Abstract
We examined the hypothesis that insulin resistance in skeletal muscle promotes the development of atherogenic dyslipidemia, associated with the metabolic syndrome, by altering the distribution pattern of postprandial energy storage. Following ingestion of two high carbohydrate mixed meals, net muscle glycogen synthesis was reduced by ≈60% in young, lean, insulin-resistant subjects compared with a similar cohort of age–weight–body mass index–activity-matched, insulin-sensitive, control subjects. In contrast, hepatic de novo lipogenesis and hepatic triglyceride synthesis were both increased by >2-fold in the insulin-resistant subjects. These changes were associated with a 60% increase in plasma triglyceride concentrations and an ≈20% reduction in plasma high-density lipoprotein concentrations but no differences in plasma concentrations of TNF-α, IL-6, adiponectin, resistin, retinol binding protein-4, or intraabdominal fat volume. These data demonstrate that insulin resistance in skeletal muscle, due to decreased muscle glycogen synthesis, can promote atherogenic dyslipidemia by changing the pattern of ingested carbohydrate away from skeletal muscle glycogen synthesis into hepatic de novo lipogenesis, resulting in an increase in plasma triglyceride concentrations and a reduction in plasma high-density lipoprotein concentrations. Furthermore, insulin resistance in these subjects was independent of changes in the plasma concentrations of TNF-α, IL-6, high-molecular-weight adiponectin, resistin, retinol binding protein-4, or intraabdominal obesity, suggesting that these factors do not play a primary role in causing insulin resistance in the early stages of the metabolic syndrome.
Keywords: type 2 diabetes, nonalcoholic fatty liver disease, adipocytokines, abdominal obesity, atherogenic dyslipidemia
The metabolic syndrome is characterized by a clustering of risk factors for cardiovascular disease that include insulin resistance, abdominal obesity, atherogenic dyslipidemia, hypertension, hyperuricemia, a prothrombotic state, and a proinflammatory state (1, 2). The metabolic syndrome is estimated to afflict >50 million Americans, and approximately half of all Americans are predisposed to it (2). Individuals with the metabolic syndrome are at increased risk for the development of coronary heart disease and other diseases related to plaque buildup in artery walls, such as stroke and peripheral vascular disease, as well as type 2 diabetes mellitus (T2DM).
Abdominal obesity and insulin resistance have each been hypothesized to be the primary factors underlying the metabolic syndrome; however, the biologic mechanisms linking these and other metabolic risk factors associated with the metabolic syndrome are not fully understood and appear to be complex.
In this study we examined the hypothesis that insulin resistance in skeletal muscle may promote the development of atherogenic dyslipidemia by diverting ingested carbohydrate away from muscle glycogen storage and into hepatic de novo lipogenesis, resulting in hypertriglyceridemia. To examine this hypothesis we assessed liver and muscle triglyceride synthesis by 1H magnetic resonance spectroscopy (MRS) and liver and muscle glycogen synthesis by 13C MRS in young, lean, healthy, insulin-resistant subjects and compared them to a group of age–weight–body mass index–activity-matched, insulin-sensitive subjects after ingestion of two high-carbohydrate mixed meals. In addition, hepatic de novo lipogenesis was assessed at the same time by monitoring the incorporation of deuterium from deuterium-labeled water into plasma triglycerides (3, 4). Because intraabdominal obesity has been postulated to be at the core of the metabolic derangements and directly responsible for the atherogenic dyslipidemia associated with the metabolic syndrome, we also measured intraabdominal fat content in these two groups of subjects by MRI (5–8).
The advantage of examining this question in healthy, young, lean, insulin-resistant individuals is that they have none of the other confounding factors that are typically associated with obesity and T2DM, which have been postulated to play a major role in causing the metabolic syndrome. Furthermore previous studies have demonstrated that insulin resistance in these subjects can mostly be attributed to defects in insulin-stimulated muscle glycogen synthesis caused by defects in insulin-stimulated glucose transport/phosphorylation activity (9–11). Therefore, the role of skeletal muscle insulin resistance per se in the pathogenesis of atherogenic dyslipidemia and the metabolic syndrome can be examined at its earliest stages.
Results
We screened ≈400 young, healthy, lean, sedentary subjects with an oral glucose tolerance test and assessed insulin sensitivity in them by using the insulin sensitivity index (ISI) (12). From this screening we identified 12 insulin-resistant [lowest ISI quartile (13)] subjects and 12 insulin-sensitive [highest ISI quartile (13)] subjects who were willing to undergo additional inpatient MRS/MRI/stable isotope studies to assess liver and muscle glycogen/triglyceride synthesis and hepatic de novo lipogenesis.
Despite having large differences in the ISI, the insulin-resistant and insulin-sensitive volunteers were similar in age, body mass index, lean body mass, fat mass, and monitored physical activity (Table 1). There also were no differences in systolic or diastolic blood pressure between the groups.
Table 1.
Subject characteristics
| Subject | ISI | Age, yr | BW, kg | Height, m | BMI, kg/m2 | FM, % | LBM, kg | AFV, ml | SBP, mmHg | DBP, mmHg | Activity, miles/day |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Insulin-sensitive | 7.87 ± 0.34 | 28 ± 2 | 67.1 ± 3.6 | 1.72 ± 0.03 | 22.6 ± 0.6 | 21.8 ± 2.3 | 52.8 ± 3.6 | 340 ± 91 | 113 ± 2 | 66 ± 2 | 4.83 ± 0.45 |
| Insulin-resistant | 2.80 ± 0.20 | 23 ± 1 | 69.6 ± 2.9 | 1.71 ± 0.03 | 23.9 ± 0.6 | 26.6 ± 2.3 | 51.5 ± 3.3 | 390 ± 92 | 110 ± 4 | 66 ± 2 | 3.89 ± 0.54 |
| P value | <0.0001 | NS | NS | NS | NS | NS | NS | NS | NS | NS | NS |
BW, body weight; BMI, body mass index; FM, fat mass; LBM, lean body mass; AVF, abdominal fat volume; SBP, systolic blood pressure (1 mmHg = 133 Pa); DBP, diastolic blood pressure; NS, not significant.
