Abstract
OBJECTIVE
There is no proven primary care treatment for patients with medically unexplained symptoms (MUS). We hypothesized that a long-term, multidimensional intervention by primary care providers would improve MUS patients' mental health.
DESIGN
Clinical trial.
SETTING
HMO in Lansing, MI.
PARTICIPANTS
Patients from 18 to 65 years old with 2 consecutive years of high utilization were identified as having MUS by a reliable chart rating procedure; 206 subjects were randomized and 200 completed the study.
INTERVENTION
From May 2000 to January 2003, 4 primary care clinicians deployed a 12-month intervention consisting of cognitive-behavioral, pharmacological, and other treatment modalities. A behaviorally defined patient-centered method was used by clinicians to facilitate this treatment and the provider-patient relationship.
MAIN OUTCOME MEASURE
The primary endpoint was an improvement from baseline to 12 months of 4 or more points on the Mental Component Summary of the SF-36.
RESULTS
Two hundred patients averaged 13.6 visits for the year preceding study. The average age was 47.7 years and 79.1% were females. Using intent to treat, 48 treatment and 34 control patients improved (odds ratio [OR] = 1.92, 95% confidence interval [CI]: 1.08 to 3.40; P = 0.02). The relative benefit (relative “risk” for improving) was 1.47 (CI: 1.05 to 2.07), and the number needed to treat was 6.4 (95% CI: 0.89 to 11.89). The following baseline measures predicted improvement: severe mental dysfunction (P < 0.001), severe body pain (P = 0.039), nonsevere physical dysfunction (P = 0.003), and at least 16 years of education (P = 0.022); c-statistic = 0.75.
CONCLUSION
The first multidimensional intervention by primary care clinicians led to clinically significant improvement in MUS patients.
Keywords: medically unexplained symptoms, somatization, mental health in primary care, provider-patient relationship, satisfaction, patient-centered
Patients with medically unexplained symptoms (MUS) are frequent attenders in primary care—where the more severe, high-utilizing ones alone represent 5% to 10% of all outpatients.1 Although most such data are based on the Diagnostic and Statistical Manual of Mental Disorders, 4th edition (DSM-IV), Somatoform diagnoses,2 we recently demonstrated that over 75% of distressed, high-utilizing MUS patients did not have either a full or abridged DSM-IV Somatoform diagnosis.3 Because their symptoms are physical, MUS patients fill primary care waiting rooms with complaints like headaches, fatigue, and back pain. Unfortunately, provider-patient relationships (PPR) become strained by fruitless searches for an organic disease4 and when doctors overlook the patient's personal and psychological distress.5 In the literature review that informed the intervention reported here, we found no empirically validated treatment to guide the medical physicians who conduct almost all care.6
Using cognitive-behavioral, pharmacological, and other treatment modalities found useful in specialty care, we adapted them for primary care providers. To facilitate this treatment, we integrated patient-centered methods. We hypothesized that the first comprehensive primary care intervention for patients with MUS6 would lead to improved mental health 12 months postbaseline.
METHODS
Study Design
We conducted a 12-month randomized controlled trial for 206 high-utilizing MUS patients from 5/00 to 1/03.6–8 Subjects were randomized to our treatment protocol or to usual care. Four nurse practitioners (NP) conducted the treatment, and we collected outcome measures at baseline, 6 months, and 12 months. We evaluated our primary endpoint 12 months postbaseline: an increase of 4 points or more on the mental component summary (MCS) of the Short Form-36 (SF-36).9
Subjects and Settings
Patient inclusion criteria were being 18 to 65 years old, a member of the HMO for at least 2 years, able to speak English, literate, not under care by a mental health professional more often than once/month, having access to a telephone, and planning to be in the HMO for at least 1 year. Exclusion criteria were pregnancy, substance use disorders, suicidal ideation, organic mental syndromes, and psychosis.
With precedent for replacing physicians as primary providers,10 the 4 NPs had no prior experiences in mental health, as a primary provider, or as a case manager. We trained them in an 84-hour program over 10 weeks, at least 1/3 of the time devoted to teaching primary care diagnosis and treatment. In addition to the intervention,7,11,12 we trained them to use the PrimeMD to assist in identifying psychiatric disorders (not used in evaluating outcomes),13 and in managing suicidal ideation and common psychiatric problems. Supervision occurred every 1 to 3 weeks.
