recombinant poxviruses
The recombinant vaccinia viruses designated rV-CEA(16) and rV-CEA/B7-1(17) have been described. rV-CEA/TRICOM contains the murine B7-1, ICAM-1, and LFA-3 genes in combination with the human gene CEA as described elsewhere.(18) The recombinant vaccinia viruses designated rV-LacZ, rV-LacZ/B7-1, and rV-LacZ/TRICOM were constructed in a similar manner, and contain the LacZ gene encoding β-galactosidase (referred as β-gal). The recombinant fowlpox virus rF-CEA/TRICOM contains the murine B7-1, ICAM-1, and LFA-3 genes in combination with the human gene CEA as described elsewhere.(18) The recombinant fowlpox containing the murine GM-CSF gene (designated rF-GM-CSF) has been described.(19) Therion Biologics Corporation (Cambridge, MA) kindly provided all orthopox viruses as part of a Collaborative Research and Development Agreement with NCI.
Peptides
The H-2Kb restricted peptides β-gal (beta galactosidase96–103, DAPIYTNV(20)), OVA (ovalbumin257–264, SIINFEKL(21)) and the H-2Db restricted peptides CEA (CAP-M8, CEA526–533, EAQNTTYL(21)) and VSV-NP (NP52–59, RGYVYQGL(21)) were purchased (Synpep, Dublin, CA).
Anti-CTLA-4 mAb
Anti CTLA-4 mAb (9H10)(22) was a gift from J. Allison (University of California, Berkeley, CA). Control Purified Syrian Hamster IgG2 kappa was obtained from BD Pharmingen, San Diego, CA.
Vaccination modalities
Non-self antigen system
C57BL/6 mice were vaccinated with buffer (HBSS), β-gal peptide (100 μg) emulsified in Incomplete Freunds adjuvant (IFA), rV-LacZ, rV-LacZ/B7-1, or rV-LacZ/TRICOM. In another experiment, mice were vaccinated with rV-LacZ and administered anti-CTLA-4 mAb I.P. over a 6-day period (100 μg on day 0, 50 μg on days 3 and 6). As control, mice were vaccinated with rV-LacZ in combination with isotype control mAb. To examine the effects of GM-CSF, mice were vaccinated with rV-LacZ/TRICOM admixed with rF-GM-CSF. Finally, mice were vaccinated with rV-LacZ/TRICOM admixed with rF-GM-CSF and administered anti-CTLA-4 mAb. All recombinant vaccinia viruses were given s.c. at 1×108 pfu/mouse and rF-GM-CSF was given at 1×107 pfu/mouse. After 30 days, splenocytes were harvested (n=3/group) and used to determine T-cell avidity. These assays were repeated at least one time with similar results. To facilitate comparisons between groups, indicated data are reproduced in more than one figure.
Self antigen system
CEA-Tg mice were vaccinated with buffer (HBSS), rV-CEA, rV-CEA/B7-1, or rV-CEA/TRICOM. In another experiment, mice were vaccinated with rV-CEA and administered anti-CTLA-4 mAb and/or rF-GM-CSF as described above.
Determination of CD8+ T-cell precursor frequency
To evaluate the frequency of β-gal–specific CTL in C57BL/6 mice (n = 3/group) after vaccination, splenocytes were stained with FITC-conjugated anti-CD3e mAb (Pharmingen), CyChrome-conjugated anti-CD8 mAb (Pharmingen) and PE -conjugated β-gal/H-2Kb-tetramer (NIH Tetramer Facility, NIAID, Bethesda, MD). To evaluate the frequency of CEA-specific CTL in CEA-Tg mice after vaccination, splenocytes were stained with FITC-conjugated anti-CD3e mAb, CyChrome-conjugated anti-CD8 mAb and PE-conjugated CEA/H-2Kb-tetramer (iTAg, Beckman Coulter, Fullerton, CA). Immunofluorescence staining was performed after Fc receptor-blocking with anti-CD16/CD32 mAb (Pharmingen). The immunofluorescence was compared with the appropriate isotype-matched controls and analyzed with Cellquest software using a FACSCalibur cytometer (Becton-Dickinson, Mountain View, CA). Results were depicted as percent CD8+/tetramer+ T cells of CD3+ T cells. Precursor frequency was also expressed as percent tetramer+ T cells of CD8+ T cells (Table 1).
Table 1.
Effect of multiple costimulatory modalities to enhance CTL avidity
| Vaccine Modality | Precursor Frequency/105 CD8 T-cells | Tetramer dissociation time (Min) | Peptide concentration for CTL (nM) | ||
|---|---|---|---|---|---|
| Buffer (HBSS) | 800 | ND | NA | ||
| β-gal peptide/IFA | 1500 | 50 | 720 | ||
| rV-LacZ | 2650 | 1X | 85 | 160 | 1X |
| rV-LacZ/B7-1 | 3800 | 1.4X | 127 | 27 | 6X |
| rV-LacZ/TRICOM | 5900 | 2.2X | 300 | 3 | 53.3X |
| rV-LacZ + Isotype control mAb | 2424 | 1X | 70 | ND | |
| rV-LacZ + CTLA-4 mAb | 4191 | 1.7X | 90 | ND | |
| rV-LacZ/B7-1 + Isotype control mAb | 3393 | 1.4X | 118 | ND | |
| rV-LacZ/TRICOM + Isotype control mAb | 5709 | 2.4X | 235 | ND | |
| rV-LacZ/TRICOM + CTLA-4 mAb | 8536 | 3.5X | 310 | ND | |
| rV-LacZ/TRICOM + GM-CSF | 8200 | 1.4X | 360 | 0.05 | 3,200X |
| rV-LacZ/TRICOM + GM-CSF + CTLA-4 mAb | 13500 | 5.1X | 475 | 0.003 | 53,333X |
| Buffer (HBSS) | 133 | ND | NA | ||
| rV-CEA | 321 | 1X | 88 | 510 | 1X |
| rV-CEA/B7-1 | 584 | 1.8X | 135 | 110 | 4.6X |
| rV-CEA/TRICOM | 769 | 2.4X | 233 | 5 | 102X |
| rV-CEA | 455 | 1X | 70 | 950 | 1X |
| rV-CEA + CTLA-4 mAb | 784 | 1.7X | 105 | 237 | 4X |
| rV-CEA/B7-1 | 674 | 1.5X | 120 | 135 | 7X |
| rV-CEA/TRICOM + CTLA-4 mAb | 1303 | 4X | 370 | 0.4 | 1,275X |
| rV-CEA/TRICOM + GM-CSF | 1289 | 4X | 315 | 0.6 | 850X |
| rV-CEA/TRICOM + GM-CSF + CTLA-4 mAb | 1690 | 5.3X | 480 | 0.02 | 25,500X |
Determination of CD8+ T-cell avidity: tetramer dissociation
Tetramer decay/dissociation was performed largely as described by Holmberg et al.(23) Briefly, splenocytes were stained with FITC-conjugated anti-CD3e mAb, CyChrome-conjugated anti-CD8 mAb and PE-conjugated-tetramer as described above. Cells were washed twice and kept on ice until they were mixed with appropriate excess anti-Kb or Db and then incubated at 37°C to allow tetramer dissociation. Dissociation was followed for 0–180 minutes, at which time the cells were fixed with Cytofix (Pharmingen). Results were expressed as the percentage of tetramer positive cells over time. To normalize groups within each experiment, data was also expressed as the percentage of maximum tetramer binding over time. Finally, the natural logarithm of the normalized data was plotted against time. The dissociation half-life of each tetramer was derived from the slope of the natural logarithm, and was expressed in minutes.
Determination of CD8+ T-cell avidity: cytotoxicity assay
To evaluate the avidity of β-gal–specific CTL in C57BL/6 mice after vaccination, splenocytes were pooled and dispersed into single-cell suspensions, and then stimulated with β-gal peptide (10 μg/ml). To evaluate the frequency of CEA-specific CTL in CEA-Tg mice after vaccination, splenocytes were pooled and dispersed into single-cell suspensions, and then stimulated with CEA peptide (10 μg/ml). Six days later, bulk lymphocytes were separated by centrifugation through a Ficoll-Hypaque gradient. Using these recovered lymphocytes, tumor-killing activity was tested as described previously.(16) Briefly, the recovered lymphocytes (2.4 x105 cells/well) and 111In-labeled target tumor cells (EL-4, 3×103 cells/well) were coincubated at a constant E:T ratio (80:1) in the presence of diminishing concentrations of peptide (10 μm-0μm) for 5 hours (96-well U-bottom plates), and radioactivity in supernatants was measured using a γ-counter (Corba Autogamma, Packard Instruments, Downers Grove, IL). In a separate plate, lymphocytes and target cells (E:T 80:1) were coincubated with control peptides: OVA for the β-gal peptide and VSV-NP for the CEA peptide. The percentage of tumor lysis was calculated as follows: % Tumor lysis = [(experimental cpm − spontaneous cpm)/(maximum cpm − spontaneous cpm)] × 100. Non-specific 111 In release in response to each control peptide was subtracted from that induced by the appropriate antigen peptide. The data were averaged and graphed as Δ% specific lysis. To normalize groups within each experiment, data were also expressed as percentage of maximum lysis vs. peptide concentration. Finally, the natural logarithm of the normalized data was plotted against peptide concentration. The avidity of each T-cell population was defined as the negative log of the peptide concentration that resulted in 50% maximal target lysis (5) and was expressed in nM.
Statistical analysis
Significant differences were statistically evaluated using Analysis of Variance (ANOVA) with repeated measures using Statview 4.1 (Abacus Concepts Inc, Berkeley, CA). Correlation coefficients were determined using Statview. Evaluation of survival patterns in mice bearing MC38-CEA+ tumors was performed by the Kaplan-Meier method.
