Abstract
Clinically, we most often associate Wernicke's encephalopathy (WE) with an alcohol abusing population. However, it is important to consider other causes of malnutrition and vitamin deficiency as risk factors for the development of this disorder. We present a case of a 51-year-old man with schizophrenia and malnutrition who presented with delirium, ophthalmoplegia, and seizures. He responded rapidly to the administration of IV thiamine. Because of the high rate of mortality and morbidity, WE should be high on the differential of any patient at risk for malnutrition or with ophthalmoplegia, regardless of alcohol history. This is particularly important in psychiatric patients where the syndrome may be masked and thus treatment delayed.
Wernicke's encephalopathy (WE) is most commonly associated with heavy alcohol consumption, but is also seen in other clinical settings where malnutrition and vitamin deficiencies occur.1–11 Data from autopsy series note the prevalence of nonalcoholic WE to range from 0.8% to 2.8%.12,13 In one report, 20% of cases were diagnosed before death.3 Patients with psychiatric disorders often have poor dietary habits, malnutrition, and high prevalence of alcoholism, predisposing them to WE.14 Wernicke's symptoms may be overlooked or obscured by psychiatric illness. We present a case of nonalcohol-associated WE in a patient with chronic schizophrenia.
CASE
A 51-year-old independently living white male with chronic paranoid schizophrenia was admitted to the hospital following 2 witnessed generalized tonic-clinic seizures. Paramedics measured his blood glucose at 60 mg/dL. He had a second seizure in the emergency department. He had no history of neurological abnormalities, gait disturbances, or tardive dyskinesia, and at baseline was well functioning, and performing his activities of daily living. Three months before his admission, he stopped his chlorpromazine resulting in increased isolation and paranoia, decreased appetite, and a 40-pound weight loss. He had a remote alcohol abuse history but no history of alcohol dependence, delirium tremens, or seizures. As per his parents and case worker who visited him regularly, he had remained alcohol free the past several years and reported no evidence of him drinking alcohol during the weeks before admission. He had no tobacco or illicit drug use. Review of system was negative for paresthesia, paresis, chest pain, or shortness of breath.
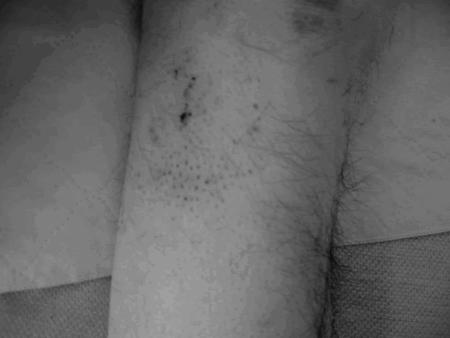
His examination was notable for a temperature of 36.6°F, blood pressure 95/74, heart rate 118, respiration 20, with a room air oxygen saturation of 97%. He was markedly disoriented, writhing with agitation, emaciated, and disheveled. He had mild dysarthria, a flat affect, randomly responded “not much, no, or yes” to questions, and increased nonpurposeful head and arm movements. Pupils were equal, round, and reactive to light, with marked limitation of bilateral vertical and horizontal gaze with no nystagmus. His neck and extremity tone was increased, and deep tendon reflexes could not be elicited. The plantar responses were flexor. He was unable to stand, ambulate, or cooperate with cerebellar, sensory, or motor examination due to confusion but was able to move all 4 extremities. He had a 3 cm forehead laceration with ecchymosis, multiple perifollicular petechiae on his posterior legs (Fig. 1). He had no changes in gums or tooth mobility. He had no appreciable ocular or retinal abnormalities.
FIGURE 1.
Follicular petechiae on the patient's lower extremities.
Laboratory studies revealed hyponatremia, hypochloremia, an anion gap acidosis, moderate renal failure, and mild rhabdomyolysis (Table 1). His CBC values were within normal range except for a mildly increased white blood cells (WBC) of 13.5 with 85% neutrophils. His MCV, methylmalonic acid, homocysteine, urine dipstick and microanalysis, urine drug screen, blood cultures, prothrombin time (by INR), partial thromboplastin time, troponin, electrocardiogram, arterial blood gas, chest radiograph were normal, and cerebral spinal fluid (CSF) fluid were unrevealing. On admission he had a mildly elevated aspartate aminotransaminase (AST) and total bilirubin but normal alanine aminotransaminase, alkaline phosphatase, total protein and albumin. His anion gap, AST, and bilirubin normalized on hospital day 2, but his albumin fell to 1.9 g/dL following hydration. A blood alcohol level was not obtained. A noncontrast head computed tomography (CT) showed mild cortical atrophy but no evidence of intracranial bleed or trauma.