Subject Identification
Following IRB approval and subjects' informed consent, we identified patients who had 8 or more visits per year for the last 2 years to any providers. Using a reliable procedure,8 trained physician chart raters identified patients whose primary problem was MUS. We defined patients with MUS as having no documented organic disease to explain symptoms of at least 6 months duration.6,8 Because there was a delay of up to 9 months after ratings, charts that met the study entry criterion as primary MUS 8 were independently reviewed a final time by one of the authors (R.C.S.) just prior to recruitment to ensure that predominant organic disease had not supervened and that high utilization persisted. The patients entered into study had at least 50% of visits designated primary MUS.
Randomization/Blinding
Eligible patients were recruited, from 5/00 to 1/02. Our statistician (J.C.G.) had no information about participants and used a computerized random number generator (SAS Software, v. 9, SAS Institute Inc., Cary, NC) to create subject assignments that were stratified by physician and blocked by time to ensure balance for each physician. Although interviewers were blinded, it was not possible to blind patients, NPs, or usual care physicians to treatment or control status.
Intervention
As the “process” to enhance treatment,6 NPs used a behaviorally defined, 5-step patient-centered method to establish a positive PPR and communicate effectively (Model 1) and a 3-step patient-centered method to inform and motivate patients specifically about treatment (Model 2) 11,14 (see Appendix). Nurse practitioners deployed treatment in a collaborative stepped-care fashion, also useful in primary care depression.15 Treatment included antidepressants, reduction/elimination of controlled substance medications that were ineffective, exercise, relaxation training, physical therapy, and comorbid organic disease management. Most referrals were to mental health professionals, usually for improved patients who would benefit from counseling. Treatment entailed twelve scheduled patient visits (20 min each) in weeks 1, 2, 3, 5, 8, 12, 16, 22, 28, 36, 44, and 52, but additional visits could occur. Telephone contact (5 to 10 min) was scheduled between visits. Controls received usual care from 21 HMO physicians.
Study outcomes and other measures
The World Health Organization Composite International Diagnostic Interview (WHO-CIDI) was used to make DSM-IV diagnoses at baseline.16 At baseline, 6 months, and 12 months, we also obtained: SF-36—the MCS, the physical component summary (PCS),9 and the 8 SF-36 subscales (mental health [MH], role emotional [RE], social functioning [SF], vitality [VT], general health [GH], bodily pain [BP], role physical [RP], and physical functioning [PF])17—the MCS comprises the first 4 subscales and the PCS the last 4; the brief disability inventory (PF and RP combined)18; Center for Epidemiological Studies-Depression Scale (CES-D)19; Psychosomatic Symptom Checklist (PSC)20; the Spielberger State Anxiety Scale (SSAS)21; and a satisfaction with the PPR Questionnaire (see Web Appendix).14,22 We also obtained antidepressant and controlled substance (anti-anxiety agents, narcotics, and sedative-hypnotics) medication use from nursing documentation forms (treatment) and from chart review (controls).
Statistical Method/Sample Size
Baseline characteristics of patients in the treatment and control groups were compared by the Wilcoxon rank sum test or t-test for continuous variables, and by the Chi-square test for categorical variables. Following intent to treat, logistic regression was used to assess correlates of a ≥4 point improvement in MCS as outcome from a list of prespecified candidate variables that included a dummy variable for treatment group, and baseline characteristics. Variables identified in univariable testing (P≤.20) were considered in a preliminary logistic model in which we retained variables significant at P≤.10. Candidate variables excluded at the initial stage were then added to the model to ascertain if significant improvement in its predictive power would be realized. In addition, prespecified interactions were tested for significance. Odds ratios (OR) and associated 95% confidence intervals were computed for all independent variables in the final model. For continuous predictors we used 1−SD decrements to present comparisons. Regression diagnostics were used to reveal any outlying or influential observations, and the Hosmer–Lemeshow goodness-of-fit test and c-statistic were used to gauge the reliability of the model.
We hypothesized that 50% in the treatment group and 30% in the control group would exhibit at least a 4-point improvement on the MCS. Based on 2-tailed testing (α = 0.05), to detect this difference with 80% power required a sample of 103 patients per group.