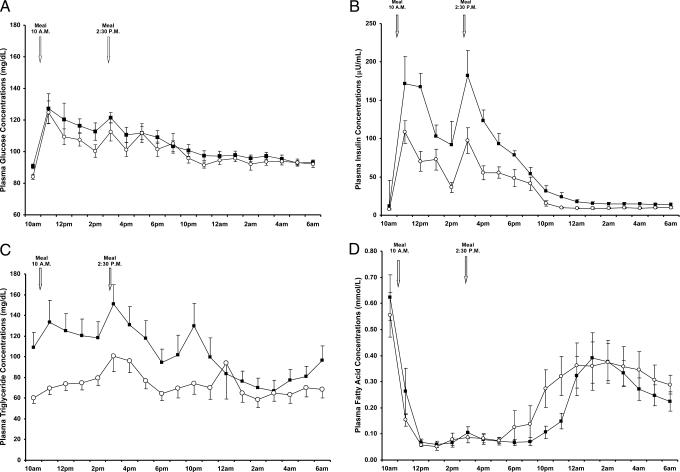
Fasting plasma glucose, triglyceride, and insulin concentrations were higher in the insulin-resistant subjects compared with the insulin-sensitive subjects (Table 2). After the mixed meals, postprandial plasma glucose concentrations were similar in the two groups (Fig. 1A). In contrast, postprandial plasma concentrations of insulin and triglycerides were markedly increased in the insulin-resistant subjects (Fig. 1 B and C). There were no differences in fasting or postprandial plasma fatty acid concentrations between the two groups, except from 10 to 11 p.m., when plasma fatty acid concentrations were lower in the insulin-resistant subjects compared with the insulin-sensitive subjects (Fig. 1D).
Table 2.
Fasting plasma metabolite and hormone concentrations
| Glucose, mg/dl | Insulin, microunits/ml | Triglyceride, mg/dl | Total cholesterol, mg/dl | LDL, mg/dl | HDL, mg/dl | VLDL SP, nmol/liter | LDL LP, nmol/liter | Uric acid, mg/dl | CRP, mg/liter | |
|---|---|---|---|---|---|---|---|---|---|---|
| Insulin-sensitive | 84.1 ± 1.7 | 7.6 ± 0.6 | 53 ± 7 | 182 ± 12 | 93 ± 9 | 78 ± 5 | 38.6 ± 4.0 | 446.4 ± 34.2 | 3.9 ± 0.3 | 0.47 ± 0.16 |
| Insulin-resistant | 90.6 ± 1.5 | 12.1 ± 1.2 | 86 ± 13 | 157 ± 5 | 77 ± 6 | 62 ± 3 | 23.8 ± 2.9 | 311.9 ± 46.0 | 5.2 ± 0.5 | 1.02 ± 0.24 |
| P value | 0.009 | 0.003 | 0.03 | NS | NS | 0.01 | 0.007 | 0.03 | 0.04 | 0.07 |
SP, small particles; LP, large particles; CRP, C-reactive protein; NS, not significant.
Fig. 1.
Plasma concentrations of glucose (A), insulin (B), triglyceride (C), and fatty acids (D) in insulin-sensitive (○) and insulin-resistant (■) participants before and after the mixed meals.
Fasting plasma high-density lipoprotein (HDL) cholesterol concentrations were 20% lower in the insulin-resistant subjects (Table 2), but there were no differences in total cholesterol or low-density lipoprotein (LDL) between the two groups. The concentrations of small very-low-density lipoprotein (VLDL) and large LDL particles were decreased by 38% and 30%, respectively, in the insulin resistant subjects (Table 2). Plasma concentrations of uric acid were increased by ≈30% in the insulin-resistant subjects compared with the insulin-sensitive control subjects (Table 2). In contrast, there were no differences in plasma concentrations of high-molecular-weight (HMW) adiponectin, IL-6, resistin, plasminogen activator inhibitor-1, retinol binding protein-4 (RBP-4), or TNF-α between the two groups (Table 3).
Table 3.
Fasting plasma adipocytokine concentrations
| HMW adiponectin, μg/ml | IL-6, pg/ml | Resistin, ng/ml | TNF-α, pg/ml | PAI-1, ng/ml | RBP-4, μg/ml | TTR, μg/ml | |
|---|---|---|---|---|---|---|---|
| Insulin-sensitive | 5.8 ± 2.5 | 0.91 ± 0.20 | 11.0 ± 1.1 | 1.49 ± 0.12 | 13.8 ± 3.9 | 29.5 ± 3.3 | 134 ± 8 |
| Insulin-resistant | 3.5 ± 0.7 | 1.31 ± 0.23 | 12.5 ± 0.9 | 1.49 ± 0.17 | 13.2 ± 2.5 | 28.1 ± 3.1 | 149 ± 11 |
| P value | NS | NS | NS | NS | NS | NS | NS |
PAI-1, plasminogen activator inhibitor-1; TTR, transthyretin; NS, not significant.