RESULTS
Peptide vs. vector
Studies were conducted in the β-gal model to compare the ability of β-gal peptide vs. rV-LacZ to generate T-cell responses of different or similar avidities. As seen in Figure 1A, rV-LacZ enhanced tetramer positive CD8+ T cells in vaccinated mice by approximately 2-fold compared with peptide-vaccinated mice. This was accompanied by a slight increase in tetramer dissociation time in the rV-LacZ group. Furthermore, lysis was enhanced 4-fold in rV-LacZ vaccinated mice (Figure 1B and C, open vs. closed squares, Table 1).
Figure 1. Relationship of vaccine-mediated costimulation to antigen-specific T-cell precursor frequency and T-cell avidity.
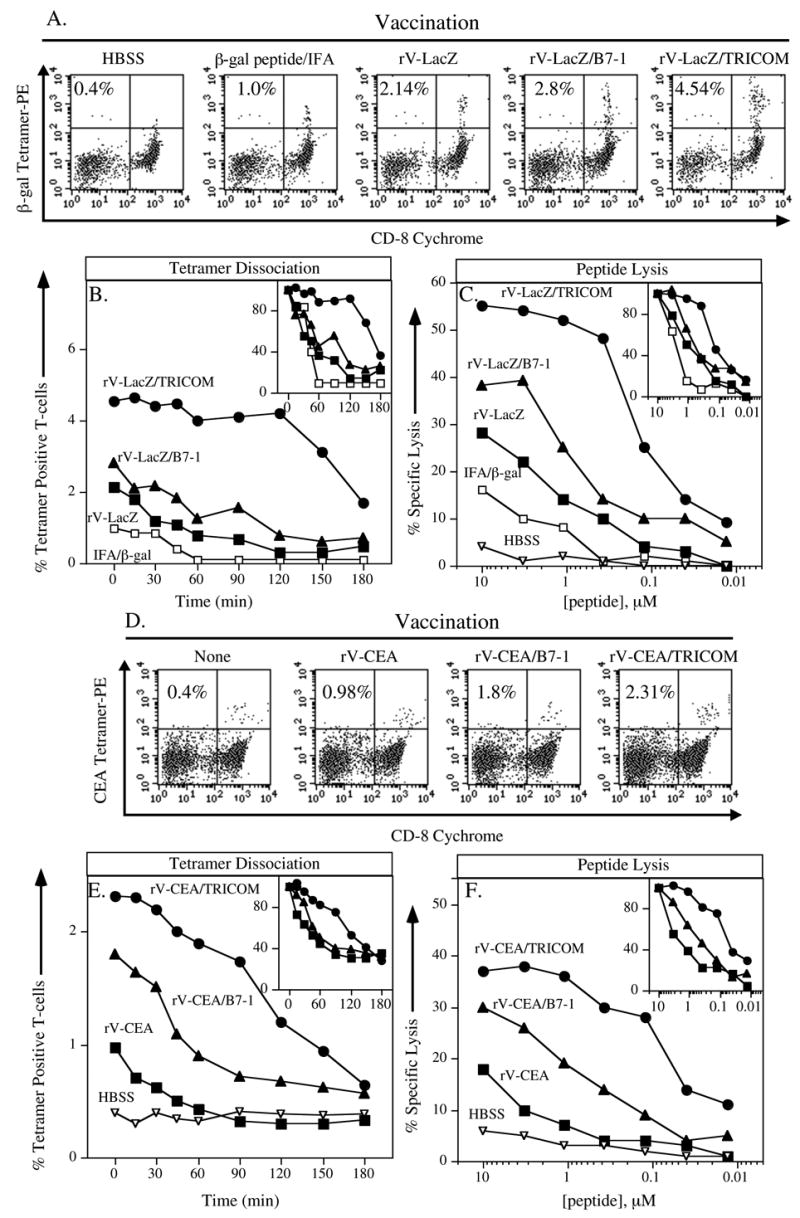
Panels A–C: C57BL/6 mice were vaccinated with buffer (HBSS, inverted open triangles), β-gal peptide emulsified in Incomplete Freunds adjuvant (IFA, open squares), rV-LacZ (closed squares), rV-LacZ/B7-1 (closed triangles), or rV-LacZ/TRICOM (closed circles). After 30 days, splenocytes were harvested. Panel A: β-gal–specific CD8+ T-cell precursor frequency as determined by tetramer staining. Inset number indicates percent β-gal tetramer+/CD8+ T cells of CD3+ T cells. Panel B: β-gal–specific CD8+ T-cell avidity as determined by tetramer dissociation. To normalize groups within each experiment, data was also expressed as the percentage of maximum tetramer binding over time (Inset panel). Panel C: β-gal–specific CD8+ T-cell avidity as determined by CTL assay. Inset panel depicts results that are normalized as the percentage of maximum lysis.(11) Panels D–F: CEA-Tg mice were vaccinated with either buffer (HBSS, inverted open triangles), rV-CEA (closed squares), rV-CEA/B7-1 (closed triangles), or rV-CEA/TRICOM (closed circles). After 30 days, splenocytes were harvested. Panel D: CEA-specific CD8+ T-cell precursor frequency as determined by tetramer staining. Inset number indicates percent CEA tetramer+/CD8+ T cells of CD3+ T cells. Panel E: CEA-specific CD8+ T-cell avidity as determined by tetramer dissociation. Inset panel depicts results that are normalized as the percentage of maximum tetramer binding. Panel F: CEA-specific CD8+ T-cell avidity as determined by CTL assay. Inset panel depicts results that are normalized as the percentage of maximum lysis.
Vector-driven costimulation
We next compared CTL responses in mice vaccinated with rV-LacZ, rV-LacZ/B7-1, and rV-LacZ/TRICOM (rV-LacZ/B7-1/ICAM-1/LFA-3). We have previously shown, using other models and T-cell proliferation assays, that vectors containing three costimulatory molecules are more efficient than vectors devoid of, or containing one or two costimulatory molecules in generating greater quantities of T cells.(18) This is extended here with the analysis of tetramer binding T cells; there is a modest increase in the number of tetramer binding T cells with more costimulation (Figure 1A). Profound differences are seen, however, when one compares tetramer dissociation (Figure 1B) and quantitative lytic activity (Figure 1C) of rV-LacZ and rV-LacZ/TRICOM. Indeed, while there is approximately a 2-fold increase in precursor frequency among the three vectors, there is a 6-fold increase in avidity in rV-LacZ vs. rV-LacZ/B7-1, and a 53-fold increase in avidity comparing rV-LacZ with rV-LacZ/TRICOM (Figure 1A–C, Table 1). One could speculate that the in-vitro stimulation of T cells with peptide-pulsed APC, required to obtain enough cells for cytolytic titrations, could skew the population toward higher or lower avidity T cells. To control for this, we analyzed tetramer dissociation from T cells directly obtained from spleens and after the 1 week in-vitro stimulation. While there was, as expected, an expansion of tetramer positive T cells post in-vitro stimulation (Figure 2A and B), there was no difference observed in tetramer dissociation prior to or post in-vitro stimulation (Figure 2C and D).
Figure 2. Effect of in-vitro stimulation on T-cell precursors and avidity.

C57BL/6 mice were vaccinated with rV-LacZ (open squares) or rV-LacZ/TRICOM (closed circles). After 30 days, splenocytes were harvested (n=3/group). β-gal–specific CD8+ T-cell avidity was determined by tetramer dissociation from T cells isolated directly from splenocytes (Panels A and C) or after a 7-day in-vitro stimulation with β-gal peptide (Panels B and D). To normalize groups within each experiment, data was also expressed as the percentage of maximum tetramer binding (Panels C and D).
While β-gal is a well-established experimental foreign antigen, we then sought to determine if similar findings would be obtained with the use of a weaker antigen in a “self” system. To this end, studies were performed with recombinant vector constructs containing the CEA transgene in CEA-Tg mice, in which CEA is expressed as a self antigen in fetal and normal GI tissues.(14, 24) Tetramer positive T cells from splenocytes of immunized mice increased approximately 2-fold with the use of rV-CEA/B7-1 or rV-CEA/TRICOM vs. rV-CEA (Figure 1D). Tetramer dissociation, however, increased substantially, from 88 minutes with the use of rV-CEA, to 135 minutes for rV-CEA/B7-1, to 233 minutes with the use of rV-CEA/TRICOM as immunogen (Figure 1E). This was accompanied, moreover, by a 100-fold increase in functional avidity (510 nM for rV-CEA, to 110 nM for rV-CEA/B7-1, to 5 nM for rV-CEA/TRICOM (Figure 1F, Table 1).
Effect of positive vs. negative costimulatory regulation on T-cell avidity
Anti-CTLA-4 antibody has been shown to have anti-tumor effects in several models using moderately antigenic and highly immunogenic tumors.(13, 25) The growth of poorly immunogenic tumors, however, is not profoundly influenced by the sole use of anti-CTLA-4 mAb.(26) Studies were first conducted here to define how to employ anti-CTLA-4 antibody with a live recombinant viral vector. These studies revealed that administering anti-CTLA-4 mAb at the same time as rV-LacZ/TRICOM (day 0) was superior to administering it on day 3, −3, or −6 in relation to vaccine, in terms of inducing enhanced β-gal–specific IFN-γ production and lysis by CD8+ T cells (rV-LazZ/TRICOM, 1900 pg/ml/24h vs rV-LacZ/TRICOM + anti-CTLA-4 mAb, 3400 pg/ml/24h).