Selection of Candidate Variables
(1) Demographic factors: age at study entry, gender, marital status (married vs other), educational status (16 or more years of formal schooling vs less than 16 years). (2) Clinical measures of initial severity: subscales of the SF-36 that were associated with the primary outcome, but not strongly intercorrelated (Pearson correlation, r < 0.7 in absolute value)—MH, RE, SF, VT, BP. Because CES-D and SSAS were correlated with MH (r = 0.80), neither scale was used in model building. (3) Chart review factors: the major explanations (MUS vs non-MUS) for symptoms at each visit. (4) The WHO-CIDI provided the following full and abridged DSM-IV psychiatric diagnoses: (a) Non-Somatoform Disorders—major depression, bipolar disorder, dysthymia, generalized anxiety disorder, agoraphobia, social phobia, specific phobia, posttraumatic stress disorder, obsessive compulsive disorder, and panic disorder; the presence of any one of these diagnoses defined “non-somatoform positive,” also called “psychiatric comorbidity;” (b) Somatoform disorders—somatization disorder (SD), conversion disorder, pain disorder, and hypochondriasis. We also identified abridged SD based on 4 symptoms in men and 6 in women.23
Total satisfaction and 4 subscales (open-endedness, confidence, empathy, general satisfaction) were derived from the satisfaction questionnaire by simple summation of items and rescaling to a 0 to 100 range.14,22 Higher scores reflect greater satisfaction. Because postbaseline satisfaction scales were skewed, we dichotomized each scale at 80, approximating the benchmark of the HMO. Logistic regression was used to evaluate the effect of treatment on improved satisfaction at 12 months (i.e., a score ≥80) controlling for baseline satisfaction.
Finally, from our nursing documentation form, and chart review of controls, we created 2 binary treatment variables: initiating (or changing) antidepressants to full dosage; and reduction of controlled substance medications that had been ineffective, defined as a decreased dose of any 1 controlled substance by at least 25%.
RESULTS
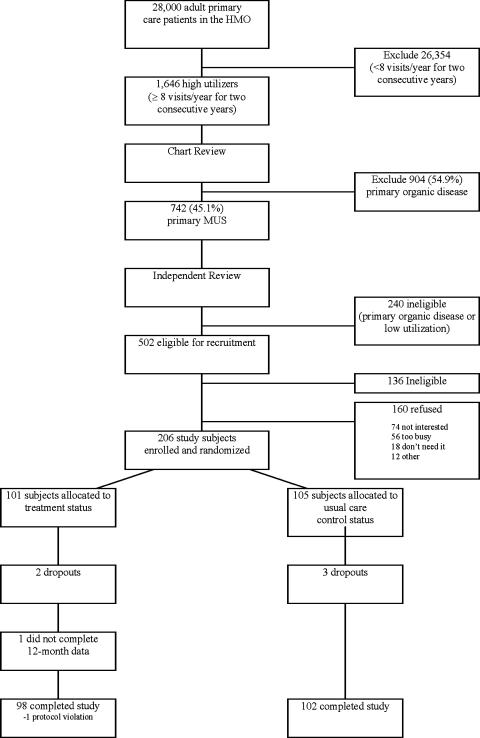
Figure 1 demonstrates participant flow and a recruitment rate of 206 of 366 subjects (56.3%) meeting inclusion criteria. There was no statistically significant difference between the enrolled subjects and the refusals on all available clinical and demographic baseline measures: age, gender, co-pay status, mean number of visits, and percentage of MUS symptoms. Of 206 subjects entered into study, 200 (98 treatment; 102 controls) successfully completed it (97% retention rate).
FIGURE 1.
A flow diagram of study eligibility and enrollment for a randomized, controlled trial. HMO, health maintenance organization; MUS, medically unexplained symptoms
Outcomes and Estimation
Table 1 shows that there was no statistically significant difference between treatment and controls. The PSC did not differ from normal or aid understanding in our analyses; we relied upon the PCS to assess physical status. Baseline satisfaction scores are presented at the bottom of Table 1 and generally are quite high on a scale from 0 to 100, corresponding to average scores from 4.0 to 4.25 on a 5-point scale. Correlation among the 4 factors was 0.97 and the Cronbach α for each of the 4 factors varied from 0.86 to 0.94.
Table 1.
