Abstract
Bilateral facial paralysis is a rare condition and therefore represents a diagnostic challenge. We report the case of a 34-year-old healthy woman with sequential bilateral facial paralysis as a sole manifestation of sarcoidosis. She initially presented with an isolated left sided Bell's palsy without any symptoms to suggest alternative diagnoses. Within a month there was progression to peripheral facial paresis on the contra lateral side, prompting a diagnosis of Lyme disease. Her physical examination and chest x-ray did not reveal any clinical evidence of sarcoidosis. After failing to respond to an empiric trial of intravenous ceftriaxone for a presumptive diagnosis of Lyme disease, computed tomography scan of the chest was ordered which demonstrated bilateral hilar lymphadenopathy. Bronchoscopic biopsy confirmed a diagnosis of sarcoidosis. The patient then made a complete recovery on steroid therapy. We discuss the differential diagnosis of facial diplegia and focus on the clinical presentation, diagnosis and treatment of neurosarcoidosis.
Keywords: Neurosarcoidosis, sarcoidosis, bilateral facial palsy
Bilateral simultaneous facial paralysis is a rare clinical entity, which unlike the unilateral presentation, is seldom secondary to Bell's palsy. Adour found only 3 bilateral cases in a consecutive series of 1,000 patients with Bell's palsy.1 Simultaneous onset is defined as the involvement of the opposite side within 30 days of the onset of the first side. It is most often a special finding in a symptom complex of a systemic disease, occurring in 0.3% to 2.0% of facial palsy cases.2
In this paper, we present a case of sarcoidosis presenting as bilateral facial nerve paralysis and discuss the differential diagnosis of acquired simultaneous bilateral facial nerve paralysis in adults. Although few cases have been reported with bilateral facial paralysis as the initial manifestation of this disease,3–5 it brings up an important point for discussion of the bilateral facial palsy.
CASE PRESENTATION
A 34-year African american female presented to her primary care physician with a 3-day history of left sided peripheral facial paralysis. She complained of bilateral headaches, a 20 lb weight loss over the prior month, and an alteration of her taste sensation. She was on no medications and had no prior illness apart from a recent viral infection. She worked as a teacher and denied any tobacco, alcohol, or drug abuse. A diagnosis of Bell's palsy with a Grade V (House-Brackmann grading system) weakness was made and sent home with 40 mg of prednisone and acyclovir. However, she presented to the emergency room 3 days later with a new right peripheral facial palsy and headache but with improvement of her left peripheral facial paresis.
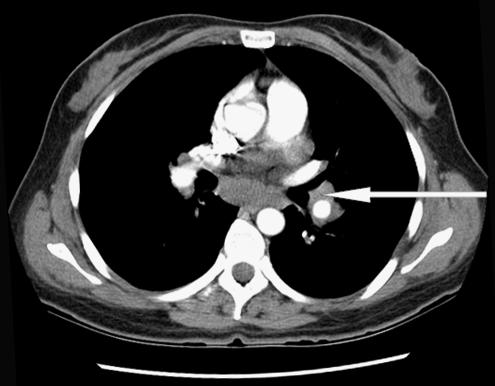
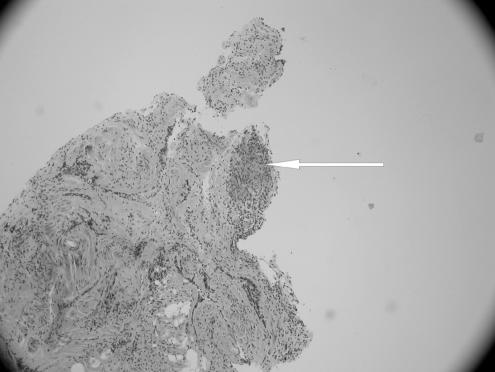
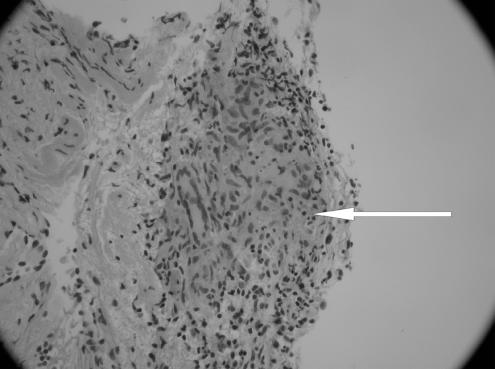
On examination in the emergency room, the patient was in no distress. Her temperature was 99.6°F, pulse 90 bpm, respiratory rate 20/min, and blood pressure 124/86 mm Hg. The head, eyes, ears, nose, and throat were all normal. The neck was supple, with a midline trachea. There was no thyromegaly, parotid gland enlargement, adenopathy, or mucosal rugosities. Auscultation of the heart and lungs was normal. The abdomen was soft and nontender, without organomegaly, bowel sounds were normal. The parotid glands were not enlarged and neurological examination revealed a bilateral peripheral facial paralysis with a Grade V weakness on the right side and a Grade III weakness on the left side. Her taste sensation was not tested and there was evidence of Bell's phenomenon. Complete blood count, comprehensive metabolic panel, chest radiography, and brain computed tomography (CT) scan showed no abnormality. Lumbar puncture was performed and the cerebrospinal fluid (CSF) analysis showed the following: protein 124 mg/dL, glucose of 41 mg/dL, RBC