Abstract
We have previously reported that organ cultured coronary arteries from market-age pigs (6–9 months of age) exhibit an enhanced contraction to the atherosclerotic-associated peptide, endothelin-1 (ET-1). The objective of this study was to investigate the interaction of 17β-estradiol with ET-1 in organ cultured coronary arteries from older female pigs (3–4 years old). A cumulative-concentration response relationship (1 × 10−9 M to 3 × 10−7 M) was generated to ET-1, and the isometric tension measured in fresh and organ cultured (4 days at 37°C) arterial rings that were each pre-incubated for 50 minutes in different concentrations (1 × 10−9 M to 1 × 10−5 M) of 17β-estradiol. Compared to freshly used arteries, culturing induced a 2-fold increase in tension development to ET-1 (3 × 10−7 M). Although 17β-estradiol previously relaxed pre-constricted (with a 60 mM KCl solution) arteries, it did not affect the constrictive response to ET-1. Also, using an ET-1 ELISA we found that 17β-estradiol did not effect ET-1 production in intact arteries. Our results indicate that 17β-estradiol does not attenuate the production and constrictive properties of ET-1 in coronary arteries demonstrating a dedifferentiated cell phenotype.
Keywords: endothelin-1, 17β-estradiol, porcine, organ culture, female
1. Introduction
Endothelin-1 (ET-1) is a potent vasoconstrictive and mitogenic peptide whose synthesis is increased with cardiovascular diseases such as atherosclerosis, hypertension, and congestive heart failure (Deedwania, 1999; Miyauchi and Masaki, 1999; Schiffrin and Touyz, 1998). We previously found that when coronary arteries are organ cultured there is a 2-fold increase in both the myoplasmic calcium concentration and isometric tension to ET-1 (Hill et al., 2000). This increased tension development was attributed to the enhanced function of the ETA receptor subtype. Organ culture has been previously used to induce the dedifferentiation and proliferation of vascular smooth muscle and endothelial cells which is an early event in vascular injuries such as coronary artery disease (Alm et al., 2002; Hill et al., 2001; Hill et al., 2000; Ozaki and Karaki, 2002). This shift to a more synthetic, proliferative phenotype also occurs with an increase in age (Kadziolka and Gasior, 1983; Nikkari et al., 1990; Shyu et al., 2002).
Once women reach menopause there is a rapid increase in the development of coronary artery disease which is thought to be partially due to the decline in plasma 17β-estradiol levels (Koudy and Suparto, 2004; Mendelsohn and Karas, 2005; Rossi et al., 2002); therefore, age may influence the cardiovascular benefits of 17β-estradiol. For example, Lopez-Jarmillo et al. (2004) found that 17β-estradiol induced arterial dilation only in young women, and not postmenopausal women or those demonstrating coronary heart disease. Therefore, it is important to consider the age of the experimental animals because the vascular responses to sex steroids in coronary arteries changes with age/puberty in pigs and humans (Chatrath et al., 2003; Zhao et al., 2005). The enhanced ET-1-induced contraction in organ cultured coronary arteries we previously described was done using market-age pigs which were approximately 6–9 months of age (Hill et al., 2000). Because puberty in pigs is typically reached by 6 months of age (Sell et al., 1996), in this study we want to determine if an increase in the age of the pigs (3–4 years of age) increases the vascular reactivity to ET-1. At this age there is a decline in reproductive viability for females, thus the typical herd life of these pigs is 2–5 years (Koketsu et al., 1999; Thomas et al., 1999). This increase in age is important because it is associated with vascular endothelial damage and an increased production of ET-1 by the endothelium (Brandes et al., 2005). The increase in ET-1 synthesis can accelerate atherosclerotic development by inducing cell proliferation (Dong et al., 2005; Kizawa et al., 2001) and produce coronary spasms, which may lead to a myocardial attack (Pernow et al., 2000). Because advanced age may influence the vascular benefit of 17β-estradiol, the objective of this study was to determine if 17β-estradiol can attenuate the synthesis and constrictive function of ET-1, especially in a proliferative disease model (i.e. organ culture) using older female pigs.
