Abstract
An infant’s ability to process auditory signals presented in rapid succession (i.e. rapid auditory processing abilities [RAP]) has been shown to predict differences in language outcomes in toddlers and preschool children. Early deficits in RAP abilities may serve as a behavioral marker for language-based learning disabilities. The purpose of this study is to determine if performance on infant information processing measures designed to tap RAP and global processing skills differ as a function of family history of specific language impairment (SLI) and/or the particular demand characteristics of the paradigm used. Seventeen 6- to 9-month-old infants from families with a history of specific language impairment (FH+) and 29 control infants (FH−) participated in this study. Infants’ performance on two different RAP paradigms (head-turn procedure [HT] and auditory-visual habituation/recognition memory [AVH/RM]) and on a global processing task (visual habituation/recognition memory [VH/RM]) was assessed at 6 and 9 months. Toddler language and cognitive skills were evaluated at 12 and 16 months. A number of significant group differences were seen: FH+ infants showed significantly poorer discrimination of fast rate stimuli on both RAP tasks, took longer to habituate on both habituation/recognition memory measures, and had lower novelty preference scores on the visual habituation/recognition memory task. Infants’ performance on the two RAP measures provided independent but converging contributions to outcome. Thus, different mechanisms appear to underlie performance on operantly conditioned tasks as compared to habituation/recognition memory paradigms. Further, infant RAP processing abilities predicted to 12- and 16-month language scores above and beyond family history of SLI. The results of this study provide additional support for the validity of infant RAP abilities as a behavioral marker for later language outcome. Finally, this is the first study to use a battery of infant tasks to demonstrate multi-modal processing deficits in infants at risk for SLI.
Introduction
Specific language impairment (SLI)1 is characterized by the failure to develop normal language skills in the absence of an apparent cause; individuals have normal intelligence, hearing and no known neurological impairments. SLI affects approximately 7% of all children (Tomblin, Zhang, Buckwalter & Catts, 1997) and more than 50% of children with SLI continue on to develop reading problems similar to those seen in dyslexics (Bishop & Snowling, 2004; Stark, Bernstein, Condino, Bender, Tallal & Catts, 1984; Tallal, 1988; Tomblin, Zhang, Buckwalter & Catts, 2000), and, conversely, a significant number of dyslexic individuals show deficits in parameters of oral language (e.g. Byrne, 1981; Joanisse, Manis, Keating & Seidenberg, 2000). Longitudinal studies as well as family genetic studies demonstrate considerable overlap in etiology and co-occurrence between specific oral language disorders and reading problems, such as dyslexia (Catts, 1993; Flax, Realpe-Bonilla, Hirsch, Brzustowicz, Bartlett & Tallal, 2003; Rissman, Curtiss & Tallal, 1990; cf. Leonard, 1998; Snow, Burns & Griffin, 1998). While a large body of research has been dedicated to uncovering the etiology of SLI, much of the literature has focused on the grammatical development of impaired individuals and studies have reported persistent difficulties with morphology and syntax. In English, SLI individuals have difficulties with function words (e.g. ‘the’) and unstressed morphological endings (e.g. ‘ed’) (Clahsen, 1992; Rice & Wexler, 1996; see Leonard 1998). While SLI children understand the concept of ‘pastness’ and ‘plurality’ they have difficulty in producing and comprehending morphologically complex words (such as booked and books). Theories proposed to account for SLI range from arguments for specific deficits in learning grammatical rules (grammar-specific model) (Gopnik & Crago, 1991) to limitations in processing capacity or working memory (Gathercole & Baddeley, 1990; Joanisse & Seidenberg, 1998; Schul, Stiles, Wulfeck & Townsend, 2004). Another consistent deficit implicated, and thought to be causal, in children with language or reading deficits is poor phonological processing (SLI: Bird, Bishop & Freeman, 1995; Elliott & Hammer, 1988; Elliott, Hammer & Scholl, 1989; Gathercole & Baddeley, 1990; Stark & Tallal, 1981; Sussman, 1993; Whitehurst & Fischel, 1994; Reading: Scarborough, 1990; Wagner & Torgesen, 1987). However, the precise nature and origin of these deficits remains the focus of intense study and theoretical debate. A controversial issue is the question of whether phonological deficits are ‘speech-specific’, or whether lower-level sensory-processing mechanisms such as rapid auditory processing abilities (RAP) play a crucial role in setting up the phonological building blocks of human language (see Fitch & Tallal, 2003; also see Ramus, 2003; Rosen, 2003). Considerable research has recently been directed towards the contribution of basic mechanisms such as attention, perception, and memory to this process (e.g. Farmer & Klein, 1995; Habib, 2003; Hari & Renvall, 2001; Hoffman & Gillam, 2004; Gillam & Hoffman, 2004; Webster & Shevell, 2004; see Fitch & Tallal, 2003, and Tallal, 2004, for a review). Evidence suggests that language acquisition has both ‘hard-wired’ characteristics (such as statistical learning; e.g. Maye, Werker & Gerken, 2002; Saffran, Aslin & Newport, 1996) and is also influenced significantly by environmental factors (e.g. immediate language environment; Hart & Risely, 1995).
However, critical areas of knowledge remain elusive particularly since most studies have examined the etiology of these language-specific deficits in children and adults who have already been diagnosed with SLI. The inconsistent findings between early deficits in perceptual abilities and later language problems may be related to the lack of prospective studies assessing developmental changes in the association between auditory processing and language outcomes. Rather than concentrate on the study of disorders solely at their end-state in school-aged children and adults it becomes essential to study disorders in early infancy, and longitudinally, to understand how alternative developmental pathways might lead to different phenotypical outcomes (Karmiloff-Smith, 1998). Longitudinal studies with infants offer insights into the etiology of language impairment and disentangle the precursors of SLI from problems that occur concurrently. In the prospective, longitudinal study described here we investigate RAP abilities early in development (at 6 and 9 months) using two different paradigms, and examine its association with toddler expressive and receptive language abilities.
The mechanism by which RAP abilities influence language acquisition may be associated with how speech sounds are represented, assembled and refined in the infant brain. Difficulties in processing brief, rapid successive auditory cues could lead to distorted neural boundaries that may result in degraded categorical and phonemic representations (Elbro, 1990, 1998; Godfrey, Syrdal-Lasky, Millay & Knox, 1981; Werker & Tees, 1987). These indistinct and perhaps over-inclusive representations may lead to expressive and/or receptive language delays in early childhood, and subsequent reading, writing, and spelling deficits due to poor phonographic (spoken) to orthographic (visual/written) mapping (Benasich & Read, 1998; Tallal, 2000; Tallal, Miller, Jenkins & Merzenich, 1997). Indeed, research has shown that individuals with deficits in phonological, syntactic and/or grammatical processing also exhibit difficulties in discriminating phonetic contrasts with fast formant transitions, such as/ba/versus/da/(Godfrey et al., 1981; Kraus, McGee, Carrell, Zecker, Nicol & Koch, 1996; Reed, 1989; Stark & Heinz, 1996; Werker & Tees, 1987) as well as transient non-speech stimuli (Fitch, Miller & Tallal, 1997; McAnally & Stein, 1996, 1997; Nagarajan, Mahncke, Salz, Tallal, Roberts & Merzenich, 1999; Reed, 1989; Tallal & Piercy, 1973a, 1973b, 1974, 1975; Tallal, Stark & Mellits, 1985a, 1985b; Witton, Talcott, Hansen, Richardson, Griffiths, Rees, Stein & Green, 1998; Witton, Stein, Stoodley, Rosner & Talcott, 2002; Wright, Lombardino, King, Puranik, Leonard & Merzenich, 1997). Given that RAP deficits are evident in experiments using both speech and non-speech stimuli, it seems likely that the processing limitations exhibited by SLI individuals are present before the onset of spoken language, and recent studies support that view (Benasich & Heim, 2005; Benasich & Leevers, 2002; Tallal, 2004).
Developmental studies of infants at risk for language disorders and associations with RAP abilities
While prior studies have shown that very young infants (2-month-olds) have the ability for detecting transient auditory signals (Aslin, 1989; Eilers, Morse, Gavin & Oller, 1981; Irwin, Ball, Kay, Stillman & Rosser, 1985; Jusczyk, Pisoni, Walley & Murray, 1980; Morrongiello & Trehub, 1987) and that these skills are measurable very early on in development, prospective longitudinal studies tracing the contribution of early perceptual processing abilities to language acquisition are few in number. In a series of developmental studies Benasich and colleagues have shown that the RAP threshold of infants born into families with a history of language impairment (thus at increased risk of developing language problems, see Choudhury & Benasich, 2003) were significantly poorer than those of age-matched peers from control families without such a history (Benasich & Tallal, 1996, 2002; Spitz, Tallal, Flax & Benasich, 1997). More importantly, infant RAP abilities were predictive of receptive and expressive language at 16 months (on the MacArthur Communicative Inventory), and at 24 and 36 months (on the Preschool Language Scale and language subsets of the Stanford Binet Intelligence Scale); infants with poorer RAP thresholds were developing language more slowly than those with better thresholds (Benasich & Tallal, 2002; Benasich, Thomas, Choudhury & Leppänen, 2002; Benasich & Spitz, 1998). Furthermore, early RAP abilities were more robust predictors of language at 2 and 3 years of age than family history: infants’ 7.5-month RAP threshold accounted for 21% of the variance in 3-year language abilities (Benasich & Tallal, 2002). These studies show that it is possible to successfully assess RAP skill using behavioral techniques from a very young age.
Assessing RAP abilities in infants
Most behavioral studies assessing early perceptual skills, such as RAP, use a battery of assessments that typically include operantly conditioned head-turning paradigms (HT) (also referred to as visually reinforced conditioned head-turn paradigms, see Kuhl, 1985; Morrongiello, 1990, for a review) and habituation-recognition memory tasks (H/RM). Both have been shown to be reliable and valid measures of infant information processing (see Colombo, 1993, 2002; Rose, Feldman & Jankowski, 2003, 2004a, 2004b, for review of habituation and recognition memory measures; Schneider & Trehub, 1985, for review of operantly conditioned HT measures).
The HT paradigm utilizes the infant’s proclivity to turn their heads in the direction of a sound source (a part of the general orienting reflex) (see Kuhl, 1985; Morrongiello, 1990, for reviews). Operantly conditioned HT paradigms exploit this initially reflexive response by training infants to turn their head when a repetitive sound (the standard signal) is changed to another (the target signal). The response is typically reinforced by presenting visual stimuli that are of interest to infants (e.g. animated toys). Successful performance requires an infant to focus on the auditory signal, identify the target signal and reliably demonstrate that a contingency between the target signal and the reinforcer has been learned.
Infant RAP abilities are tested using the HT paradigm by manipulating the temporal properties of the auditory signal (e.g. changing the VOT in a CV paradigm or reducing the intrastimulus interval, ISI, within a tone-pair so that signals appear closer together in time; see Benasich & Read, 1998; Benasich & Leevers, 2002, for review). Theoretically, the HT procedure does not assess RAP abilities during the training phase; infants are trained with sufficiently large ISIs, or obviously discrepant VOTs, so as to allow the infant to clearly identify the target from the standard signal. However, individual variability during the training phase may be apparent in the number of trials required to acquire the contingency. This variability could be attributed to differences in attention, speed of encoding and/or the ability to consolidate information into memory (Colombo, 1993, 2002; Kavsek, 2004; Nelson, 2002; Rose et al., 2003, 2004a, 2004b). Test sessions are explicitly designed to assess RAP ability by manipulating temporal properties of the signal. Cognitive processes such as sustained attention, the ability to maintain and generalize a learned association, and inhibition of attention also play important roles in performance during a test session.
Habituation and recognition memory paradigms are reliable measures of infant perceptual processing and have been shown to be predictive of later cognitive abilities, accounting for up to 25% of the variance on language proficiency measures, academic achievement indexes, and intelligence test scores at ages 8 and 11 years (Rose & Feldman, 1995, 1996, 1997; Rose, Feldman, Wallace & Cohen, 1991a; Rose, Feldman, Wallace & McCarton, 1991b; Sigman, Cohen & Beckwith, 1997; for reviews see Bornstein & Sigman, 1986; McCall & Carriger, 1993; Rose et al., 2003, 2004a, 2004b; Rose & Tamis-LeMonda, 1999). These tasks are based on the tendency of infants to differentially fixate novel as compared to familiar visual stimuli. Theoretical accounts of habituation are mainly derived from the comparator theory (Sokolov, 1963) that states that the magnitude of attention (assessed by the duration of visual orientation to a stimulus) is an index of the degree of discrepancy between an internal representation of a stimulus and the actual stimulus. As the internal representation increasingly resembles the displayed stimulus, the infant’s attention begins to wane. This waning of attention is the habituation function, or the rate of habituation, and is thought to reflect speed or efficiency of information encoding and storage (Caron & Caron, 1968; Colombo & Mitchell, 1988, 1990; Malcuit, Pomerleau & Lamarre, 1988; for reviews see Bornstein & Sigman, 1986; McCall & Carriger, 1993; McCall & Mash, 1995), as well as sustained attention and inhibition (Colombo, 2002; Colombo, Shaddy, Richman, Maikranz & Blaga, 2004; Sigman, Cohen, Beckwith, Asarnow & Parmelee, 1991; McCall & Mash, 1995). Introduction of a novel stimulus (during the test phase) produces a recovery of attention reflecting both discrimination of the novel from the familiarized stimulus and memory for the familiar stimulus (Colombo, 1993, 2002).
Auditory-visual habituation and recognition memory paradigms have been primarily used to examine visual discrimination; however, studies have quite successfully used this paradigm to assess infant auditory processing (Colombo & Bundy, 1983; Lewkowicz, 2000, 2003; Lewkowicz & Turkewitz, 1980, 1981). Most studies of this type habituate infants to compound auditory-visual stimulus and then test with the same visual stimulus coupled with a novel auditory stimulus. Novelty preference (and in some cases familiarity preference) is taken as an index of auditory discrimination abilities. In studies of RAP, novelty preference has been shown to be a reliable index of infants’ ability to discriminate between fast transient auditory stimuli (such as CV contrasts) (Benasich, 1998; Benasich & Leevers, 2002). Relatively little, however, is known about how different parameters of the habituation recognition memory paradigm, such as rate of habituation, number of trials needed to habituate or initial fixation duration, all of which have been shown to be important in characterizing infant processing skills, may be related to the ability to process transient auditory cues and subsequent language development.
