Abstract
BACKGROUND
Angiotensin converting enzyme-inhibitors (ACEI) and angiotensin-II-receptor blockers (ARB) are equally efficacious in reducing mortality after MI, although the latter are far more costly. Little is known about their relative use after MI in typical care settings, and about their relative effectiveness outside the clinical trial setting.
OBJECTIVES
To assess temporal trends in the relative use of ACEI and ARB after myocardial infarction, and to test for differences in 1-year survival between users of these drug classes.
DESIGN
Retrospective closed cohort study.
PATIENTS
Medicare beneficiaries who survived > 90 days after myocardial infarction, had full prescription drug coverage, and who filled a prescription for either ACEI or ARB within 90 days of myocardial infarction.
MEASUREMENTS
Relative use of ACEI versus ARB over time. Adjusted relative 1-year mortality between ACEI and ARB users.
RESULTS
Between 1995 and 2004, 14,190 patients met inclusion criteria. Mean age was 80 years, 75% were female, and 90% were white. Overall, 88% received an ACEI, and 12% an ARB, with the proportion receiving an ARB increasing from 2% (1995) to 25% (2004; P<.001). Multivariate-adjusted 1-year mortality did not differ between ARB and ACEI users (HR: 1.04; 95% confidence interval: 0.88 to 1.22). The findings were similar for new users of ACEI/ARB, and for those with preexisting heart failure.
CONCLUSIONS
ARB users had the same 1-year mortality after myocardial infarction as ACEI users in routine care. Use of more costly ARB has increased dramatically over time, to a quarter of ACEI/ARB users, despite the lack of a therapeutic advantage for most patients.
Keywords: coronary heart disease, epidemiology, health services and outcomes research, preventive cardiology
The mortality benefit of angiotensin converting enzyme-inhibitor (ACEI) therapy after myocardial infarction has been established in numerous clinical trials. While the earliest data were based on patients with reduced left-ventricular function,1–3 more recent evidence has established the efficacy of ACEI in reducing cardiovascular mortality and morbidity in patients without heart failure.4 ACEI efficacy has also been established for several other indications such as primary and secondary prevention of diabetic nephropathy, and for antihypertensive treatment. The ACEI class presented with a favorable side-effect profile: the clinically most relevant side-effect, cough, occurred in <10% of patients in most trials, and the rate difference of cough between ACEI and the comparator group was <5% in nearly all of them. This side-effect, albeit annoying for the patient, is benign and completely reversible upon withdrawal.5 Both hyperkalemia and (temporary) increases in renal parameters can be detected early through appropriate clinical monitoring. The most dreaded side-effect of ACEI treatment, angioedema, is extremely rare.5 Several studies have established the favorable cost effectiveness of ACEI treatment for various indications.6–9
In 1995, angiotensin-II-receptor blockers (ARB) became available for clinical use, and their effectiveness has been established for similar indications.10 While the risk of treatment-associated hyperkalemia and renal dysfunction is similar between these classes, ARB are unlikely to cause cough even in patients who developed this side-effect under ACEI therapy.11,12 The biggest difference between the 2 drug classes, however, is their cost: ARB are substantially more expensive than ACEI of comparable dose.13
In clinical practice, it is often assumed that ARB and ACEI can be used interchangeably, except for the small proportion of patients who develop ACEI-associated cough. Few studies have compared these classes head to head, and none have shown superiority for ARB over ACEI.14,15 While this evidence indicates equal efficacy between ACEI and ARB in trial settings, the relative effectiveness of these drug classes in real populations and more typical health care settings is unknown. We conducted the present study to evaluate trends in the relative utilization of ACEI and ARB and to test whether mortality differed between patients taking ARB versus ACEI after MI in 2 large eastern states of the United States.
METHODS
For this study, we used medical claims data from 4 different sources: the complete Medicare Part A and B claims from New Jersey and Pennsylvania (1994 to 2004), the New Jersey Medicaid program (1994 to mid-2000), the New Jersey Pharmaceutical Assistance for the Aged and Disabled (PAAD) program, and the Pennsylvania Pharmaceutical Assistance Contract for the Elderly (PACE; 1994 to 2004) program. These means-tested programs (Medicaid, PAAD, PACE) provide comprehensive prescription drug coverage for eligible elderly patients in New Jersey or Pennsylvania. Neither program had any restrictions or prior-authorization programs in place for ARB. We identified all patients hospitalized for at least 3 days who were discharged with an ICD-9 diagnosis code of 410.xx. This method of selecting patients with acute myocardial infarction for study had a positive predictive value of 94% in a recently published validation study.16 Patients were required to have been an active participant in their respective benefit programs for >1 year before MI to be included. We further required all study patients to be discharged within 30 days and to survive at least 90 days from the first day of their myocardial infarction hospitalization.