Baseline Characteristics of the Study Participants
| Characteristic | Treatment Group (n = 101) No. (%) | Control Group (n = 105) No. (%) | P value |
|---|---|---|---|
| Gender, female | 83 (82.2) | 80 (76.2) | .291 |
| Marital status, married | 76 (75.3) | 72 (68.6) | .287 |
| Education, < 16 years | 82 (81.2) | 76 (72.4) | .135 |
| Mean (SD) | Mean (SD) | ||
| Age, years | 48.8 (8.5) | 46.6 (9.1) | .064 |
| Mental component summary (MCS) | 46.5 (11.6) | 48.6 (11.9) | .198 |
| Physical component summary (PCS) | 37.4 (10.2) | 35.5 (10.5) | .188 |
| Depression, CES-D | 15.9 (12.7) | 15.3 (11.8) | .719 |
| Spielberger Anxiety Scale, SSAS | 39.8 (20.4) | 38.5 (19.3) | .656 |
| Physical Symptoms List, PSC | 22.6 (14.1) | 23.5 (16.2) | .657 |
| Total Satisfaction Score | 79.7 (16.0) | 77.5 (20.6) | .398 |
| Open endedness | 74.9 (21.8) | 74.9 (22.6) | .981 |
| Confidence | 83.0 (16.2) | 79.6 (21.1) | .194 |
| Empathy | 81.5 (15.0) | 78.9 (20.8) | .317 |
| General satisfaction | 80.1 (19.7) | 77.4 (24.6) | .395 |
CES-D, Center for Epidemiological Studies-Depression scale; PSC, Psychosomatic Symptom Checklist; MCS, mental component summary of the SF-36; PCS, physical component summary of the SF-36; SSAS, spielberger state anxiety scale. On the SF-36 higher scores indicate better health while higher scores on the CES-D, PSC, and SSAS indicate worse mental health.
Individual providers had no differential impact on the outcome and were evaluated as one treatment group. Patients in the treatment group were more likely to improve than those in usual care: 48 (49.0%) vs 34 (33.3%) (OR = 1.92, 95% CI: 1.08 to 3.40). The relative benefit (relative “risk” for improving) was 1.47 (95% CI: 1.05 to 2.07), and the number needed to treat was 6.4 (95% CI: 0.89 to 11.89); i.e., 1 additional patient improved for every 6 to 7 patients treated.
Higher education and marital status were associated with improvement. The mental health (MH), role-emotional (RE), social function (SF), and vitality (VT) subscales were all strongly correlated with outcome, with lower scores at baseline (more severe) indicative of higher likelihood of improvement. Neither the presence of a Somatoform diagnosis nor the presence of psychiatric comorbidity was associated with improvement; 60.2% of MUS patients had psychiatric comorbidity.3
The multivariable logistic model contained treatment, education, mental health (MH), physical function (PF), and body pain (BP) (Table 2). A lower MH score (more severe dysfunction) at baseline was associated with higher odds of improvement, whereas a lower PF score at baseline had a lower likelihood of improvement. Baseline predictors of improvement should be distinguished from the treatment process variables that predict an actual response.
Table 2.
Multivariable Logistic Regression of Likelihood of Improvement
| Variable | Odds Ratio (95% CI)* | P value |
|---|---|---|
| Treatment group | 2.16 (1.14 to 4.10) | .019 |
| Education, at least 16 years | 2.39 (1.13 to 5.06) | .022 |
| Mental health (SD = 19.70) | 2.14 (1.49 to 3.09) | < 0.001 |
| Physical function (SD = 26.13) | 0.56 (0.38 to 0.82) | .003 |
| Body pain (SD = 20.48) | 1.51 (1.02 to 2.23) | .039 |
OR for continuous variables presented for a 1-SD lower score; c-statistic = 0.75; Hosmer–Lemeshow test (P = .81).
Secondary Outcomes
The CES-D improved from baseline to 12 months in the treatment group (3.17, 95% CI: 1.27 to 5.08) but not in the usual care group (1.73, 95% CI: −0.14 to 3.60). Disability scores were equivalent for treatment and controls at baseline (P = 0.28) but significantly different at 12 months (P = 0.02); also, changes within treatment from baseline to 12 months were statistically significant (P = 0.001), but the corresponding changes in controls were not (P = 0.26). We also found that the use of an antidepressant to full doses occurred in 65 of 95 (68.4%) treatment subjects compared with 20 of 101 (19.8%) of control subjects; P < 0.001. Similarly, 26 of 37 (70.3%) treatment patients decreased use of controlled substances compared with 6 of 42 (14.3%) controls; P < 0.001. A very high proportion of patients in the treatment group scored 80 or more on all satisfaction scales at 6 and 12 months, with marked increases from baseline (P < 0.0001 to P < 0.01).