Finally while both head-turn and habituation measures have been shown to be associated with RAP abilities and are predictive of later language abilities (Benasich & Tallal, 1996, 2002), little is known about how performances on these two different paradigms are related and if they provide converging evidence for RAP abilities. Examination of the association between HT and H/RM measures would provide important information about how infants process perceptually brief auditory signals and allow assessment of the contribution of the particular demand characteristics of each paradigm in predicting language and cognitive abilities. In this study, we investigate performance on the HT and H/RM task with stimuli designed to assess RAP abilities. Our main aim is to assess if performance on these two paradigms can be attributed to similar cognitive processes and are therefore overlapping measures, or whether each taps different processes, thus providing independent and/or additive converging evidence for the role of RAP abilities in the acquisition of language. The present study is an extension of earlier studies investigating the association between infant RAP and later language abilities (Benasich & Tallal, 1996, 2002). In previous studies we have used a two-alternative forced-choice paradigm to measure RAP. Here, however, an operantly conditioned Go/No-Go head-turn paradigm is used and the addition of habituation/recognition memory measures to also assess RAP, in the same infants, allows examination of the associations and predictability of these two paradigms. A new cohort of infants from families with a history of language-based learning disorders (FH+) were recruited, and their performance on a battery of information processing tasks was compared to that of control children without such a history (FH−). Based on previous research which has shown that FH+ infants have poorer RAP abilities compared to FH− infants (Benasich & Spitz, 1998; Benasich & Tallal, 1996, 1998, 2002), we hypothesized that group differences may be apparent when processing transient stimuli on both HT and H/RM tasks, but not when processing stimuli that do not require RAP abilities to be utilized. Further, as little is known about associations between performance on HT and H/RM tasks, we posit that if infants are using related cognitive processes and/or strategies to resolve differences on the HT and H/RM task then there should be an association between the dependent measures from the two paradigms. Finally, we examine the language development of both groups of infants until 16 months of age and consider the predictive association between infant RAP abilities and language outcomes.
Methods
Participants
Forty-six families with infants younger than 6 months of age (19 males and 26 females) were recruited from urban and suburban communities in New Jersey and were assigned to one of two groups based on parental report of family history of SLI: the family history positive group (FH+) and the family history negative group (FH−). The FH+ group consisted of 17 full-term normal birthweight healthy infants (7 males and 10 females). In this group at least one nuclear family member (typically an older sibling) had a clinical diagnosis of SLI (see below for a description of the SLI group and inclusion criteria, as well as Choudhury & Benasich, 2003). The FH− group consisted of 29 infants (12 males and 17 females) with no reported family history of SLI or of dyslexia, learning disability, attention deficit disorder, pervasive developmental disorder, or autism. Prematurity and/or LBW were exclusionary criteria and comparison of the FH+ and FH− groups on birthweight and gestational age revealed no group differences (see Table 1), hence these factors are not included in the analyses presented here.
Table 1.
Infant birthweight, gestational age and parental age, education and socioeconomic status by group (FH+ versus FH−)
| FH+
|
FH−
|
||||
|---|---|---|---|---|---|
| Mean | (std) | Mean | (std) | p | |
| Gestational age (weeks) | 39.9 | 1.0 | 39.9 | 1.0 | .95 |
| Birth weight (grams) | 3682 | 312 | 3468 | 451 | .82 |
| Maternal age (years, months) | 34, 1 | 4, 6 | 33, 5 | 4, 2 | .74 |
| Maternal education1 (years) | 15.27 | .57 | 15.41 | .63 | .84 |
| Paternal age (years, months) | 36, 11 | 4, 4 | 35, 1 | 5, 6 | .87 |
| Paternal education1 (years) | 15.27 | .82 | 15.36 | .53 | .69 |
| Hollingshead score2 | 57.4 | 6.2 | 55.9 | 9.1 | .46 |
Note: Parental education is reported in the number of years of schooling; 12 years is equivalent to a high school diploma, 14 years is equivalent to completion of a two-year college or technical degree and 16 years is equivalent to completion of a four-year undergraduate program.
57.4 corresponds to the category of major professional while 55.9 corresponds to the category of minor professional.
Selection criteria for FH+ group
Infants from FH+ families were recruited from local newspaper birth announcements and pediatric clinics. In order to be classified as FH+, families provided clinical reports of expressive and receptive language scores and a general cognitive score for at least one affected child or parent (the ‘proband’). If language scores on any standardized language assessment (e.g. the CELF, PLS or TOLD) for the proband were at least one standard deviation below the age-appropriate mean, and performance on standardized tests of general cognitive ability (e.g. Stanford Binet, or any of the Wechsler intelligence scales) was within the normal range, the family was recruited into the FH+ group. While a substantial body of research has shown that SLI is highly co-morbid with dyslexia (Bishop & Snowling, 2004; Flax et al., 2003), care was taken to ensure that probands had a primary diagnosis of ‘oral language deficits’ congruent with the definition of SLI, and not a reading deficit such as dyslexia. This was relatively easy since the majority (15 out of 17) were sibling probands (mean age 3 years) who had not yet started to read. Two parents were included as SLI but they demonstrated an oral language deficit, and in addition one of these two parents had a reading deficit. Families with children who received a primary diagnosis of attention deficit disorder, or families with children who had language impairments because of hearing loss, neurological disorders, oral motor impairment, a diagnosis of pervasive developmental disorder or autism were not included in this sample. Control families reported no known history of language or learning impairments in the nuclear or the extended family (grandparents, aunts and uncles).
Procedure
All children visited the lab at 6, 9, 12, 16 and 24 months of age. Data presented here are from the 6-, 9-, 12- and 16-month visits and are restricted to infants with complete data at either 6 or 9 months and follow-up data at 12 and 16 months. At the 6- and 9-months sessions, a battery of tasks assessing infant information processing (HT, auditory-visual and visual habituation–recognition memory task) as well as the Bayley Scale of Infant Development, Mental Development Index (MDI) were administered. When their children were 12 months mothers completed a parent report inventory of language development; the MacArthur Communicative Development Inventory. At the 16-month visit toddlers received a battery of standardized language and cognitive measures; in all cases the Preschool Language Scale (PLS-3) was given first followed by the MDI (see Table 2 for complete schedule of assessments at each age).
Table 2.
Schedule of assessments for each visit
| Age at visit | Tasks |
|---|---|
| 6–9 months mean age: 6 mos, 17 days (SD:15 days) |
|
| 12 months mean age: 12 mos, 16 days (SD: 14 days) |
|
| 16 months mean age: 16 mos, 14 days (SD: 16 days) |
|
Measures
Conditioned head-turn task
A Go/No-Go Operant Head Turn (HT) procedure (based on Morrongiello & Trehub, 1987; Trehub, Schneider & Henderson, 1995) was used to assess processing of rapidly presented non-linguistic auditory stimuli.
HT stimuli
HT stimuli were two 70 ms complex tones with a fundamental frequency of 100 or 300 Hz with 15 harmonics (6 dB roll-off per octave). The tones were presented as paired stimuli: the standard stimulus was a 100–100 Hz tone-pair and the target stimulus was a 100–300 Hz tone-pair. The ISI was either 500 ms, 300 ms or 70 ms depending on the training or testing phase of the task. All tone-pairs were presented at 62 dB SPL (Figure 1).
Figure 1.
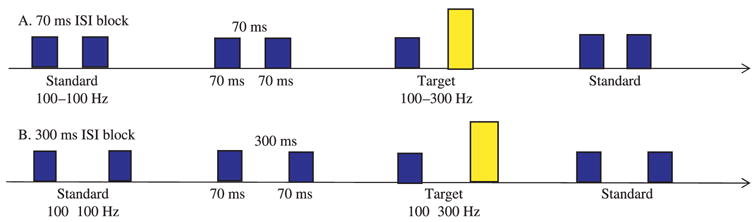
Schematic representation of stimuli used for the Go/No-Go HT procedure.
HT procedure
The infant was seated on the mother’s lap and opposite one of the experimenters (Experimenter A). To the infant’s left, and at an angle of 60 degrees, was a reinforcement box with a dark plexiglass cover. The box was divided into four quadrants each of which contained a toy that could be illuminated and animated. A loudspeaker was placed behind the reinforcement box. Experimenter A and parent both wore headphones carrying music to mask the nature of the trials presented to the infant. Throughout the session Experimenter A engaged the infant with silent puppet play in order to maintain attention and a forward orientation. The infant’s looking patterns and direction of gaze were videotaped with a video camera hidden behind Experimenter A. The video signal was also displayed on TV monitors in an adjacent room where the infant’s head position and central fixations were monitored by Experimenter B. All trials were initiated and all responses were coded by Experimenter B using National Instruments Labview 4.0.1 for Macintosh computers. The proportion of turns on target trials (hits versus misses) and standard trials (false alarms versus correct rejections) as well as latency to hits and false alarms were recorded. A LED light on the back wall signaled the onset and offset of each trial to both experimenters.
Infants were operantly conditioned to make a 60° head-turn to the left to a target tone sequence (i.e. 100–300 Hz) embedded within a standard repeating sequence (e.g. 100–100 Hz). Infants were trained on tone-pairs with ISI of 500 ms, well above the RAP threshold for all infants, until a predetermined criterion (four out of five correct responses in a row) was attained thus verifying successful training. During the test phase, two blocks of different ISIs were presented in a standard order: all infants received a 70 ms ISI block first and then a 300 ms ISI block. Twenty trials (10 target trials and 10 standard or catch trials) were randomly presented for each block.
HT performance was rescored offline by the experimenter to eliminate trials when the infant had begun to turn before the stimulus onset and to obtain intra- and inter-rater reliabilities. All infants included in these analyses had seven or more target trials (at least 70%) and seven or more standard/change trials (at least 70%) for both the 70 and the 300 ISI blocks. The dependent variables were calculated from rescored data. The d-prime (d′; the difference between the hit and false alarm distributions; Green & Swets, 1966) was used as an index for detecting the target tone at each ISI as well as the number of hits, misses, false alarms and correct rejections. The latency between onset of target auditory signal and a head-turn (latency to hits) and the latency between onset of the signal for standard trials and a head-turn (latency to false alarms) was also calculated. Spearman’s correlations were used to calculate on-line and off-line agreements for 15 infants. Average intra-rater reliability was 0.98 for hits, false alarms, misses and correct rejections. Pearson’s correlation was used to assess on- and off-line agreement for latency for hits and latency for false alarms; average agreement for the latencies for each trial type was 0.95.
Habituation/recognition-memory
Infants received three infant-controlled habituation-recognition memory tasks: one visual habituation-recognition memory task (VH/RM) with face stimuli, and two auditory visual habituation-recognition memory tasks (AVH/RM1 and AVH/RM2) with abstract visual slides coupled with tone-pairs.
VH/RM stimuli
VH/RM stimuli were paired black and white slides of faces with neutral affect and comparable amounts of contour and areas of illumination (previously shown to be equally salient and discriminated by children of the ages used in this experiment, see Benasich & Tallal, 1996). Infants were habituated to slides of an identical pair of faces and tested with the familiarized face and a novel face. Left and right positions were counterbalanced during the test phase. The pre- and post-test stimulus was an abstract pattern (Table 3).
Table 3.
Habituation recognition memory stimuli
| Task | Habituation visual stimuli | Test visual stimuli | Habituation auditory stimuli | Test auditory stimuli |
|---|---|---|---|---|
| VH/RM |
|

|
None | None |
| AVH/RM1 |

|

|
400–400 Hz tone pair
70 ms ISI |
400–600 Hz tone pair
70 ms ISI |
| AVH/RM2 |

|

|
400–400 Hz tone pair
300 ms ISI |
400–600 Hz tone pair
300 ms ISI |
AVH/RM stimuli
In both AVH/RM tasks infants were habituated to compound auditory-visual stimuli. Auditory discrimination was assessed in the test phase by changing the auditory component of the compound stimuli while keeping the visual component constant. The visual stimuli for the tasks were identical pairs of slides: for AVH/RM1 a black and white starburst pattern and for AVH/RM2 a black and red checkerboard pattern was used. Visual patterns were coupled with auditory tone-pairs. In both tasks, habituation tone-pairs were 400–400 Hz complex tones and test tone-pairs were 400–600 Hz complex tones. All tones were 70 ms long with a fundamental frequency of 400 or 600 Hz with 15 harmonics (6 dB roll-off per octave). During AVH/RM1 an ISI of 70 ms was used as the ISI for both the habituation and test phases while AVH/RM2 used an ISI of 300 ms. All tone-pairs were presented at 62 dB SPL. A photograph of a woman’s face served as the pre- and post-test stimulus for both tasks.
Habituation procedure
Infants were seated in a standard infant seat approximately 30 inches in front of two screens approximately 60 cm square (subtending ~ 35° of visual angle). A small light was used to attract attention to the midline before a pair of visual stimuli was projected on to the screen. The auditory stimuli were generated by a National Instruments digital signal processing virtual instrument (via Labview 4.0.1) and presented via a Realistic Minimus 77 speaker (Radio Shack) placed centrally on top of the display apparatus. A midline camera, hidden behind the display apparatus, captured infant looking and transmitted the signal to a TV display in the adjacent room with the experimenter. The experimenter operated the program and coded all looking patterns on-line.
Each infant received a standard sequence: pre-test, habituation, test, and post-test. During pre- and post-test, the same stimulus was shown for 10 seconds. Habituation consisted of consecutive discrete trials based on the infant’s individual looks. The infant began to accumulate looking time when judged to be looking at the visual stimulus for a minimum of 300 ms. To terminate a trial the infant had to look away from the stimulus for a minimum of 1.5 sec. A single look with these parameters was defined as a trial. The mean looking times of the first two habituation trials constituted the baseline and was automatically set at 100%. Habituation continued until the infants looking time declined to less than or equal to 50% of baseline looking for two consecutive trials (criterion).
Immediately following habituation the infant was presented with test trials. In the VH/RM task infants were tested with the habituation stimuli paired with a novel stimulus in a counterbalanced design. Each stimuli pair was presented for 10 sec. In the AVH/RM tasks, the visual stimuli remained constant from habituation through to the test phase; only the auditory stimulus changed. The infant was tested twice with the familiarized auditory-visual stimuli and twice with the novel stimuli in the following sequence: Novel, Familiar, Familiar, Novel. Each trial lasted 8 seconds and included six presentations of the tone stimuli (onset to onset time = 1.3 secs).
Across tasks, the dependent variables for the habituation phase were: number of trials to criterion, the percent of response decrement (percent decrement = A−B/A × 100, where A = the mean of the first two trials and B = the mean of the last two trials) and rate of habituation in real looking time (habituation slope; the best-fit linear regression function) were also calculated. The dependent variable for the recognition memory test was percent novelty preference (N/(F+N) × 100 = %N; where N = seconds of fixation to the novel stimulus and F = seconds of fixation to the familiarized stimulus). On-line and off-line agreements were calculated with 16 infants for total looking times for each trial on the habituation and the recognition memory phases of the assessment. Pearson’s correlation revealed an average agreement across trials of 0.97.
Standardized cognitive measure
Cognitive outcomes were assessed with the Bayley Scales of Infant Development – Mental Development Index (MDI) (Bayley, 1993) at 6, 9 and 16 months. The MDI consists of a range of items, administered according to age, assessing various cognitive abilities such as search, social interaction, imitation, vocalization, and puzzle completion. The scale provides a standardized score with a mean of 100 and a standard deviation of 16 points.