From enrollment files, we defined each patient's age, gender, and race (white, black, other). We searched all medical claims from the year before myocardial infarction for all diagnosis codes, and defined all recorded comorbid conditions (hypertension, coronary artery disease, congestive heart failure, cerebrovascular disease, peripheral artery disease, atrial fibrillation, arthritis, diabetes, chronic kidney disease, end-stage renal disease [maintenance dialysis or kidney transplantation], chronic obstructive pulmonary disease, alcohol abuse, dementia, depression, other mental disease, any malignancy, obesity), as well as several health care utilization indicators (number of hospital days, physician visits, prescriptions for different generic drugs filled, any nursing home stay). We also ascertained whether each patient had filled any prescriptions for the following cardiovascular medications during the year before myocardial infarction: α-receptor blockers, ACEI, ARB, antiplatelet drugs (ticlopidine, clopidogrel), β-receptor blockers, calcium-channel blockers, centrally acting antihypertensives, diuretics, statins, nitrates, and warfarin. We recorded the days of hospitalization for MI, and whether angiography or revascularization procedure was performed. Between discharge and 90 days postadmission, we ascertained any filled prescriptions for the same medications listed above. In this study, we compared relative utilization and outcomes between ACEI and ARB users rather than versus nonusers of these drugs.17
The main analysis considered all patients who fulfilled the stated inclusion criteria; a secondary analysis focused on patients who had not used any ACEI or ARB during the year before myocardial infarction (new users). We also stratified by the presence of congestive heart failure during the preceding year.
Patient characteristics were compared between ACEI and ARB users using t-tests for continuous variables and χ2 tests for categorical variables. We compared 1-year all-cause mortality between ACEI and ARB users using univariate and multivariate Cox proportional hazards regression. A priori, we decided to build full multivariate models including all available variables. Hazards ratios were calculated with 95% confidence interval (CI). We used the SAS for Windows software (release 8.2) for all statistical analyses (The SAS Institute, Cary, NC).
RESULTS
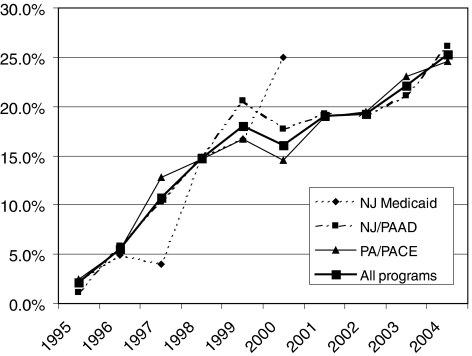
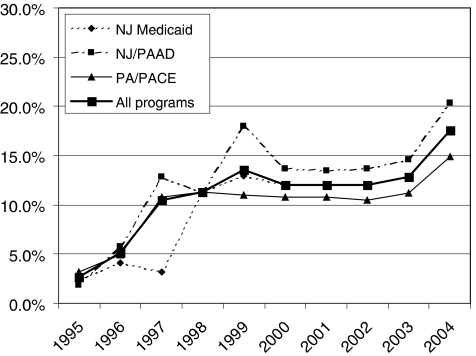
We identified 43,416 patients with myocardial infarction who were active participants in 1 of the drug entitlement programs for at least a year; 30,741 (70.8%) survived at least 90 days postmyocardial infarction. We excluded 980 patients who were hospitalized for more than 30 days, which left 29,761 patients for study. Of these, 14,612 (49.1%) filled a prescription for an ACEI or ARB within 90 days after admission for myocardial infarction. Four hundred and twenty-two patients filled a prescription for both an ACEI and an ARB. Overall, between 1994 and 2004, 12,485 (88%) patients received an ACEI and 1,705 (12%) received an ARB. Figure 1 shows the trend in relative utilization of ARB versus ACEI during the study period: while ARB were only used in 2.1% of patients in 1995, this proportion increased to 25.3% in 2004. Trends were similar for new users (Fig. 2): in 2004, 17.6% of patients received an ARB without prior ACEI use, up from 2.7% in 1995.
FIGURE 1.
Trends in ARB use as a proportion of all angiotensin-blocking drugs (i.e., ACEI or ARB), all users. ACEI, angiotensin converting enzyme inhibitor; ARB, angiotensin-II-receptor blocker; NJ, New Jersey; PA. Pennsylvania; PAAD, Pharmaceutical Assistance for the Aged and Disabled; PACE, Pharmaceutical Assistance Contract for the Elderly.
FIGURE 2.
Trends in ARB use as a proportion of all angiotensin-blocking drugs (i.e., ACEI or ARB), new users. ACEI, angiotensin converting enzyme inhibitor; ARB, angiotensin-II-receptor blocker; RAAS, renin angiotensin aldosterone system; NJ, New Jersey; PA, Pennsylvania; PAAD, Pharmaceutical Assistance for the Aged and Disabled; PACE, Pharmaceutical Assistance Contract for the Elderly.