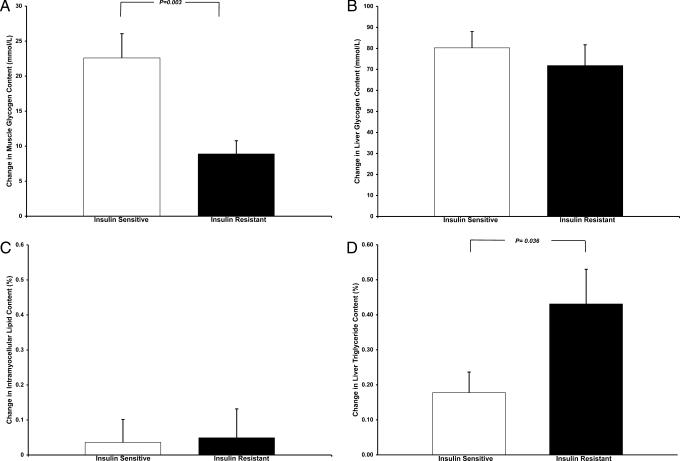
Basal concentrations of muscle glycogen content were similar in the insulin-sensitive (87.1 ± 5.7 mmol/liter muscle) and insulin-resistant (84.1 ± 4.6 mmol per liter of muscle, P = 0.69) subjects. After the mixed meals, however, net muscle glycogen synthesis was 61% lower in the insulin-resistant subjects compared with the insulin-sensitive subjects (Fig. 2A). In contrast, there were no differences between the two groups in basal liver glycogen concentration (insulin-sensitive, 131.6 ± 9.2 mmol per liter of liver, vs. insulin-resistant, 125.0 ± 17.7 mmol per liter of liver; P = 0.74) or net liver glycogen synthesis after ingestion of the meals (Fig. 2B).
Fig. 2.
13C MRS measurements of changes in muscle (A) and liver (B) glycogen concentrations and 1H MRS measurements of changes in intramyocellular triglyceride (C) and hepatic triglyceride (D) content in insulin-sensitive and insulin-resistant subjects after the mixed meals.
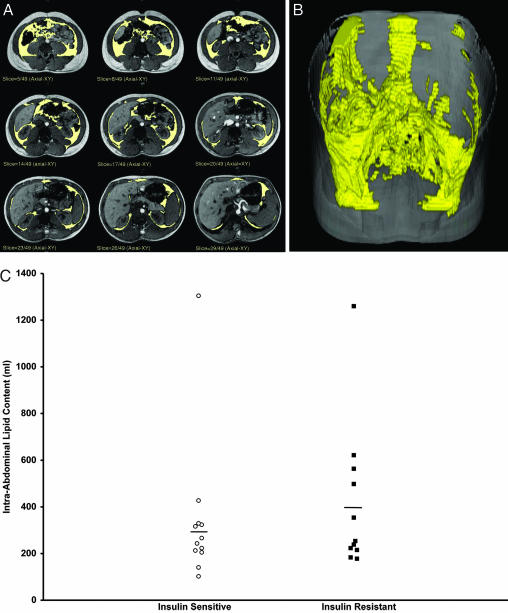
There were no differences in the basal concentrations of hepatic triglyceride in the two groups (insulin-sensitive, 0.65 ± 0.14%, vs. insulin-resistant, 0.76 ± 0.20%; P = 0.63); however, net hepatic triglyceride synthesis was ≈2.5-fold greater in the insulin-resistant subjects than the insulin-sensitive subjects after the carbohydrate meals (Fig. 2D). There were no changes in intramyocellular triglyceride content after the meals in either group (Fig. 2C). The mean (Table 1) and distribution (Fig. 3C) of intraabdominal fat volume assessed by MRI were similar between the two groups, whether or not the single outlier in each group was excluded from analysis.
Fig. 3.
MRI imaging of intraabdominal fat of several slices through the abdomen in a subject (A), three-dimensional reconstruction of intraabdominal fat in the same subject (B), and intraabdominal fat volume in the insulin-sensitive and insulin-resistant subjects (C).
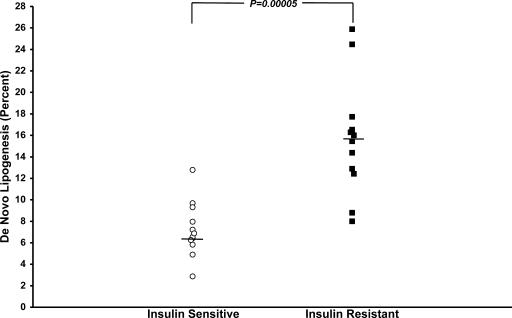
Postprandial fractional hepatic de novo triglyceride lipogenesis, as assessed by the incorporation of deuterated water into plasma triglyceride, was increased by 2.2-fold in the insulin-resistant subjects (15.7 ± 1.5%) compared with the insulin-sensitive subjects (7.2 ± 0.7%, P = 0.00005) (Fig. 4).
Fig. 4.
Fractional de novo lipogenesis in insulin-sensitive and insulin-resistant subjects after the two high-carbohydrate mixed meals.
Discussion
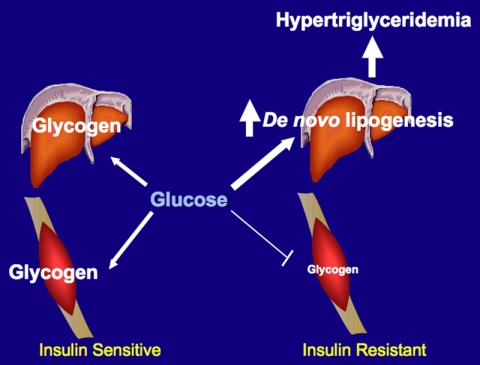
We found that the pattern of stored energy distribution derived from two high-carbohydrate meals was markedly different in young, lean, insulin-resistant individuals compared with young, lean, insulin-sensitive individuals. In contrast to the young, lean, insulin-sensitive subjects, who stored most of their ingested energy in liver and muscle glycogen, the young lean insulin-resistant subjects had a marked defect in muscle glycogen synthesis and diverted much more of their ingested energy into hepatic de novo lipogenesis, resulting in increased plasma triglycerides, lower HDL, and increased hepatic triglyceride synthesis(Fig. 5).