Table 1.
Laboratory Values
| Variable | Values |
|---|---|
| Chemistry values on admission | |
| Sodium (mmol/L) | 130 |
| Potassium (mmol/L) | 3.4 |
| Chloride (mmol/L) | 89 |
| Total CO2 (mmol/L) | 16 |
| BUN (mg/dL) | 37 |
| Creatinine (mg/dL) | 1.9 |
| Glucose (mg/dL) | 108 |
| Calcium (mg/dL) | 10.4 |
| Magnesium (mg/dL) | 1.7 |
| Phosphorous (mg/dL) | 3.0 |
| Albumin (g/dL) | 4.1 |
| CK (U/L) | 2443 (normal range, 49 to 397) |
| Lactic acid (mmol/L) | 2.0 |
| Prealbumin (mg/L)—obtained after rehydration | 93 (normal range, 180 to 445) |
| AST (U/L) | 84.0 (normal range 15 to 41) |
| ALT (U/L) | 31.0 (normal range 13 to 48) |
| Alk phos (U/L) | 72 (normal range 53 to 128) |
| Bilirubin direct (mg/dL) | 0.5 (normal range<0.4) |
| Bilirubin total (mg/dL) | 2.7 (normal range 0.3 to 1.2) |
| Total protein (g/dL) | 7.2 (normal range 6.1 to 7.9) |
| Variable | Values |
|---|---|
| Hematologic values on admission | |
| Hematocrit (%) | 46.8 |
| Mean corpusule volume (fL) | 95.1 |
| Reticulocyte count (%) | 1.0 |
| Sedimentation rate (mm/hr) | 19 |
| White cell count (K/cu mm) | 13.5 |
| Differential (%) | |
| Neutrophils | 85 |
| Lymphocyte | 8 |
| Monocyte | 8 |
| Eosinophil | 0 |
| Basophil | 0 |
| Platelet count (K/cu mm) | 156 |
| Tests | Finding |
|---|---|
| Lumbar puncture findings | |
| CSF appearance | Clear |
| CSF cells count | |
| WBC (/cu mm) | 2 |
| RBC (/cu mm) | 6 |
| Protein (mg/dL) | 30 (normal range, 15 to 45) |
| Glucose (mg/dL) | 73 (normal range, 40 to 70) |
| Gram stain and culture | Negative |
| Acid fast bacilli culture | Negative |
| Fungal culture | Negative |
| CMV IgG, Elisa | Not detected |
| HSV viral culture | No HSV isolated |
| Tests | Result |
|---|---|
| Other infectious serology tests | |
| RPR | Nonreactive |
| HIV-1/HIV-2 antibody | Nonreactive |
| Tests | Result |
|---|---|
| Other chemistry tests | |
| TSH (μIU/mL) | 0.620 |
| Homocysteine (μmol/L) | 9.8 (normal range, 4 to 12) |
| Methylmalonic acid (μmol/L) | 0.13 (normal range,<0.40) |
| Phenytoin (μg/mL)-day 2 of hospitalization | 14.9 |
| Total CO2 (mmol/L)-9 hours after admission | 26 |
| Vitamin A (mg/L) | 0.18 (normal range, 0.30 to 1.20) |
| Vitamin E, α form (mg/L) | 4.1 (normal range, 5.5 to 18.0) |
| Vitamin K (ng/mL) | 0.51 (normal range, 0.10 to 2.20) |
| Vitamin D (ng/mL) | 18.0 (normal range, 10 to 68) |
| Vitamin C (mg/dL) | 0.6 (normal range, 0.4 to 2.0) |
AST, aspartate aminotransaminase; ALT, alanine aminotransaminase; WBC, whitw blood cells; RBC, red blood cells; TSH, thyroid-stimulating hormone; CSF, cerebrospinal fluid.