Possible Mechanisms
Satisfaction
At odds with the literature,24 there were no significant correlations at baseline or 12 months, among all patients, when total satisfaction and all 4 subscales were compared with patients' mental and physical health status (MCS, PCS, CES-D, SSAS, PSC).
Although satisfaction increased following treatment, no satisfaction scale at 6 months was independently associated with an improved MCS at 12 months (P values 0.15 to 0.69).24,25 Further against a mediating role, for improvement on the MCS at 12 months, the OR for treatment, adjusted for open-ended satisfaction at 6 months, was 1.74—a < 10% reduction from the unadjusted OR of 1.92.25 There was evidence that satisfaction was associated with the increased use of antidepressants in full doses (P = 0.037) and reduction of controlled substance medications (P = 0.043).
Antidepressants
Treatment also was strongly associated with using antidepressants in full doses (P < 0.0001), and the latter was associated with an improved MCS at 12 months (P = 0.012). When we controlled for antidepressant use, the primary association of treatment with an improved MCS disappeared (P = 0.22), the OR falling from 1.98 to 1.52 (23% reduction). Similarly, when controlling for treatment, the effect of antidepressants on improved MCS disappeared (P = 0.11). Among improved treatment patients, 37/46 (80%) took full doses, but the remaining 9 subjects (20%) did not. There was no association of the reduction in controlled substances with the improved MCS at 12 months (P = 0.68).
DISCUSSION
We confirmed the hypothesis that our intervention would lead to clinically significant improvement in mental function. A 4-point increase on the MCS corresponds to the mental improvement observed following a combined mitral/aortic valve replacement.9 In addition, patients' depression, satisfaction, physical disability, use of antidepressants, and non-use of controlled substances improved.
We believe the success of this study stemmed from addressing what many consider the basic problem with MUS patients, the personal dimension.1,4,23 By enhancing antidepressant use and by otherwise reducing psychological distress, MUS patients coped better with their symptoms. Patients who do best are those who cope best, not those who focus on eliminating physical symptoms.26
There were limitations. First, our results may not be representative of patients with lower utilization, with more severe co-morbid organic diseases, and with lower education. Second, the 43.7% who refused could have differed, but the baseline similarities between treatment and control groups are against this. Third, our data were not classified in such a way that we could evaluate treatment response in the various MUS syndromes such as IBS. Finally, full cost-effectiveness studies will require many more subjects,27 and utilization could not be evaluated as an outcome because the protocol prescribed a fixed number of visits for treatment patients. We do know, though, that utilization was similar in treatment and control groups (14.2 vs 12.3, respectively; P < 0.05). But, the total amount of contact, when including telephone calls, was almost certainly greater, a useful part of the intervention itself.
With no treatment precedent in medicine for MUS patients,6 this study sought, as its main objective, to determine if a primary care method was even feasible and effective (and at what “dose”). Our data raised the following questions for future study. (1) Can using providers more skilled in primary care reduce training time? (2) With the impact of treatment occurring by 3 to 6 months, will fewer visits suffice? (3) Can outcomes be improved by selecting just the more severe MUS population? Clearly, much work remains before the field has an efficient, refined, and generalizable approach and, ultimately, effective dissemination. For the present, the study supports establishing a strong PPR, frequent visits, antidepressants, and CBT principles.6
When we controlled for satisfaction, the association of treatment with improved mental function was unchanged, indicating that satisfaction did not mediate it,25 i.e., there was no longitudinal relationship of satisfaction and mental health status.24 Importantly, though, our questionnaire addressed satisfaction only with general communication and PPR skills (model 1), but it did not concern the more specific informing and motivating skills we used for treatment (model 2). We hypothesize that satisfaction with specific treatment-related patient-centered skills would have to be measured to evaluate whether satisfaction with the PPR could be significantly related to mental health outcome.