Fig. 5.
Schematic of whole-body energy distribution after high-carbohydrate mixed meals in insulin-sensitive and insulin-resistant individuals.
These data have several important implications for the role of skeletal muscle insulin resistance in the pathogenesis of the metabolic syndrome and T2DM. First, the data are consistent with the hypothesis that insulin resistance in skeletal muscle promotes atherogenic dyslipidemia by promoting the conversion of energy derived from ingested carbohydrate into hepatic de novo lipogenesis and increased VLDL production. This hypothesis is further supported by studies in mice with muscle-specific inactivation of the insulin receptor gene, which have been shown to have increased plasma triglycerides and increased adiposity as a result of muscle-specific insulin resistance (14).
Second, increased hepatic de novo lipogenesis resulting in hypertriglyceridemia also can explain the lower HDL levels in the insulin-resistant subjects by the following mechanism: In the presence of increased plasma VLDL concentrations and normal activity of cholesteryl ester transfer protein, VLDL triglycerides can be exchanged for HDL cholesterol, where a VLDL particle donates a molecule of triglyceride to an HDL particle in return for one of the cholesteryl ester molecules from HDL. This process leads to a cholesterol-rich VLDL remnant particle that is atherogenic and a triglyceride-rich, cholesterol-depleted HDL particle (15). The triglyceride-rich HDL particle can undergo further modification, including hydrolysis of its triglyceride, which leads to the dissociation of the apo A-I protein. The free apo A-I in plasma is cleared more rapidly than apo A-I associated with HDL particles, leading to reductions in the amount of circulating apo A-I, HDL cholesterol, and the number of HDL particles (15).
These data also demonstrate that skeletal muscle insulin resistance predates hepatic insulin resistance and that hepatic triglyceride synthesis is increased in these insulin-resistant subjects after high-carbohydrate meals, which may predispose them to nonalcoholic fatty liver disease (NAFLD). The absence of hepatic steatosis in this group of insulin-resistant subjects is consistent with recent studies by our group demonstrating the relatively low prevalence of hepatic steatosis in young, lean, healthy subjects of different ethnic backgrounds with the important exception of Asian Indian males who have a marked increase in the prevalence of hepatic steatosis (13). Although it is believed that hepatic reesterification of fatty acids accounts for the majority of newly synthesized triglyceride, these results suggest that increased hepatic de novo lipogenesis precede the development of adipose tissue insulin resistance, which subsequently leads to increased flux of fatty acids to the liver (16, 17). This hypothesis is supported by our previous findings of similar basal and insulin suppressed rates of whole body and subcutaneous fat lipolysis in similar groups of insulin-sensitive and insulin-resistant subjects (12). NAFLD is strongly linked to hepatic insulin resistance, and recent studies have demonstrated that NAFLD-induced hepatic insulin resistance is a major factor responsible for the transition from normoglycemia to fasting hyperglycemia and T2DM (18–23). The cellular signal driving this increased hepatic de novo lipogenesis can most likely be attributed to hyperinsulinemia promoting increased expression of the sterol regulatory element binding protein-1c in the liver, which coordinately regulates transcription of all of the key enzymes involved in lipogenesis (24). Indeed, assuming a portal vein–artery concentration gradient for insulin of ≈3, portal vein insulin concentrations can be estimated to exceed 500 microunits/ml in the insulin-resistant subjects
Increased export of triglyceride from the liver to the peripheral and visceral adipose tissue in the form of VLDL may also predispose these insulin-resistant individuals to abdominal obesity (25). Although abdominal obesity has been postulated to play the major role in causing the atherogenic dyslipidemia and insulin resistance associated with the metabolic syndrome (5–7), we did not observe any significant differences in the mean volume of intraabdominal fat between these two groups. These data suggest that abdominal obesity develops later in the course of the metabolic syndrome along with NAFLD and that it is likely more a consequence of insulin resistance in skeletal muscle rather than a primary cause of insulin resistance and atherogenic dyslipidemia (5–7). These results are consistent with recent observations in lipodystrophic patients and mice (20) as well as rodent models of hepatic insulin resistance (18) that have disassociated intraabdominal adiposity from insulin resistance and instead have implicated hepatic steatosis in causing hepatic insulin resistance associated with the metabolic syndrome and T2DM (22, 23, 26).
Finally, alterations in plasma adipocytokine concentrations have also been proposed to play a major role in the development of insulin resistance associated with the metabolic syndrome and T2DM. However, we observed no differences in circulating concentrations of HMW adiponectin, IL-6, resistin, TNF-α, or RBP-4 in the two groups (27–29). Taken together these data suggest that alterations in the concentrations in these plasma adipocytokines do not play a primary role in the early development of insulin resistance and atherogenic dyslipidemia in these young, lean, insulin-resistant subjects and that the observed alterations in the concentrations of these adipocytokines in the metabolic syndrome and T2DM are likely to be secondary to other factors, such as obesity.