Employing the optimal dose scheduling, we then compared positive costimulation (rV-LacZ/B7-1) vs. negative costimulatory signal regulation (rV-LacZ + anti-CTLA-4), using rV-LacZ as a control. Both vaccination strategies elicited slightly higher tetramer positive T cells in vaccinated mice than the use of rV-LacZ alone (Figure 3A). Vaccination with rV-LacZ/B7-1 resulted in T cells with a slightly higher tetramer dissociation constant than vaccination with rV-LacZ + anti-CTLA-4 mAb (127 minutes vs. 90 minutes) (Figure 3C, Table 1).
Figure 3. Evaluation of positive vs. negative costimulation strategies on antigen-specific T-cell precursor frequency and T-cell avidity.
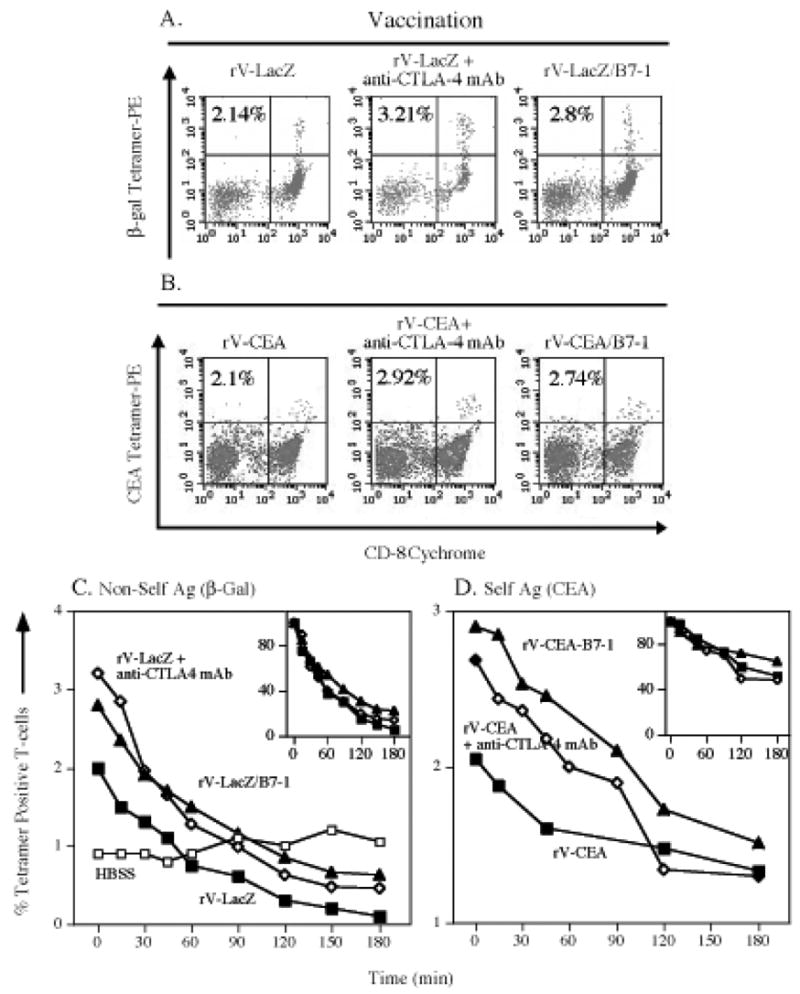
Panels A and C: C57BL/6 mice were vaccinated with rV-LacZ (closed squares), rV-LacZ in combination with anti-CTLA-4 mAb (open diamonds) or rV-LacZ/B7-1 (closed triangles). Panels B and D: CEA-Tg mice were vaccinated with rV-CEA (closed squares), rV-CEA in combination with anti-CTLA-4 mAb (open diamonds) or rV-CEA/B7-1 (closed triangles). After 30 days, splenocytes were harvested. Panel A: β-gal–specific CD8+ T-cell precursor frequency as determined by tetramer staining. Inset number indicates percent β-gal tetramer+/CD8+ T- cells of CD3+ T-cells. Panel B: CEA-specific CD8+ T-cell precursor frequency as determined by tetramer staining. Inset number indicates percent CEA-tetramer+/CD8+ T cells of CD3+ T cells. Panel C: β-gal–specific CD8+ T-cell avidity as determined by tetramer dissociation. Panel D: CEA-specific CD8+ T-cell avidity as determined by tetramer dissociation. Inset panels depict results that are normalized as the percentage of maximum tetramer binding.
We next evaluated the manipulation of positive vs. negative costimulatory signals in the CEA self-antigen system. Vaccination of mice with rV-CEA/B7-1, or rV-CEA + anti-CTLA-4 mAb, gave T cells with similar tetramer positive precursor frequencies, and those frequencies were only slightly higher than those of mice vaccinated with rV-CEA (Figure 3B). Tetramer dissociation was also slightly higher for T cells from mice vaccinated with rV-CEA/B7-1 than T cells from mice receiving rV-CEA + anti-CTLA-4 mAb, and both dissociations were greater than T cells from rV-CEA vaccinated mice (Figure 1D). This was accompanied, however, by clear differences in lytic ability between the groups (135 nM for rV-CEA/B7-1, 237 nM for rV-CEA + anti-CTLA-4 mAb, and 950 nM for rV-CEA, Table 1).
Combined use of positive and negative costimulatory strategies for optimal T-cell avidity
We then asked if the combined use of positive and negative costimulatory strategies would enhance T-cell avidity. Toward this goal, mice were vaccinated with rV-LacZ/TRICOM ± anti-CTLA-4 mAb. As seen in Figure 4A, there was an increase in tetramer positive T cells with the addition of anti-CTLA-4 mAb to rV-LacZ/TRICOM. There was no effect of isotype control mAb on any parameters measured (Table 1). There was, moreover, a clear advantage in the use of the combination of rV-LacZ/TRICOM + anti-CTLA-4 in terms of both tetramer dissociation (Figure 4B) and quantitative lytic activity (Figure 4C). Similar findings were seen in the self-antigen system. While there was less than a 2-fold increase in CEA-specific tetramer positive T cells from mice vaccinated with rV-CEA/TRICOM vs. rV-CEA/TRICOM + anti-CTLA-4 (Figure 4D), there was a profound difference in tetramer dissociation (Figure 4E), and a 10-fold difference in functional avidity in T cells receiving both rV-CEA/TRICOM and anti-CTLA-4 vs. rV-CEA/TRICOM alone (Figure 4F, Table 1).
Figure 4. Contribution of anti-CTLA-4 mAb to TRICOM costimulation on antigen-specific T-cell precursor frequency and T-cell avidity.
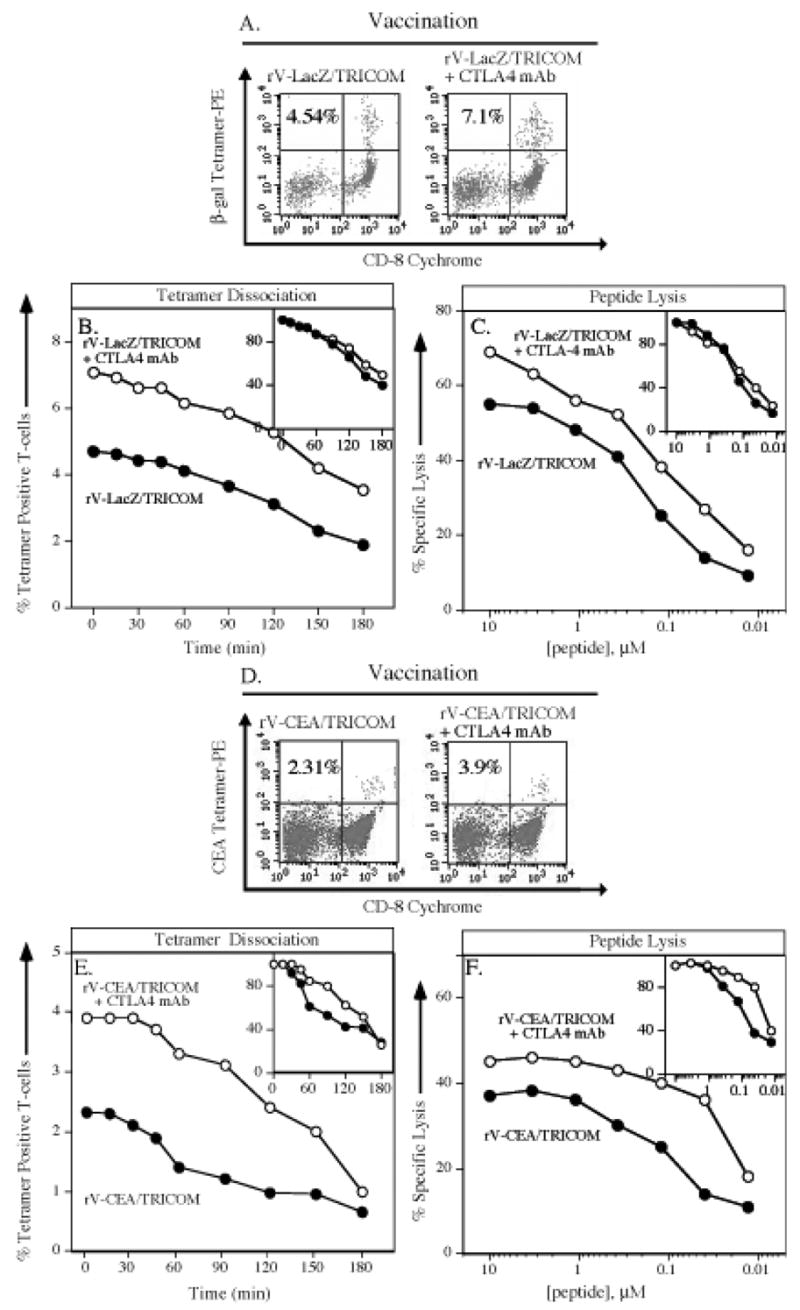
Panels A–C: C57BL/6 mice were vaccinated with rV-LacZ/TRICOM (closed circles) or rV-LacZ/TRICOM in combination with anti-CTLA-4 mAb (open circles). After 30 days, splenocytes were harvested. Panel A: β-gal specific CD8+ T-cell precursor frequency as determined by tetramer staining. Inset number indicates percent β-gal tetramer+/CD8+ T cells of CD3+ T cells. Panel B: β-gal–specific CD8+ T-cell avidity as determined by tetramer dissociation. Inset panel depicts results that are normalized as the percentage of maximum tetramer binding. Panel C: β-gal–specific CD8+ T-cell avidity as determined by CTL assay. Inset panel depicts results that are normalized as the percentage of maximum lysis. Panels D–F: CEA-Tg mice were vaccinated with rV-CEA/TRICOM (closed circles) or rV-CEA/TRICOM in combination with anti-CTLA-4 mAb (open circles). After 30 days, splenocytes were harvested. Panel D: CEA-specific CD8+ T-cell precursor frequency as determined by tetramer staining. Inset number indicates percent CEA tetramer+/CD8+ T cells of CD3+ T cells. Panel E: CEA-specific CD8+ T-cell avidity as determined by tetramer dissociation. Inset panel depicts results that are normalized as the percentage of maximum tetramer binding. Panel F: CEA-specific CD8+ T-cell avidity as determined by CTL assay. Inset panel depicts results that are normalized as the percentage of maximum lysis.