of 18/μL, WBC 22/μL, acid-fast, Gram stain, Lyme polymerase chain reaction (PCR), VDRL (Venereal Disease Research Laboratory) test and oligoclonal bands were all negative. Cerebrospinal fluid angiotensin converting enzyme (ACE) level was not requested; however, serum ACE level was 65 (ref range 9 to 67) and HIV antibody test was negative. Because of a history of deer foraging near the school where she taught, she was sent home on intravenous (IV) ceftriaxone with a presumptive diagnosis of Lyme disease. After 2 days of therapy with ceftriaxone, she returned because of severe headaches at which point a repeat lumbar puncture and magnetic resonance imaging (MRI) were obtained. The CSF analysis was within the normal range except for a raised protein level at 75. Malignant cells were not seen on cytology; MRI of the head was nondiagnostic. Borrelia burgdorferi immunoglobulins were not detected on 3 separate analyses and serum ACE level was 45 (ref range 9 to 67). A chest x-ray (CXR) performed again 3 weeks after her initial presentation showed hilar prominence with the chest CT confirming mediastinal and hilar lymphadenopathy (see Fig. 1). The patient then underwent bronchoscopy with lymph node biopsy, which revealed noncaseating epitheloid granuloma (see Figs. 2 and 3). Because of the clinical presentation compatible with neurosarcoidosis, laboratory support of central nervous system (CNS) inflammation, exclusion of other possible causes and evidence of systemic sarcoidosis, she was diagnosed with probable neurosarcoidosis as per the criteria for diagnosis of neurosarcoidosis (see Table 2).6,7 She was started on IV methylprednisone of 1 g/d for 5 days and then on 60 mg of prednisone. She improved on this regimen with complete resolution of her facial palsies and headaches after 6 weeks of steroid therapy. Her steroids were slowly tapered after 8 weeks of therapy and she continues to do well off steroids after 1 year of follow-up.
FIGURE 1.
Computed tomography scan of the chest, showing hilar adenopathy.
FIGURE 2.
Transbronchial biopsy, showing a single noncaseating granuloma (hematoxylin-eosin stain) (× 40 magnification).
FIGURE 3.
High power magnification showing Giant cell and the noncaseating epithlioid granuloma (× 200 magnification).
Table 2.
Criteria for the Diagnosis of Neurosarcoidosis
| Definite |
| Clinical presentation compatible with neurosarcoidosis |
| Exclusion of other possible causes |
| Positive nervous system histology |
| Probable |
| Clinical presentation compatible with neurosarcoidosis |
| Laboratory support of CNS inflammation* |
| Exclusion of other possible causes |
| Evidence of systemic sarcoidosis† |
| Possible |
| Clinical presentation compatible with neurosarcoidosis |
| Exclusion of other possible causes |
High concentration of CSF protein and high numbers of cells, the presence of oligoclonal bands, or MRI evidence compatible with neurosarcoidosis.
Positive histology or at least 2 indirect indicators from gallium scan, chest imaging, and serum angiotensin-converting-enzyme. Reproduced with permission from Oxford University Press.16
CNS, central nervous system; CSF, cerebrospinal fluid; MRI, magnetic resonance imaging
DISCUSSION
The etiology of facial paralysis includes many conditions such as congenital, traumatic, infectious, neurological, metabolic, neoplastic, toxic, vascular, and idiopathic. Unlike unilateral facial paralysis, where the cause is mostly idiopathic (over 50%), bilateral facial palsy is less often idiopathic (under 20%). We list the differential diagnosis of acquired simultaneous bilateral facial nerve paralysis of peripheral origin in adults in Table 1.8,9 In a review of reported cases over a period of 10 years, Teller and Murphy10 show that Lyme disease is responsible for 36% of the cases for facial diplegia. Guillain-Barre syndrome (5%), trauma (4%), sarcoidosis (0.9%), and AIDS (0.9%) are other seen causes.
Table 1.