Important patient characteristics, stratified by ACEI versus ARB use, are shown in Table 1. ARB users were slightly older and more likely to be female than users of ACEI. They also tended to have more diagnosed comorbidity, concomitant use of a greater number of other prescription drugs, and more physician visits in the year before myocardial infarction (Table 1). Of the 12,485 users of ACEI after myocardial infarction, 51.8% had received an ACEI and 3.7% an ARB during the year before myocardial infarction. By contrast, in the ARB group, 26.2% had received an ACEI and 52.7% an ARB in the previous year (P<.001). The duration of the index hospital stay was slightly shorter for ARB users, but their use of several cardiovascular medications after discharge was greater than in ACEI patients (Table 2). New ARB users were more likely to be female than new ACEI users. Of all comorbid conditions, only diagnoses of chronic obstructive pulmonary disease (P=.01) and hypertension were greater in ARB users (P<.001; Table 1).
Table 1.
Patient Characteristics Before Myocardial Infarction
| Variable (N (%)) or (mean (± SD)) | All Users (N = 14,612) | New Users (N = 6,385) | ||||
|---|---|---|---|---|---|---|
| ACEI (N = 12,485) | ARB (N = 2,127) | P-value | ACEI (N = 5,724) | ARB (N = 661) | P-value | |
| New Jersey PAAD | 4,504 (83.9) | 866 (16.1) | — | 2,037 (87.4) | 294 (12.6) | — |
| New Jersey Medicaid | 1,046 (89.9) | 117 (10.1) | — | 489 (92.4) | 40 (7.6) | — |
| Pennsylvania PACE | 6,935 (85.8) | 1,144 (4.2) | — | 3,198 (90.7) | 327 (9.3) | — |
| Age | 79.9 (± 7.1) | 80.4 (± 7.1) | .003 | 80.0 (± 7.0) | 80.2 (± 7.1) | .46 |
| Male gender | 3,156 (25.3) | 382 (18.0) | <.001 | 1,506 (26.3) | 132 (20.0) | <.001 |
| White race | 11,182 (89.6) | 1,905 (89.6) | .74 | 5,174 (90.4) | 588 (89.0) | .46 |
| Black race | 810 (6.5) | 144 (6.8) | 330 (5.8) | 42 (6.4) | ||
| Other race | 493 (4.0) | 78 (3.7) | 220 (3.8) | 31 (4.7) | ||
| Nursing home in year before MI | 1,045 (8.4) | 151 (7.1) | .05 | 396 (6.9) | 29 (4.4) | .01 |
| Hospital days in year before MI | 4.6 (± 10.3) | 4.6 (± 9.6) | .74 | 3.1 (± 8.2) | 2.4 (± 6.3) | .01 |
| Number of physician visits in year before MI | 9.7 (± 7.1) | 11.0 (± 7.3) | <.001 | 8.8 (± 6.5) | 10.0 (± 6.9) | <.001 |
| Coronary artery disease | 10,217 (81.8) | 1,823 (85.7) | <.001 | 4,420 (77.2) | 529 (80.0) | .10 |
| Congestive heart failure | 4,899 (39.2) | 938 (44.1) | <.001 | 1,448 (25.3) | 187 (28.3) | .10 |
| Cerebrovascular disease | 2,992 (24.0) | 592 (27.8) | <.001 | 1,217 (21.3) | 143 (21.6) | .82 |
| Peripheral vascular disease | 2,778 (22.3) | 480 (22.6) | .75 | 1,067 (18.6) | 127 (19.2) | .72 |
| Hypertension | 8,164 (65.4) | 1,622 (76.3) | <.001 | 3,212 (56.1) | 426 (64.5) | <.001 |
| Atrial fibrillation | 2,198 (17.6) | 439 (20.6) | <.001 | 713 (12.4) | 95 (14.4) | .16 |
| Obesity | 356 (2.9) | 88 (4.1) | .001 | 113 (2.0) | 19 (2.9) | .12 |
| Diabetes | 5,820 (46.6) | 1,051 (49.4) | .02 | 2,303 (40.2) | 269 (40.7) | .82 |
| Arthritis | 4,246 (34.0) | 770 (36.2) | .05 | 2,073 (36.2) | 245 (37.1) | .67 |
| Chronic kidney disease | 434 (3.5) | 113 (5.3) | <.001 | 110 (1.9) | 18 (2.7) | .16 |
| Dialysis or kidney transplantation | 82 (0.7) | 26 (1.2) | .04 | 30 (0.5) | 4 (0.6) | .69 |
| Chronic obstructive pulmonary disease | 3,600 (28.8) | 703 (33.1) | <.001 | 1,520 (26.6) | 205 (31.0) | .01 |