GM-CSF
GM-CSF has been employed in numerous preclinical and clinical studies to enhance vaccine efficacy by recruiting DC to regional nodes and thus increasing the potency of regional APC. While numerous studies have determined that greater quantities of Ag-specific T cells can be generated by using GM-CSF (recombinant protein or vector-driven rF-GM-CSF) alone with vaccine,(27, 28) none has evaluated if there is any effect of GM-CSF on the avidity of those T cells. Ahlers et al. have reported that when mice received peptide vaccines in combination with multiple cytokines, including GM-CSF, IL-12, and TNF-α, optimal induction of CTL was achieved, although the avidity of the CTL elicited by the peptide vaccination was not directly affected by the exogenous cytokine administration.(28) To examine the effect of GM-CSF with poxvirus vaccines, mice were thus vaccinated with rV-LacZ/TRICOM ± rF-GM-CSF. As seen in Figure 5A, a slight increase in tetramer binding precursor frequency is observed. There is, however, a substantial increase in tetramer dissociation (360 vs. 300 minutes; Figure 5B) and a 60-fold increase in lytic ability (0.05 nM vs. 3 nM; Figure 5C and Table 1) in the use of rV-LacZ/TRICOM + GM-CSF as compared with rV-LacZ/TRICOM alone. Similar results were seen in the self antigen system. Only a slight increase in tetramer positive T cells was seen with the addition of GM-CSF to rV-CEA/TRICOM (Figure 5D). Tetramer dissociation, however, increased from 233 minutes to 315 minutes with the addition of GM-CSF (Figure 5E), and lysis increased 10-fold with the addition of GM-CSF to rV-CEA/TRICOM in vaccination (Figure 5F and Table 1).
Figure 5. Contribution of GM-CSF to TRICOM-mediated costimulation on antigen-specific T-cell precursor frequency and T-cell avidity.

Panels A–C: C57BL/6 mice were vaccinated with rV-LacZ/TRICOM (closed circles) or rV-LacZ/TRICOM in combination with rF-GM-CSF (open triangles). After 30 days, splenocytes were harvested. Panel A: β-gal–specific CD8+ T-cell precursor frequency as determined by tetramer staining. Inset number indicates percent β-gal tetramer+/CD8+ T cells of CD3+ T cells. Panel B: β-gal–specific CD8+ T-cell avidity as determined by tetramer dissociation. Inset panel depicts results that are normalized as the percentage of maximum tetramer binding. Panel C: β-gal–specific CD8+ T-cell avidity as determined by CTL assay. Inset panel depicts results that are normalized as the percentage of maximum lysis. Panels D–F: CEA-Tg mice were vaccinated with rV-CEA/TRICOM (closed circles) or rV-CEA/TRICOM in combination with rF-GM-CSF (open triangles). After 30 days, splenocytes were harvested. Panel D: CEA-specific CD8+ T-cell precursor frequency as determined by tetramer staining. Inset number indicates percent CEA tetramer+/CD8+ T cells of CD3+ T cells. Panel E: CEA-specific CD8+ T-cell avidity as determined by tetramer dissociation. Inset panel depicts results that are normalized as the percentage of maximum tetramer binding. Panel F: CEA-specific CD8+ T-cell avidity as determined by CTL assay. Inset panel depicts results that are normalized as the percentage of maximum lysis.
Combined costimulatory modalities
Finally we asked if the combined use of rV-LacZ/TRICOM, anti-CTLA-4 mAb, and r-GM-CSF would result in a still higher avidity T-cell response. Compared with the use of rV-LacZ/TRICOM alone, a 2-fold increase in tetramer positive precursor frequency is seen with the addition of anti-CTLA-4 antibody and rF-GM-CSF (Figure 6A). There is a substantial increase, however, in tetramer dissociation (300 minutes for rV-LacZ/TRICOM vs. 475 minutes for the triple combination) (Figure 6B). This is associated with a 1,000-fold increase in functional avidity of resulting CTL when comparing vaccination with rV-LacZ/TRICOM (3.0 nM) vs. vaccination with rV-LacZ/TRICOM + rF-GM-CSF + anti-CTLA-4 (0.003 nM) (Figure 6C and Table 1).
Figure 6. Effects of combined use of anti-CTLA-4 mAb, GM-CSF, and TRICOM-mediated costimulation on antigen-specific T-cell precursor frequency and T-cell avidity.
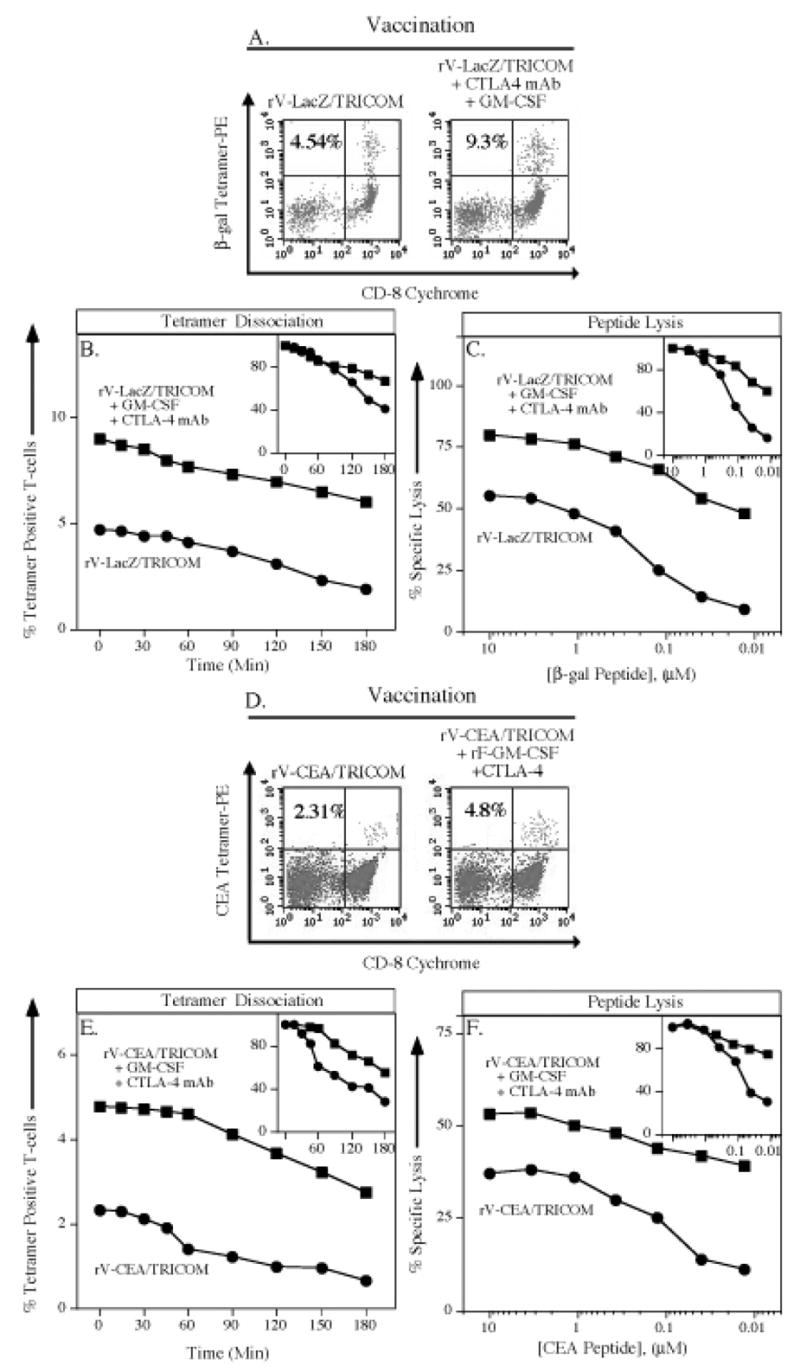
Panels A–C: C57BL/6 mice were vaccinated with rV-LacZ/TRICOM (closed circles) or rV-LacZ/TRICOM in combination with rF-GM-CSF and anti-CTLA-4 mAb (closed squares). After 30 days, splenocytes were harvested. Panel A: β-gal–specific CD8+ T-cell precursor frequency as determined by tetramer staining. Inset number indicates percent β-gal tetramer+/CD8+ T cells of CD3+ T cells. Panel B: β-gal–specific CD8+ T-cell avidity as determined by tetramer dissociation. Inset panel depicts results that are normalized as the percentage of maximum tetramer binding. Panel C: β-gal–specific CD8+ T-cell avidity as determined by CTL assay. Inset panel depicts results that are normalized as the percentage of maximum lysis. Panels D–F: CEA-Tg mice were vaccinated with rV-CEA/TRICOM (closed circles) or rV-CEA/TRICOM in combination with rF-GM-CSF and anti-CTLA-4 mAb (closed squares). After 30 days, splenocytes were harvested. Panel D: CEA-specific CD8+ T-cell precursor frequency as determined by tetramer staining. Inset number indicates percent CEA tetramer+/CD8+ T cells of CD3+ T cells. Panel E: CEA-specific CD8+ T-cell avidity as determined by tetramer dissociation. Inset panel depicts results that are normalized as the percentage of maximum tetramer binding. Panel F: CEA-specific CD8+ T-cell avidity as determined by CTL assay. Inset panel depicts results that are normalized as the percentage of maximum lysis.