2. Methods
2.1 Artery preparation
Pig hearts were obtained from a local meat plant (staffed with an onsite United States Department of Agriculture veterinarian who confirmed the sex and age of the pigs). The hearts were from female Yorkshire pigs 3 to 4 years of age (550–600 lbs), which have given more than five litters and are cycling (showing regular heat cycles); thus these are “retired” breeding sows. Farmers cull these pigs for slaughter because of reproductive failures, and reduced litter size (Koketsu et al., 1999; Thomas et al., 1999). For comparison, the average pig life span is 15–17 years and puberty is typically reached by 6 months of age (Sell et al., 1996).
The right coronary artery was finely dissected out of the heart in a cold, low calcium solution (concentration in mM: 138 NaCl, 5 KCl, 0.2 CaCl2, 1 MgCl2, 10 HEPES, 10 Glucose, and pH 7.4). The dissected artery was temporarily stored in RPMI 1640 medium (supplemented with 2 gm/L of NaHCO3, and adjusted to a pH of 7.4) at 2 to 40 C before its experimental use. Those arteries to be organ cultured were placed in a 35 mm Petri dish containing 20 mL of RPMI 1640 medium (phenol-free) and incubated at 37°C and 5% CO2 for 4 days (Hill et al., 2001; Hill et al., 2000). The segments were inspected daily and if a change in pH occurred (from 7.4) the culture medium was replaced.
2.2 Measuring isometric tension
Coronary arteries were sectioned into 3 mm rings and, when required, the endothelium was removed by gently rubbing the luminal surface of the arterial ring with a toothpick (Hill et al., 2000). The rings were suspended in 25 mL organ baths containing an oxygenated (95% O2: 5% CO2) modified Kreb’s solution (PSS; composition in mM: 138 NaCl, 5 KCl, 2 CaCl2, 1 MgCl2, 10 HEPES, 10 glucose) having a pH of 7.4 and kept at 37°C. Artery rings were placed under 2 grams tension for 30 minutes before determining the length-tension relationship for each arterial ring. A length-tension relationship was determined by initially stretching the rings 50–100 μm, and then applying a depolarizing 60 mM KCl solution (composition in mM: 83 NaCl, 60 KCl, 2 CaCl2, 1 MgCl2, 10 HEPES, 10 glucose; pH 7.4) to the rings to induce a contraction. This procedure was repeated until the next maximum contraction observed was not more than 10% of the previous stretch (Hill et al., 2000). After determining the length-tension relationship, the arterial rings were allowed to relax in PSS for 30 minutes. Rings were then exposed to a 80 mM KCl solution (the composition is similar to the 60 mM KCl solution except 63 mM NaCl and 80 mM KCl was used); this contraction was used as an internal control for the experiment. For the relaxation experiments, rings were pre-constricted with a 60 mM KCl solution and a concentration-response relationship was generated to 17β-estradiol (1 × 10−9 M to 1 × 10−4 M) and its vehicle (ethanol). For the constriction experiments, rings were incubated in separate organ baths each having a different 17β-estradiol concentration (1 × 10−9 M to 1 × 10−5 M, respectively) for 50 minutes before a cumulative concentration response relationship was generated to endothelin-1 (ET-1; 1 × 10−9 M to 3 × 10−7 M). As previously done by Hill et al. (2000), the endothelium was removed to maximize the ET-1 contraction. The isometric tension was amplified by a Transbridge 4M amplifier (World Precision Instruments, Florida) and the data recorded by the Windaq data acquisition system (Dataq Instruments, Ohio).
2.3 Measuring endothelin-1 production
The arterial rings were opened longitudinally to expose the luminal side. Each artery segment was extensively washed in PSS in five serial Petri dishes placed on ice, and one at room temperature, to get rid of any residual ET-1. The washed artery segments were incubated at 37°C in a humidified 5% CO2 incubator using a four-well multidish (Nunclon, Denmark) containing 1 mL of phenol-free RPMI media. A time control experiment was initially done to determine the proper incubation time for maximum ET-1 production and secretion into the media. For this experiment the media samples were collected at 4, 8, 12, and 24 hours and stored in liquid nitrogen until the ET-1 ELISA was performed. The weight of the artery segments from each respective well was recorded after blotting on kimwipes to remove excess culture media. The incubation time for maximum ET-1 production (8 hours as determined from the time control experiment) was used in the subsequent experiments to investigate the effect of 17β-estradiol against ET-1 production. The wash and incubation protocol described above was followed, and artery segments were incubated for 8 hours under 7 different treatments. The treatment for each well containing culture media and an artery segment, unless indicated, were as follows: (1) no artery segment, (2) media + artery, (3) the ethanol vehicle, (4) 1 × 10−6 M 17β-estradiol, (5) 1 × 10−6 M 17β-estradiol, 1 × 10−5 M 1-aminobenzotriazole, and 1 × 10−5 M 2, 4, Dinitrocatechol, (6) 1 × 10−5 M 1-aminobenzotriazole, and (7) 1 × 10−5 M 2, 4, Dinitrocatechol.