| Malignancy | 765 (6.1) | 113 (5.3) | .14 | 333 (5.8) | 47 (7.1) | .18 |
| Dementia | 959 (7.7) | 153 (7.1) | .43 | 397 (6.9) | 32 (4.8) | .04 |
| Depression | 1,598 (12.8) | 288 (13.5) | .35 | 714 (12.4) | 81 (12.3) | .87 |
| Alcohol abuse | 333 (2.7) | 52 (2.4) | .55 | 119 (2.1) | 13 (2.0) | .85 |
| Other mental disease | 1,040 (8.3) | 165 (7.8) | .37 | 454 (7.9) | 47 (7.1) | .46 |
| Prescriptions filled before MI | ||||||
| Total number of different drugs | 10.9 (± 6.2) | 12.8 (± 6.6) | <.001 | 9.3 (± 5.6) | 10.5 (± 6.0) | <.001 |
| α-receptor blocker | 696 (5.6) | 153 (7.2) | .003 | 314 (5.5) | 40 (6.1) | .55 |
| ACE inhibitor | 6,462 (51.8) | 558 (26.2) | <.001 | — | — | — |
| ARB | 462 (3.7) | 1,121 (52.7) | <.001 | — | — | — |
| ACE inhibitor or ARB | 6,761 (54.2) | 1,466 (68.9) | <.001 | — | — | — |
| Antiplatelet drugs (ticlopidine, clopidogrel) | 665 (5.3) | 230 (10.8) | <.001 | 202 (3.5) | 43 (6.5) | <.001 |
| β-receptor blocker | 3,010 (24.1) | 645 (30.3) | <.001 | 1,590 (27.8) | 203 (30.7) | .11 |
| Calcium-channel blocker | 5,789 (46.3) | 1,096 (51.5) | <.001 | 2,635 (46.0) | 347 (52.5) | .002 |
| Centrally acting antihypertensive drug | 593 (4.8) | 142 (6.7) | <.001 | 232 (4.1) | 33 (5.0) | .25 |
| Diuretic | 1,371 (11.0) | 324 (15.2) | <.001 | 447 (7.8) | 65 (9.8) | .07 |
| Statin | 2,951 (23.6) | 731 (34.4) | <.001 | 1,062 (18.6) | 181 (27.4) | <.001 |
| Nitrate | 5,479 (43.9) | 996 (46.8) | .003 | 2,006 (33.1) | 254 (38.4) | .09 |
| Warfarin | 1,527 (12.2) | 294 (13.8) | .04 | 473 (8.3) | 63 (9.5) | .27 |
ACEI, angiotensin converting enzyme-inhibitors; ARB, angiotensin-II-receptor blockers; MI, myocardial infarction; PAAD, Pharmaceutical Assistance for the Aged and Disabled; PACE, Pharmaceutical Assistance Contract for the Elderly.
Table 2.
Health Care Utilization between Admission and 90 d After Myocardial Infarction
| Variable | All Users (N = 17,646) | New Users (N = 6,938) | ||||
|---|---|---|---|---|---|---|
| ACEI (N = 15,601) | ARB (N = 2,045) | P-value | ACEI (N = 6,393) | ARB (N = 545) | P-value | |
| Myocardial infarction hospitalization | ||||||
| Hospitalization length of stay | 9.8 (± 5.1) | 9.5 (± 5.2) | <.001 | 10.1 (± 5.2) | 10.4 (± 5.5) | .26 |
| Angiography or revascularization procedure | 1,641 (13.1) | 364 (17.1) | <.001 | 872 (15.2) | 135 (20.4) | <.001 |
| Prescriptions filled within 90 d after MI | ||||||
| α-receptor blocker | 299 (2.4) | 84 (4.0) | <.001 | 114 (2.0) | 21 (3.2) | .04 |
| Antiplatelet drugs (ticlopidine, clopidogrel) | 2,326 (18.6) | 629 (29.6) | <.001 | 1,067 (18.6) | 183 (27.7) | <.001 |
| β-receptor blocker | 4,330 (34.7) | 1,022 (48.1) | <.001 | 2,095 (36.6) | 328 (49.6) | <.001 |
| Calcium-channel blocker | 3,285 (26.3) | 675 (31.7) | <.001 | 1,273 (22.2) | 179 (27.1) | .005 |
| Centrally acting antihypertensive drug | 266 (2.1) | 86 (4.0) | <.001 | 83 (1.5) | 14 (2.1) | .18 |
| Diuretic | 996 (8.0) | 272 (12.8) | <.001 | 343 (6.0) | 59 (8.9) | .003 |
| Fibrate | 191 (1.5) | 37 (1.7) | .47 | 68 (1.2) | 10 (1.5) | .47 |
| Statin | 4,101 (32.9) | 930 (46.7) | <.001 | 1,856 (32.4) | 266 (40.2) | <.001 |
| Nitrate | 8,395 (67.2) | 1,411 (66.3) | .41 | 3,672 (64.2) | 429 (64.9) | .70 |
| Warfarin | 2,395 (19.2) | 412 (19.4) | .84 | 1,048 (18.3) | 134 (20.3) | .22 |
ACEI, angiotensin converting enzyme-inhibitors; ARB, angiotensin-II-receptor blockers.