If one evaluates the various modalities employed to enhance T-cell costimulation, these studies demonstrate a 5.1-fold increase in tetramer positive T cells in the use of rV-LacZ as a vaccine vs. vaccination with rV-LacZ/TRICOM + rF-GM-CSF + anti-CTLA-4 (Table 1). If one evaluates T-cell avidity, however, these differences are greatly magnified. Dissociation of tetramer positive T cells increases from 85 minutes for mice vaccinated with rV-LacZ to 475 minutes for mice vaccinated with rV-LacZ/TRICOM + rF-GM-CSF + anti-CTLA-4 mAb (Table 1). Most strikingly, avidity as measured by quantitative lysis increases from 160 nM for CTL of mice vaccinated with rV-LacZ to 0.003 nM for CTL of mice vaccinated with rV-LacZ/TRICOM + rF-GM-CSF + anti-CTLA-4, a 53,000-fold increase (Table 1). These results are even further magnified if one uses as a starting point CTL from mice vaccinated with β-gal peptide in IFA adjuvant (Table 1). It should also be stated that there was a high degree of statistical correlation between tetramer dissociation and quantitative lysis (R2 = 0.96), as a measure of CTL avidity (calculated from Table 1).
We then examined the single vs. triple modality vaccination strategy in the CEA self-antigen system. As seen in Figure 6D, there was a 2-fold increase in tetramer positive T-cell frequency from mice vaccinated with rV-CEA/TRICOM + GM-CSF + anti-CTLA-4 vs. mice vaccinated with rV-CEA/TRICOM alone. There is a substantial difference (475 vs. 300 minutes), however, in CEA tetramer dissociation between the two groups (Figure 6E). This is accompanied by a 250-fold increase in lysis of targets: 5 nM for CTL of rV-CEA/TRICOM vaccinated mice vs. 0.02 from CTL of mice vaccinated with rV-CEA/TRICOM + GM-CSF + anti-CTLA-4 (Figure 6F). Moreover, if one compares the use of rV-CEA alone as a vaccine with that of rV-CEA/TRICOM + GM-CSF + anti-CTLA-4, there is a 5.3-fold increase in tetramer positive T cells, but functional avidity of CTL from vaccinated mice goes from 510 nM to 0.02 nM (a 25,000-fold increase; see Table 1). As with the β-gal system, there was a high degree of correlation (R2 = 0.98) in the CEA self system in the measurement of tetramer dissociation vs. quantitative lysis (calculated from Table 1).
Tumor therapy
Studies were conducted to determine if the increases in avidity of CTL observed employing the various vaccination strategies would result in any advantage in anti-tumor activity. A stringent CEA-Tg mouse model was used for these studies, one in which we have previously shown that CEA/TRICOM vaccine alone has no significant anti-tumor effect.(29) As can be seen in Figure 7, 40% (4 of 10) of the mice receiving the CEA/TRICOM vaccine regimen with rF-GM-CSF and anti-CTLA-4 mAb (closed circles) remained alive and apparently healthy through the 22-week observation period. However, only 10% (1 of 10) of the mice that received the vaccine regimen with rF-GM-CSF (closed triangles) survived past 6 weeks (P=0.04). None of the mice that received no therapy (open squares) or anti-CTLA-4 mAb alone (closed diamonds) survived past 6 weeks. When comparing the tumor volumes of mice 28 days post tumor transplant (inset panel), 7 of 10 mice receiving the vaccine regimen with rF-GM-CSF and CTLA-4 mAb had tumor volumes ≤400mm3, while only 2 of 10 mice that received the vaccine regimen with rF-GM-CSF, and 1 of 10 that received anti-CTLA-4 mAb alone, had tumor volumes ≤400mm3.
Figure 7. Tumor therapy with CEA/TRICOM vaccine, GM-CSF and anti-CTLA-4 mAb.
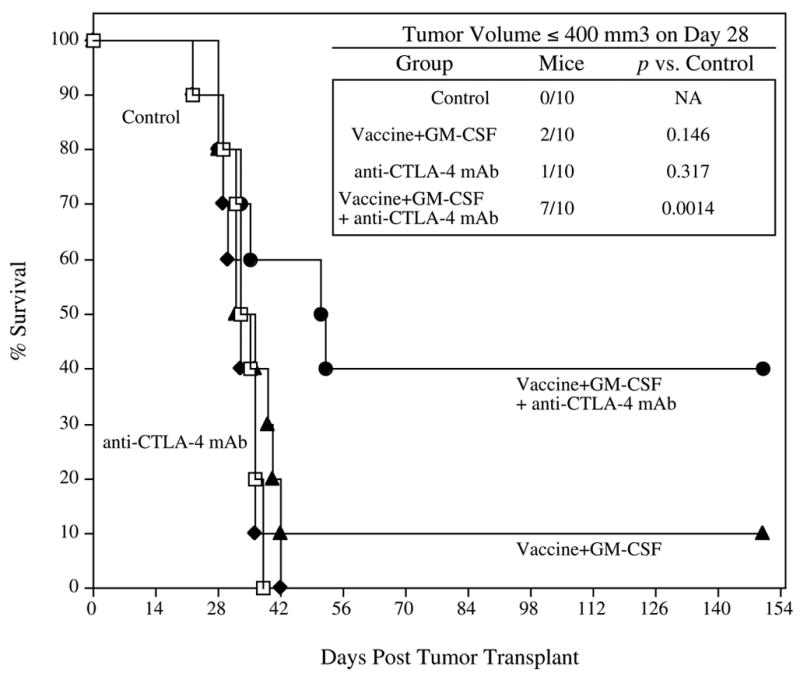
CEA-Tg mice (n=10/group) were transplanted s.c. with MC38-CEA+ tumors on day 0. Four days later, mice received rV-CEA/TRICOM prime vaccination admixed with rF-GM-CSF and were administered anti-CTLA-4 mAb, followed by three weekly boosts with rF-CEA/TRICOM admixed with rF-GM-CSF (closed circles). Another group of CEA-Tg mice received rV-CEA/TRICOM prime vaccination admixed with rF-GM-CSF followed by three weekly boosts with rF-CEA/TRICOM admixed with rF-GM-CSF (closed triangles). Another group of mice received anti-CTLA-4 mAb alone (closed diamonds). A control group received buffer (HBSS, open squares). Mice in each group were monitored weekly for tumor size and survival. Inset panel: Tumor volumes ≤ 400 mm3.
Since CEA is expressed in normal GI epithelium in CEA-Tg mice at levels similar to those in humans, and CEA protein is found in the serum of CEA-Tg mice at levels seen in human colon carcinoma patients, studies were undertaken to determine if there was any evidence of autoimmunity observed in mice cured of their tumor following the triple modality strategy (CEA/TRICOM + GM-CSF + anti-CTLA-4 mAb) shown in Figure 7. Mice cured of tumor were followed for 5 months; non-vaccinated age matched controls were also employed. There were no differences evident in any symptoms in either group, including body weight (vaccine group average weight at day 150 was 25±2g vs. 24.3±2g for the age matched control group). Vaccinated mice developed anti-CEA, anti-vaccinia virus and anti-fowlpox Ig responses. However, vaccinated and cured mice did not develop any detectable antibody responses above control mice to murine B7-1, ICAM-1, LFA-3 or GM-CSF. Finally, mice vaccinated and cured of tumor did not develop any detectable antibodies to nuclear ribonuclear protein, histone, double stranded or single stranded DNA, SCL-70 (DCA topoisomease 1), and no circulating immune complexes were observed. Thus, no evidence of autoimmunity was observed in mice that received the CEA/TRICOM vaccine regimen with rF-GM-CSF and anti-CTLA-4 mAb.
Discussion
Recent studies in both animal models and clinical studies have demonstrated that functional T-cell avidity is a major determinant for anti-viral and anti-tumor immunity.(30–33) Vaccine approaches to tumor or viral immunotherapy should therefore focus on modalities that can selectively induce and expand (a) greater levels of tumor antigen-specific T-cell precursor frequencies and, more importantly, (b) higher avidity T cells. Two major methods have been proposed to analyze avidity of T cells; tetramer dissociation and lysis of target cells pulsed with different peptide concentrations. There is, however, contradiction in the literature as to whether these two methods always coincide.(23, 34–38) Recent studies(39) have also suggested that CD107A may be a marker for high avidity human T cells, but no corresponding marker has been identified for murine cells. While several studies have shown how to select for high avidity T cells from vaccinated mice or patients, few studies have demonstrated a method of inducing higher avidity T cells in vivo. In those studies, this has mainly been accomplished by altering the amino acid sequence of the test antigen.(1, 5–9, 40–42) In only one previous study has costimulation been employed to enhance avidity;(11) in that study, B cells pulsed with peptide and overexpressing costimulatory molecules were shown to induce higher avidity T cells than uninfected peptide-pulsed B cells. In the studies reported here, we have employed seven different vaccine strategies and two independent methods to measure avidity. Moreover, we have compared results in a foreign and a self-antigen system.