Language measures
Two language measures were used; the MacArthur Communicative Development Inventories (CDI; Fenson, Dale, Reznick, Thal, Bates, Hartung, Pethick & Reilly, 1993) and the Preschool Language Scale-3 (PLS-3; Zimmerman, Steiner & Pond, 1992). The CDI is a parent report measure of child comprehension and expression and provides a valid and efficient means of assessing communicative gestures and play, early imitation, language comprehension, language production, and the early stages of grammatical development. The CDI provides age- and gender-normed language scores and has been shown to be stable over time, as well as highly correlated with other language and communication measures. At 12 months parents completed the Words and Gestures inventory (infant version) of the CDI, which catalogues the number of words and phrases their infant understands (comprehension) and produces (expression). At 16 months, in addition to Words and Gestures, parents also completed the Words and Sentences inventory (toddler version). The latter inventory catalogues the number of words and phrases an infant uses and presents percentile scores for individual infants. The percentile ranks range from 5 to 99. Preliminary analyses of the 16-month data comparing both inventories revealed a near perfect correlation for the number of words produced on the Words and Gestures and the Words and Sentences inventory (r = .98, p < .00); therefore at 16 months, the data presented here utilize information from the toddler version of the CDI.
The Preschool Language Scale-3 (PLS-3) provides age-normed language scores for children from birth to 7 years of age and was administered in the laboratory at 16 months. The PLS-3 assesses receptive (Auditory Comprehension) and expressive (Expressive Communication) language skills and yields standard scores (Mean = 100, SD = 15), percentile ranks, and age scores for the subscales as well as a Total Language Score. In these analyses standard scores for the two subscales, Auditory Comprehension and Expressive Communication, were used.
Preliminary analysis and data reduction: age-related changes on infant information processing tasks
Preliminary analyses revealed no differences between FH+ and FH− groups on gender distribution or socio-environmental variables (e.g. parental education, income or socioeconomic status as assessed by the Hollingshead Four Factor index) (Table 1). The male to female ratio in both groups was the same (41% males to 59 % females) and based on the Hollingshead, 85% of the families reported being in the highest social strata while the remaining 15% were from the middle social strata.
Preliminary analyses were also conducted to assess age-related changes (6 to 9 months) in infant information processing (HT, VH/RM, AVH/RM). Results from a mixed factorial ANOVA (Group by Age) revealed no age effects for the HT task or any of the H/RM tasks; infants’ performance on the head-turn and the habituation-recognition memory paradigms for these tasks did not improve from 6 to 9 months. Intercorrrelations revealed significant associations between performance on 6- and 9-month measures on a number of variables (Table 4), suggesting modest short-term reliability across age. Given that earlier reports have shown that the predictive validity for performance on infant information processing is most robust between 6 and 9 months of age (Colombo, 1993), the data presented here are restricted to the first successful visit for which infants completed the information processing battery (overall mean age 6 months and 18 days). However, six out of the 46 infants included here were assessed at 9 months for the first time; these families were not able to attend the 6-month visit for varying reasons (e.g. family emergencies, infant illness and weather-related emergencies). Two of the six infants were from FH+ families while four were from FH− families. There were no differences between these six infants and their respective groups on infant information processing variables, demographic or outcome variables. All variables used in subsequent analyses were normally distributed without significant skew or kurtosis. For group differences analyses, data were checked for homogeneity of variance using the appropriate statistical method (Levene’s test of homogeneity of variance or Mauchly’s test of Sphericity). Analyses revealed no violations of the assumption of homogeneity or sphericity.
Table 4.
Intercorrelations between 6- and 9-month performance on HT, AVH/RM and VH/RM tasks
| 6–9-month intercorrelations (Pearson’s r) | p | |
|---|---|---|
| HT | ||
| Number of trials to criterion | .40 | .24 |
| D′ score (70 ms ISI) | .28 | .23 |
| Latency to hits (70 ms ISI) | .59 | .04 |
| Latency to false alarms (70 ms ISI) | .69 | .02 |
| D′ score (300 ms ISI) | .24 | .23 |
| Latency to hits (300 ms ISI) | .82 | .00 |
| Latency to false alarms (300 ms ISI) | .73 | .04 |
| AVH/RM1 (70 ms) | ||
| Number of trials to criterion | .51 | .01 |
| Habituation slope (real time) | .36 | .04 |
| % Decrement | .05 | .71 |
| % Novelty preference | .30 | .18 |
| AVH/RM2 (300 ms) | ||
| Number of trials to criterion | .33 | .04 |
| Habituation slope (real time) | .74 | .05 |
| % Decrement | .01 | .91 |
| % Novelty preference | .22 | .29 |
| VH/RM | ||
| Number of trials to criterion | .80 | .04 |
| Habituation slope (in real time) | .32 | .16 |
| % Decrement | .11 | .56 |
| % Novelty preference | .36 | .04 |
Results
Performance on HT and AVH/RM paradigms
In order to assess performance on the infant HT and AVH/RM paradigms on 70 and 300 ms ISI conditions, repeated measures analyses and chi-square analyses were conducted for each paradigm. The data from the control group are presented first followed by comparisons to the FH+ group. An ANOVA procedure was used to assess differences on RAP measures based on group status (FH+ versus FH−). Performance on the VH/RM measure was also assessed in order to consider processing differences between groups that were not related to fast transient processing.
The conditioned head-turn task
On the HT paradigm, 34 of the 46 infants successfully learned the contingency and passed criterion with the 500 ms ISI. Twenty-three of the 29 (80%) control infants successfully achieved criterion while 11 of 17 (65%) FH+ infants reached criterion (χ2 (1) = 1.86, n.s.). There were no group differences in the number of trials to criterion (FH− = 13 trials, FH+ = 12 trials; F(1, 32) = 0.203, n.s.). In the following section data are presented for only those children who successfully learned the task.
In order to assess if the d′ scores at 70 ms and 300 ms ISIs were significantly different from chance,2 one-sample t-test analyses were conducted for each group (Table 5). Results indicate that the FH− control group showed significant discrimination of target from non-target tone-pairs on both the 70 and 300 ms ISI blocks (70 ms ISI, t(22) = 7.164, p < .001; 300 ms ISI, t(22) = 3.328, p < .001). Repeated measures analysis found no differences in performance between the two blocks (t(21) = −0.828, n.s.). However, the pattern of discriminability was different for the FH+ group. While infants were able to discriminate target from non-target tone-pairs on the 300 ms ISI block (t(10) = 2.80, p < .01), they performed at chance levels on the 70 ms ISI block (t(10) = 0.768, n.s.). Repeated measures analyses showed that FH+ infants performed significantly better on the 300 ms ISI block than on the 70 ms ISI block (t(10) = 2.22, p < .05) (Table 5). Finally, a two-way ANOVA (Group × ISI) revealed an interaction (F(1, 32) = 5.80, p < .05), confirming that FH+ infants had significantly poorer d′ scores for the 70 ms ISI block compared to FH− infants (Table 5).
Table 5.
Means and standard deviations for HT, AVH/RM, and VH/RM by family history of SLI
| FH+
|
FH−
|
|||||
|---|---|---|---|---|---|---|
| Mean | (std) | Mean | (std) | p | Partial η2 | |
| HT | N = 11 | N = 23 | ||||
| Number of trials to criterion1 | 12.27 | 7.0 | 13.4 | 6.6 | .20 | – |
| D′ score (70 ms ISI)2 | 0.186 | 0.803 | 0.753 | 0.504 | .05 | 0.2 |
| D′ score (300 ms ISI) | 0.860 | 1.018 | 0.619 | 0.892 | ||
| AVH/RM2 | N = 17 | N = 29 | ||||
| Number of trials to criterion (70 ms) | 5.8 | 1.8 | 5.9 | 2.4 | .01 | 0.2 |
| Number of trials to criterion (300 ms) | 7.2 | 3.8 | 4.9 | 1.1 | ||
| Habituation slope (70 ms) | −1.54 | 1.45 | −1.46 | 1.46 | .05 | 0.1 |
| Habituation slope (300 ms) | −1.14 | 0.85 | −2.05 | 1.53 | ||
| % Decrement (70 ms) | 66.20 | 7.83 | 70.80 | 9.20 | .05 | 0.2 |
| % Decrement (300 ms) | 66.52 | 6.89 | 75.22 | 8.81 | ||
| % Novelty preference (70 ms) | 56.01 | 12.00 | 62.60 | 11.20 | .01 | 0.1 |
| % Novelty preference (300 ms) | 60.28 | 12.23 | 61.12 | 11.33 | ||
| VH/RM1 | N = 17 | N = 29 | ||||
| Number of trials to criterion | 7.65 | 3.58 | 7.25 | 4.23 | ns | – |
| Slope (in real time) | −1.58 | 2.12 | −1.94 | 2.10 | ns | – |
| % Decrement | 73.62 | 7.21 | 68.67 | 8.04 | .05 | 0.1 |
| % Novelty preference | 50.13 | 16.62 | 59.60 | 13.20 | .05 | 0.1 |
Note: Number of trials to criterion from the HT paradigm and the VH/RM paradigm do not have an ISI component and therefore results presented here are from one-way ANOVA procedures.
A two-way ANOVA procedure was used to assess interactions between Group (FH+/FH−) and ISI (70/300ms). Statistics from the main model are presented here.
The auditory visual habituation tasks
Habituation trials
Performance on the habituation trials was assessed by examining group differences on the number of trials needed to habituate (trials to criterion), the rate of habituation (habituation slope) and percent decrement. Three mixed factorial ANOVA models (group by ISI) were used to assess performance differences between FH+ and FH− infants. In all models, group (FH+/FH−) was used as the between subject variable and either trials to criterion, habituation slope or percent decrement for 70 and 300 ms ISI task was the within subjects factor (Table 5). Results revealed two significant Group × ISI interactions for trials to criterion and habituation slope. A closer examination of the univariate data revealed that infants in the FH+ group needed significantly more trials to habituate (trials to criterion F(1, 42) = 34.18, p < .01) and had slower rates of learning (habituation slope, F(1, 42) = 4.22, p < .05) compared to FH− infants for the 300 ms ISI tasks only. Findings from the ANOVA analysis with percent decrement revealed a main effect for group (F(1, 42) = 11.67, p < .05), favoring FH− infants.
Test trials
In both the 70 ms and the 300 ms ISI tasks the dependent variable, percent novelty preference, was used as an index of discriminability and a score of 54% or greater was taken to indicate significant discrimination (Fagan, 1990, 1991; Fagan & Dettermann, 1992). Results revealed that both FH+ and FH− groups showed significant discrimination of the novel tone-pair for both 70 and 300 ms ISI tasks (FH+, 70 ms ISI, t(17) = 3.5, p < .05, 300 ms ISI, t(17) = 2.2, p < .05; FH−, 70 ms ISI, t(35) = 7.3, p < .05, 300 ms ISI, t(35) = 5.1, p < .05). A mixed factorial ANOVA procedure (Group × ISI) was used to test for interactions between family history of SLI and RAP abilities. Findings revealed a significant interaction (F(1, 42) = 7.12, p < .01) such that there were no differences between 70 and 300 ms ISI tasks for the FH− infants, whereas FH+ infants showed lower percent novelty preference on the 70 ms ISI as compared to the 300 ms ISI task.
The visual habituation task
Performance on a VH/RM task that assessed visual discrimination of faces was used to consider processing differences between groups that may not be related to RAP abilities. As with the AVH/RM tasks, performance on the visual paradigm can be parsed into habituation trials (trials to criterion, percent decrement and habituation slope) and test trials (percent novelty preference). A one-way ANOVA revealed no group differences on trials to criterion (F(1, 44) = 0.30, n.s.) or the habituation slope (F(1, 44) = 0.10, n.s.) (Table 5). However, significant group differences were found for percent decrement favoring the FH+ group (F(1, 44) = 4.24, p < .05). Performance on the test trials was also found to be significantly different by group (F(1, 44) = 4.56, p < .05); the mean percent novelty preference scores for the FH+ group were signi-ficantly lower and at chance levels (i.e. 50%) compared to the FH− group (Table 5). This effect was a result of a subset of the FH+ group showing a familiarity preference: eight out of the 17 (47%) infants in the FH+ group showed a clear familiarity preference even after they had been habituated to the stimuli, while only four out of 27 (14%) infants in the FH− group showed a familiarity preference, χ2 (1) = 4.71, p < .05.
Converging evidence for RAP abilities from HT and AV tasks
In order to assess performance on HT and AVH/RM tasks by group, a mixed-factorial MANOVA (Task × Group × ISI) was conducted after all outcome variables for both tasks were converted to standardized scores (z-scores) (Table 6). In the MANOVA model Paradigm and ISI were entered as repeated measures variables and Group was entered as the between subjects factor. Results indicated a significant three-way interaction (F(1, 32) = 4.91, p < .05). Given that earlier results indicated that performance on the 300 ms ISI was significantly better than performance for the 70 ms ISI conditions for each task (HT and AV), we were interested in understanding how the two groups compared on their performance on the two different tasks within the same ISI. Therefore two mixed-factorial ANOVAs (Task × Group) were run separately for each condition (70 ms ISI and 300 ms ISI) in order to interpret the earlier three-way interaction. The first model compared performance on the 70 ms ISI conditions and the second model compared performance on the 300 ms ISI conditions.
Table 6.
Standardized score means and standard deviations of d′ and novelty preference for head-turn and auditory visual habituation paradigms by group
| FH+ (n = 11)
|
FH− (n = 23)
|
|||
|---|---|---|---|---|
| Mean | (std) | Mean | (std) | |
| z-scores for 70 ms ISI | ||||
| HT: d′ | −0.58 | 1.2 | 0.27 | 0.8 |
| AVH/RM: % Novelty preference | −0.19 | 0.78 | 0.20 | 1.21 |
| z-scores for 300 ms ISI | ||||
| HT: d′ | 0.27 | 1.16 | −0.01 | 1.02 |
| AVH/RM: % Novelty preference | −0.14 | 1.11 | 0.21 | 0.93 |
Note: Data are limited to all infants who successfully learned the HT task.
Analysis of the 70 ms ISI condition revealed a significant main effect for group (F(1, 32) = 5.84, p < .05). Infants from FH+ families had significantly lower scores on both the AVH/RM and the HT paradigms for the 70 ISI condition compared to infants from control families (Figure 2). There was no task effect (F(1, 32) = 0.46, p > .05) and no significant interaction (F(1, 32) = 0.98, p > .05). Findings from the 300 ms ISI condition revealed no significant group effect, task effect or interaction: Performance on the HT and AVH/RM tasks for both groups of infants was comparable (Figure 2).
Figure 2.
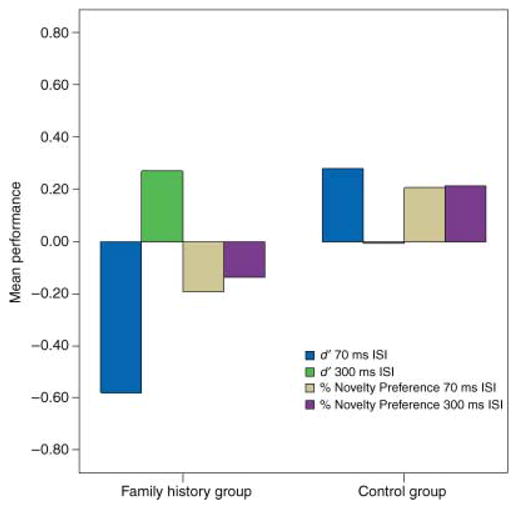
Mean performance (z-score) on head-turn and auditory-visual habituation/recognition memory tasks for 70 and 300 ms ISI conditions by group.