In summary, these data support the hypothesis that insulin resistance in skeletal muscle, due to decreased muscle glycogen synthesis, promotes atherogenic dyslipidemia by diverting energy derived from ingested carbohydrate away from muscle glycogen synthesis into increased hepatic de novo lipogenesis. These findings have important implications for understanding the mechanism by which insulin resistance in skeletal muscle promotes the development of the metabolic syndrome, NAFLD, T2DM, and the associated cardiovascular disease. These data also suggest that reversing defects in insulin-stimulated glucose transport in skeletal muscle to reverse insulin resistance in this organ might be the best way to prevent the development of the metabolic syndrome at its earliest stages of development.
Materials and Methods
Participants.
Between 2005 and 2007 we studied ≈400 young, healthy, lean, nonsmoking, sedentary volunteers from the New Haven community. All subjects were in excellent health, taking no medications, lean, nonsmoking, of normal birth weight, and sedentary as defined by a physical activity questionnaire (30) and subsequently, by 3 days of physical activity monitoring using a pedometer (Pedometer 342; Sportline). Blood pressure was measured in the resting state by a digital 300 Vital Signs Monitor (Welch Allyn, Skaneateles Falls, NY). Subjects were excluded if they had any metal implants, body piercing, or history of claustrophobia.
Yale University Human Investigation Committee approved the protocol, and written consent was obtained from each subject after the purpose, nature, and potential complications of the studies were explained.
ISI.
Whole-body insulin sensitivity was assessed with a 3-hr, 75-g oral glucose tolerance test in combination with the ISI (12, 31, 32). Twenty minutes after insertion of an antecubital i.v. line, fasting blood samples were collected for determination of plasma glucose, insulin, fatty acid, C-reactive protein, HMW adiponectin, resistin, IL-6, RBP-4, and TNF-α concentrations. The dextrose drink (Glucola; Curtin Matheson Scientific, Houston, TX) was administered, and blood samples were collected at 10, 20, 30, 60, 90, 120, 150, and 180 min for determination of plasma glucose, fatty acid, and insulin concentrations. The ISI measures the overall effects of insulin to stimulate glucose disposal and inhibit glucose production. From this cohort, 12 insulin-resistant subjects (four male and eight female; two African American, eight Caucasian, and two Hispanic) as defined by an ISI of <3.90 × 10−4 dl/min per microunit/ml (lower quartile of the ISI distribution) and 12 insulin-sensitive subjects (five male and seven female; one black and 11 Caucasian) as defined as having an ISI of >6.06 × 10−4 dl/min per microunit/ml (top quartile of ISI distribution) volunteered to be studied further as described below (13).
All qualifying subjects subsequently underwent dual-energy x-ray absorptiometry scanning (QDR-4500 W; Hologic, Bedford, MA) to assess lean body mass and fat mass (33), a complete medical history, a physical examination, and blood tests to verify that the following were normal: blood and platelet counts, electrolytes, aspartate amino transferase, alanine amino transferase, blood urea nitrogen, creatinine, prothrombin time, partial prothrombin time, cholesterol, and triglyceride.
Dietary Regimen.
To ensure that all participants were weight-stable and following a regular dietary regimen, each participant answered a questionnaire about their usual daily food intake and eating habits over the past 12 months. For 3 days before the study, the participants received all their meals from the General Clinical Research Center (GCRC) Metabolic Kitchen (34–37). The diet was eucaloric [≈35 kcal (1 cal = 4.18 J) per kilogram of body weight per day] and contained 55% carbohydrate, 10% protein, and 35% fat.
Experimental Protocol.
On day 1, the subjects were admitted to the GCRC at 5 p.m. and had dinner at 7 p.m. (33% of their daily caloric requirements). The subjects remained fasting until the first part of the test meal the following day (day 2). Baseline MRS measurements of lipid and glycogen content in muscle and liver were performed on day 2 starting at 6:30 a.m. After completion of these baseline MRS measurements, the subjects were returned to the GCRC for the test meals and determination of de novo lipogenesis as described below. At 7 p.m. the postprandial MRS measurements of lipid and glycogen content in muscle and liver were started. To avoid physical activity, the subjects were encouraged to remain in bed throughout the study, and they were brought to and from the Yale Magnetic Resonance Research Center in a wheelchair.
De Novo Lipogenesis.
After completion of the baseline MRS measurements, the liquid test meals were served. These meals were prepared by the GCRC Metabolic Kitchen for each subject and contained all of the required daily energy (35 kcal per kilogram of body weight; 55% carbohydrate, 10% protein, and 35% fat) with an additional 25% of the daily energy requirements added in the form of sucrose. The meals were equal in size and first portion was served at 10 a.m., and the second was served at 2:30 p.m. Each meal was consumed within 15–20 min. Between the two test meals at noon and at 2 p.m., the loading doses of deuterium-labeled water (3 ml per kilogram of body water; 99.8%; Cambridge Isotopes, Cambridge, MA) was given in two portions of equal size. To maintain constant deuterium enrichment in plasma water, deuterium-labeled drinking water (0.45% enrichment) was given ad libitum for the remainder of the study. The incorporation of deuterium into lipids during administration of deuterium-labeled water was used to determine the fractional synthetic rate of fatty acids as described previously (3, 4). Blood was collected to assess de novo lipogenesis before the first dose of deuterium-labeled water and hourly from 10 p.m. until 6 a.m. (4). After centrifugation, plasma was split into two parts, one part was stored at −20°C until analysis, and the other part was processed immediately by ultracentrifugation for purification and removal of chylomicrons and plasma triglyceride extraction (3, 4, 38). Deuterium enrichment in palmitate and plasma water was measured by gas chromatography–mass spectrometry (5971A Mass Selective Detector; Hewlett–Packard, Wilmington, DE) as previously described (3, 4, 38), and fractional rates of de novo lipogenesis were calculated as previously described (39).