Numerous preclinical studies have demonstrated anti-tumor effects in some rodent models with the use of anti-CTLA-4 antibody. (26, 43–48) Several theories have been put forth(12, 13) as to the role of anti-CTLA-4 mAb, but the mechanism has not been fully elucidated. Data have been presented(49) that the antibody inhibits the activity of CD4+CD25+CTLA-4+ Treg cells, while other data refute this.(50) Another theory states, “Preferential inhibition of T cells bearing high avidity TCRs by CTLA-4 could slow antigen driven selection thereby preventing high avidity T cell clones from dominating the response during its early stages. Thus, the attenuation model would predict that in the absence of CTLA-4 mediated inhibition, high affinity T cell clones would outcompete weaker clones….”(13) To date, however, no experimental data have demonstrated that the use of anti-CTLA-4 antibody results in the generation of higher avidity CTL. The studies reported here are the first to show that the administration of anti-CTLA-4 mAb with vaccine will actually increase the avidity of antigen-specific T cells (Figures 3, 4 and 6, Table 1).
GM-CSF has been employed in many vaccine strategies, many with indications of enhancing levels of T-cell responses and anti-tumor effects.(51–55). These include the use of GM-CSF in whole tumor cell, anti-id, peptide, and vector-based vaccines. The mechanism of action of GM-CSF has been shown to be the recruitment of dendritic cells to regional nodes(19, 27, 53, 56, 57) Since dendritic cells are, de facto, APC rich in the expression of costimulatory molecules, the use of GM-CSF with vaccines could be considered still another strategy to enhance costimulation. Previous preclinical studies have shown that the use of four daily doses of recombinant GM-CSF or one dose of rF-GM-CSF (as employed in these studies) results in enhanced recruitment of dendritic cells to regional nodes, with increases in the quantity of antigen-specific T cells generated.(27) The studies reported here are the first to show that the administration of GM-CSF with vaccine will actually also increase the avidity of antigen-specific T cells (Figures 5 and 6, Table 1).
We show here that the avidity of T cells for a specific β-gal epitope was enhanced by the use of a rV-LacZ vs. peptide in adjuvant (Figure 1, Table 1). This result could be attributable to at least three factors: (a) helper epitopes in the LacZ gene delivered by rV-LacZ, (b) non-specific help by the vaccinia virus proteins, and (c) better cytosolic expression and presentation of the epitope by the recombinant vaccinia virus. However, employing the same recombinant poxvirus vector, additional studies showed the avidity of T cells produced by vaccination with rV-LacZ/TRICOM was greater than that of rV-LacZ/B7-1, which was greater than rV-LacZ (Figure 1, Table 1).
It is interesting to note that the differences observed in enhancing precursor frequency or avidity employing the different vaccine strategies were quite similar for the non-self (LacZ) and self (CEA) systems. There were, however, both greater numbers of T cells and more avid T cells seen for each vaccine using the foreign antigen LacZ model compared with the CEA self model. For example, rV-LacZ vaccination resulted in a precursor frequency of 2,650 tetramer positive cells/105 CD8 cells, while rV-CEA vaccination resulted in 321 tetramer positive cells/105 CD8 cells (Table 1). Likewise, the combined vaccination regimen of rV-LacZ/TRICOM + GM-CSF + anti-CTLA-4 precursor frequency was 13,500 tetramer positive cells/105 CD8 cells vs. 1,690 frequency for the CEA-based combination therapy. Quantitative cytolytic avidities were always stronger for the non-self antigen: 160 nM for rV-LacZ vs. 510 nM for rV-CEA. Furthermore, employing the TRICOM/GM-CSF/anti-CTLA-4 strategy, cytolytic avidity was approximately 10-fold greater in the non-self than the self system (0.003 for the LacZ vaccinations vs. 0.02 for the CEA vaccinations, Table 1).
The vaccine strategies employed here are all currently being evaluated clinically as single agents or in combination with a second agent. CEA/TRICOM vaccines and PSA/TRICOM vaccines (± GM-CSF) are currently in clinical trials in patients with advanced carcinoma(58–61) These Phase I studies are providing preliminary evidence of objective clinical response, drops in tumor markers, with increased survival.(62, 63) Anti-CTLA-4 has been in clinical trials with a peptide vaccine in melanoma with indications of anti-tumor activity accompanied by severe autoimmunity.(64) Perhaps the use of lower doses of anti-CTLA-4 with more potent TRICOM vectors will achieve anti-tumor activity with less severe autoimmunity.
The studies reported here also provide evidence that multiple strategies can be employed in combination to enhance T-cell avidity; to our knowledge, these are the first studies to demonstrate such a phenomenon. These studies also demonstrate that the difference between success and failure in an anti-tumor situation can hinge on just such additive effects. It should be pointed out that similar strategies can be employed in the development of vaccines directed against many viral-mediated diseases, either in the prevention or therapeutic setting.
Unfortunately, many therapeutic anti-cancer and anti-viral vaccine regimens to date have employed one or at most two vaccine strategies. The strategies reported here, alone or in combination, do not require the use of costly and labor-intensive ex-vivo manipulation and/or expansion of dendritic cells or T cells, and can readily be employed as pre-vialed reagents. Hopefully, the studies reported here and by others will provide the proof of concept to institute more sophisticated and rigorous vaccine strategies employing such multiple modalities.
Acknowledgments
We thank Marion Taylor for his excellent technical assistance. Drs. Dennis Panicali, Gail Mazzara, Alicia Gomez Yafal, and Linda Gritz of Therion Biologics Corporation are thanked for kindly providing all Orthopox virus vectors. We thank Debra Weingarten for her editorial assistance in the preparation of this manuscript.
References
- 1.Alexander-Miller MA, Leggatt GR, Berzofsky JA. Selective expansion of high- or low-avidity cytotoxic T lymphocytes and efficacy for adoptive immunotherapy. Proc Natl Acad Sci U S A. 1996;93:4102. doi: 10.1073/pnas.93.9.4102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Berzofsky JA, Ahlers JD, Derby MA, Pendleton CD, Arichi T, Belyakov IM. Approaches to improve engineered vaccines for human immunodeficiency virus and other viruses that cause chronic infections. Immunol Rev. 1999;170:151. doi: 10.1111/j.1600-065x.1999.tb01336.x. [DOI] [PubMed] [Google Scholar]
- 3.Berzofsky JA, Ahlers JD, Belyakov IM. Strategies for designing and optimizing new generation vaccines. Nat Rev Immunol. 2001;1:209. doi: 10.1038/35105075. [DOI] [PubMed] [Google Scholar]
- 4.Snyder JT, Alexander-Miller M, Berzofsky J, Belyakov IM. Molecular mechanisms and biological significance of CTL avidity. Current HIV Research. 2003;1:287. doi: 10.2174/1570162033485230. [DOI] [PubMed] [Google Scholar]
- 5.Derby M, Alexander-Miller M, Tse R, Berzofsky J. High-avidity CTL exploit two complementary mechanisms to provide better protection against viral infection than low-avidity CTL. J Immunol. 2001;166:1690. doi: 10.4049/jimmunol.166.3.1690. [DOI] [PubMed] [Google Scholar]
- 6.Nugent CT, Morgan DJ, Biggs JA, Ko A, Pilip IM, Pamer EG, Sherman LA. Characterization of CD8+ T lymphocytes that persist after peripheral tolerance to a self antigen expressed in the pancreas. J Immunol. 2000;164:191. doi: 10.4049/jimmunol.164.1.191. [DOI] [PubMed] [Google Scholar]
- 7.Yee C, Savage PA, Lee PP, Davis MM, Greenberg PD. Isolation of high avidity melanoma-reactive CTL from heterogeneous populations using peptide-MHC tetramers. J Immunol. 1999;162:2227. [PubMed] [Google Scholar]
- 8.Zheng L, Fisher G, Miller RE, Peschon J, Lynch DH, Lenardo MJ. Induction of apoptosis in mature T cells by tumour necrosis factor. Nature. 1995;377:348. doi: 10.1038/377348a0. [DOI] [PubMed] [Google Scholar]
- 9.Snyder JT, I, Belyakov M, Dzutsev A, Lemonnier F, Berzofsky JA. Protection against lethal vaccinia virus challenge in HLA-A2 transgenic mice by immunization with a single CD8+ T-cell peptide epitope of vaccinia and variola viruses. J Virol. 2004;78:7052. doi: 10.1128/JVI.78.13.7052-7060.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tsang KY, Zhu M, Even J, Gulley J, Arlen P, Schlom J. The infection of human dendritic cells with recombinant avipox vectors expressing a costimulatory molecule transgene (CD80) to enhance the activation of antigen-specific cytolytic T cells. Cancer Res. 2001;61:7568. [PubMed] [Google Scholar]