Cognitive and language outcomes
Group differences on standardized cognitive (MDI) and language measures (PLS-3 and CDI) were assessed using the ANOVA procedure. Results indicate that all infants performed within the normal range on the Bayley Scale (MDI) in both FH+ and FH− groups (range 85 to 109), and t-test analysis revealed no difference between FH+ and FH− groups on the MDI at either 6 months (F(1, 44) = 0.67, n.s.), 9 months (F(1, 44) = 0.85, n.s.) or 16 months (F(1, 34) = 0.03, n.s.) (Table 7). Analyses of the language measures revealed significant group differences at 12 and 16 months. Parents reported that FH− infants had a larger number of gestures on the CDI 12-month inventory (CDI: Words and Gestures) compared to FH+ infants (F(1, 44) = 2.05, p < .05) (Table 7), and that at 16 months (CDI: Words and Sentences) FH+ infants had smaller vocabularies (F(1, 34) = 5.01, p < .05) and produced fewer irregular words compared to FH− infants (F(1, 34) = 5.33, p < .05) (Table 7). Similar results were found on the PLS-3; FH+ infants had lower scores on the expressive subscale (F(1, 33) = 4.10, p < .05), while there were no significant group differences on the auditory-comprehension subscale of the test (Table 7).3
Table 7.
Means and standard deviations for 6- and 16-month MDI scores and 12- and 16-month CDI scores
| FH+
|
FH−
|
||||||
|---|---|---|---|---|---|---|---|
| Mean | (std) | Mean | (std) | F | p | Partial η2 | |
| Bayley Scale | |||||||
| MDI – 6 months | 96.5 | 5.39 | 95.2 | 5.27 | .647 | ns | – |
| MDI – 9 months | 99.9 | 6.97 | 97.7 | 6.32 | .854 | ns | – |
| MDI – 16 months | 100.8 | 11.5 | 100.7 | 9.3 | .003 | ns | – |
| CDI 12 m | |||||||
| Gestures % | 44 | 31 | 57 | 26 | 2.05 | .02* | 0.1 |
| Words % | 40 | 31 | 42 | 32 | .06 | ns | – |
| Phrases % | 42 | 27 | 49 | 26 | .69 | ns | – |
| Comprehension % | 45 | 26 | 52 | 23 | .62 | ns | – |
| CDI 16 m | |||||||
| % Production | 23 | 23 | 45 | 25 | 5.01 | .03* | 0.2 |
| % Irregular words | 61 | 6 | 72 | 12 | 5.33 | .02* | 0.2 |
| % Sentence complexity | 82 | 9 | 84 | 20 | .984 | ns | – |
| PLS 16 m | |||||||
| Expressive communication | 106.7 | 18.7 | 115.8 | 16.8 | 4.10 | .05* | 0.1 |
| Auditory comprehension | 116.3 | 13.3 | 114.7 | 12.0 | 0.05 | ns | – |
Predictions from infant information processing abilities to language development at 12 and 16 months
Associations
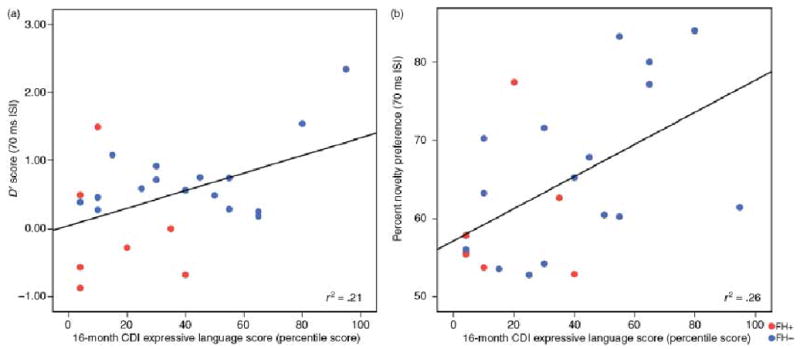
In the final set of analyses, RAP abilities were used to predict language outcomes at 12 and 16 months of age. Preliminary analyses revealed no association among HT, AVH/RM and VH/RM parameters (Pearson’s Product Moment Correlations ranged from 0.09 to 0.27, p > .15), thus enabling us to enter the appropriate parameter from each assessment into the same regression model. However, correlations revealed a number of significant positive associations between infant information processing variables and language measures. At 12 months, percent novelty preference from the 300 ms ISI condition was related to the number of gestures produced and the comprehension score of the CDI (Table 8). At 16 months, performance on the 70 ms ISI conditions for both HT (d′) and AVH/RM (percent novelty preference) paradigms was associated with the CDI production scores (Table 8 and Figure 3) and the expressive language subscale of the PLS-3 (Table 8 and Figure 4); infants with larger d′ and novelty preference scores had larger vocabularies on the CDI and higher expressive scores on the PLS-3. D-prime (d′) for the 70 ms ISI HT test was also associated with the number of irregular words produced. No associations were found between infant information processing and performance on the MDI of the Bayley scales.
Table 8.
Pearson Product Moment Correlations (r) between infant information processing measures and language abilities at 12 and 16 months
| Conditioned head-turn
|
Auditory-visual habituation
|
|||
|---|---|---|---|---|
| D′ 70 ms ISI | D′ 300 ms ISI | % Novelty preference 70 ms ISI | % Novelty preference 300 ms ISI | |
| CDI 12 m | ||||
| % Gestures | .169 | .117 | .147 | .457* |
| % Words | .355 | .336 | .231 | .133 |
| % Phrases | −.226 | .147 | .152 | .295 |
| % Comprehension | .006 | .232 | .076 | .413* |
| CDI 16 m | ||||
| % Production | .463* | .033 | .561* | .115 |
| % Irregular words | .454* | .194 | .147 | .147 |
| % Sentence complexity | .097 | .361 | −.102 | .287 |
| PLS 16 m | ||||
| Auditory comprehension | .239 | .184 | .306 | .006 |
| Expressive communications | .428* | −.071 | .416* | −.106 |
p < .05.
Note: Data are limited to all infants who successfully learned the HT task. FH+ n = 11, FH− n = 23.
Figure 3.
Associations between CDI expressive language scores at 16 months and d′ and percent novelty preference.
Figure 4.
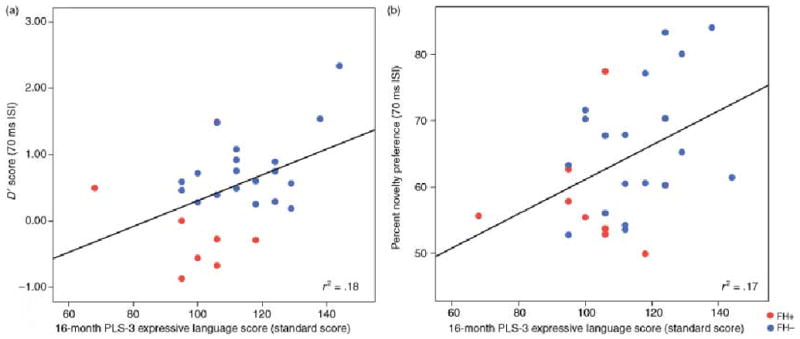
Associations between PLS-3 expressive language scores at 16 months and d′ and percent novelty preference.
Given that group differences also emerged on the visual habituation and recognition memory task, the association between VH/RM and language outcomes was examined. Results revealed no significant associations; neither language comprehension nor production at 12 or 16 months of age was associated with performance on the visual habituation and recognition memory task.
Predictions from 6 to 16 months
Finally two regression analyses were conducted to investigate the predictive association between infant RAP processing skills on the HT and the AVH/RM paradigms, group status (family history) and language production at 16 months (Table 9). In the first regression analysis, the dependent variable was the number of words produced on the 16-month CDI while the second analysis assessed performance on the 16-month expressive sub-scale of the PLS-3. In both analyses, group status (FH+/FH−), 70 ms ISI d′ and percent novelty preference scores were entered as independent variables. Stepwise regression analysis method was used to assess the unique contribution of each independent variable in predicting language abilities.
Table 9.
Hierarchical stepwise regression analyses predicting to expressive language (CDI and the PLS-3) at 16 months from performance on infant information processing measures of RAP (70 ms ISI novelty preference and D′) at 6 months
| Unstandardized
|
||||||||
|---|---|---|---|---|---|---|---|---|
| Beta | Std error | Standard beta | t | p | Model F | Model p | Adj. R2 | |
| CDI (Expression) | ||||||||
| Model 1: | 4.22 | .05 | .12 | |||||
| Family history of SLI | 21.75 | 10.58 | .35 | 2.05 | .04 | |||
| Model 2: | 7.99 | .01 | .39 | |||||
| % Novelty preference | 1.22 | 0.43 | .48 | 2.87 | .01 | .24 | ||
| D′ | 15.07 | 6.03 | .42 | 2.50 | .02 | .15 | ||
| Family history of SLI | na | na | .14 | 0.66 | ns | ns | ||
| PLS-3 (Expressive Communication) | ||||||||
| Model 1: | 3.60 | .06 | .09 | |||||
| Family history of SLI | 12.08 | 6.37 | .34 | 1.90 | .06 | |||
| Model 2: | 6.93 | .01 | .35 | |||||
| D′ | 10.30 | 3.97 | .45 | 2.60 | .02 | .20 | ||
| % Novelty preference | .68 | .28 | .42 | 2.41 | .03 | .15 | ||
Note: Data are limited to all infants who successfully learned the HT task: FH+ n = 11, FH− n = 23.
As indicated in Table 9, both percent novelty preference and d′ scores entered significantly together accounting for 39% of the variance in 16-month language production on the CDI. Twenty-four percent (24%) of the variance was accounted for by performance on the AVH/RM task and 15% by the 70 ms ISI d′ score. When family history was entered alone, it accounted for 12% of the variance but group status did not add to the prediction of language abilities at 16 months when included in the model with RAP measures (d′ and percent novelty preference) (Table 9).
On the PLS-3 both percent novelty preference and d′ scores entered significantly together, accounting for 35% of the variance. Twenty percent of the variance was accounted for by the 70 ms ISI d′ score, whereas 15% was accounted for by performance on the AVH/RM task. Even when entered alone, family history of SLI did not contribute significantly to 16-month language abilities on the PLS-3 (Table 9).
Discussion
Performance on infant information processing tasks has been shown to be predictive of later cognitive and language abilities (Benasich & Tallal, 1996, 2002; Bornstein & Sigman, 1986; Colombo, 1993, 2002; Rose et al., 2003, 2004a, 2004b). Earlier studies have reported that approximately 25% of variance in later cognitive abilities (Kavsek, 2004; McCall & Carriger, 1993) and 50% of the variance on language assessments (Benasich & Tallal, 2002) can be accounted for by performance on early information processing measures. Results from the present study parallel and extend previous findings and yet again demonstrate the importance of RAP skills for the acquisition of language (Benasich & Tallal, 1996, 2002; Benasich et al., 2002). In the current study, rapid auditory processing abilities, assessed by the HT and the AVH/RM paradigms, accounted for 35–39% of the variance in 16-month expressive language skills. Interestingly, but perhaps unsurprisingly (given the extensive LI literature in older children), the current study also revealed differences between infants at risk for language delay (i.e. from FH+ families) and controls on information processing abilities that are not related to RAP skills. For example, infants from FH+ groups performed differently on the visual-habituation task; 47% of infants in the FH+ group showed a familiarity preference during the recognition memory test compared to 14% of infants in the control group. A significant familiarity response is thought to index a more immature, but still discriminative, response that has been interpreted as an indication of the need for additional processing time (Rose, 1981, 1983). FH+ infants also required significantly more trials to habituate and had more shallow slopes on the AVH/RM 300 ms ISI task compared to control infants. Taken together these findings seem to implicate more global, perhaps attentional differences between children from FH+ families and their FH− peers. These findings are discussed in more detail below.
Mechanisms underlying performance on infant information processing tasks
In this study we examined infants’ performance on two different information-processing tasks (HT and H/RM) in an attempt to disambiguate the role of one basic perceptual mechanism, RAP, from other cognitive processes in supporting language acquisition. One of our main findings is that the two different infant information-processing tasks predicted independently to 16-month language abilities. Further, performance on these tasks was not associated with each other. While this is not an unusual finding in the infant literature – cognitive measures administered concurrently are frequently not associated with each other (Colombo, 1993, 2002; Colombo et al., 2004) – it is surprising because both these measures were designed to assess RAP abilities and each shows systematic differences between controls and children at higher risk for SLI. The most common explanation for the lack of association among infancy measures may be the lack of test-retest reliability of the measures. However, given that in all three infant information-processing tasks administered in this study there was a statistically significant, albeit modest, association across 6- and 9-month performance we do not consider reliability to be the reason for the lack of association observed here. A close examination of the data and the demand characteristics of the tasks reveal two possible alternative explanations. First, task complexity and thus differences in the cognitive demands levied by HT and AVH/RM tasks may be associated with individual differences in performance profiles (i.e. some children are not able to even learn the HT task while very easily completing the AVH/RM task). Second, such individual differences may be highlighted by the fact that the underlying cognitive processes engaged by the HT and the AVH/RM are somewhat independent. In the following section we explore these differences more closely.
Task demands
In both the head-turn procedure and the auditory-visual habituation task the infant’s general orienting response (OR) plays an important role in allocating attention to relevant stimuli. In the HT paradigm the OR is activated by a target auditory signal that is coupled with age-appropriate rewards (e.g. animated toys), and in the habituation task the OR is activated by an auditory signal that directs the child’s visual attention to a stimulus. However, this is where the similarities between the two paradigms end and the different demand characteristics of each task necessitate different behaviors in order to achieve successful performance.
The head-turn paradigm is based on operant conditioning principles (Bornstein & Arterberry, 1999; Werker, Shi, Desjardins, Pegg, Polka & Patterson, 1998). In this paradigm an arbitrary auditory signal (the target tone-pair) is coupled with the onset of an appetitive reinforcer (animated toys). The target signal is interspersed within a random series of non-target auditory stimuli and there is no way for the infant to predict when the target tone will occur. Therefore, the infant is required to constantly monitor the auditory environment for the occurrence of the target signal. In most cases, the repeated pairing of the target tone with the reward will lead to the acquisition of a conditioned response; the infant learns that the target auditory signal predicts a reward (as in the traditional stimulus–response conditioning paradigms). To be successful in this task the infant is required to maintain a certain level of vigilance throughout the session and develop a relatively strong representation of the target stimuli.