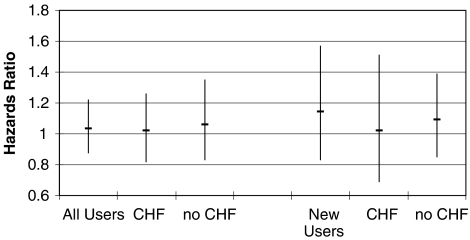
Of the 14,612 patients in the all-user cohort, 2,287 died within the first year after myocardial infarction (16.1%). In univariate analysis, there was no difference in 1-year mortality between ARB (referent) and ACEI users (HR=0.92; 95% CI: 0.80 to 1.05; Fig. 3). After multivariate adjustment, the finding of no association remained (HR=1.04; 95% CI: 0.88 to 1.22). Restricting the analyses to 6,385 new-users of ACEI or ARB, 891 deaths were observed within the first year postmyocardial infarction (14.3%). As before, there was no univariate (HR=1.07; 95% CI: 0.85 to 1.36) or multivariate association (HR=1.09; 95% CI: 0.85 to 1.39) between ARB versus ACEI use and 1-year mortality. These findings were essentially unchanged for patients with versus without prior diagnosis of congestive heart failure (Fig. 3).
FIGURE 3.
Hazard ratios with 1-year mortality of ARB use compared with ACEI use after myocardial infarction. ACEI, angiotensin converting enzyme inhibitor; ARB, angiotensin-II-receptor blocker; CHF, previous diagnosis of congestive heart failure; New Users, no ACEI or ARB use in 365 days before admission for myocardial infarction. ARB, reference group.
DISCUSSION
The present study provides evidence of increasing use of ARB compared with ACEI after myocardial infarction, reaching > 25% of patients receiving either class in 2004. This percentage is substantially greater than the <10% of patients who develop cough or another side-effect associated with ACEI, but not with ARB use. Furthermore, among new users, 18% were prescribed an ARB without any prior evidence of ACEI use. Similar to findings from head-to-head trials that failed to establish superiority of ARB treatment over ACEI after myocardial infarction,14,15 this study showed all-cause mortality at 1 year to be similar between patients receiving ARB versus ACEI. Although this was an observational study, the sample size was adequate to detect small differences. The CIs surrounding our estimated effects were tight and demonstrated that even a 15% improvement in mortality with ARB, corresponding to the lower 95% confidence limit of both new-user and all-user analyses, was extremely unlikely. This finding persisted whether the patients had prediagnosed CHF or whether they were new users of ACEI or ARB. The findings were also unchanged after removal of patients who switched or added a compound from the respective other class of RAAS inhibitors during follow-up. These findings raise important issues for clinical treatment decisions, taking into account relative effectiveness, risks, and cost.
ACEI have long been a mainstay of therapy for secondary prevention after MI, management of hypertension, treatment of congestive heart failure, and prevention of kidney damage. The availability of ARB has provided clinicians with yet another therapeutic choice for these indications. While ARB share many clinical features with the older ACEI class, they were expected to provide several theoretical and practical advantages. Angiotensin II is not only produced by angiotensin converting enzyme (kininase II), but by other enzymes, so that such angiotensin II production would not be completely blocked with ACEI. Thus, it was hoped that ARB use would lead to a more complete blockade of angiotensin II effects by targeting the AT1 receptor rather than the messenger. Further, ACEI, but not ARB, prevent bradikinin degradation and allow it to accumulate, leading to the known side-effects of ACEI therapy such as cough, rash, and angioedema.18 As a result, the most notable side-effect of ACEI, a dry and nonproductive cough, is considerably less frequent with ARB therapy. Several studies have demonstrated that most patients who experienced cough with ACEI can be safely switched to ARB. Similarly, angioedema, a rare consequence of ACEI therapy, is less likely to occur with ARB. Other side-effects such as hypotension, hyperkalemia, or worsening of renal function do not differ between these classes.