1H MRS and 13C MRS.
All MRS measurements were performed on a whole-body, 4.0-T Medspec (Bruker, Billerica, MA) system. Muscle glycogen and lipid content were measured in the calf muscle by using an 8.5-cm-diameter, circular 13C surface coil with twin, orthogonal circular 13-cm 1H quadrature coils. The probe was tuned and matched, and scout images of the lower leg were obtained to ensure correct positioning of the subject and to define an adequate volume for localized shimming using the FASTMAP procedure (40). 13C MRS spectra were acquired in a (60 × 30 × 60)-mm3 volume placed within the gastrocnemius/soleus muscles to measure glycogen content. Protons were decoupled during the 25-ms acquisition time with a WALTZ-4, and localization of the volume was performed with a three-dimensional adiabatic outer-volume suppression.
After the 20-min acquisition for the glycogen spectra, localized 1H spectra were acquired to assess the muscle lipid content from a (10 × 10 × 10)-mm3 voxel centered in the soleus muscle using a stimulated echo acquisition mode sequence, with three modules of water suppression with the chemical-shift selective imaging sequence. The total lipid content was estimated from comparison of a water-suppressed lipid spectrum and a lipid-suppressed water spectrum, with the appropriate peak for each spectrum on-resonance. The intramyocellular lipid and water resonances were corrected for T1 and T2 relaxation, and intramyocellular lipid content was expressed as a percentage of the water content.
The glycogen and triglyceride content in the liver was measured with a coil assembly composed of a 12-cm, circular 13C coil and twin (13 × 9)-cm elliptical 1H radio frequency coils arranged in quadrature. The probe was secured on the abdomen over the liver with Velcro straps, and a nonmagnetic pneumatic expansion bellows were used for gating the MRS acquisition to the respiratory movements. After imaging the liver and localized shimming with the FASTMAP method with respiration gating (40), 13C MRS for liver glycogen content was acquired with an adiabatic half passage pulse and with WALTZ-4 decoupling during the acquisition time.
The liver triglyceride content was measured by 1H respiratory-gated stimulated echo acquisition mode sequence spectroscopy in a (15 × 15 × 15)-mm3 voxel (41–43). Acquisition was synchronized to the respiratory cycle and triggered at the end of expiration. A water-suppressed lipid spectrum and a lipid-suppressed water spectrum were acquired, and a minimum of two lipid spectra and two water spectra were time-averaged to minimize variations due to chest movements. This sequence was repeated in a second location of the liver to account for liver inhomogeneity. A minimum of eight spectra was acquired for each subject and the total lipid content was averaged and calculated as previously described (44).
Abdominal Fat by MRI.
A MRI of the trunk was acquired in each subject by using a Siemens Sonata 1.5-T Instrument (multibreathold T1-weighted acquisition; field of view, 38.0 × 38.0 cm; matrix, 256 × 256; in-plane resolution, 1.48 mm; 50 contiguous slices; slice thickness, 5-mm). After slice intensity homogeneity correction, intraabdominal fat was interactively segmented in each slice by using the Yale BioImage Arbor Suite software package (www.bioimagesuite.org) and the segmentation map was summed for the entire trunk to generate volume measurements (33).
Analytical Methods.
Plasma glucose concentrations were measured by using a YSI STAT 2700 Analyzer (Yellow Springs Instrument Co., Yellow Springs, CA). Plasma concentrations of insulin, resistin, and HMW adiponectin were measured with double-antibody RIA kits (Linco, St. Louis, MO), and plasma concentrations of IL-6 and TNF-α were measured with Quantine High-Sensitivity kits (R&D Systems, Minneapolis, MN). Plasma fatty acid and triglyceride concentrations were determined by using a microfluorimetric method (45). RBP4 and transthyretin (TTR) were measured by quantitative Western blotting (46) with protein standards prepared with purified full-length human recombinant RBP4 and purified human plasma TTR (Sigma, St. Louis, MO). Immunodetection was performed with polyclonal antibodies to human RBP4 (DakoCytomation, Glostrup, Denmark) and TTR (DakoCytomation). Plasma VLDL and LDL particle concentrations were determined by using MRS (LipoScience, Raleigh, NC).
Statistical Analysis.
Statistical analyses were performed by using the StatView package (Abacus Concepts, Berkeley, CA). Wilcoxon Rank Sum test or unpaired Student's t tests were performed where appropriate to detect statistical differences between insulin-resistant and insulin-sensitive subjects. Paired t tests were performed to detect statistical differences within individuals. All data are expressed as mean ± SEM.
Acknowledgments
We thank Dr. Henry Ginsberg and Colleen Ngai for assistance with setting up the method for VLDL kinetics. We also thank Andrea Belous; Peter Brown; Carolyn Canonica, B.S.; Donna Caseria, R.D.; Christopher Cunningham, B.S.; Donna Dione; James Dziura, Ph.D.; Donna D'Eugenio, R.N.; Robin DeGraaf, Ph.D.; Aida Groszmann; Yanna Kosover; Graeme Mason, Ph.D.; Terry Nixon; Hedy Sarafino; Christine Simpson; Irina Smolgovsky; Mikhail Smolgovsky; and the staff of the Yale/New Haven Hospital General Clinical Research Center for expert technical assistance with the studies and the volunteers for participating in this study. This work was supported by U.S. Public Health Service Grants R01 AG-23686 (to K.F.P.), P01 DK-068229 (to G.I.S.), R01 DK-43051 (to B.B.K.), P30 DK-45735, R01 EB-006494 (to X.P.), and M01 RR-00125; the Yamanouchi USA Foundation; and a Distinguished Clinical Scientist Award from the American Diabetes Association (to G.I.S.).