The conditioned head-turn paradigm provides reliable data about perception because infants actively and voluntarily respond to the change in stimuli and thereby directly communicate their perceptions to the experimenter. In contrast, habituation tasks draw minimally on infant motor ability and voluntary control and instead habituate the infant’s OR to an oft-repeated presentation of an auditory (or visual) stimulus (Honey & Good, 2000). Habituation reflects a lack of discrepancy between the current stimulus and the memory trace based on experience with the stimulus (Bornstein, 1985; Colombo, 1993, 2002; Sokolov, 1963). This habituation function is measured behaviorally by a decrease in attention allocated to the oft-presented stimulus. Habituation measures of both the decrement of attention and the recovery of attention have been adopted widely in experimental studies of infant perception and cognition, and have proved to be the most versatile and fruitful testing method.
Mechanisms underlying performance
Given the different demand characteristics of the HT and the habituation tasks it is plausible that they engage different cognitive processes. For example, in the HT paradigm processes governing associative learning, the ability to maintain and generalize the learned contingency, vigilance (or sustained attention), inhibition of attention to non-relevant events and the efficiency with which information is moved from working memory into either short-term or even long-term memory may be necessary for successful performance. While there are no studies that have examined the cognitive processes necessary for performance on infant HT (or the Go/No-Go task which is analogous to the conditioned HT task used here), findings from behavioral and imaging (e.g. fMRI) studies with older children and adults may shed some light on the possible neural substrates supporting performance in these measures. For example, fMRI studies of young children’s performance on Go/No-Go tasks indicate that, while functional proficiency in the detection of the ‘go’ stimuli may be achieved earlier than the inhibition of the response to ‘no-go’ stimuli, prefrontal (specifically ventral and dorsal prefrontal circuitry) and parietal activation of multiple cortical regions integrated in functional subsystems may be necessary for successful performance on both the ‘go’ and the ‘no-go’ components of this task (Casey, Trainor, Orendi, Schubert, Nystrom, Giedd, Castellanos, Haxby, Noll, Cohen, Forman, Dahl & Rapoport, 1997; Durston, Thomas, Yang, Ulag, Zimmerman & Casey, 2002; Durston, Tottenham, Thomas, Davidson, Eigsti, Yang, Ulag & Casey, 2003; Fiducia & O’Leary 1990; Mesulam, 1990; Posner & Petersen, 1990; also see Parasuraman, Warm & See, 1998, for review of brain regions supporting attention and inhibition processes). In humans, fronto-parietal circuitries have been shown to be associated with working memory (Casey, Cohen, Jezzard, Turner, Noll, Trainor, Giedd, Kaysen, Hertz-Panier & Rapoport, 1995; Casey et al., 1997; Goldman-Rakic, 1987), vigilance (Cabeza & Nyberg, 2000; Lawrence, Ross, Hoffmann, Garavan & Stein, 2003; Paus, Zatorre, Hofle, Caramanos, Gottman, Petrides & Evans, 1997; Parasuraman et al., 1998; Posner & Petersen, 1990) and inhibition of pre-potent responses (Casey et al., 1997; Diamond, 1990; Diamond & Goldman-Rakic, 1989; Goldman-Rakic, 1987).
The habituation paradigm, on the other hand, may tap into more basic perceptual processes (see Colombo, 1993, 2002, for a discussion of possible underlying mechanisms; Richards, 2003; Rose et al., 2004a, 2004b; Snyder, Webb & Nelson, 2002). A number of infant studies have combined behavioral and brain-imaging techniques (e.g. EEG/ERP responses) in an attempt to identify processes involved during habituation-recognition memory tasks (deRegnier, Georgieff & Nelson, 1997; Richards, 2003; Nelson & Collins, 1991, 1992; Nelson & deRegnier, 1992; Nelson & Salapetek, 1986; Snyder et al., 2002). For example, Synder and colleagues (2002) describe separate waveforms associated with stimulus repetition and response to familiarity (these are analogous to habituation to an oft repeated stimulus and allocation of attention to a new stimulus during the recognition memory test of the paradigm, respectively). Findings suggest that processes associated with stimulus familiarity may be reflected in the mid-latency negative component (Nc), while the long latency slow wave component (SW) may be generated by processes related to stimulus repetition (i.e. habituation). Studies suggest that there are different neuronal subsystems supporting the two processes; the Nc is most likely to be subserved by areas in the prefron-tal cortex (associated with attention) and the anterior cingulate (Nelson & Collins 1991, 1992; Richards, 2003) whereas the SW seems to be generated by processes closely associated with the medial temporal lobe (mostly involved in the updating of short-term perceptual memory) (Goldman, Shapiro & Nelson, 2004; Nelson & Dukette, 1998; Richards, 2003; Synder et al., 2002).
Animal lesion studies have also helped to clarify the role of specific brain areas. For example, Goldman-Rakic and colleagues (Goldman-Rakic, Isseroff, Schwartz & Bugbee, 1983; Goldman & Rosvold, 1972) have shown that damage to the caudate nucleus in both infant and juvenile monkeys results in impaired performance on tasks requiring memory for location (such as delayed response, i.e. finding an object that has been hidden in a specific location following a delay, as well as delayed alternation, finding an object that had been previously hidden in a second location). Lesions to the mediodorsal nucleus of the thalmus in infant monkeys also impairs performance on these types of memory for location tasks (Isseroff, Rosvold, Galkin & Goldman-Rakic, 1982). The ventromedial prefrontal cortex has strong projections to the amygdala and to thalamic nuclei other than the mediodorsal nucleus but does not project to the caudate. These pathways have been implicated in visual recognition memory (Goldman et al., 1971; Mishkin, 1964) as well as to selective attention in humans (Luria, 1966; Milner, 1971). Bachevalier and colleagues (e.g. Alvarado, Wright & Bachevalier, 2002; Bachevalier, Beauregard & Alvarado, 1999; Bachevalier & Mishkin, 1986; Pascalis & Bachevalier, 1999) have shown that amygdalo-hippocampal lesions in infant monkeys result in deficits in a preferential looking task, a task commonly used for infants in the recognition memory paradigm.
Given the differences in the demand characteristics and the possibility that different brain circuitry may support performance on the HT and the habituation paradigms, it is not surprising that infants’ performance on these two measures may provide independent but converging contributions to outcome, as is evident in our data. For example, even if we are to highlight the role of memory processes to these two tasks, the literature suggests that each may be subserved by different memory systems. For example, performance on the head-turn task seems to be more influenced by the infant’s ability to consolidate and retain information; this means that information must be moved from working to short-term to long-term memory. This type of memory may be similar to explicit memory (Nelson, 1995) and may require adequate development of the hippocampus and related cortical areas such as the inferotemporal cortical area ‘TE’. On the other hand, the habituation paradigm may be more dependent on the ability to maintain information in short-term or in working memory and then being able to retrieve and effectively use that information for comparison purposes (i.e. showing a novelty preference). Nelson (1995) hypothesized that this is a form of ‘pre-explicit’ memory and requires only the functioning of the hippocampus and not the surrounding areas. In fact, Bachevalier, Brickson and Hagger (1993) showed that lesioning area TE in infant monkeys had no effect on visual paired preference comparisons, while bilateral removal of the hippocampus and the amygdala disrupted performance on the task (i.e. infant monkeys failed to show a novelty preference).
Processing differences between infants with a family history of SLI and their peers
While different underlying mechanisms seem to support performance on the HT and the AVH/RM tasks, this study also found differences in information processing abilities based on group status. Infants from families with a history of SLI (FH+) were found to have less efficient RAP abilities and also showed differences in global processing and attention abilities compared to infants from control families. These results are consistent with prior studies that have reported RAP deficits in infants from families with a history of SLI compared to control infants (Benasich & Tallal, 1996, 2002). All participants were prelinguistic and thus did not have diagnoses of language delay or impairment. However, as suggested by prior aggregation studies, by virtue of being born into a family with a language-based learning disability the FH+ infants seem to be at greater risk for non-normative language development (Choudhury & Benasich, 2002). While the results of this study support the notion that infants born into families with a history of SLI are at higher risk for LI, results of the regression analysis clearly implicate RAP abilities from infancy as a predictor of future language abilities above and beyond family history of SLI. The percentage of infants with poorer RAP processing is indeed significantly higher in groups of infants with a family history of such disorders; however, RAP thresholds across both paradigms are the most robust predictors of outcome. This finding supports previous results reported in an earlier study with a different and smaller sample of infants (Benasich & Tallal, 2002).
A closer analysis of each group’s performance on the two different measures implicates not only differences in RAP but also other cognitive processes. While results of the regression analysis show that only RAP variables were predictive of language outcome, group differences were also found on visual habituation paradigms; significantly more FH+ infants exhibited a familiarity preference during the test trials despite no differences in the number of trials to criterion, slope or decrement. That FH+ infants showed a familiarity preference implies that these infants were discriminating the novel stimulus from the familiarized one but that something about their processing led them to extend their looking to the familiar. One of the major drawbacks of the habituation paradigm is that one cannot determine why a certain percentage of children show a familiarity preference even after having been successfully habituated. A possible explanation may involve disruption in the ability to disengage attention from the familiar and inhibit responding to it (also known as sticky fixation). Habituation and recognition memory paradigms require infants to disengage attention from a familiar stimulus and shift attention to one that is more informative (in this case unfamiliar) (McCall, 1994; McCall & Mash, 1995; Rose, Feldman & Jankowski, 2003, 2004a, 2004b; Sigman et al., 1997). It may be that infants who show a clear familiarity preference have dif-ficulties with inhibition of attention. Another hypothesis is that infants showing significant familiarity responses may have a slower trajectory of growth in information processing abilities including the encoding and storing of information and/or subsequent retrieval during a recognition memory task. This response has been seen in pre-terms as compared to full-term infants and a novelty response can be elicited from pre-terms by increasing the infant’s exposure time (and thus opportunity for processing) to the stimulus (Rose, 1981, 1983; Rose, Feldman, McCarton & Wolfson, 1988). Conversely, one may produce a familiarity response in a full-term infant by decreasing the amount of time the stimulus is available for inspection. However, this effect is more pronounced in a paradigm that has fixed habituation trials rather than the infant control procedure used here (Benasich & Behar, 1992). While we are unable to speak to these hypotheses directly there are some indications from our follow-up data (24 months) that children with a family history of SLI may differ in their attentional abilities, especially inhibitory control, during a problem-solving task (Badridze, Choudhury & Benasich, 2004). An alternative explanation may be related to temperamental differences; infants that show a familiarity preference may be developing an expectation in which they anticipate a particular type of stimuli and are ‘somewhat overwhelmed’ by this violation (i.e. the novel stimulus). It is possible that if the test stimuli were presented for an extended period of time, more than is needed for habituation, such infants would increase their looks to the novel with time (i.e. these infants may need more time to ‘warm up’ to the novel stimulus). There is some evidence that infants who show a familiarity preference may in fact differ in other ways, as their mothers report that they are more difficult to soothe and that they are more readily distressed by novel events (Mannix, Yue, Choudhury, Badridze & Benasich, 2004).
Additionally, our findings show that FH+ infants took longer to habituate to the stimuli presented during the AVH/RM 300 ms ISI paradigm. This finding taken together with results from the VH paradigm suggests processing differences in the FH+ group that are not limited to RAP abilities and are congruent with research that has shown multimodal processing differences in language-impaired adults and children; including difficulties in motor coordination (Bishop & Edmundson, 1987; Johnston, Stark, Mellits & Tallal, 1981; Robinson, 1991; Stark & Tallal, 1981; Viholainen, Ahonen, Cantell, Lyytinen & Lyytinen, 2002), automatization of skills (Nicolson, Fawcett & Dean, 2001), tactile and visual perception (Stoodley, Talcott, Carter, Witton & Stein, 2000; Stein, 1999; Stein & Talcott, 1999; Talcott, Hansen, Assoku & Stein, 2000; Witton et al., 1998; Livingstone, Rosen, Drislane & Galaburda, 1991; Powell & Bishop, 1992) and working memory (Botting & Conti-Ramsden, 2001; Dollaghan & Campbell, 1998; Gathercole & Baddeley, 1990). Finally, given that the infants in this study are not themselves language impaired as they are too young for such diagnoses, it remains to be seen how many will have language or learning difficulties and how these differences will become manifest.
In conclusion, the data presented here demonstrate that habituation and recognition memory as well as operantly conditioned head-turn paradigms provide evidence for the importance of infant rapid auditory processing abilities to later language. Our results show that infants who are poorer at this basic pre-linguistic processing are less advanced in their linguistic development, whether or not they have a family history of such disorders. It is also clear that the underlying cognitive processes and perhaps neural substrates that are engaged during each of these tasks differ in a number of respects. We have just begun to identify how these tasks differ and what consequence this might have for elucidation of the sensory and cognitive processes studied. Additional information will be gained in future studies by including additional and converging data gained via EEG/ERPs using similar stimuli (Benasich, Thomas, Choudhury & Leppänen, 2001; Benasich, Choudhury, Friedman, Realpe-Bonilla, Chojnowska & Gou, 2005).
Acknowledgments
This research was supported by Grant RO1-HD29419 from NICHD with additional support from the Charles A. Dana Foundation and the Elizabeth H. Solomon Center for Neurodevelopmental Research. We thank the parents and children who participated in this study.
Footnotes
The term SLI is used to refer to developmental language disorders as described above and will be used instead of other terms such as the broader terms, language-based learning impairments (LLI) or developmental dysphasia.
d′ scores were tested against the null hypothesis that d′ = 0 because at the group-level d′ scores of zero show that infants are not able to discriminate the target tone-pair from the non-target tone-pair.
3 Performance on the Expressive Communication subscale of the PLS and the expressive score of the CDI were correlated; PLS-3 expressive scores were associated to parent report of the number of words produced on the CDI at 12 and 16 months (r = .53 and r = .81, p < .05, respectively) and the number of irregular words produced (r = .46, p < .05).