The media samples collected from each respective well were stored in liquid nitrogen. ET-1 production was quantified using the protocol from the ET-1 ELISA kit (Alpco Diagnostics, New Hampshire). An auto strip washer - ELX50 (Biotek Instruments Inc., Vermont) and Micro plate autoreader EL311 (Biotek Instruments Inc., Vermont) were used for the washing and reading of the ELISA plate, respectively. The ET-1 production from each sample was divided by the weight of each respective artery segment to calculate the ET-1 production per gram of tissue.
2.4 Chemicals
17β-Estradiol, 1-Aminobenzotriazole (Cyto-P450 inhibitor), 2, 4-dinitrocatechol (COMT inhibitor), and RPMI media were all purchased from Sigma Aldrich (St. Louis, MO). Endothelin-1 was purchased from American Peptide Company (Sunnyvale, CA).
2.5 Statistical Analysis
Data are expressed as the mean ± S.E. for the number (n) of the animals within each group. Quantification of ET-1 using ELISA was done by constructing a standard curve with the known standards from the ET-1 ELISA kit. Although there was no significant effect associated with the ethanol vehicle, to be precise the net relaxation or constriction was obtained by subtracting out the non-significant effect of the vehicle. Arterial constriction is expressed as a percentage (%) of the maximum response to 80 mM KCl. Analysis of data was conducted using a paired student’s t test and a two-way ANOVA when comparing two groups or more than two groups, respectively. The EC50 values (-log of the effective concentration to generate a 50% response) were calculated and analyzed using GraphPad Prism 2.0 (GraphPad Software Inc., San Diego, CA). Statistical analysis of the data was performed using SigmaStat (Jandel Scientific Software, San Rafael, CA). Significance was defined as p < 0.05.
3. Results
A typical tracing of the concentration-response relationship to 17β-estradiol (1 × 10−9 M to 1 × 10−4 M) is shown in figure 1. The coronary arterial rings were initially pre-constricted by exposing them to a 60 mM KCl solution. Next, a concentration response relationship (1 × 10−9 M to 1 × 10−4 M) was generated to 17β-estradiol in fresh (n=5) and organ cultured arterial rings (n=5) in the presence and absence of their endothelium. Organ cultured arteries generated less relaxation to 17β-estradiol (1 × 10−5 M to 1 × 10−4 M) than the paired freshly used arteries in the presence of an intact endothelium (figure 2a), but not after removal of the endothelium (figure 2b). There was no difference (p > 0.05) in the 17β-estradiol-induced relaxation in the presence or absence of their endothelium for both the fresh and organ cultured arterial rings
Fig. 1.
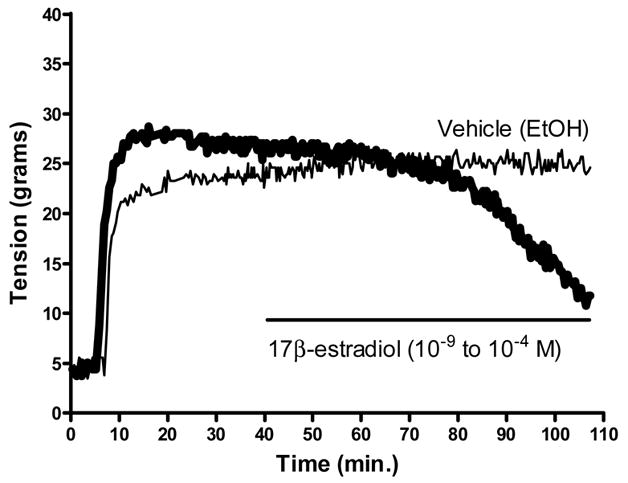
Representative tracing depicted the 17β-estradiol-mediated relaxation of a pre-constricted (60 mM KCl) coronary artery segment. No relaxation was present using ethanol (EtOH) as the vehicle for 17β-estradiol. The horizontal line indicates the time period for the generation of the 17β-estradiol concentration response relationship.