When we controlled for antidepressant use, the association of treatment with an improved MCS disappeared, and, when controlling for treatment, the effect of antidepressants disappeared. While antidepressants were a key factor, they were not the only one in the multidimensional treatment that contributed to improved mental function. The nonantidepressant aspects of the treatment must be invoked to explain improvement in the 20% of treatment patients who did not take full doses of antidepressants. Further, we hypothesize that these other treatment factors led also to the tripling of antidepressant use itself, an unusual achievement in medicine.28 Further study is needed to pinpoint the key components of our multidimensional treatment package.
Like others, we found that not all MUS patients were depressed and that, instead, they seemed to exist on a spectrum of severity.3,29,30 We conceptualize MUS as the unit of interest 31 and propose that it is a general warning signal of underlying psychological distress, of which depression is an advanced manifestation.3,29 The important point for primary care is that unexplained symptoms obscure the patient's psychic distress by misdirecting providers into the organic disease realm.4,5 This creates a serious problem: high-utilizing MUS patients are one of the most common conditions in all of medicine.3 Medically unexplained symptoms also are the major mode of presentation of (co-morbid) depression.3,29
CONCLUSION
We present the first multidimensional, primary care approach to the common, costly problem of distressed, high-utilizing MUS patients and show that it was effective. Much work remains, however, before definitive treatment guidelines can be identified.
Acknowledgments
Supported by NIMH grant MH 57099. Orally presented as a research abstract at the annual meetings of the Society of General Internal Medicine (May 2004), the American Academy on Physician and Patient Research Forum (October 2004), and the Academy of Psychosomatic Medicine (November 2004). We are grateful for the unflagging support and cooperation from colleagues at Blue Cross Network, Lansing, MI, 48824. We also thank the Michigan Public Health Institute for their always effective and timely assistance in our data gathering. We have no conflicts of interest.
Appendix
The data-gathering and relationship-building skills from the first model are integrated throughout the second one in plentiful amounts. Both models are adapted from R.C. Smith, patient-centered interviewing: An evidence-based method, Lippincott Williams & Wilkins, 2002; see Chapters 3 and 9, especially.
Model #1—“General/Basic Model”
A patient-centered interviewing method for establishing effective communication and relationships
Step 1—Setting the stage for the interview
Welcome the patient
Use the patient's name
Introduce self and identify specific role
Ensure patient readiness and privacy
Remove barriers to communication
Ensure comfort and put the patient at ease
Step 2—Chief Complaint/Agenda Setting
Indicate time available
Indicate own needs, e.g., to do physical exam
Obtain list of all issues patient wants to discuss, e.g., specific symptoms, requests, expectations, understanding
Summarize and finalize the agenda; negotiate specifics if too many agenda items
Step 3—Opening the HPI
Open-ended beginning question
“Nonfocusing” open-ended skills (Attentive Listening): silence, neutral utterances, nonverbal encouragement
Obtain additional data from nonverbal sources: nonverbal cues, physical characteristics, autonomic changes, accouterments, and environment
Step 4—Continuing the Patient-Centered HPI
Obtain description of the physical symptoms (Focusing open-ended skills)
Develop the more general personal/psychosocial context of the physical symptoms (Focusing open-ended skills)
Develop an emotional focus (Emotion-seeking skills)
Address the emotion(s) (Emotion-handling skills)
Expand the story to new chapters (focused open-ended skills, emotion-seeking skills, emotion-handling skills)
Step 5—Transition to the Doctor-Centered Process
Brief summary
Check accuracy
Indicate that both content and style of inquiry will change if the patient is ready
Model #2—“Treatment Model”
A patient-centered interviewing method for informing and motivating patients to take healthy treatment actions*
Step 1—Establish an Information Base and Motivate the Patient
Determine patient's knowledge base, specific situation, and readiness for change or acceptance of a new recommendation
Provide clear information about the adverse potential of the present situation and the benefits from the recommended changes
Make brief, clear recommendation of the change proposed, e.g., start antidepressants and an exercise program, stop addicting medications and alcohol
-
Motivate the patient
Inform of health and other benefits from action
Incorporate knowledge of patient's personality to enhance acceptance of recommendations
Emphasize patient's capacity for change
Emphasize that help is available
Indicate that past failures do not bode poorly
Check understanding and desire for change
Step 2—Commitment
Obtain explicit commitment to the new treatment
Set expectations for success
Reaffirm commitment
(Manage decision against advice)
Step 3—Negotiate a Specific Plan
Develop full understanding of the habit to be changed in the patient's life
Involve patient actively in plan to ensure appropriate levels of shared decision-making about how to handle all relevant diagnostic/treatment issues
Check understanding and reaffirm plan
Set regular follow-up
*We applied this model with all potentially difficult decisions about diagnosis and treatment. We used it to guide our negotiations of patients' preferences and, in its fullest extent, with patients who were resistant to and/or nonadherent with recommendations, e.g., take SSRIs, increase physical activity, stop taking narcotics and tranquilizers, begin exercise.