References
- Alvarado MC, Wright AA, Bachevalier J. Object and spatial relational memory in adult rhesus monkeys is impaired by neonatal lesions of the hippocampal formation but amygdaliod cortex. Hippocampus. 2002;12:421–433. doi: 10.1002/hipo.1115. [DOI] [PubMed] [Google Scholar]
- Aslin RN. Discrimination of frequency transitions by human infants. Journal of the Acoustical Society of America. 1989;86:582–590. doi: 10.1121/1.398237. [DOI] [PubMed] [Google Scholar]
- Bachevalier J, Beauregard M, Alvarado M. Long-term effect of neonatal damage to the hippocampal formation and amygdaliod cortex on object discrimination learning and object recognition memory in monkeys. Behavioral Neuroscience. 1999;113:1127–1151. doi: 10.1037//0735-7044.113.6.1127. [DOI] [PubMed] [Google Scholar]
- Bachevalier J, Brickson M, Hagger C. Limbic-dependent recognition memory in monkeys develops early in infancy. Neuroreport: An International Journal for the Rapid Communication of Research in Neuroscience. 1993;4 (1):77–80. doi: 10.1097/00001756-199301000-00020. [DOI] [PubMed] [Google Scholar]
- Bachevalier J, Mishkin M. Visual recognition impairment follows ventromedial but not dorsolateral pre-frontal lesions in monkeys. Behavioral Brain Research. 1986;20 (3):249–261. doi: 10.1016/0166-4328(86)90225-1. [DOI] [PubMed] [Google Scholar]
- Badridze N, Choudhury N, Benasich AA. Antecedents of object mastery motivation at 24 months in children with and without family history of language impairment. Poster presented at CNS conference; San Francisco, CA. 2004. Apr, [Google Scholar]
- Bayley N. The Bayley Scales of Infant Development. 2. New York: Psychological Corporation; 1993. [Google Scholar]
- Benasich AA. Temporal integration as an early predictor of speech and language development. In: Von Euler C, Lundberg I, Llinas R, editors. Basic mechanisms in cognition and language – with special reference to phonological problems in dyslexia. Vol. 70. Oxford: Elsevier Science; 1998. pp. 123–142. [Google Scholar]
- Benasich AA, Behar II. The Fagan test of infant intelligence: a critical review. Journal of Applied Developmental Psychology. 1992;13:153–171. [Google Scholar]
- Benasich AA, Choudhury N, Friedman JT, Realpe-Bonilla T, Chojnowska C, Gou Z. The infant as a prelinguistic model for language learning impairments: predicting from event-related potentials to behavior. Neuropsychologia. 2005 doi: 10.1016/j.neuropsychologia.2005.06.004. 27 July, e-pub. ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benasich AA, Heim S. Disorders of language. In: Cicchetti D, Cohen DJ, editors. Developmental psychopathology. 2. New York: John Wiley & Sons; 2005. [Google Scholar]
- Benasich AA, Leevers HJ. Processing of rapidly presented auditory cues in infancy: implications for later language development. In: Fagan J, Haynes H, editors. Progress in infancy research. Vol. 3. Mahwah, NJ: Erlbaum; 2002. pp. 245–288. [Google Scholar]
- Benasich AA, Read H. Representation: picture or process? In: Sigel IE, editor. Theoretical perspectives in the development of representational (symbolic) thought. Mahwah, NJ: Lawrence Erlbaum Associates; 1998. pp. 33–160. [Google Scholar]
- Benasich AA, Spitz RV. Insights from infants: temporal processing abilities and genetics contribute to language development. In: Willems G, Whitmore K, editors. A neurodevelopmental approach to specific learning disorders. London: MacKeith Press; 1998. pp. 191–210. [Google Scholar]
- Benasich AA, Tallal P. An operant conditioning paradigm for assessing auditory temporal processing in 6- to 9-month-old infants. Tallal P, Galaburda AM, Llinás RR, Von Euler C, editors. Temporal information processing in the nervous system: Special reference to dyslexia and dysphasia. The New York Academy of Sciences. 1993;682:312–314. doi: 10.1111/j.1749-6632.1993.tb22978.x. [DOI] [PubMed] [Google Scholar]
- Benasich AA, Tallal P. Auditory temporal processing thresholds, habituation, and recognition memory over the first year. Infant Behavior and Development. 1996;19:339–357. [Google Scholar]
- Benasich AA, Tallal P. Infant processing of auditory temporal information: links to family history and later language outcome. Society for Neuroscience Abstracts. 1998;24:819. [Google Scholar]
- Benasich AA, Tallal P. Infant discrimination of rapid auditory cues predicts later language impairment. Behavioral Brain Research. 2002;136:31–49. doi: 10.1016/s0166-4328(02)00098-0. [DOI] [PubMed] [Google Scholar]
- Benasich AA, Thomas JJ, Choudhury N, Leppänen PHT. The importance of rapid auditory processing abilities to early language development: evidence from converging methodologies. Developmental Psychobiology. 2002;40:278–292. doi: 10.1002/dev.10032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bird D, Bishop D, Freeman N. Phonological awareness and literacy development in children with expressive phonological impairments. Journal of Speech and Hearing Research. 1995;38:446–462. doi: 10.1044/jshr.3802.446. [DOI] [PubMed] [Google Scholar]
- Bishop DVM, Edmundson A. Language-impaired 4-year-olds: distinguishing transient from persistent impairment. Journal of Speech and Hearing Disorders. 1987;52:156–173. doi: 10.1044/jshd.5202.156. [DOI] [PubMed] [Google Scholar]
- Bishop DVM, Powell RP. Clumsiness and perceptual problems in children with specific language impairment. Developmental Medicine and Child Neurology. 1992;34 (9):755–765. doi: 10.1111/j.1469-8749.1992.tb11514.x. [DOI] [PubMed] [Google Scholar]
- Bishop DVM, Snowling MJ. Developmental dyslexia and specific language impairement: same or different? Psychological Bulletin. 2004;130:858–888. doi: 10.1037/0033-2909.130.6.858. [DOI] [PubMed] [Google Scholar]
- Bornstein MH. Habituation of attention as a measure of visual information processing in human infants: summary, systematization, and synthesis. In: Gottlieb G, Krasnegor NA, editors. Measurement of audition and vision in the first year of postnatal life: A methodological overview. Westport, CT: Ablex Publishing; 1985. pp. 253–300. [Google Scholar]
- Bornstein MH, Arterberry ME. Perceptual development. In: Bornstein MH, Lamb ME, editors. Developmental psychology: An advanced textbook. 4. Mahwah, NJ: Lawrence Erlbaum Associates; 1999. pp. 231–274. [Google Scholar]
- Bornstein MH, Sigman MD. Continuity in mental development from infancy. Child Development. 1986;57:251–274. doi: 10.1111/j.1467-8624.1986.tb00025.x. [DOI] [PubMed] [Google Scholar]
- Botting N, Conti-Ramsden G. Non-word repetition and language development in children with specific language impairment (SLI) International Journal of Language and Communication Disorders. 2001;36:421–432. doi: 10.1080/13682820110074971. [DOI] [PubMed] [Google Scholar]
- Byrne B. Deficient syntactic control in poor readers: is a weak phonetic memory code responsible? Applied Psycholinguistics. 1981;2:201–212. [Google Scholar]
- Cabeza R, Nyberg L. Imaging cognition II: An empirical review of 275 PET and fMRI studies. Journal of Cognitive Neuroscience. 2000;12:1–47. doi: 10.1162/08989290051137585. [DOI] [PubMed] [Google Scholar]
- Caron RF, Caron AJ. The effects of repeated exposure and stimulus complexity on visual fixation in infants. Psychonomic Science. 1968;10:207–208. [Google Scholar]
- Casey BJ, Cohen JD, Jezzard P, Turner R, Noll DC, Trainor TJ, Giedd J, Kaysen D, Hertz-Panier L, Rapoport JL. Activation of prefrontal cortex in children during a nonspatial working memory task with functional MRI. Neuroimaging. 1995;2:221–229. doi: 10.1006/nimg.1995.1029. [DOI] [PubMed] [Google Scholar]
- Casey BJ, Trainor RJ, Orendi JL, Schubert AB, Nystrom LE, Giedd JN, Castellanos FX, Haxby JV, Noll DC, Cohen JD, Forman SD, Dahl RE, Rapoport JL. A developmental functional MRI study of prefrontal activation during performance of a Go-No-Go task. Journal of Cognitive Neuroscience. 1997;9:835–847. doi: 10.1162/jocn.1997.9.6.835. [DOI] [PubMed] [Google Scholar]
- Catts H. The relationship between speech-language impairments and reading disabilities. Journal of Speech and Hearing Research. 1993;36:948–958. doi: 10.1044/jshr.3605.948. [DOI] [PubMed] [Google Scholar]
- Choudhury N, Benasich AA. The influence of family history and other risk factors on language development. Journal of Speech, Language and Hearing Research. 2003;46:261–272. doi: 10.1044/1092-4388(2003/021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clahsen H. The grammatical characterization of developmental dysphasia. Linguistics. 1992;27:897–920. [Google Scholar]
- Colombo J. Infant cognition: Predicting later intellectual functioning. Newbury Park, CA: Sage Publications; 1993. [Google Scholar]
- Colombo J. Infant attention grows up: the emergence of a developmental cognitive neuroscience perspective. Current Directions in Psychological Science. 2002;11 (6):196–200. [Google Scholar]
- Colombo J, Bundy RS. Infant response to auditory familiarity and novelty. Infant Behavior and Development. 1983;6 (3):305–311. [Google Scholar]
- Colombo J, Mitchell DW. Infant visual habituation: in defense of an information processing analysis. European Bulletin of Cognitive Psychology. 1988;8:455–461. [Google Scholar]
- Colombo J, Mitchell DW. Individual differences in early visual attention: fixation time and information processing. In: Colombo J, Fagen JW, editors. Individual differences in infancy: Reliability, stability, prediction. Hillsdale, NJ: Lawrence Erlbaum Associates; 1990. pp. 193–227. [Google Scholar]
- Colombo J, Shaddy DJ, Richman WA, Maikranz JM, Blaga OM. The developmental course of habituation in infancy and preschool outcome. Infancy. 2004;5:1–38. [Google Scholar]
- deRegnier RO, Georgieff MK, Nelson CA. Visual event-related brain potentials in 4-month-old infants at risk for neurodevelopmental impairments. Developmental Psychobiology. 1997;30 (1):11–28. doi: 10.1002/(sici)1098-2302(199701)30:1<11::aid-dev2>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- Diamond A. The development and neural basis of memory functions as indexed by the A-not-B and delayed response tasks in human infants and infant monkeys. In: Diamond A, editor. The development and neural bases of higher cognitive functions. New York: Academy of Science Press; 1990. pp. 267–317. [DOI] [PubMed] [Google Scholar]
- Diamond A, Goldman-Rakic P. Comparison of human infants and rhesus monkeys on Piaget’s A-not-B task: evidence for dependence on dorso-lateral prefrontal cortex. Experimental Brain Research. 1989;74:24–40. doi: 10.1007/BF00248277. [DOI] [PubMed] [Google Scholar]
- Dollaghan C, Campbell TF. Nonword repetition and child language impairment. Journal of Speech, Language and Hearing Research. 1998;41:1136–1146. doi: 10.1044/jslhr.4105.1136. [DOI] [PubMed] [Google Scholar]
- Durston S, Thomas KM, Yang Y, Ulug AM, Zimmerman RD, Casey BJ. A neural basis for the development of inhibitory control. Developmental Science. 2002;5 (4):F9–F16. [Google Scholar]
- Durston S, Tottenham NT, Thomas KM, Davidson MC, Eigsti IM, Yang Y, Ulug AM, Casey BJ. Differential patterns of striatal activation in young children with and without ADHD. Biological Psychiatry. 2003;53 (10):871–878. doi: 10.1016/s0006-3223(02)01904-2. [DOI] [PubMed] [Google Scholar]
- Eilers RE, Morse PA, Gavin WJ, Oller DK. Discrimination of voice onset time in infancy. Journal of the Acoustical Society of America. 1981;70:955–965. [Google Scholar]
- Elbro C. Differences in dyslexia: A study of reading strategies and deficits in a linguistic perspective. Copenhagen: Munksgaard International Publishers; 1990. [Google Scholar]
- Elbro C. Early linguistic abilities and reading development: a review and a hypothesis. Reading and Writing. 1996;8 (6):453–485. [Google Scholar]
- Elbro C. When reading is ‘readn’ or ‘somthn’: ‘distinctnes’ of phonological representations of lexical items in normal and disabled readers. Scandinavian Journal of Psychology. 1998;39 (3):149–153. doi: 10.1111/1467-9450.393070. [DOI] [PubMed] [Google Scholar]
- Elliott LL, Hammer MA. Longitudinal changes in auditory discrimination in normal children and children with language-learning problems. Journal of Speech and Hearing Disorders. 1988;53:467–474. doi: 10.1044/jshd.5304.467. [DOI] [PubMed] [Google Scholar]
- Elliott LL, Hammer MA, Scholl ME. Fine-grained auditory discrimination in normal children and children with language-learning problems. Journal of Speech and Hearing Research. 1989;32:112–119. doi: 10.1044/jshr.3201.112. [DOI] [PubMed] [Google Scholar]
- Fagan JF. The paired-comparison paradigm and infant intelligence. Annals of the New York Academy of Sciences. 1990;608:337–364. doi: 10.1111/j.1749-6632.1990.tb48902.x. [DOI] [PubMed] [Google Scholar]
- Fagan JF. The Fagan Test of Infant Intelligence. Cleveland, OH: Infantest Corporation; 1991. [Google Scholar]
- Fagan JF, Detterman DK. The Fagan Test of Infant Intelligence: a technical summary. Journal of Applied Developmental Psychology. 1992;13 (2):173–193. [Google Scholar]
- Farmer ME, Klein R. The evidence for a temporal processing deficit linked to dyslexia: a review. Psychonomic Bulletin Review. 1995;2 (4):460–493. doi: 10.3758/BF03210983. [DOI] [PubMed] [Google Scholar]
- Fenson LS, Dale P, Reznick JS, Thal D, Bates E, Hartung JP, Pethick S, Reilly JS. Technical manual for the MacArthur Communicative Development Inventory. San Diego, CA: Singular Press; 1993. [Google Scholar]
- Fiducia D, O’Leary DS. Development of behavior attributed to the frontal lobes and the relationship to other cognitive functions. Developmental Neuropsychology. 1990;6 (2):85–94. [Google Scholar]
- Fitch RH, Miller S, Tallal P. Neurobiology of speech perception. Annual Review of Neuroscience. 1997;20:331–353. doi: 10.1146/annurev.neuro.20.1.331. [DOI] [PubMed] [Google Scholar]
- Fitch RH, Tallal P. Neural mechanisms of language-based learning impairments: insights from human populations and animal models. Behavioral and Cognitive Neuroscience Review. 2003;2:155–178. doi: 10.1177/1534582303258736. [DOI] [PubMed] [Google Scholar]
- Flax JF, Realpe-Bonilla T, Hirsch LS, Brzustowicz LM, Bartlett CW, Tallal P. Specific language impairment in families: evidence for co-occurrence with reading impairments. Journal of Speech, Language and Hearing Research. 2003;46:530–543. doi: 10.1044/1092-4388(2003/043). [DOI] [PubMed] [Google Scholar]