Fig. 2.
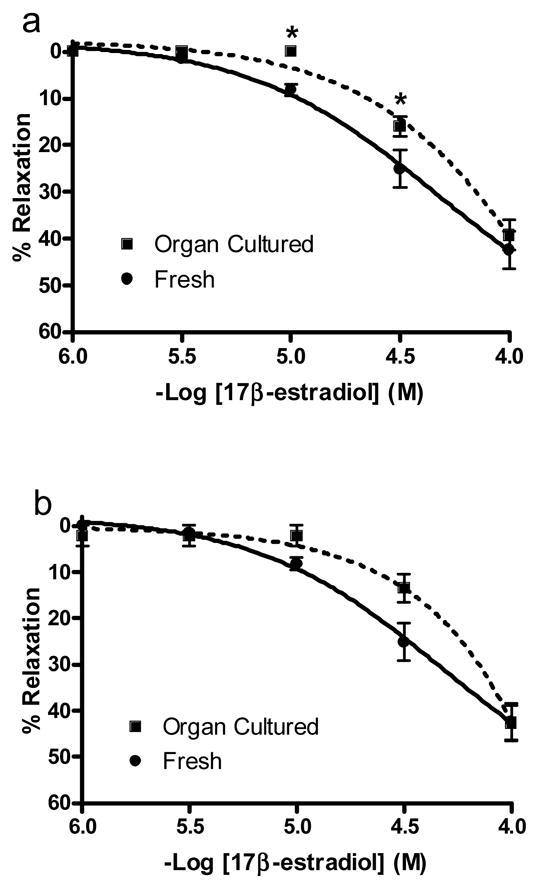
17β-estradiol-induced relaxation of fresh and organ cultured arterial rings. As depicted in figure 1, rings were initially pre-constricted with 60 mM KCl. This was followed by the generation of a cumulative concentration response relationship to 17β-estradiol (1 × 10−9 M to 10−4 M). Only 1 × 10−6 M to 10−4 M is shown because no relaxation was present at a lower concentration. (a) Arterial rings with an intact endothelium. (b) Denuded arterial rings (endothelium removed). The 17β-estradiol-induced relaxation is expressed as percentage (%) of the maximum contractile response to 60 mM KCl. The * indicates a significant difference (p < 0.05) between freshly used and organ cultured rings. The values are expressed as mean ± S.E. (n=5 pigs for each group).
The 17β-estradiol-induced vasorelaxation was investigated against endothelin-1 (ET-1) to determine if the 17β-estradiol-induced vasorelaxation could counteract the constrictive effect of ET-1. Each arterial ring was initially incubated in an organ bath having a different 17β-estradiol concentration (1 × 10−9 M to 1 × 10−5 M) or ethanol (vehicle control) for 50 minutes. After the 17β-estradiol incubation a cumulative concentration-response relationship (1 × 10−9 M to 3 × 10−7 M) to ET-1 was generated in fresh (n=4) and organ cultured arterial rings (n=4). Without 17β-estradiol exposure, the potency (EC50) of ET-1 was 1.86 ± 0.77 × 10−8 M and 1.75 ± 0.82 × 10−8 M in fresh and organ cultured arterial rings, respectively. Linear regression analysis of the concentration response curves (3 × 10−9 M to 3 × 10−8 M) indicated a significant difference between slopes for the fresh (28.33 ± 4.37, r2 = 0.98) and organ cultured rings (62.36 ± 6.58, r2 = 0.99). There also a significant increase in the maximum tension developed (Tmax) to 3 × 10−7 M ET-1 with organ culture. Organ culture increased the Tmax from 52.11 ± 4.53% (fresh rings) to 113.68 ± 6.47% (organ cultured rings) of their maximum response to 80 mM KCl (figure 3a). This infers that the increase in Tmax in organ cultured arteries is due to the enhanced response of ET-1 receptors. These results are in agreement with Hill et al. (2000) who used young market-age pigs (6 to 9 months) to demonstrate that organ culture induces the functional upregulation of the ETA receptor subtype for ET-1. The enhanced Tmax to 3 × 10−7 M ET-1 in organ cultured arterial rings is unaffected by the short-term (50 minutes) exposure to 17β-estradiol (figure 3b). Similarly, 17β-estradiol did not affect the ET-1-induced contraction (Tmax) in freshly obtained arteries (figure 3b).