Differential Diagnosis of Acquired Bilateral Peripheral Facial Palsy
| Trauma | Skull fractures |
| Parotid surgery | |
| Mastoid surgery | |
| Infection | Postinfluenza |
| Infectious mononucleosis | |
| HIV infection | |
| Lyme disease, Banwarth's syndrome | |
| Guillain–Barre syndrome | |
| Syphilis | |
| Brainstem encephalitis | |
| HTLV-1 infection | |
| Poliomyelitis | |
| Metabolic | Diabetes |
| Acute porphyria | |
| Neoplastic | Acute leukemia |
| Acoustic neuroma | |
| Autoimmune | Sarcoidosis |
| Amyloidosis | |
| Neurological | Multiple sclerosis |
| Pseudobulbar and bulbar palsy | |
| Parkinson's disease | |
| Idiopathic | Bell's palsy |
HTLV, human T-cell lymphotrophic virus.
Diagnosis workup for a patient presenting with bilateral facial paralysis depends upon the history. The history should include time sequence of onset, prior history of facial paralysis, recent viral or upper respiratory tract infection, recent camping or hiking, otological symptoms, change in taste, facial numbness, vesicles, or recent immunization.
The first priority in the workup is to rule out a life-threatening disease such as leukemia or Guillain-Barre syndrome. If these are suspected, the patient should be admitted to the hospital for close observation. The physical examination should be complete with emphasis on the neurological and head and neck portions of the exam. Workup should include complete blood count, fluorescent treponemal antibody test, HIV test, fasting glucose, erythrocyte sedimentation rate, Lyme titer, and antinuclear antibody level measurement. Lumbar puncture after a CT scan and also special facial nerve function tests could be performed. Magnetic resonance imaging is useful in the demonstration of seventh cranial nerve lesions, tumor cell infiltration, and widening of the internal acoustic canal. Also, the areas that are most important to visualize are the central nervous system, skull base, meninges, and cerebellopontine angle, which are best imaged by enhanced MRI. A discussion of Lyme disease, Guillain-Barre syndrome, and sarcoidosis follows.
The most common infectious cause of facial diplegia is Lyme disease, caused by Borrelia burgdorferi, named after the person who first isolated it. It commonly begins in the summer with a skin lesion, erythema migrans. The diagnosis is made by an immunologic assay using antibody titers against the spirochete. Treatment with an antibiotic should be started immediately and not delayed until there is serological confirmation. The type of therapy is determined in part by the clinical features and stage of the disease.
Guillain-Barre syndrome or ascending inflamatory demyelinating polyneuropathy (AIDP) presents as a progressive development of palsy of the voluntary muscles of the legs, arms, trunk, and face. The most commonly affected cranial nerves are IX, X, and VII. In 27% to 50% of the cases, the facial nerve is involved.11 Fifty percent of the patients with a facial paralysis have bilateral involvement. Prognosis is rather good and therapy consists of plasma exchanges and administration of IV Immunoglobulins within 10 days of the onset of symptoms.
Our patient was finally diagnosed as having bilateral facial paralysis as the presenting feature of neurosarcoidosis. Involvement of the facial nerve is a relatively common neurological finding in sarcoidosis. However, bilateral involvement is very uncommon and is even more unusual as the presenting complaint. In our patient, her response to steroids in the absence of other treatments such as antituberculous medications confirmed the diagnosis of neurosarcoidosis. Meningitis, myelopathy, optic neuropathy, cerebral mass lesions, and polyneuropathy can also be manifestations of neurosarcoidosis.6,12–14 Diagnosis is made by blood analysis, biopsy of the affected organ, and enlargement of lymph nodes on chest CT. With neurosarcoidosis, the CSF protein level is usually elevated whereas the CSF glucose level is usually within the reference range or slightly low. A predominantly lymphocytic cerebrospinal fluid pleocytosis is common.13 The concentration of ACE in the CSF is high in more than half of the patients with neurosarcoidosis.15 However, high concentrations of ACE in CSF are not specific to neurosarcoidosis, as elevated levels have also been reported in CNS infections and malignant tumors. These levels are especially useful in the monitoring of disease activity and treatment response, rather than being specifically useful diagnostically.6 Whole-body gallium scanning remains a useful indicator of systemic disease, which although, a relatively nonspecific measure, it does add, a diagnostic probability. Ga-67 citrate is taken up at sites of active sarcoidosis and also by other inflammatory and malignant diseases, including tuberculosis and lymphomas, but the pattern of uptake among patients with active sarcoidosis is well recognized. Accordingly, as a component of the investigations relevant to the diagnosis of extra thoracic sarcoidosis, it can be very helpful, although constrained by the limitations imposed by subjective interpretation.7
Therapy for facial paralysis secondary to sarcoidosis consists of administration of corticosteroids.6,14 These agents usually suppress inflammation and may relieve acute symptoms. Especially CNS mass lesions may be responsive to corticosteroid therapy.6,14 Whether corticosteroids change the natural course of neurosarcoidosis is unknown. Nevertheless, corticosteroid therapy is recommended in most cases of neurosarcoidosis as spontaneous recovery cannot be predicted.6,14 An initial dose of prednisone 1 mg/kg/d is typically recommended.6 In severe cases, high doses of IV methylprednisolone may be used for a few days to achieve a good clinical response in severely ill or deteriorating patients.6
The prognosis for bilateral facial palsy is dependent on the underlying etiology. If the etiology can be identified and successfully managed, the prognosis is excellent. The prognosis may be worse with age over 60 years, diabetes mellitus, hypertension, pain, decreased tearing, and the degree of denervation demonstrated on electrical testing. Resolution of bilateral facial paralysis may be nonsimultaneous.