- Gathercole SE, Baddeley AD. Phonological memory deficits in language disordered children: is there a causal connection? Journal of Memory and Language. 1990;29:336–360. [Google Scholar]
- Gillam RB, Hoffman LM. Information processing and language learning in children with specific language impairment. In: Verhoeven L, van Balkom H, editors. Classification of developmental language disorders: Theoretical issues and clinical implications. Hove, UK: Lawrence Erlbaum; 2004. pp. 137–157. [Google Scholar]
- Godfrey JJ, Syrdal-Lasky AK, Millay KK, Knox CM. Performance of dyslexic children on speech perception tests. Journal of Experimental Child Psychology. 1981;32:401–424. doi: 10.1016/0022-0965(81)90105-3. [DOI] [PubMed] [Google Scholar]
- Goldman DZ, Shapiro EG, Nelson CA. Measurement of vigilance in 2-year-old children. Developmental Neuropsychology. 2004;25 (3):227–250. doi: 10.1207/s15326942dn2503_1. [DOI] [PubMed] [Google Scholar]
- Goldman PS, Rosvold H. The effects of selective caudate lesions in infant and juvenile rhesus monkeys. Brain Research. 1972;43 (1):53–66. doi: 10.1016/0006-8993(72)90274-0. [DOI] [PubMed] [Google Scholar]
- Goldman PS, Rosvold HE, Vest B, Galkin TW. Analysis of the delayed-alternation deficit produced by dorso-lateral pre-frontal lesions in the rhesus monkey. Journal of Comparative Physiology and Psychology. 1971;77:212–220. doi: 10.1037/h0031649. [DOI] [PubMed] [Google Scholar]
- Goldman-Rakic PS. Development of cortical circuitry cognitive function. Child Development. 1987a;58:601–622. [PubMed] [Google Scholar]
- Goldman-Rakic PS. Circuitry of primate prefrontal cortex and regulation of behavior by representational memory. In: Plum F, editor. Handbook of physiology: The nervous system: Section 1. Higher functions of the brain Part 1. Vol. 5. Bethesda: American Physiological Society; 1987b. pp. 373–417. [Google Scholar]
- Goldman-Rakic PS, Isseroff A, Schwartz ML, Bugbee NM. The neurobiology of cognitive development. In: Mussen P, editor. Handbook of child psychology: Biology and infancy development. Vol. 2. New York: Wiley; 1983. pp. 281–344. [Google Scholar]
- Gopnik M, Crago M. Familial aggregation of a developmental language disorder. Cognition. 1991;39:1–50. doi: 10.1016/0010-0277(91)90058-c. [DOI] [PubMed] [Google Scholar]
- Green DM, Swets JA. Signal detection theory and psychophysics. New York: Wiley; 1966. [Google Scholar]
- Habib M. Rewiring the dyslexic brain. Trends in Cognitive Sciences. 2003;7:330–333. doi: 10.1016/s1364-6613(03)00164-5. [DOI] [PubMed] [Google Scholar]
- Hari R, Renvall H. Impaired processing of rapid stimulus sequences in dyslexia. Trends in Cognitive Sciences. 2001;5:525–532. doi: 10.1016/s1364-6613(00)01801-5. [DOI] [PubMed] [Google Scholar]
- Hart B, Risely TR. Meaningful differences in the everyday experience of young American children. Baltimore, MD: Brookes; 1995. [Google Scholar]
- Hoffman LM, Gillam RB. Verbal and spatial information processing constraints in children with and without specific language impairment. Journal of Speech, Language and Hearing Research. 2004;47 (1):114–125. doi: 10.1044/1092-4388(2004/011). [DOI] [PubMed] [Google Scholar]
- Hollingshead AB. The Four-Factor Index of Social Status. Department of Sociology; Yale University, New Haven, CT 06520: 1975. [Google Scholar]
- Honey RC, Good M. Associative components of recognition memory. Current Opinion in Neurobiology. 2000;10:200–204. doi: 10.1016/s0959-4388(00)00069-6. [DOI] [PubMed] [Google Scholar]
- Irwin RJ, Ball AKR, Kay N, Stillman JA, Rosser J. The development of auditory temporal acuity in children. Child Development. 1985;56:614–620. [PubMed] [Google Scholar]
- Isseroff A, Rosvold HE, Galkin TW, Goldman-Rakic PS. Spatial memory impairments following damage to the mediodorsal nucleus of the thalamus in rhesus monkeys. Brain Research. 1982;232 (1):97–113. doi: 10.1016/0006-8993(82)90613-8. [DOI] [PubMed] [Google Scholar]
- Joanisse MF, Manis FR, Keating P, Seidenberg MS. Language deficits in dyslexic children: speech perception, phonology, and morphology. Journal of Experimental Child Psychology. 2000;77:30–60. doi: 10.1006/jecp.1999.2553. [DOI] [PubMed] [Google Scholar]
- Joanisse MF, Seidenberg MS. Specific language impairment in children: an impairment in grammar or processing? Trends in Cognitive Sciences. 1998;2 (7):240–246. doi: 10.1016/S1364-6613(98)01186-3. [DOI] [PubMed] [Google Scholar]
- Johnston R, Stark R, Mellits E, Tallal P. Neurological status of language-impaired and normal children. Annals of Neurology. 1981;10:159–163. doi: 10.1002/ana.410100206. [DOI] [PubMed] [Google Scholar]
- Jusczyk PW, Pisoni DB, Walley A, Murray J. Discrimination of relative onset time of two-component tones by infants. Journal of the Acoustical Society of America. 1980;67:262–270. doi: 10.1121/1.383735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karmiloff-Smith A. Development itself is the key to understanding developmental disorders. Trends in Cognitive Sciences. 1998;2:389–398. doi: 10.1016/s1364-6613(98)01230-3. [DOI] [PubMed] [Google Scholar]
- Kavsek M. Predicting later IQ from infant visual habituation and dishabituation: a meta-analysis. Applied Developmental Psychology. 2004;25:369–393. [Google Scholar]
- Kraus N, McGee TJ, Carrell TD, Zecker SG, Nicol TG, Koch DB. Auditory neurophysiologic responses and discrimination deficits in children with learning problems. Science. 1996;273:971–973. doi: 10.1126/science.273.5277.971. [DOI] [PubMed] [Google Scholar]
- Kuhl PK. Methods in the study of infant speech perception. In: Gottlieb G, Krasnegor NA, editors. Measurement of audition and vision in the first year of postnatal life: A methodological overview. Norwood, NJ: Ablex Publishing Company; 1985. pp. 233–251. [Google Scholar]
- Lawrence NS, Ross TJ, Hoffmann R, Garavan H, Stein EA. Multiple neural networks mediate sustained attention. Journal of Cognitive Neuroscience. 2003;15 (7):1028–1038. doi: 10.1162/089892903770007416. [DOI] [PubMed] [Google Scholar]
- Liberman AM. Speech: A special code. Cambridge, MA: MIT Press; 1996. [Google Scholar]
- Leonard LB. Children with specific language impairment. Cambridge, MA: The MIT Press; 1998. [Google Scholar]
- Lewkowicz DJ. Infants’ perception of the audible, visible, and bimodal attributes of multimodal syllables. Child Development. 2000;71 (5):1241–1257. doi: 10.1111/1467-8624.00226. [DOI] [PubMed] [Google Scholar]
- Lewkowicz DJ. Learning and discrimination of audiovisual events in human infants: the hierarchical relation between intersensory temporal synchrony and rhythmic pattern cues. Developmental Psychology. 2003;39 (5):795–804. doi: 10.1037/0012-1649.39.5.795. [DOI] [PubMed] [Google Scholar]
- Lewkowicz DJ, Turkewitz G. Cross-modal equivalence in early infancy: auditory-visual intensity matching. Developmental Psychology. 1980;16 (6):597–607. [Google Scholar]
- Lewkowicz DJ, Turkewitz G. Intersensory interaction in newborns: modification of visual preferences following exposure to sound. Child Development. 1981;52 (3):827–832. [PubMed] [Google Scholar]
- Livingstone MS, Rosen GD, Drislane FW, Galaburda AM. Physiological and anatomical evidence for a magnocellular defect in developmental dyslexia. Proceedings of the National Academy of Sciences. 1991 doi: 10.1073/pnas.88.18.7943. [Published erratum appears in Proceedings of the National Academy of Sciences USA, 1993, 90, 2556.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luciana M, Nelson CA. Assessment of neuropsychological function through use of the Cambridge Neuropsychological Testing Automated Battery: performance in 4- to 12-year-old children. Developmental Neuropsychology. 2002;22 (3):595–624. doi: 10.1207/S15326942DN2203_3. [DOI] [PubMed] [Google Scholar]
- Luria AR. Higher cortical functions in man. rev. New York: Basic Books; 1980. [Google Scholar]
- McAnally KI, Stein JF. Auditory temporal coding in dyslexia. Proceedings of the Royal Society of London. 1996;263:961–965. doi: 10.1098/rspb.1996.0142. [DOI] [PubMed] [Google Scholar]
- McAnally KI, Stein JF. Scalp potentials evoked by amplitude-modulated tones in dyslexia. Journal of Speech, Language and Hearing Research. 1997;40 (4):939–945. doi: 10.1044/jslhr.4004.939. [DOI] [PubMed] [Google Scholar]
- McCall RB. What process mediates predictions of childhood IQ from infant habituation and recognition memory? Speculations on the roles of inhibition and rate of information processing. Intelligence. 1994;18 (2):107–125. [Google Scholar]
- McCall RB, Carriger MS. A meta-analysis of infant habituation and recognition memory performance as predictors of later IQ. Child Development. 1993;64:57–79. [PubMed] [Google Scholar]
- McCall RB, Mash CW. Infant cognition and its relation to mature intelligence. In: Vasta R, editor. Annals of child development: A research annual. Vol. 10. London: Jessica Kingsley; 1995. pp. 27–56. [Google Scholar]
- McCroskey RL, Kidder HC. Auditory fusion among learning disabled, reading disabled, and normal children. Journal of Learning Disabilities. 1980;13 (2):69–76. doi: 10.1177/002221948001300205. [DOI] [PubMed] [Google Scholar]
- Malcuit G, Pomerleau A, Lamarre G. Habituation, visual fixation and cognitive activity in infants: a critical analysis and attempt at a new formulation. European Bulletin of Cognitive Psychology. 1988;8:415–440. [Google Scholar]
- Mannix M, Yue N, Choudhury N, Badridze N, Benasich AA. Self-regulation and maternal behavior as antecedents of performance on visual habituation and recognition memory tasks; Biennial meeting of the International Society for Infancy Studies; Chicago, IL. 2004. [Google Scholar]
- Maye J, Werker J, Gerken L. Infant sensitivity to distributional information can affect phonetic discrimination. Cognition. 2002;82 (3):B101–B111. doi: 10.1016/s0010-0277(01)00157-3. [DOI] [PubMed] [Google Scholar]
- Mesulam MM. Large-scale neurocognitive networks and distributed processing for attention, language, and memory. Annals of Neurology. 1990;28 (5):597–613. doi: 10.1002/ana.410280502. [DOI] [PubMed] [Google Scholar]
- Milner B. Interhemispheric differences in the localization of psychological processes in man. British Medical Bulletin. 1971;27:272–277. doi: 10.1093/oxfordjournals.bmb.a070866. [DOI] [PubMed] [Google Scholar]
- Mishkin M. Preservation of central sets after frontal lesions in monkeys. In: Warren JM, Akert K, editors. The frontal granular cortex and behavior. New York: McGraw-Hill; 1964. pp. 219–241. [Google Scholar]
- Morrongiello BA. The study of individual differences in infants: auditory processing measures. In: Colombo J, Fagen J, editors. Individual differences in infancy: Reliability, stability, prediction. Hillsdale, NJ: Lawrence Erlbaum Associates; 1990. pp. 271–320. [Google Scholar]
- Morrongiello BA, Trehub SE. Age-related changes in auditory temporal perception. Journal of Experimental Child Psychology. 1987;44:413–426. doi: 10.1016/0022-0965(87)90043-9. [DOI] [PubMed] [Google Scholar]
- Nagarajan S, Mahncke H, Salz T, Tallal P, Roberts T, Merzenich MM. Proceedings of the National Academy of Sciences USA. 1999. Cortical auditory signal processing in poor readers; pp. 6483–6488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson CA. The ontogeny of human memory: a cognitive neuroscience perspective. Developmental Psychology. 1995;31 (5):723–738. [Google Scholar]
- Nelson CA. The ontogeny of human memory: a cognitive neuroscience perspective. In: Johnson MH, Munakata Y, Gilmore R, editors. Brain development and cognition: A reader. 2. Malden, MA: Blackwell Publishers; 2002. pp. 151–178. [Google Scholar]
- Nelson CA, Collins PF. Event-related potential and looking-time analysis of infants’ responses to familiar and novel events: implications for visual recognition memory. Developmental Psychology. 1991;27 (1):50–58. [Google Scholar]
- Nelson CA, Collins PF. Neural and behavioral correlates of visual recognition memory in 4- and 8-month-old infants. Brain and Cognition. 1992;19 (1):105–121. doi: 10.1016/0278-2626(92)90039-o. [DOI] [PubMed] [Google Scholar]
- Nelson CA, deRegnier R. Neural correlates of attention and memory in the first year of life. Developmental Neuropsychology. 1992;8 (2–3):119–134. [Google Scholar]
- Nelson CA, Dukette D. A cognitive neuroscience perspective on the relation between attention and memory development. In: Richards JE, editor. Cognitive neuroscience of attention: A developmental perspective. Mahwah, NJ: Lawrence Erlbaum Associates; 1998. pp. 327–362. [Google Scholar]
- Nelson CA, Salapatek P. Electrophysiological correlates of infant recognition memory. Child Development. 1986;57 (6):1483–1497. [PubMed] [Google Scholar]
- Nicolson RI, Fawcett AJ, Dean P. Developmental dyslexia: the cerebellar deficit hypothesis. Trends in Neurosciences. 2001;24:508–511. doi: 10.1016/s0166-2236(00)01896-8. [DOI] [PubMed] [Google Scholar]
- Pascalis O, Bachevalier J. Neonatal aspiration lesions of the hippocampal formation impair visual recognition memory when assessed by paired-comparison task but not by delayed nonmatching-to-sample task. Hippocampus. 1999;9:609–616. doi: 10.1002/(SICI)1098-1063(1999)9:6<609::AID-HIPO1>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- Parasuraman R, Warm JS, See JE. Brain systems of vigilance. In: Parasuraman R, editor. The attentive brain. Cambridge, MA: The MIT Press; 1998. pp. 221–256. [Google Scholar]
- Paus T, Zatorre RJ, Hofle N, Caramanos Z, Gottman J, Petrides M, Evans AC. Time-related changes in neural systems underlying attention and arousal during the performance of an auditory vigilance task. Journal of Cognitive Neuroscience. 1997;9 (3):392–408. doi: 10.1162/jocn.1997.9.3.392. [DOI] [PubMed] [Google Scholar]
- Posner MI, Petersen SE. The attention system of the human brain. Annual Review of Neuroscience. 1990;13:25–42. doi: 10.1146/annurev.ne.13.030190.000325. [DOI] [PubMed] [Google Scholar]
- Powell RP, Bishop DV. Clumsiness and perceptual problems in children with specific language impairment. Developmental Medicine and Child Neurology. 1992;34 (9):755–765. doi: 10.1111/j.1469-8749.1992.tb11514.x. [DOI] [PubMed] [Google Scholar]
- Ramus F. Developmental dyslexia: specific phonological deficit or general sensorimotor dysfunction? Current Opinion in Neurobiology. 2003;13 (2):212–218. doi: 10.1016/s0959-4388(03)00035-7. [DOI] [PubMed] [Google Scholar]