- 11.Oh S, Hodge JW, Ahlers JD, Burke DS, Schlom J, Berzofsky JA. Selective induction of high avidity CTL by altering the balance of signals from APC. J Immunol. 2003;170:2523. doi: 10.4049/jimmunol.170.5.2523. [DOI] [PubMed] [Google Scholar]
- 12.Allison JP, Chambers C, Hurwitz A, Sullivan T, Boitel B, Fournier S, Brunner M, Krummel M. A role for CTLA-4-mediated inhibitory signals in peripheral T cell tolerance? Novartis Found Symp. 1998;215:92. doi: 10.1002/9780470515525.ch7. [DOI] [PubMed] [Google Scholar]
- 13.Egen JG, Kuhns MS, Allison JP. CTLA-4: new insights into its biological function and use in tumor immunotherapy. Nat Immunol. 2002;3:611. doi: 10.1038/ni0702-611. [DOI] [PubMed] [Google Scholar]
- 14.Eades-Perner AM, van der Putten H, Hirth A, Thompson J, Neumaier M, von Kleist S, Zimmermann W. Mice transgenic for the human carcinoembryonic antigen gene maintain its spatiotemporal expression pattern. Cancer Res. 1994;54:4169. [PubMed] [Google Scholar]
- 15.Robbins PF, Kantor JA, Salgaller M, Hand PH, Fernsten PD, Schlom J. Transduction and expression of the human carcinoembryonic antigen gene in a murine colon carcinoma cell line. Cancer Res. 1991;51:3657. [PubMed] [Google Scholar]
- 16.Kantor J, Irvine K, Abrams S, Kaufman H, DiPietro J, Schlom J. Antitumor activity and immune responses induced by a recombinant carcinoembryonic antigen-vaccinia virus vaccine. J Natl Cancer Inst. 1992;84:1084. doi: 10.1093/jnci/84.14.1084. [DOI] [PubMed] [Google Scholar]
- 17.Kalus RM, Kantor JA, Gritz L, Gomez Yafal A, Mazzara GP, Schlom J, Hodge JW. The use of combination vaccinia vaccines and dual-gene vaccinia vaccines to enhance antigen-specific T-cell immunity via T-cell costimulation. Vaccine. 1999;17:893. doi: 10.1016/s0264-410x(98)00275-8. [DOI] [PubMed] [Google Scholar]
- 18.Hodge JW, Sabzevari H, Yafal AG, Gritz L, Lorenz MG, Schlom J. A triad of costimulatory molecules synergize to amplify T-cell activation. Cancer Res. 1999;59:5800. [PubMed] [Google Scholar]
- 19.Kass E, Panicali DL, Mazzara G, Schlom J, Greiner JW. Granulocyte/macrophage-colony stimulating factor produced by recombinant avian poxviruses enriches the regional lymph nodes with antigen-presenting cells and acts as an immunoadjuvant. Cancer Res. 2001;61:206. [PubMed] [Google Scholar]
- 20.Overwijk WW, Surman DR, Tsung K, Restifo NP. Identification of a Kb-restricted CTL epitope of beta-galactosidase: potential use in development of immunization protocols for “self” antigens. Methods. 1997;12:117. doi: 10.1006/meth.1997.0461. [DOI] [PubMed] [Google Scholar]
- 21.Hodge JW, Rad AN, Grosenbach DW, Sabzevari H, Yafal AG, Gritz L, Schlom J. Enhanced activation of T cells by dendritic cells engineered to hyperexpress a triad of costimulatory molecules. J Natl Cancer Inst. 2000;92:1228. doi: 10.1093/jnci/92.15.1228. [DOI] [PubMed] [Google Scholar]
- 22.Krummel MF, Allison JP. CD28 and CTLA-4 have opposing effects on the response of T cells to stimulation. J Exp Med. 1995;182:459. doi: 10.1084/jem.182.2.459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Holmberg K, Mariathasan S, Ohteki T, Ohashi PS, Gascoigne NR. TCR binding kinetics measured with MHC class I tetramers reveal a positive selecting peptide with relatively high affinity for TCR. J Immunol. 2003;171:2427. doi: 10.4049/jimmunol.171.5.2427. [DOI] [PubMed] [Google Scholar]
- 24.Kass E, Schlom J, Thompson J, Guadagni F, Graziano P, Greiner JW. Induction of protective host immunity to carcinoembryonic antigen (CEA), a self-antigen in CEA transgenic mice, by immunizing with a recombinant vaccinia-CEA virus. Cancer Res. 1999;59:676. [PubMed] [Google Scholar]
- 25.Egen JG, Allison JP. Cytotoxic T lymphocyte antigen-4 accumulation in the immunological synapse is regulated by TCR signal strength. Immunity. 2002;16:23. doi: 10.1016/s1074-7613(01)00259-x. [DOI] [PubMed] [Google Scholar]
- 26.Leach DR, Krummel MF, Allison JP. Enhancement of antitumor immunity by CTLA-4 blockade. Science. 1996;271:1734. doi: 10.1126/science.271.5256.1734. [DOI] [PubMed] [Google Scholar]
- 27.Kass E, Parker J, Schlom J, Greiner JW. Comparative studies of the effects of recombinant GM-CSF and GM-CSF administered via a poxvirus to enhance the concentration of antigen-presenting cells in regional lymph nodes. Cytokine. 2000;12:960. doi: 10.1006/cyto.2000.0684. [DOI] [PubMed] [Google Scholar]
- 28.Ahlers JD, I, Belyakov M, Matsui S, Berzofsky JA. Mechanisms of cytokine synergy essential for vaccine protection against viral challenge. Int Immunol. 2001;13:897. doi: 10.1093/intimm/13.7.897. [DOI] [PubMed] [Google Scholar]
- 29.Kudo-Saito C, Schlom J, Hodge JW. Intratumoral vaccination and diversified subcutaneous/intratumoral vaccination with recombinant poxviruses encoding a tumor antigen and multiple costimulatory molecules. Clin Cancer Res. 2004;10:1090. doi: 10.1158/1078-0432.ccr-03-0145. [DOI] [PubMed] [Google Scholar]
- 30.Zeh HJ, 3rd, Perry-Lalley D, Dudley ME, Rosenberg SA, Yang JC. High avidity CTLs for two self-antigens demonstrate superior in vitro and in vivo antitumor efficacy. J Immunol. 1999;162:989. [PubMed] [Google Scholar]
- 31.Bullock TN, Mullins DW, Colella TA, Engelhard VH. Manipulation of avidity to improve effectiveness of adoptively transferred CD8(+) T cells for melanoma immunotherapy in human MHC class I-transgenic mice. J Immunol. 2001;167:5824. doi: 10.4049/jimmunol.167.10.5824. [DOI] [PubMed] [Google Scholar]
- 32.Yang S, Linette GP, Longerich S, Haluska FG. Antimelanoma activity of CTL generated from peripheral blood mononuclear cells after stimulation with autologous dendritic cells pulsed with melanoma gp100 peptide G209-2M is correlated to TCR avidity. J Immunol. 2002;169:531. doi: 10.4049/jimmunol.169.1.531. [DOI] [PubMed] [Google Scholar]
- 33.Dutoit V, Rubio-Godoy V, Dietrich PY, Quiqueres AL, Schnuriger V, Rimoldi D, Lienard D, Speiser D, Guillaume P, Batard P, Cerottini JC, Romero P, Valmori D. Heterogeneous T-cell response to MAGE-A10(254–262): high avidity-specific cytolytic T lymphocytes show superior antitumor activity. Cancer Res. 2001;61:5850. [PubMed] [Google Scholar]
- 34.Derby MA, Wang J, Margulies DH, Berzofsky JA. Two intermediate-avidity cytotoxic T lymphocyte clones with a disparity between functional avidity and MHC tetramer staining. Int Immunol. 2001;13:817. doi: 10.1093/intimm/13.6.817. [DOI] [PubMed] [Google Scholar]
- 35.Palermo B, Campanelli R, Mantovani S, Lantelme E, Manganoni AM, Carella G, Da Prada G, della Cuna GR, Romagne F, Gauthier L, Necker A, Giachino C. Diverse expansion potential and heterogeneous avidity in tumor-associated antigen-specific T lymphocytes from primary melanoma patients. Eur J Immunol. 2001;31:412. doi: 10.1002/1521-4141(200102)31:2<412::aid-immu412>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- 36.Rubio-Godoy V, Dutoit V, Rimoldi D, Lienard D, Lejeune F, Speiser D, Guillaume P, Cerottini JC, Romero P, Valmori D. Discrepancy between ELISPOT IFN-gamma secretion and binding of A2/peptide multimers to TCR reveals interclonal dissociation of CTL effector function from TCR-peptide/MHC complexes half-life. Proc Natl Acad Sci U S A. 2001;98:10302. doi: 10.1073/pnas.181348898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dutoit V, Rubio-Godoy V, Doucey MA, Batard P, Lienard D, Rimoldi D, Speiser D, Guillaume P, Cerottini JC, Romero P, Valmori D. Functional avidity of tumor antigen-specific CTL recognition directly correlates with the stability of MHC/peptide multimer binding to TCR. J Immunol. 2002;168:1167. doi: 10.4049/jimmunol.168.3.1167. [DOI] [PubMed] [Google Scholar]
- 38.Echchakir H, Dorothee G, Vergnon I, Menez J, Chouaib S, Mami-Chouaib F. Cytotoxic T lymphocytes directed against a tumor-specific mutated antigen display similar HLA tetramer binding but distinct functional avidity and tissue distribution. Proc Natl Acad Sci U S A. 2002;99:9358. doi: 10.1073/pnas.142308199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rubio V, Stuge TB, Singh N, Betts MR, Weber JS, Roederer M, Lee PP. Ex vivo identification, isolation and analysis of tumor-cytolytic T cells. Nat Med. 2003;9:1377. doi: 10.1038/nm942. [DOI] [PubMed] [Google Scholar]