CONCLUSION
Unilateral peripheral facial palsies are usually idiopathic (presumably virally related), whereas bilateral palsies usually reflect an underlying systemic pathology. Patients with bilateral facial palsies need thorough assessment and follow-up. Sarcoidosis presenting with bilateral facial paralysis is very rare. Facial paralysis with sarcoidosis is reflective of central nervous system involvement, and is an indication for steroid therapy and potentially more intensive immunosuppressive therapies.
REFERENCES
- 1.Adour KK, Byl FM, Hilsinger RL, Kahn ZM, Sheldon MI. The true nature of Bell's palsy: analysis of 1,000 consecutive patients. Laryngoscope. 1978;88:787–801. doi: 10.1002/lary.1978.88.5.787. [DOI] [PubMed] [Google Scholar]
- 2.Stahl N, Ferit T. Recurrent bilateral peripheral facial palsy. J Laryngol Otol. 1989;103:117–9. doi: 10.1017/s0022215100108199. [DOI] [PubMed] [Google Scholar]
- 3.McIntosh WE, Brenner JF, Aschenbrenner JE. Bilateral facial paralysis as the sole presenting feature of sarcoidosis: report of a case. J Am Osteopath Assoc. 1987;87:245–7. [PubMed] [Google Scholar]
- 4.George MK, Pahor AL. Sarcoidosis: a cause for bilateral facial palsy. Ear Nose Throat J. 1991;70:492–3. [PubMed] [Google Scholar]
- 5.Haydar A, Hujairi NM, Tawil A. Bilateral facial paralysis: what's the cause? Med J Am. 2003;179:553. doi: 10.5694/j.1326-5377.2003.tb05685.x. [DOI] [PubMed] [Google Scholar]
- 6.Hoitsma E, Faber CG, Drent M, et al. Neurosarcoidosis: a clinical dilemma. Lancet Neurol. 2004;3:397–407. doi: 10.1016/S1474-4422(04)00805-1. [DOI] [PubMed] [Google Scholar]
- 7.Zajicek JP, Scolding NJ, Foster O, et al. Central nervous system sarcoidosis—diagnosis and management. Q J Med. 1999;92:103–17. doi: 10.1093/qjmed/92.2.103. [DOI] [PubMed] [Google Scholar]
- 8.Steenerson RL. Bilateral facial paralysis. Am J Otol. 1986;7:99–103. [PubMed] [Google Scholar]
- 9.Gevers G, Lemkens P. Bilateral simultaneous facial paralysis—differential diagnosis and treatment options. Acta Otorhinolaryngologica Belg. 2003;57:139–46. [PubMed] [Google Scholar]
- 10.Teller D, Murphy T. Bilateral facial paralysis: a case presentation and literature review. J Otolaryngol. 1992;21:44–46. [PubMed] [Google Scholar]
- 11.May M. The Facial Nerve. New York: Thieme Inc; 1986. p. 181. Chapter 9. [Google Scholar]
- 12.Mayock RL, Bertrand P, Morrison CE, et al. Manifestations of sarcoidosis: analysis of 145 patients with a review of nine series selected from the literature. Am J Med. 1963;35:67–89. doi: 10.1016/0002-9343(63)90165-7. [DOI] [PubMed] [Google Scholar]
- 13.Delaney P. Neurologic manifestations in sarcoidosis: review of the literature, with a report of 23 cases. Ann Intern Med. 1977;87:336–45. doi: 10.7326/0003-4819-87-3-336. [DOI] [PubMed] [Google Scholar]
- 14.Stern BJ, Krumholz A, Johns C, et al. Sarcoidosis and its neurological manifestations. Arch Neurol. 1985;42:909–17. doi: 10.1001/archneur.1985.04060080095022. [DOI] [PubMed] [Google Scholar]
- 15.Oksanen V, Fyhrquist F, Somer H, et al. Angiotensin converting enzyme in cerebrospinal fluid: a new assay. Neurology. 1985;35:1220–3. doi: 10.1212/wnl.35.8.1220. [DOI] [PubMed] [Google Scholar]