Abbreviations
- HMW
high molecular weight
- HDL
high-density lipoprotein
- ISI
insulin sensitivity index
- LDL
low-density lipoprotein
- MRS
magnetic resonance spectroscopy
- NAFLD
nonalcoholic fatty liver disease
- RBP-4
retinol binding protein-4
- T2DM
type 2 diabetes mellitus
- VLDL
very-low-density lipoprotein.
Footnotes
Conflict of interest statement: B.B.K. has a research grant from Takeda Pharmaceuticals and is an inventor on a patent for RBP-4.
References
- 1.Reaven GM. Diabetes. 1988;37:1595–1607. doi: 10.2337/diab.37.12.1595. [DOI] [PubMed] [Google Scholar]
- 2.Grundy SM, Brewer HB, Jr, Cleeman JI, Smith SC, Jr, Lenfant C. Circulation. 2004;109:433–438. doi: 10.1161/01.CIR.0000111245.75752.C6. [DOI] [PubMed] [Google Scholar]
- 3.Previs SF, Hazey JW, Diraison F, Beylot M, David F, Brunengraber H. J Mass Spectrom. 1996;31:639–642. doi: 10.1002/(SICI)1096-9888(199606)31:6<639::AID-JMS336>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 4.Diraison F, Pachiaudi C, Beylot M. J Mass Spectrom. 1997;32:81–86. doi: 10.1002/(SICI)1096-9888(199701)32:1<81::AID-JMS454>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- 5.Peiris AN, Sothmann MS, Hoffmann RG, Hennes MI, Wilson CR, Gustafson AB, Kissebah AH. Ann Intern Med. 1989;110:867–872. doi: 10.7326/0003-4819-110-11-867. [DOI] [PubMed] [Google Scholar]
- 6.Abate N, Garg A, Peshock RM, Stray-Gundersen J, Grundy SM. J Clin Invest. 1995;96:88–98. doi: 10.1172/JCI118083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Carr DB, Utzschneider KM, Hull RL, Kodama K, Retzlaff BM, Brunzell JD, Shofer JB, Fish BE, Knopp RH, Kahn SE. Diabetes. 2004;53:2087–2094. doi: 10.2337/diabetes.53.8.2087. [DOI] [PubMed] [Google Scholar]
- 8.Nieves DJ, Cnop M, Retzlaff B, Walden CE, Brunzell JD, Knopp RH, Kahn SE. Diabetes. 2003;52:172–179. doi: 10.2337/diabetes.52.1.172. [DOI] [PubMed] [Google Scholar]
- 9.Rothman DL, Magnusson I, Cline G, Gerard D, Kahn CR, Shulman RG, Shulman GI. Proc Natl Acad Sci USA. 1995;92:983–987. doi: 10.1073/pnas.92.4.983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Perseghin G, Price TB, Petersen KF, Roden M, Cline GW, Gerow K, Rothman DL, Shulman GI. N Engl J Med. 1996;335:1357–1362. doi: 10.1056/NEJM199610313351804. [DOI] [PubMed] [Google Scholar]
- 11.Petersen KF, Dufour S, Shulman GI. PLoS Med. 2005;2:e233. doi: 10.1371/journal.pmed.0020233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Petersen KF, Dufour S, Befroy D, Garcia R, Shulman GI. N Engl J Med. 2004;350:664–671. doi: 10.1056/NEJMoa031314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Petersen KF, Dufour S, Feng J, Befroy D, Dziura J, Dalla Man C, Cobelli C, Shulman GI. Proc Natl Acad Sci USA. 2006;103:18273–18277. doi: 10.1073/pnas.0608537103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kim JK, Michael MD, Previs SF, Peroni OD, Mauvais-Jarvis F, Neschen S, Kahn BB, Kahn CR, Shulman GI. J Clin Invest. 2000;105:1791–1797. doi: 10.1172/JCI8305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Krauss RM, Siri PW. Endocrinol Metab Clin N Am. 2004;33:405–415. doi: 10.1016/j.ecl.2004.03.016. [DOI] [PubMed] [Google Scholar]
- 16.Klein S. J Clin Invest. 2004;113:1530–1532. doi: 10.1172/JCI22028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Reaven GM, Hollenbeck C, Jeng CY, Wu MS, Chen YD. Diabetes. 1988;37:1020–1024. doi: 10.2337/diab.37.8.1020. [DOI] [PubMed] [Google Scholar]
- 18.Kim JK, Gavrilova O, Chen Y, Reitman ML, Shulman GI. J Biol Chem. 2000;275:8456–8460. doi: 10.1074/jbc.275.12.8456. [DOI] [PubMed] [Google Scholar]
- 19.Kim JK, Fillmore JJ, Chen Y, Yu C, Moore IK, Pypaert M, Lutz EP, Kako Y, Velez-Carrasco W, Goldberg IJ, et al. Proc Natl Acad Sci USA. 2001;98:7522–7527. doi: 10.1073/pnas.121164498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Petersen KF, Oral EA, Dufour S, Befroy D, Ariyan C, Yu C, Cline GW, DePaoli AM, Taylor SI, Gorden P, Shulman GI. J Clin Invest. 2002;109:1345–1350. doi: 10.1172/JCI15001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Samuel VT, Liu ZX, Qu X, Elder BD, Bilz S, Befroy D, Romanelli AJ, Shulman GI. J Biol Chem. 2004;279:32345–32353. doi: 10.1074/jbc.M313478200. [DOI] [PubMed] [Google Scholar]