The theoretical advantages of ARB led to 2 randomized controlled trials that compared these classes in patients after myocardial infarction. The Valsartan in Acute Myocardial Infarction (VALIANT) trial enrolled 14,703 high-risk MI patients with evidence of reduced left ventricular systolic dysfunction and randomized them to captopril, valsartan, or both. Valsartan was shown to be as effective as captopril using formal noninferiority analysis. Combination therapy increased the rate of adverse events without conferring any additional benefit.14 The Optimal Therapy in Myocardial Infarction with the Angiotensin II Antagonist Losartan (OPTIMAAL) trial randomized 5,477 patients to receive either losartan or captopril. In that trial, there was a trend toward superiority of captopril, and noninferiority for losartan compared with captopril could not be shown.15
From a quality assurance point of view, the proportion of patients who should receive ARB rather than ACEI treatment depends on the frequency with which patients experience a side-effect from ACEI therapy that could be avoided with use of an ARB. Most ACEI trials and comparative trials of ACEI versus ARB have found that <5% of patients experienced ACEI-related cough, and the risk of angioedema is very small (<1%). In VALIANT, the respective rates of cough and angioedema were 5% and 0.5% in the captopril group and 1.7% and 0.2% in the valsartan treatment arm, respectively. Fewer than half of the patients experiencing cough discontinued the study drug. In OPTIMAAL, although the reported rates of cough were considerably higher in both treatment arms (18.7% for captopril and 9.3% for losartan), discontinuation rates due to cough were similar compared with VALIANT: only 4.1% (captopril) and 1.7% (losartan) of patients stopped the study drug. Angioedema rates were similar in OPTIMAAL and VALIANT. Taking into account the risks of these adverse events with ACEI and ARB, it is likely that of all patients receiving an angiotensin-blocking drug, no more than 10% should receive an ARB. It is possible, though, that slightly more than 10% of patients in our specific study sample of predominantly older women experienced ACEI-related cough, since it has been shown that both age and female gender are associated with this side-effect of ACEI therapy.19 Regardless, it seems plausible that the > 25% of ARB use found in our study constitutes overuse of this class: clinicians appear to be increasingly inclined to prescribe ARB without trying an ACEI first: roughly 18% of the patients receiving an RAAS blocking drug in 2004 received an ARB with no evidence of prior ACEI use. The annual treatment cost for the average dose in VALIANT, for example, are $237 for captopril therapy (50 mg t.i.d.) and $1,143 for valsartan (80 mg b.i.d.).13 Some insurance and prescription drug benefits programs have established prior-authorization policies for ARB in an attempt to restrict their use to patients who cannot receive ACEI. The effectiveness of these policies has not been studied. Presumably, ARB are a good target for prior-authorization programs, similar to cyclooxygenase-2 inhibitors, as their difference to ACEI lies solely in the safety profile and tolerability.20 An systematic evaluation of ARB versus ACEI use in state Medicaid programs throughout the United States found that prior authorization programs for ARBs were ineffective unless they required a previous trial of an ACEI.21 While prior-authorization or similar policy tools could be implemented to increase appropriate therapeutic choices, it is uncertain whether savings from such programs would actually offset their implementation costs. Thus, building awareness among the prescribers of these drugs about these issues, maybe through academic detailing programs, might be an alterative means to improving appropriate use of ARB in the near future.
Thus, from a policy perspective, assuming equal effectiveness between ACEI and ARB, a greater risk of selected side-effects with ACEI, and substantially greater cost with ARB, the treatment approach appears clear: in patients who present with an indication for an angiotensin-blocking drug, ACEI should be the effective and inexpensive first-line treatment. In addition to the situation after MI, several systematic reviews have concluded that ACEI and ARB do not differ in efficacy for reducing all-cause mortality or hospitalizations in patients with CHF or high-risk MI, also suggesting that ARB should be reserved for second line use.22,23 Others have even observed that while ACEI have been shown to reduce all-cause mortality, such effectiveness has not yet been demonstrated for ARB.24 Thus, ARB as more expensive niche drugs are important for patients who develop a side-effect such as cough, rash, or angioedema. For side-effects such as hypotension, decline in renal function, or increase in serum potassium concentrations, a switch to ARB is not indicated as these are equally likely with ARB therapy. In the absence of any financial constraint, one could argue that ARBs—with equal efficacy and fewer side-effects—should be used for most or all patients; however, prescription drug costs remain a major concern throughout the health care system and must be taken into account in weighing choices between drug classes.