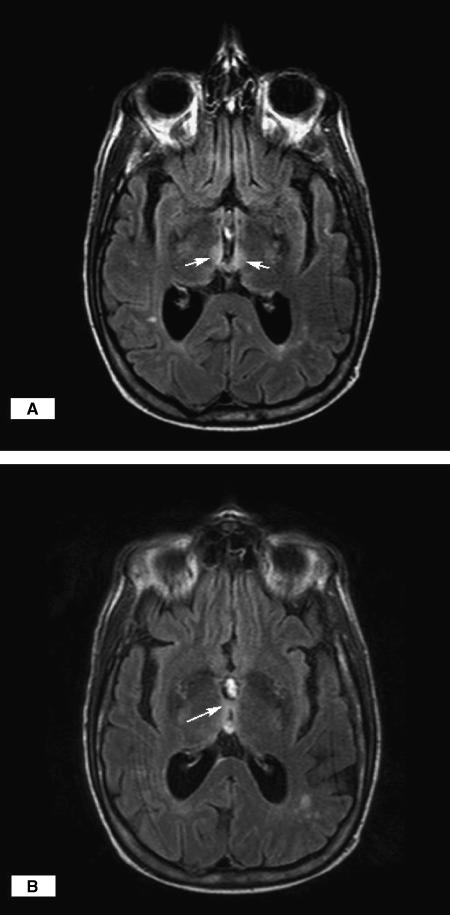
He was admitted with seizures, mild rhabdomyolysis, metabolic acidosis (thought secondary to his seizures), and laboratory and clinical features of malnutrition, scurvy, andWE. He was treated with intravenous 0.9% saline with 5% dextrose, thiamine, multivitamins, and phosphenytoin. Several hours after receiving 100 mg IV thiamine, his mental status, dyskinesia, rigidity, and ophthalmoplegia improved; however, marked vertical and horizontal nystagmus developed. He experienced a third witnessed generalized tonic-clonic seizure. An awake, interictal electroencephalogram (EEG) showed mild generalized slowing. A cranial magnetic resonace imaging (MRI) with gadolinium demonstrated moderate cortical atrophy and scattered bihemispheric subcortical and deep white matter nonenhancing foci. These changes were interpreted as consistent with chronic small vessel ischemic changes. Further review of the MRI revealed increased FLAIR T2 signal involving the medial aspect of the anterior thalamus extending into the hypothalamus and the massa intermedia (Fig. 2A and B), all consistent with his clinical diagnosis of WE. His vitamin analysis revealed low vitamin A, slightly low vitamin E, and C (Table 1).
FIGURE 2.
(A and B) Increased signal on axial T2 images involving the medial aspect of the thalamus and the massa intermedia. The arrows on “A” indicate the hyperintensity in the medial thalamus and anterior hypothalamus. The arrow on “B” indicates the hyperintensity in the massa intermedia.
Olanzapine was initiated for his schizophrenia. The thiamine dose was maintained at 100 mg/d throughout hospitalization and continued upon discharge. A repeat EEG on hospital day 4 showed improvement. On day 14, he was discharged at his baseline functioning mental status with mild ataxia, and persistent vertical nystagmus.
DISCUSSION
Our patient presented with decompensated chronic schizophrenia, acute delirium, ophthalmoplegia, malnutrition, and new seizures without evidence of trauma, concurrent alcohol use, or other medication toxicity. He also had physical exam and laboratory abnormalities consistent with mild dehydration with hypotension, tachycardia and mild azotemia, and severe malnutrition and multiple vitamin deficiencies. The differential diagnosis of acute delirium in schizophrenia is broad but the presence of seizures or focal neurological finding should suggest possible medication toxicity, substance abuse, trauma, or nutritional deficiency syndromes.15 Additionally, he had mild hypoglycemia, hyponatremia, hypochloremia, and hypomagnesemia, values not considered to be in the range to explain his clinical presentation. Given the course of our patient's presentation of ophthalmoplegia, and mental confusion which all improved rapidly with IV thiamine, we feel this is most supportive of a diagnosis ofWE. This diagnosis was also supported by typical findings of WE on MRI. Of note, vitamin E depletion could present with gaze paresis, areflexia, and increased muscle tone but the near normal lab value and improvement with IV thiamine would not be expected.16 While it is always difficult to completely exclude recent alcohol abuse as a contributor to his presentation, the observations of his family and case worker, and the absence of macrocytosis and meaningful elevations in AST make it less likely.