- 40.Sarobe P, Pendleton CD, Akatsuka T, Lau D, Engelhard VH, Feinstone SM, Berzofsky JA. Enhanced in vitro potency and in vivo immunogenicity of a CTL epitope from hepatitis C virus core protein following amino acid replacement at secondary HLA-A2.1 binding positions. J Clin Invest. 1998;102:1239. doi: 10.1172/JCI3714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tangri S, Ishioka GY, Huang X, Sidney J, Southwood S, Fikes J, Sette A. Structural features of peptide analogs of human histocompatibility leukocyte antigen class I epitopes that are more potent and immunogenic than wild-type peptide. J Exp Med. 2001;194:833. doi: 10.1084/jem.194.6.833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gray PM, Parks GD, Alexander-Miller MA. A novel CD8-independent high-avidity cytotoxic T-lymphocyte response directed against an epitope in the phosphoprotein of the paramyxovirus simian virus 5. J Virol. 2001;75:10065. doi: 10.1128/JVI.75.21.10065-10072.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.van Elsas A, Sutmuller RP, Hurwitz AA, Ziskin J, Villasenor J, Medema JP, Overwijk WW, Restifo NP, Melief CJ, Offringa R, Allison JP. Elucidating the autoimmune and antitumor effector mechanisms of a treatment based on cytotoxic T lymphocyte antigen-4 blockade in combination with a B16 melanoma vaccine: comparison of prophylaxis and therapy. J Exp Med. 2001;194:481. doi: 10.1084/jem.194.4.481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.van Elsas A, Hurwitz AA, Allison JP. Combination immunotherapy of B16 melanoma using anti-cytotoxic T lymphocyte-associated antigen 4 (CTLA-4) and granulocyte/macrophage colony-stimulating factor (GM-CSF)-producing vaccines induces rejection of subcutaneous and metastatic tumors accompanied by autoimmune depigmentation. J Exp Med. 1999;190:355. doi: 10.1084/jem.190.3.355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hernandez J, Ko A, Sherman LA. CTLA-4 blockade enhances the CTL responses to the p53 self-tumor antigen. J Immunol. 2001;166:3908. doi: 10.4049/jimmunol.166.6.3908. [DOI] [PubMed] [Google Scholar]
- 46.Hurwitz AA, Foster BA, Kwon ED, Truong T, Choi EM, Greenberg NM, Burg MB, Allison JP. Combination immunotherapy of primary prostate cancer in a transgenic mouse model using CTLA-4 blockade. Cancer Res. 2000;60:2444. [PubMed] [Google Scholar]
- 47.Hurwitz AA, Yu TF, Leach DR, Allison JP. CTLA-4 blockade synergizes with tumor-derived granulocyte-macrophage colony-stimulating factor for treatment of an experimental mammary carcinoma. Proc Natl Acad Sci U S A. 1998;95:10067. doi: 10.1073/pnas.95.17.10067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yang YF, Zou JP, Mu J, Wijesuriya R, Ono S, Walunas T, Bluestone J, Fujiwara H, Hamaoka T. Enhanced induction of antitumor T-cell responses by cytotoxic T lymphocyte-associated molecule-4 blockade: the effect is manifested only at the restricted tumor-bearing stages. Cancer Res. 1997;57:4036. [PubMed] [Google Scholar]
- 49.Read S, Malmstrom V, Powrie F. Cytotoxic T lymphocyte-associated antigen 4 plays an essential role in the function of CD25(+)CD4(+) regulatory cells that control intestinal inflammation. J Exp Med. 2000;192:295. doi: 10.1084/jem.192.2.295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sutmuller RP, van Duivenvoorde LM, van Elsas A, Schumacher TN, Wildenberg ME, Allison JP, Toes RE, Offringa R, Melief CJ. Synergism of cytotoxic T lymphocyte-associated antigen 4 blockade and depletion of CD25(+) regulatory T cells in antitumor therapy reveals alternative pathways for suppression of autoreactive cytotoxic T lymphocyte responses. J Exp Med. 2001;194:823. doi: 10.1084/jem.194.6.823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Aarts WM, Schlom J, Hodge JW. Vector-based vaccine/cytokine combination therapy to enhance induction of immune responses to a self-antigen and antitumor activity. Cancer Res. 2002;62:5770. [PubMed] [Google Scholar]
- 52.Aruga A, Tanigawa K, Aruga E, Yu H, Chang AE. Enhanced adjuvant effect of granulocyte-macrophage colony-stimulating factor plus interleukin-12 compared with either alone in vaccine-induced tumor immunity. Cancer Gene Ther. 1999;6:89. doi: 10.1038/sj.cgt.7700010. [DOI] [PubMed] [Google Scholar]
- 53.Disis ML, Bernhard H, Shiota FM, Hand SL, Gralow JR, Huseby ES, Gillis S, Cheever MA. Granulocyte-macrophage colony-stimulating factor: an effective adjuvant for protein and peptide-based vaccines. Blood. 1996;88:202. [PubMed] [Google Scholar]
- 54.Dranoff G, Jaffee E, Lazenby A, Golumbek P, Levitsky H, Brose K, Jackson V, Hamada H, Pardoll D, Mulligan RC. Vaccination with irradiated tumor cells engineered to secrete murine granulocyte-macrophage colony-stimulating factor stimulates potent, specific, and long-lasting anti-tumor immunity. Proc Natl Acad Sci U S A. 1993;90:3539. doi: 10.1073/pnas.90.8.3539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kurane S, Arca MT, Aruga A, Krinock RA, Krauss JC, Chang AE. Cytokines as an adjuvant to tumor vaccines: efficacy of local methods of delivery. Ann Surg Oncol. 1997;4:579. doi: 10.1007/BF02305540. [DOI] [PubMed] [Google Scholar]
- 56.Kwak LW, Young HA, Pennington RW, Weeks SD. Vaccination with syngeneic, lymphoma-derived immunoglobulin idiotype combined with granulocyte/macrophage colony-stimulating factor primes mice for a protective T-cell response. Proc Natl Acad Sci U S A. 1996;93:10972. doi: 10.1073/pnas.93.20.10972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kielian T, Nagai E, Ikubo A, Rasmussen CA, Suzuki T. Granulocyte/macrophage-colony-stimulating factor released by adenovirally transduced CT26 cells leads to the local expression of macrophage inflammatory protein 1alpha and accumulation of dendritic cells at vaccination sites in vivo. Cancer Immunol Immunother. 1999;48:123. doi: 10.1007/s002620050556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.An Open Label Pilot Study to Evaluate the Safety and Tolerability of PANVAC-V (Vaccinia) and PANVAC-F (Fowlpox) in Combination with Sargramostim in Patients with Metastatic Adenocarcinoma. National Institutes of Health Clinical Trials Database. 2004 www.clinicaltrials.gov.
- 59.A Phase I/II Pilot Study of Sequential Vaccinations with rFowlpox-PSA (L155)-TRICOM (PROSTVAC-F/TRICOM) Alone, or in Combination with rVaccinia-PSA (L155)-TRICOM (PROSTVAC-V/TRICOM), and the Role of GM-CSF, in Patients with Prostate Cancer. National Institutes of Health Clinical Trials Database. 2004 www.clinicaltrials.gov.
- 60.Phase II Randomized Study of Vaccinia-PSA-TRICOM Vaccine, Fowlpox-PSA-TRICOM Vaccine, and Sargramostim (GM-CSF) Versus Empty Vector Control in Patients With Metastatic Androgen-Independent Prostate Cancer. National Institutes of Health Clinical Trials Database. 2004 www.clinicaltrials.gov.
- 61.A Phase II Randomized, Double Blind, Controlled Study to Evaluate the Safety and Efficacy of PROSTVAC®-VF/TRICOM™ in Combination with GM-CSF in Patients with Androgen-Independent Adenocarcinoma of the Prostate. National Institutes of Health Clinical Trials Database. 2004 www.clinicaltrials.gov TBC-PRO-002.
- 62.Arlen PM, Gulley J, Dahut W, Skarupa L, Morin S, Pazdur M, Todd N, Panicalli D, Tsang KY, Schlom J. A phase I study of sequential vaccinations with recombinant Fowlpox-PSA (L155)-TRICOM (rF)alone, or in combination with recombinant vaccinia-PSA (L155)-TRICOM (rV), and the role of GM-CSF, in patients (Pts) with prostate cancer. Annual Meeting Proceedings, American Society of Clinical Oncology; Boston, MA. 2004. [Google Scholar]
- 63.Marshall JL, Gulley JL, Arlen PM, Beetham PK, Tsang KY, Slack R, Hodge JW, Doren S, Hwang J, Fox E, Odogwu L, Park S, Panicali D, Schlom J. A phase I study of sequential vaccinations with Fowlpox-CEA (6D)-TRICOM (B7-1/ICAM-1/LFA-3) alone and sequentially with Vaccinia-CEA (6D)-TRICOM, with and without GM-CSF, in patients with CEA-expressing carcinomas. Journal of Clinical Oncology. 2004 doi: 10.1200/JCO.2005.10.206. In-Press. [DOI] [PubMed] [Google Scholar]
- 64.Phan GQ, Yang JC, Sherry RM, Hwu P, Topalian SL, Schwartzentruber DJ, Restifo NP, Haworth LR, Seipp CA, Freezer LJ, Morton KE, Mavroukakis SA, Duray PH, Steinberg SM, Allison JP, Davis TA, Rosenberg SA. Cancer regression and autoimmunity induced by cytotoxic T lymphocyte-associated antigen 4 blockade in patients with metastatic melanoma. Proc Natl Acad Sci U S A. 2003;100:8372. doi: 10.1073/pnas.1533209100. [DOI] [PMC free article] [PubMed] [Google Scholar]