The present study has certain limitations beyond the natural limitations of administrative datasets that do not include any direct information on clinical parameters. First, we only included patients who filled at least 1 prescription for an ACEI or ARB within 90 days of myocardial infarction, but did not study patients who received such a prescription, but chose not to fill it. Similarly, we do not know whether patients actually took the medications they received from the pharmacy. The outcomes study of differential mortality is observational, and patients were not randomized to receive either of the 2 classes. However, we were able to control for a large number of factors that may confound the association between ARB versus ACEI use and 1-year mortality post-MI, and our findings were similar to these reported in randomized controlled trials. Furthermore, study results remained unchanged after removal of patients who crossed over from ACEI to ARB or vice versa, a phenomenon that could lead to bias toward the null. We further assumed therapeutic equivalence within the ACEI and ARB drug classes; at least one report has challenged the validity of this assumption.25 One could argue that the elderly patients studied are not representative of all patients with myocardial infarction, especially that elderly patients may present clinically different from those enrolled in the randomized controlled trials that provided the underlying evidence. Conversely, one might argue that studying elderly patients, who are often excluded from participation in these trials, might actually be a strength. Lastly, the observed utilization patterns may vary from other populations that differ in demographic composition, case mix, or in other geographic areas.
The findings of this population based study confirm that there is equivalence in survival after myocardial infarction between patients who receive ARB versus ACEI, and that there is some evidence for increasing overutilization of ARB, which may be inappropriate given their substantially higher cost. Reduction of these opportunity costs is important from a policy perspective, and could be achieved without impairing patient safety or clinical outcomes.
Acknowledgments
Dr. Winkelmayer is supported by an American Heart Association Scientist Development Grant (AHA 0535232N). He is also a 2004 to 2006 T. Franklin Williams Scholar in Geriatric Nephrology (American Society of Nephrology-Association of Subspecialty Professors Junior Development Award in Geriatric Nephrology, jointly sponsored by the Atlantic Philanthropies, the American Society of Nephrology, the John A. Hartford Foundation, and the Association of Subspecialty Professors).
REFERENCES
- 1.Pfeffer MA, Braunwald E, Moye LA, et al. Effect of captopril on mortality and morbidity in patients with left ventricular dysfunction after myocardial infarction. Results of the survival and ventricular enlargement trial. The SAVE Investigators. N Engl J Med. 1992;327:669–77. doi: 10.1056/NEJM199209033271001. [DOI] [PubMed] [Google Scholar]
- 2.Effect of ramipril on mortality and morbidity of survivors of acute myocardial infarction with clinical evidence of heart failure. The acute infarction ramipril efficacy (AIRE) study investigators. Lancet. 1993;342:821–8. [PubMed] [Google Scholar]
- 3.Kober L, Torp-Pedersen C, Carlsen JE, et al. A clinical trial of the angiotensin-converting-enzyme inhibitor trandolapril in patients with left ventricular dysfunction after myocardial infarction. Trandolapril cardiac evaluation (TRACE) study group. N Engl J Med. 1995;333:1670–6. doi: 10.1056/NEJM199512213332503. [DOI] [PubMed] [Google Scholar]
- 4.Effects of ramipril on cardiovascular and microvascular outcomes in people with diabetes mellitus results of the HOPE study and MICRO-HOPE substudy. Heart outcomes prevention evaluation study investigators. Lancet. 2000;355:253–9. [PubMed] [Google Scholar]
- 5.Israili ZH, Hall WD. Cough and angioneurotic edema associated with angiotensin-converting enzyme inhibitor therapy. A review of the literature and pathophysiology. Ann Intern Med. 1992;117:234–42. doi: 10.7326/0003-4819-117-3-234. [DOI] [PubMed] [Google Scholar]
- 6.McMurray JJ, McGuire A, Davie AP, Hughes D. Cost-effectiveness of different ACE inhibitor treatment scenarios post-myocardial infarction. Eur Heart J. 1997;18:1411–5. doi: 10.1093/oxfordjournals.eurheartj.a015466. [DOI] [PubMed] [Google Scholar]
- 7.Golan L, Birkmeyer JD, Welch HG. The cost-effectiveness of treating all patients with type 2 diabetes with angiotensin-converting enzyme inhibitors. Ann Intern Med. 1999;131:660–7. doi: 10.7326/0003-4819-131-9-199911020-00005. [DOI] [PubMed] [Google Scholar]
- 8.Weintraub WS, Cole J, Tooley JF. Cost and cost-effectiveness studies in heart failure research. Am Heart J. 2002;143:565–76. doi: 10.1067/mhj.2002.120965. [DOI] [PubMed] [Google Scholar]
- 9.Rosen AB, Hamel MB, Weinstein MC, Cutler DM, Fendrick AM, Vijan S. Cost-effectiveness of full medicare coverage of angiotensin-converting enzyme inhibitors for beneficiaries with diabetes. Ann Intern Med. 2005;143:89–99. doi: 10.7326/0003-4819-143-2-200507190-00007. [DOI] [PubMed] [Google Scholar]
- 10.Grossman E, Messerli FH, Neutel JM. Angiotensin II receptor blockers equal or preferred substitutes for ACE inhibitors? Arch Intern Med. 2000;160:1905–11. doi: 10.1001/archinte.160.13.1905. [DOI] [PubMed] [Google Scholar]
- 11.Granger CB, McMurray JJ, Yusuf S, et al. Effects of candesartan in patients with chronic heart failure and reduced left-ventricular systolic function intolerant to angiotensin-converting-enzyme inhibitors the CHARM-Alternative trial [see comment] Lancet. 2003;362:772–6. doi: 10.1016/S0140-6736(03)14284-5. [DOI] [PubMed] [Google Scholar]
- 12.Ramsay LE, Yeo WW. ACE inhibitors, angiotensin II antagonists and cough. The Losartan cough study group. J Hum Hypertens. 1995;9(suppl. 5):S51–S54. [PubMed] [Google Scholar]
- 13. [June 23, 2005]; www.drugstore.com.