Wernicke's encephalopathy was first recognized in the 1880s as a morbid neurological condition associated with thiamine deficiency and characterized by nystagmus, abducens nerve and conjugate gaze palsies, ataxia of gait, and mental confusion, and a predictable response to thiamine.17 Seizures are uncommon in WE and their presence should suggest other possibilities such as alcohol withdrawal.17,18 Other complications include cardiomyopathy, central pontine myelinolysis, and gastrointestinal symptoms.9–11,17 Wernicke's encephalopathy is a medical emergency and if not recognized and treated early is associated with progression to irreversible Korsakoff psychosis, consisting of confabulation and anterograde memory deficits, and with 17% mortality.17,19 Following rapid treatment with thiamine, one would expect ocular signs to recover first, followed by ataxia, and variable mental status improvement.17,19 Because of a theoretical concern of precipitating WE, thiamine is usually given before glucose; however, this practice has not been substantiated.5,17,20
Thiamine is an important cofactor in the Kreb's and pentose phosphate cycle; its deficiency impairs glucose metabolism, cerebral metabolism of glutamate, and eventually the generation of proteins, DNA, neurotransmitters, and radical scavengers. The central nervous system (cerebellar vermis, and the midline mesencephalon structures) is particularly sensitive to thiamine deficiency due to cellular dependence on oxidative metabolism. Cell death occurs via necrosis and apoptosis.21 The pathologic lesions of WE are microscopic neuronal necrosis involving symmetrical lesions in the paraventricular regions of the thalamus and hypothalamus, mammillary bodies, periaqueductal region of the midbrain, floor of the fourth ventricle, and superior cerebellar vermis.17 Considerable heterogeneity exists between pathologic features and presenting signs.17,22 Even with early recognition and aggressive therapy, permanent disability often occurs due to the irreversible cytotoxic effects on specific regions of the brain.1
While alcohol abuse is the predominant risk factor for WE, numerous other conditions have been associated with its development, all of which predispose to malnutrition or vitamin deficiency (Table 2).1–11 Wernicke's encephalopathy can occur in both acute and chronic malnutrition states, as body stores of thiamine are only sufficient for up to 18 days.23 Previous published case reports suggests that there is an approximate lag time of 4 to 6 weeks from onset of thiamine-deficient diet to symptomatic presentation of WE.24–26
Table 2.
| AIDS |
| Chronic kidney disease on hemodialysis |
| Hyperemesis gravidarum |
| Postgastrointestinal surgery |
| Malignancy |
| Starvation or prolonged fasting |
| Prolonged parenteral feeding without proper supplementation |
| Transplantation |
| Anorexia nervosa or dieting |
| Uremia |
Additionally, one is less likely to recognize and treat patients with nonalcohol-associated WE. Although WE is thought to occur mainly in the alcoholic population due to poor nutritional habits, Lindboe's autopsy series of 6,964 autopsies in Norway demonstrated WE in 12 of 52 patients (23%) nonalcoholic patients.27 Among the 18 cases with clinical WE disease, all of them were diagnosed in patients with alcoholism. The most common clinical symptoms were depressed levels of consciousness and disorientation, whereas only 3 cases noted eye symptoms. Importantly, as none of the nonalcoholics were diagnosed before death, none were given thiamine therapy, whereas some of the alcoholics received routine large doses of thiamine. This study and our case report describes that WE is not routinely considered in the nonalcoholic population potentially resulting in progression of this treatable condition.