Note: the general communication and PPR skills of the first model must be extensively integrated throughout this model for it to be effective.
Supplementary Material
The following supplementary material is available for this article online at www.blackwell-synergy.com
Satisfaction with the provider-patient relationship questionnaire.
REFERENCES
- 1.deGruy F, Columbia L, Dickinson P. Somatization disorder in a family practice. J Fam Pract. 1987;25:45–51. [PubMed] [Google Scholar]
- 2.4. Washington, DC: American Psychiatric Association; 1994. American Psychiatric Association: Diagnostic and Statistical Manual of Mental Disorders. [Google Scholar]
- 3.Smith RC, Gardiner JC, Lyles JS, et al. Exploration of DSM-IV criteria in primary care patients with medically unexplained symptoms. Psychosom Med. 2005;67:123–9. doi: 10.1097/01.psy.0000149279.10978.3e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Escobar JI, Waitzkin H, Silver RC, Gara M, Holman A. Abridged somatization: a study in primary care. Psychosom Med. 1998;60:466–72. doi: 10.1097/00006842-199807000-00012. [DOI] [PubMed] [Google Scholar]
- 5.Bridges KW, Goldberg DP. Somatic presentation of DSM III psychiatric disorders in primary care. J Psychosom Res. 1985;29:563–9. doi: 10.1016/0022-3999(85)90064-9. [DOI] [PubMed] [Google Scholar]
- 6.Smith RC, Lein C, Collins C, et al. Treating patients with medically unexplained symptoms in primary care. J Gen Intern Med. 2003;18:478–89. doi: 10.1046/j.1525-1497.2003.20815.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lyles JS, Hodges A, Collins C, et al. Using nurse practitioners to implement an intervention in primary care for high utilizing patients with medically unexplained symptoms. Gen Hosp Psychiatry. 2003;25:63–73. doi: 10.1016/s0163-8343(02)00288-8. [DOI] [PubMed] [Google Scholar]
- 8.Smith RC, Korban E, Kanj M, et al. A method for rating charts to identify and classify patients with medically unexplained symptoms. Psychother Psychosom. 2004;73:36–42. doi: 10.1159/000074438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ware JE, Jr, Kosinski M, Keller SD. Boston: The Health Institute, New England Medical Center; 1994. SF-36 Physical and Mental Health Summary Scales: A User's Manual. [Google Scholar]
- 10.Horrocks S, Anderson E, Salisbury C. Systematic review of whether nurse practitioners working in primary care can provide equivalent care to doctors. Br Med J. 2002;324:819–23. doi: 10.1136/bmj.324.7341.819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Smith RC. 2. Philadelphia: Lippincott Williams & Wilkins; 2002. Patient-Centered Interviewing: An Evidence-Based Method. [Google Scholar]
- 12.Sharpe M. Cognitive behavioural therapies in the treatment of functional somatic symptoms. In: Mayou R, Bass C, Sharpe M, editors. Treatment of Functional Somatic Symptoms. Oxford: Oxford University Press; 1995. pp. 122–43. [Google Scholar]
- 13.Spitzer RL, Williams JBW, Kroenke K, et al. Utility of a new procedure for diagnosing mental disorders in primary care—the PRIME-MD Study. JAMA. 1994;272:1749–56. [PubMed] [Google Scholar]
- 14.Smith RC, Lyles JS, Mettler J, et al. The effectiveness of intensive training for residents in interviewing. A randomized, controlled study. Ann Intern Med. 1998;128:118–26. doi: 10.7326/0003-4819-128-2-199801150-00008. [DOI] [PubMed] [Google Scholar]
- 15.Katon W, Von Korff M, Lin E, et al. Stepped collaborative care for primary care patients with persistent symptoms of depression—a randomized trial. Arch Gen Psychiatry. 1999;46:1109–15. doi: 10.1001/archpsyc.56.12.1109. [DOI] [PubMed] [Google Scholar]