- 14.Pfeffer MA, McMurray JJ, Velazquez EJ, et al. Valsartan, captopril, or both in myocardial infarction complicated by heart failure, left ventricular dysfunction, or both. N Engl J Med. 2003;349:1893–906. doi: 10.1056/NEJMoa032292. [DOI] [PubMed] [Google Scholar]
- 15.Dickstein K, Kjekshus J. Effects of losartan and captopril on mortality and morbidity in high-risk patients after acute myocardial infarction the OPTIMAAL randomised trial. Optimal trial in myocardial infarction with angiotensin II antagonist losartan. Lancet. 2002;360:752–60. doi: 10.1016/s0140-6736(02)09895-1. [DOI] [PubMed] [Google Scholar]
- 16.Kiyota Y, Schneeweiss S, Glynn RJ, Cannuscio CC, Avorn J, Solomon DH. Accuracy of Medicare claims-based diagnosis of acute myocardial infarction estimating positive predictive value on the basis of review of hospital records. Am Heart J. 2004;148:99–104. doi: 10.1016/j.ahj.2004.02.013. [DOI] [PubMed] [Google Scholar]
- 17.Schneeweiss S, Avorn J. A review of uses of health care utilization databases for epidemiologic research on therapeutics. J Clin Epidemiol. 2005;58:323–37. doi: 10.1016/j.jclinepi.2004.10.012. [DOI] [PubMed] [Google Scholar]
- 18.McMurray JJ. Angiotensin II receptor antagonists for the treatment of heart failure what is their place after ELITE-II and Val-HeFT? J Renin Angiotensin Aldosterone Syst. 2001;2:89–92. doi: 10.3317/jraas.2001.017. [DOI] [PubMed] [Google Scholar]
- 19.Morimoto T, Gandhi TK, Fiskio JM, et al. Development and validation of a clinical prediction rule for angiotensin-converting enzyme inhibitor-induced cough. J Gen Intern Med. 2004;19:684–91. doi: 10.1111/j.1525-1497.2004.30016.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fischer MA, Schneeweiss S, Avorn J, Solomon DH. Medicaid prior-authorization programs and the use of cyclooxygenase-2 inhibitors. N Engl J Med. 2004;351:2187–94. doi: 10.1056/NEJMsa042770. [DOI] [PubMed] [Google Scholar]
- 21.Fischer MA, Choudhry NK, Winkelmayer WC. Impact of Medicaid prior authorization policies on use of angiotensin receptor blockers [Abstract] J Gen Intern Med. 2006;21:S70. [Google Scholar]
- 22.McDonald MA, Simpson SH, Ezekowitz JA, Gyenes G, Tsuyuki RT. Angiotensin receptor blockers and risk of myocardial infarction systematic review. BMJ. 2005;331:873. doi: 10.1136/bmj.38595.518542.3A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lee VC, Rhew DC, Dylan M, Badamgarav E, Braunstein GD, Weingarten SR. Meta-analysis: angiotensin-receptor blockers in chronic heart failure and high-risk acute myocardial infarction. Ann Intern Med. 2004;141:693–704. doi: 10.7326/0003-4819-141-9-200411020-00011. [DOI] [PubMed] [Google Scholar]
- 24.Epstein BJ, Gums JG. Angiotensin receptor blockers versus ACE inhibitors prevention of death and myocardial infarction in high-risk populations. Ann Pharmacother. 2005;39:470–80. doi: 10.1345/aph.1E478. [DOI] [PubMed] [Google Scholar]
- 25.Pilote L, Abrahamowicz M, Rodrigues E, Eisenberg MJ, Rahme E. Mortality rates in elderly patients who take different angiotensin-converting enzyme inhibitors after acute myocardial infarction a class effect? Ann Intern Med. 2004;141:102–12. doi: 10.7326/0003-4819-141-2-200407200-00008. [DOI] [PubMed] [Google Scholar]