It is easy to see how patients such as ours can become malnourished, but the association of WE and schizophrenia is not well documented. Patients with schizophrenia are at greater risk for WE due to minimal self-care and homelessness, predisposing them to poor dietary habits, malnutrition, and a high prevalence of alcoholism.28 Additionally, as the diagnosis of WE is primarily clinical, thiamine treatment in patients with psychiatric disorders may be delayed if given at all. The resulting brain insult may be compounded by disturbances of carbohydrate metabolism possibly from the schizophrenia itself, or acquired. It is unknown the burden of WE in patients with schizophrenia, but a review of the neuropathology literature suggests that some patients with schizophrenia demonstrate periventricular gliosis postmortem, thought due to Wernicke's disease.14
No test is diagnostic of WE and CT is only 13% sensitive.20 With severe disease, MRI imaging may demonstrate increased T2 and FLAIR signal paralleling the pathologic lesions. With appropriate therapy and clinical improvement, these imaging changes may be reversible.17,19,22,29 As repeat MRI posttherapy would not have changed patient's management so it was not done. Diffusion weighted imaging may be useful before onset of necrosis, offering a potential treatment window where memory and other functions are preserved but is not necessary in all suspected cases.22
While this case of WE appeared in the setting of a patient with active psychiatric disease, we believe it highlights the importance of recognizing nonalcoholic populations at increased risk for thiamine deficiency and WE and performing a complete and frequent neurological examination in such patients. While WE is a well-characterized syndrome in alcoholism and malnutrition, little is written of its prevalence or presentation in patients with psychiatric illness. Owing to the high rate of mortality and morbidity, WE should be considered in the evaluation of any patient with unexplained nystagmus, gaze palsies, gait ataxia, or confusion, especially if a condition associated with malnutrition is present. This is particularly important in psychiatric patients where the clinical history and syndrome may be obscured and treatment delayed.
REFERENCES
- 1.Loh Y, Watson WD, Verma A, Chang ST, Stocker DJ, Labutta RJ. Acute Wernicke's encephalopathy following bariatric surgeryclinical course and MRI correlation. Obes Surg. 2004;14:129–32. doi: 10.1381/096089204772787437. [DOI] [PubMed] [Google Scholar]
- 2.Spruill SC, Kuller JA. Hyperemesis gravidarum complicated by Wernicke's encephalopathy. Am Coll Obstetric Gynecol. 2002;99:875–7. doi: 10.1016/s0029-7844(01)01603-9. [DOI] [PubMed] [Google Scholar]
- 3.Ogershok PR, Rahman A, Nestor S, Brick J. Wernicke encephalopathy in nonalcoholic patients. Am J Med Sci. 2002;323:107–11. doi: 10.1097/00000441-200202000-00010. [DOI] [PubMed] [Google Scholar]
- 4.Kuhn J, Friedel V, Knitelius HO, Bewermeyer H. Iatrogenic Wernicke–Korsakow syndrome with unusual neurological deficits and MRI lesions. Nervenarzt. 2004;75:795–800. doi: 10.1007/s00115-004-1694-7. [DOI] [PubMed] [Google Scholar]
- 5.Koguchi K, Nakatsuji Y, Abe K, Sakoda S. Wernicke's encephalopathy after glucose infusion. Neurology. 2004;62:512. doi: 10.1212/01.wnl.0000099189.56741.a7. [DOI] [PubMed] [Google Scholar]
- 6.Kozian R, Otto FG. Subacute encephalopathy with epileptic seizures in an alcoholic patient. Psychiatrische Praxis. 2000;27:298–300. [PubMed] [Google Scholar]
- 7.Doss A, Mahad D, Romanowski CAJ. Wernicke encephalopathyunusual findings in nonalcoholic patients. J Comput Assis Tomograph. 2003;27:235–40. doi: 10.1097/00004728-200303000-00022. [DOI] [PubMed] [Google Scholar]
- 8.Coe M, Carfagnini F, Tani G, Ambrosetto P. Wernicke's encephalopathy in a childcase report and MR findings. Pediatr Radiol. 2001;31:167–8. doi: 10.1007/s002470000396. [DOI] [PubMed] [Google Scholar]