- 16.Sartorius N. Copyright World Health Organization; Composite International Diagnostic Interview (CIDI)—Core Version 1.1. [Google Scholar]
- 17.Ware JJE, Snow KK, Kosinski M, Gandek B. Boston: The Health Institute, New England Medical Center; 1993. SF-36 Health Survey—Manual and Interpretation Guide. [Google Scholar]
- 18.Von Korff M, Ustun TB, Ormel J, Kaplan I, Simon GE. Self-report disability in an international primary care study of psychological illness. J Clin Epidemiol. 1996;49:297–303. doi: 10.1016/0895-4356(95)00512-9. [DOI] [PubMed] [Google Scholar]
- 19.Radloff LS. The CES-D scale: a self-report depression scale for research in the general population. Appl Psychol Meas. 1977;1:385–401. [Google Scholar]
- 20.Chibnall J, Tait R. The Psychosomatic Symptom Checklist revisited: reliability and validity in a chronic pain population. J Behav Med. 1989;12:297–307. doi: 10.1007/BF00844873. [DOI] [PubMed] [Google Scholar]
- 21.Spielberger CD, Gorsuch RL, Lushene PR, Jacobs GA. Palo Alto, CA: Consulting Psycologists Press Inc; 1983. State-Trait Anxiety Inventory (Form Y) (“Self-Evaluation Questionnaire”) [Google Scholar]
- 22.Smith RC, Lyles JS, Mettler JA, et al. A strategy for improving patient satisfaction by the intensive training of residents in psychosocial medicine a controlled, randomized study. Acad Med. 1995;70:729–32. doi: 10.1097/00001888-199508000-00019. [DOI] [PubMed] [Google Scholar]
- 23.Escobar JI, Swartz M, Rubio-Stipec M, Manu P. Medically unexplained symptoms: distribution, risk factors, and comorbidity. In: Kirmayer LJ, Robbins JM, editors. Current Concepts of Somatization: Research and Clinical Perspectives. Washington, DC: American Psychiatric Press Inc; 1991. pp. 63–78. [Google Scholar]
- 24.Hall JA, Roter DL, Milburn MA. Illness and satisfaction with medical care. Curr Dir Psychol Sci. 1999;8:96–9. [Google Scholar]
- 25.Baron RM, Kenny DA. The moderator-mediator variable distinction in social psychological research: conceptual, strategic, and statistical considerations. J Pers Soc Psychol. 1986;51:1173–82. doi: 10.1037//0022-3514.51.6.1173. [DOI] [PubMed] [Google Scholar]
- 26.Barsky AJ, Ahern DK. Cognitive behavior therapy for hypochondriasis—a randomized controlled trial. JAMA. 2004;291:1464–70. doi: 10.1001/jama.291.12.1464. [DOI] [PubMed] [Google Scholar]
- 27.Torrance GW, Siegel JE, Luce BR. Framing and designing the cost-effectiveness analysis. In: Gold MR, Siegel JE, Russell LB, Weinstein MC, editors. Cost-Effectiveness in Health and Medicine. New York: Oxford University Press; 1996. pp. 54–81. [Google Scholar]
- 28.Lin E, Katon W, Simon G, et al. Achieving guidelines for the treatment of depression in primary care: is physician education enough? Med Care. 1997;35:831–42. doi: 10.1097/00005650-199708000-00008. [DOI] [PubMed] [Google Scholar]
- 29.Kroenke K. The interface between physical and psychological symptoms. Primary Care Companion. J Clin Psychiatry. 2003;5(Suppl 7):11–8. [Google Scholar]
- 30.Henningsen P, Zimmermann T, Sattel H. Medically unexplained physical symptoms, anxiety, and depression: a meta-analytic review. Psychosom Med. 2003;65:528–33. doi: 10.1097/01.psy.0000075977.90337.e7. [DOI] [PubMed] [Google Scholar]
- 31.Escobar JI, Gara M, Silver RC, Waitzkin G, Holman A, Compton W. Somatisation disorder in primary care. Br J Psychiatry. 1998;173:262–6. doi: 10.1192/bjp.173.3.262. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Satisfaction with the provider-patient relationship questionnaire.