- Reed MA. Speech perception and the discrimination of brief auditory cues in reading disabled children. Journal of Experimental Child Psychology. 1989;48:270–292. doi: 10.1016/0022-0965(89)90006-4. [DOI] [PubMed] [Google Scholar]
- Richards JE. Attention affects the recognition of briefly presented visual stimuli in infants: an ERP study. Developmental Science. 2003;6 (3):312–328. doi: 10.1111/1467-7687.00287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rice ML, Wexler KJ. Tense over time: the persistence of optional infinitives in English in children with SLI. In: Stringfellow A, Cahana-Amitay D, Hughes E, Zukowski A, editors. Proceedings of the 20th Annual Boston University Conference on Language Development. Somerville, MA: Cascadilla Press; 1996. [Google Scholar]
- Rissman M, Curtiss S, Tallal P. School placement outcomes of young language impaired children. Journal of Speech-Language Pathology and Audiology. 1990;14 (2):49–58. [Google Scholar]
- Robinson RJ. Causes and associations of severe and persistent speech and language disorders in children. Developmental Medicine and Child Neurology. 1991;33:943–962. doi: 10.1111/j.1469-8749.1991.tb14811.x. [DOI] [PubMed] [Google Scholar]
- Rose SA. Lags in the cognitive competence of prematurely born infants. In: Friedman SL, Sigman M, editors. Preterm birth and psychological development. New York: Academic Press; 1981. pp. 255–269. [Google Scholar]
- Rose SA. Differential rates of visual information processing in fullterm and preterm infants. Child Development. 1983;54:1189–1198. [PubMed] [Google Scholar]
- Rose SA, Feldman JF. Prediction of IQ and specific cognitive abilities at 11 years from infancy measures. Developmental Psychology. 1995;31:685–696. [Google Scholar]
- Rose SA, Feldman JF. Memory and processing speed in preterm children at eleven years: a comparison with full-terms. Child Development. 1996;67:2005–2021. [PubMed] [Google Scholar]
- Rose SA, Feldman JF. Memory and speed: their role in the relation of infant information processing to later IQ. Child Development. 1997;68:630–641. [PubMed] [Google Scholar]
- Rose SA, Feldman JF, Jankowski JJ. Infant visual recognition memory: independent contributions of speed and attention. Developmental Psychology. 2003;39 (3):563–571. doi: 10.1037/0012-1649.39.3.563. [DOI] [PubMed] [Google Scholar]
- Rose SA, Feldman JF, Jankowski JJ. Infant visual recognition memory. Developmental Review. 2004a;24 (1):74–100. doi: 10.1037/0012-1649.39.3.563. [DOI] [PubMed] [Google Scholar]
- Rose SA, Feldman JF, Jankowski JJ. Dimensions of cognition in infancy. Intelligence. 2004b;32 (3):245–262. [Google Scholar]
- Rose SA, Feldman JF, Wallace IF, Cohen P. Language: a partial link between infant attention and later intelligence. Developmental Psychology. 1991a;27:798–805. [Google Scholar]
- Rose SA, Feldman JF, Wallace IF, McCarton C. Information processing at 1 year: relation to birth status and developmental outcome during the first 5 years. Developmental Psychology. 1991b;27:723–737. [Google Scholar]
- Rose SA, Feldman JF, McCarton CM, Wolfson J. Information processing in seven-month-old infants as a function of risk status. Child Development. 1988;59:589–603. [PubMed] [Google Scholar]
- Rose SA, Feldman JF, Wallace IF. Infant information processing in relation to six-year cognitive outcomes. Child Development. 1992;63:1126–1141. [PubMed] [Google Scholar]
- Rose SA, Tamis-LeMonda CS. Visual information processing in infancy: reflections on underlying mechanisms. In: Balter L, Tamis-LeMonda CS, editors. Child psychology: A handbook of contemporary issues. Philadelphia, PA: Psychology Press/Taylor & Francis; 1999. pp. 64–84. [Google Scholar]
- Rosen S. Auditory processing in dyslexia and specific language impairment: is there a deficit? What is its nature? Does it explain anything? Journal of Phonetics. 2003;31:509–527. [Google Scholar]
- Saffran JR, Aslin RN, Newport EL. Statistical learning by 8-month old infants. Science. 1996;274:1926–1928. doi: 10.1126/science.274.5294.1926. [DOI] [PubMed] [Google Scholar]
- Scarborough HS. Very early language deficits in dyslexic children. Child Development. 1990;61:1728–1743. [PubMed] [Google Scholar]
- Schneider BA, Trehub SE. Infant auditory psychophysics: an overview. In: Gottlieb G, Krasnegor NA, editors. Measurement of audition and vision in the first year of postnatal life: A methodological overview. Norwood, NJ: Ablex Publishing Corporation; 1985. pp. 113–124. [Google Scholar]
- Schul R, Stiles J, Wulfeck B, Townsend J. How ‘generalized’ is the ‘slowed processing’ in SLI? The case of visuospatial attentional orienting. Neuropsychologia. 2004;42:661–671. doi: 10.1016/j.neuropsychologia.2003.10.010. [DOI] [PubMed] [Google Scholar]
- Sigman M, Cohen SE, Beckwith L. Why does infant attention predict adolescent intelligence? Infant Behavior and Development. 1997;20:133–140. [Google Scholar]
- Sigman M, Cohen SE, Beckwith L, Asarnow R, Parmelee AH. Continuity in cognitive abilities from infancy to 12 years of age. Cognitive Development. 1991;6:47–57. [Google Scholar]
- Snow CE, Burns MS, Griffin P. Preventing reading difficulties in young children. Washington, DC: National Academy Press; 1998. [Google Scholar]
- Snyder K, Webb SJ, Nelson CA. Theoretical and methodological implications of variability in infant brain response during a recognition memory paradigm. Infant Behavior and Development. 2002;25 (4):466–494. [Google Scholar]
- Sokolov EN. Perception and the conditioned reflex. New York: Macmillan; 1963. [Google Scholar]
- Spitz RV, Tallal P, Flax JF, Benasich AA. Look who’s talking: a prospective study of familial transmission of language impairments. Journal of Speech and Hearing Research. 1997;40:990–1001. doi: 10.1044/jslhr.4005.990. [DOI] [PubMed] [Google Scholar]
- Stark RE, Bernstein LE, Condino R, Bender M, Tallal P, Catts H. Four-year follow-up study of language impaired children. Annals of Dyslexia. 1984;34:49–68. doi: 10.1007/BF02663613. [DOI] [PubMed] [Google Scholar]
- Stark RE, Heinz JM. Perception of stop consonants in children with expressive and receptive-expressive language impairments. Journal of Speech and Hearing Research. 1996;39:676–686. doi: 10.1044/jshr.3904.676. [DOI] [PubMed] [Google Scholar]
- Stark RE, Tallal P. Selection of children with specific language deficits. Journal of Speech and Hearing Disorders. 1981;46 (2):114–122. doi: 10.1044/jshd.4602.114. [DOI] [PubMed] [Google Scholar]
- Stein J. The neurobiology of developmental dyslexia. Ottawa, ON: Canadian Dyslexia Association; 1999. [Google Scholar]
- Stein J, Talcott J. Impaired neuronal timing in developmental dyslexia – the magnocelluar hypothesis. Dyslexia: The Journal of the British Dyslexia Association. 1999;5 (2):59–77. [Google Scholar]
- Stoodley CJ, Talcott JB, Carter EL, Witton C, Stein JF. Selective deficits of vibrotactile sensitivity in dyslexic readers. Neuroscience Letters. 2000;295:13–16. doi: 10.1016/s0304-3940(00)01574-3. [DOI] [PubMed] [Google Scholar]
- Sussman JE. Perception of formant transition cues to place of articulation in children with language impairments. Journal of Speech and Hearing Research. 1993;36:1286–1299. doi: 10.1044/jshr.3606.1286. [DOI] [PubMed] [Google Scholar]
- Talcott JB, Hansen PC, Assoku EL, Stein JF. Visual motion sensitivity in dyslexia: evidence for temporal and energy integration deficits. Neuropsychologia. 2000;38 (7):935–943. doi: 10.1016/s0028-3932(00)00020-8. [DOI] [PubMed] [Google Scholar]
- Tallal P. Developmental language disorders. In: Kavanagh J, Tarkton TT, editors. Proceedings of the National Conference on Learning Disabilities. Parkton, MD: York Press; 1988. pp. 181–272. [Google Scholar]
- Tallal P. Experimental studies of language learning impairments: from research to remediation. In: Bishop DM, Leonard LB, editors. Speech and language impairments in children: Causes, characteristics, intervention and outcome. Philadelphia, PA: Psychology Press; 2000. pp. 131–156. [Google Scholar]
- Tallal P. Improving language and literacy is a matter of time. Nature Reviews Neuroscience. 2004;5 (9):721–728. doi: 10.1038/nrn1499. [DOI] [PubMed] [Google Scholar]
- Tallal P, Benasich AA. Developmental language learning impairments. Development and Psychopathology. 2002;14:559–579. doi: 10.1017/s0954579402003097. [DOI] [PubMed] [Google Scholar]
- Tallal P, Hirsch LS, Realpe-Bonilla T, Miller S, Brzustowicz LM, Bartlett C, Flax JF. Familial aggregation in specific language impairment. Journal of Speech, Language and Hearing Research. 2001;44:1172–1182. doi: 10.1044/1092-4388(2001/091). [DOI] [PubMed] [Google Scholar]
- Tallal P, Miller S, Jenkins B, Merzenich M. The role of temporal processing in developmental language-based learning disorders: research and clinical implications. In: Blachman B, editor. Foundations of reading acquisition. Hillsdale, NJ: Lawrence Erlbaum Associates; 1997. pp. 49–66. [Google Scholar]
- Tallal P, Piercy M. Defects of non-verbal auditory perception in children with developmental aphasia. Nature. 1973a;241:468–469. doi: 10.1038/241468a0. [DOI] [PubMed] [Google Scholar]
- Tallal P, Piercy M. Developmental aphasia: impaired rate of non-verbal processing as a function of sensory modality. Neuropsychologia. 1973b;11:389–398. doi: 10.1016/0028-3932(73)90025-0. [DOI] [PubMed] [Google Scholar]
- Tallal P, Piercy M. Developmental aphasia: rate of auditory processing and selective impairment of consonant perception. Neuropsychologia. 1974;12:83–93. doi: 10.1016/0028-3932(74)90030-x. [DOI] [PubMed] [Google Scholar]
- Tallal P, Piercy M. Developmental aphasia: the perception of brief vowels and extended stop consonants. Neuropsychologia. 1975;13:69–74. doi: 10.1016/0028-3932(75)90049-4. [DOI] [PubMed] [Google Scholar]
- Tallal P, Stark RE. Speech acoustic-cue discrimination abilities of normally developing and language-impaired children. Journal of the Acoustical Society of America. 1981;69 (2):568–574. doi: 10.1121/1.385431. [DOI] [PubMed] [Google Scholar]
- Tallal P, Stark RE, Mellitis D. Identification of language impaired children on the basis of rapid perception and production skills. Brain and Language. 1985a;25:314–322. doi: 10.1016/0093-934x(85)90087-2. [DOI] [PubMed] [Google Scholar]
- Tallal P, Stark RE, Mellitis D. Relationship between auditory temporal analysis and receptive language development: evidence from studies of developmental language disorders. Neuropsychologia. 1985b;23:527–536. doi: 10.1016/0028-3932(85)90006-5. [DOI] [PubMed] [Google Scholar]
- Tallal P, Stark RE, Kallman C, Mellits D. Perceptual constancy for phonemic categories: a developmental study with normal and language impaired children. Applied Psycholinguistics. 1980a;1:49–64. [Google Scholar]
- Tallal P, Stark RE, Kallman C, Mellits D. Developmental dysphasia: relation between acoustic processing deficits and verbal processing. Neuropsychologia. 1980b;18:273–284. doi: 10.1016/0028-3932(80)90123-2. [DOI] [PubMed] [Google Scholar]
- Tomblin JB, Records NL, Buckwalter P, Zhang X, Smith E, O’Brien M. Prevalence of specific language impairment in kindergarten children. Journal of Speech and Hearing Research. 1997;39:1315–1320. doi: 10.1044/jslhr.4006.1245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomblin JB, Zhang X, Buckwalter P, Catts H. The association of reading disability, behavioral disorders, and language impairment among second-grade children. Journal of Child Psychology and Psychiatry. 2000;41:473–482. [PubMed] [Google Scholar]
- Trehub SE. Temporal resolution in infancy and subsequent language development. Journal of Speech and Hearing Research. 1996;39 (6):1315–1320. doi: 10.1044/jshr.3906.1315. [DOI] [PubMed] [Google Scholar]
- Trehub SE, Schneider BA, Henderson JL. Gap detection in infants, children, and adults. Journal of the Acoustical Society of America. 1995;98:2532–2541. doi: 10.1121/1.414396. [DOI] [PubMed] [Google Scholar]
- Viholainen H, Ahonen T, Cantell M, Lyytinen P, Lyytinen H. Development of early motor skills and language in children at risk for familial dyslexia. Developmental Medicine and Child Neurology. 2002;44:761–769. doi: 10.1017/s0012162201002894. [DOI] [PubMed] [Google Scholar]
- Wagner RK, Torgesen JK. The nature of phonological processing and its causal role in the acquisition of reading skills. Psychological Bulletin. 1987;101:192–212. [Google Scholar]
- Webster RI, Shevell MI. Neurobiology of specific language impairment. Journal of Child Neurology. 2004;19 (7):471–478. doi: 10.1177/08830738040190070101. [DOI] [PubMed] [Google Scholar]
- Werker JF, Shi R, Desjardins R, Pegg JE, Polka L, Patterson M. Three methods for testing infant speech perception. In: Slater A, editor. Perceptual development: Visual auditory and speech perception in infancy. Hove, England: Psychology Press; 1998. pp. 389–421. [Google Scholar]
- Werker JF, Tees RC. Speech perception in severely disabled and average reading children. Canadian Journal of Psychology. 1987;41:48–61. doi: 10.1037/h0084150. [DOI] [PubMed] [Google Scholar]
- Whitehurst GJ, Fischel JE. Practitioner review: Early developmental language delay: what, if anything, should the clinician do about it? Journal of Child Psychology and Psychiatry. 1994;35:613–648. doi: 10.1111/j.1469-7610.1994.tb01210.x. [DOI] [PubMed] [Google Scholar]
- Witton C, Stein JF, Stoodley CJ, Rosner BS, Talcott JB. Separate influences of acoustic AM and FM sensitivity on the phonological decoding skills of impaired and normal readers. Journal of Cognitive Neuroscience. 2002;14:866–874. doi: 10.1162/089892902760191090. [DOI] [PubMed] [Google Scholar]
- Witton C, Talcott JB, Hansen PC, Richardson AJ, Griffiths TD, Rees A, Stein JF, Green GGR. Sensitivity to dynamic auditory and visual stimuli predicts nonword reading ability in both dyslexic and normal readers. Current Biology. 1998;8:791–797. doi: 10.1016/s0960-9822(98)70320-3. [DOI] [PubMed] [Google Scholar]
- Wright BA, Lombardino LJ, King WM, Puranik CS, Leonard CM, Merzenich MM. Deficits in auditory temporal and spectral resolution in language-impaired children. Nature. 1997;387:176–178. doi: 10.1038/387176a0. [DOI] [PubMed] [Google Scholar]
- Zimmerman IL, Steiner VG, Pond RE. Preschool Language Scale-3. New York: The Psychological Corporation; 1992. [Google Scholar]