- 9.Falcone N, Compagnoni A, Meschini C, Perrone C, Nappo A. Case report:central pontine myelinolysis induced by hypophosphatemia following Wernicke's encephalopathy. Neurol Sci. 2003;24:407–10. doi: 10.1007/s10072-003-0197-9. [DOI] [PubMed] [Google Scholar]
- 10.Donnino M. Gastrointestinal beriberi:a previously unrecognized syndrome. Annal Intern Med. 2004;141:898–9. doi: 10.7326/0003-4819-141-11-200412070-00035. [DOI] [PubMed] [Google Scholar]
- 11.Chimenti C. Dilated cardiomyopathy and celiac disease [reply] Italian Heart J. 2002;3:385. [PubMed] [Google Scholar]
- 12.Zubaran C, Fernandes JG, Rodnight R. Wernicke-korsakoff syndrome. Postgrad Med J. 1997;73:27–31. doi: 10.1136/pgmj.73.855.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Harper CG, giles M, Finlay-Jones R. Clinical signs in the Wernicke-Korsakoff complex; a retrospective analysis of 131 cases diagnosed at necropsy. J Neurosurg Psychiatry. 1986;49:341–5. doi: 10.1136/jnnp.49.4.341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Casanova MF. Wernicke's disease and schizophrenia:a case report and review of the literature. Int J Psychiatry Med. 1996;26:319–28. doi: 10.2190/GD76-1UP3-TR18-YPYH. [DOI] [PubMed] [Google Scholar]
- 15.Tsai HY, Lieh Yeh T, Sheei-Meei W, Chen PS, Yang YK. Starvation-induced Wernicke's encephalopathy in schizophrenia [Case Reports. Letter] Psychiatry Clin Neurosci. 2004;58:338–9. doi: 10.1111/j.1440-1819.2004.01242.x. [DOI] [PubMed] [Google Scholar]
- 16.Sokol RJ. Vitamin E deficiency and neurologic disease. Annu Rev Nutr. 1988;8:351–73. doi: 10.1146/annurev.nu.08.070188.002031. [DOI] [PubMed] [Google Scholar]
- 17.Ropper AH, Brown RH. 8. McGraw-Hill: Adams and Victor's Principles of Neurology; 2005. Diseases of the Nervous System due to Nutritional Deficiency; pp. 984–8. [Google Scholar]
- 18.Bortz JJ. Nonepileptic seizures:issues in differential diagnosis and treatment. CNS Spectrums. 1997;2 [Google Scholar]
- 19.Charness ME. Overview of chronic neurologic complications of alcohol. Uptodate online.
- 20.Chung SP, Kim SW, Yoo IS, Lim YS, Lee G. MRI as a diagnostic adjunct to WE In the ED. Am J Emerg Med. 2003;21:497–502. doi: 10.1016/s0735-6757(03)00094-9. [DOI] [PubMed] [Google Scholar]
- 21.Hack JB, Hoffman RS. Thiamine before glucose to prevent WE:examining conventional wisdom. JAMA. 1998;279 doi: 10.1001/jama.279.8.583. [DOI] [PubMed] [Google Scholar]
- 22.Doherty MJ. Diffusion abnormalities in patients with Wernicke encephalopathy. Neurology. 2002;58:655–7. doi: 10.1212/wnl.58.4.655. [DOI] [PubMed] [Google Scholar]
- 23.Ziporin ZZ, Nunes WT, Powell RC, et al. Thiamine requirement in the adult human as measured by urinary excretion of thiamine metabolites. J Nutr. 1965;85:297–304. doi: 10.1093/jn/85.3.297. [DOI] [PubMed] [Google Scholar]
- 24.Kwan MC, Lee KF, Sin SY, Chan YW, Wong AK. Wernicke's encephalopathy in a patient with hyperemesis gravidarum and thyrotoxicosis. Hong Kong Med J. 1996;2:208–10. [Google Scholar]
- 25.Kramer LD, Locke GE. Wernicke's encephalopathy. Complication of gastric plication. J Clin Gastroenterol. 1987;9:549–52. doi: 10.1097/00004836-198710000-00013. [DOI] [PubMed] [Google Scholar]
- 26.Shiozawa T, Shiota H, Shikata E, Kamei S, Mizutani T. Development of Wernicke's encephalopathy during the period of oral food intake after a subtotal colectomy for ulcerative colitis. Rinsho Shinkeigaku. 1995;35:169–74. [PubMed] [Google Scholar]
- 27.Lindboe CF, Loberg EM. Wernicke's encephalopathy in non-alcoholics. An autopsy study. J Neurol Sci. 1989;90:125. doi: 10.1016/0022-510x(89)90095-6. [DOI] [PubMed] [Google Scholar]
- 28.Spittle B, Parker J. Wernicke's encephalopathy complicating schizophrenia. Aust NZ J Psychiatry. 1993;27:638–52. doi: 10.3109/00048679309075827. [DOI] [PubMed] [Google Scholar]
- 29.Antunez E, Estruch R, Cardenal C, Nicolas JM, Fernandez-Sola J, Urbano-Marquez A. Usefulness of CT and MR imaging in the diagnosis of acute Wernicke's encephalopathy. Am J Roentgenol. 1998;171:1131–7. doi: 10.2214/ajr.171.4.9763009. [DOI] [PubMed] [Google Scholar]