- 22.Neschen S, Morino K, Hammond LE, Zhang D, Liu ZX, Romanelli AJ, Cline GW, Pongratz RL, Zhang XM, Choi CS, et al. Cell Metab. 2005;2:55–65. doi: 10.1016/j.cmet.2005.06.006. [DOI] [PubMed] [Google Scholar]
- 23.Savage DB, Choi CS, Samuel VT, Liu ZX, Zhang D, Wang A, Zhang XM, Cline GW, Yu XX, Geisler JG, et al. J Clin Invest. 2006;116:817–824. doi: 10.1172/JCI27300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Horton JD, Goldstein JL, Brown MS. J Clin Invest. 2002;109:1125–1131. doi: 10.1172/JCI15593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Eckel RH, Hernandez TL, Bell ML, Weil KM, Shepard TY, Grunwald GK, Sharp TA, Francis CC, Hill JO. Am J Clin Nutr. 2006;83:803–808. doi: 10.1093/ajcn/83.4.803. [DOI] [PubMed] [Google Scholar]
- 26.Samuel VT, Liu ZX, Wang A, Beddow SA, Geisler JG, Kahn M, Zhang XM, Monia BP, Bhanot S, Shulman GI. J Clin Invest. 2007;117:739–745. doi: 10.1172/JCI30400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wellen KE, Hotamisligil GS. J Clin Invest. 2005;115:1111–1119. doi: 10.1172/JCI25102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lazar MA. Nat Med. 2006;12:43–44. doi: 10.1038/nm0106-43. [DOI] [PubMed] [Google Scholar]
- 29.Shoelson SE, Lee J, Goldfine AB. J Clin Invest. 2006;116:1793–1801. doi: 10.1172/JCI29069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Baecke JA, Burema J, Frijters JE. Am J Clin Nutr. 1982;36:936–942. doi: 10.1093/ajcn/36.5.936. [DOI] [PubMed] [Google Scholar]
- 31.Matsuda M, DeFronzo RA. Diabetes Care. 1999;22:1462–1470. doi: 10.2337/diacare.22.9.1462. [DOI] [PubMed] [Google Scholar]
- 32.Dalla Man C, Campioni M, Polonsky KS, Basu R, Rizza RA, Toffolo G, Cobelli C. Diabetes. 2005;54:3265–3273. doi: 10.2337/diabetes.54.11.3265. [DOI] [PubMed] [Google Scholar]
- 33.Petersen KF, Hendler R, Price T, Perseghin G, Rothman DL, Held N, Amatruda JM, Shulman GI. Diabetes. 1998;47:381–386. doi: 10.2337/diabetes.47.3.381. [DOI] [PubMed] [Google Scholar]
- 34.Warram JH, Martin BC, Krolewski AS, Soeldner JS, Kahn CR. Ann Intern Med. 1990;113:909–915. doi: 10.7326/0003-4819-113-12-909. [DOI] [PubMed] [Google Scholar]
- 35.Johnson AB, Argyraki M, Thow JC, Cooper BG, Fulcher G, Taylor R. Clin Sci (London) 1992;82:219–226. doi: 10.1042/cs0820219. [DOI] [PubMed] [Google Scholar]
- 36.Krssak M, Falk Petersen K, Dresner A, DiPietro L, Vogel SM, Rothman DL, Roden M, Shulman GI. Diabetologia. 1999;42:113–116. doi: 10.1007/s001250051123. [DOI] [PubMed] [Google Scholar]
- 37.Perseghin G, Scifo P, De Cobelli F, Pagliato E, Battezzati A, Arcelloni C, Vanzulli A, Testolin G, Pozza G, Del Maschio A, Luzi L. Diabetes. 1999;48:1600–1606. doi: 10.2337/diabetes.48.8.1600. [DOI] [PubMed] [Google Scholar]
- 38.Folch J, Lees M, Sloane Stanley GH. J Biol Chem. 1957;226:497–509. [PubMed] [Google Scholar]
- 39.Diraison F, Pachiaudi C, Beylot M. Metabolism. 1996;45:817–821. doi: 10.1016/s0026-0495(96)90152-3. [DOI] [PubMed] [Google Scholar]
- 40.Gruetter R. Magn Reson Med. 1993;29:804–811. doi: 10.1002/mrm.1910290613. [DOI] [PubMed] [Google Scholar]
- 41.Frahm J, Bruhn H, Gyngell ML, Merboldt KD, Hanicke W, Sauter R. Magn Reson Med. 1989;9:79–93. doi: 10.1002/mrm.1910090110. [DOI] [PubMed] [Google Scholar]
- 42.Moonen CWT, Van Zijl PCM. J Magn Res. 1988;88:28–41. [Google Scholar]
- 43.Pauly JM, Le Roux P, Nishimura A, Macovski A. IEEE Trans Med Imaging. 1991;10:5365. doi: 10.1109/42.75611. [DOI] [PubMed] [Google Scholar]
- 44.Mayerson AB, Hundal RS, Dufour S, Lebon V, Befroy D, Cline GW, Enocksson S, Inzucchi SE, Shulman GI, Petersen KF. Diabetes. 2002;51:797–802. doi: 10.2337/diabetes.51.3.797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Miles J, Glasscock R, Aikens J, Gerich J, Haymond M. J Lipid Res. 1983;24:96–99. [PubMed] [Google Scholar]
- 46.Graham TE, Wason CJ, Bluher M, Kahn BB. Diabetologia. 2007;50:814–823. doi: 10.1007/s00125-006-0557-0. [DOI] [PubMed] [Google Scholar]