Fig. 3.
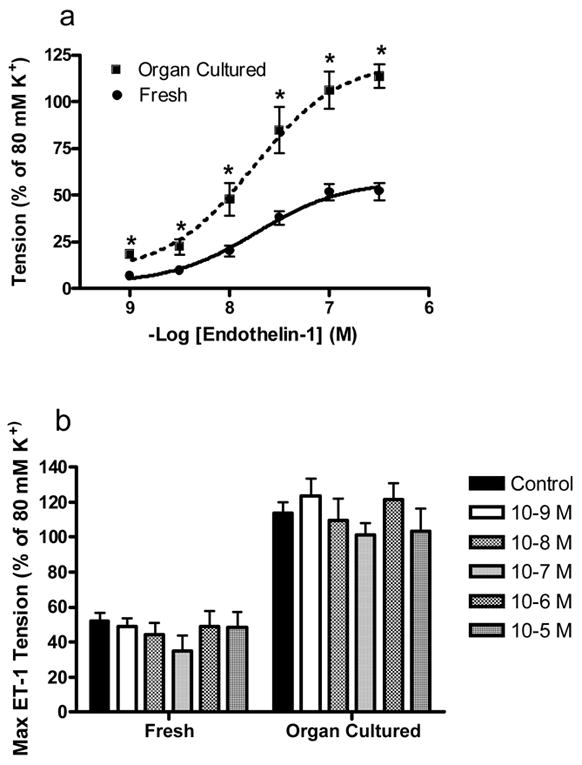
17β-estradiol has no effect on the enhanced contractile response to endothelin-1 (ET-1) in organ cultured arterial rings. (a) Organ cultured arterial rings display an enhanced response to ET-1 compared to fresh rings. A cumulative concentration response relationship was generated to ET-1 (1 × 10−9 to 3 × 10−4M). The ET-1 induced tension development is expressed as a percentage (%) of the maximum contractile response generated by 80 mM KCl. The values are expressed as mean ± S.E. (n=4 pigs for each condition). The * indicates a significant difference (p < 0.05) between freshly used and organ cultured rings. (b) Incubation in different 17β-estradiol concentrations (1 × 10−9M to 1 × 10−5 M) did not effect the contractile response to ET-1. The tension developed to 3 × 10−7 M endothelin-1 (ET-1) is expressed as percentage (%) of the maximum contractile response generated by 80 mM K+. There was no difference (p > 0.05) between groups. The values are expressed as mean ± S.E. (n=4 pigs).
Even though figure 3 suggest that a short-term exposure to 17β-estradiol does not attenuate the ET-1-induced constriction, it is still not known if 17β-estradiol could effect ET-1 production because ET-1 also induces cell proliferation (Dong et al., 2005; Kizawa et al., 2001). We investigated 17β-estradiol (1 × 10−6 M) on ET-1 production in an intact artery (n=5) using ELISA. Although previous studies have shown that 17β-estradiol inhibits ET-1 production at a low concentration of 2 × 10−9 M (Dubey et al., 2001), we used a higher concentration of 17β-estradiol (1 × 10−6 M) because we did not demonstrate a 17β-estradiol-induced attenuation of the ET-1 contraction at 1 × 10−9 M 17β-estradiol. The artery segments were incubated with 17β-estradiol for 8 hours. In addition, we also investigated the extent of 17β-estradiol metabolism in the artery segments using 1 × 10−5 M of the inhibitors, 1-aminobenzotriazole for cytochrome P450 (Cyto-P450), and 2, 4, dintrocatechol for catechol-O-methyltransferase (COMT). 17β-estradiol is metabolized to 2-hydroxyestradiol through Cyto-P450, which is then rapidly methylated to 2-methoxyestradiol through COMT (Dubey et al., 2003; Dubey and Jackson, 2001; Dubey et al., 2001; Dubey et al., 2004). The culture media was analyzed for ET-1 production and the results suggest that there is no significant difference in ET-1 production between treatments (figure 4).
Fig. 4.

17β-estradiol did not effect endothelin-1 (ET-1) production in intact arterial segments. Arterial segments were incubated for 8 hours with: (1) with no drug treatment, (2) ethanol as the vehicle control (EtOH), (3) 17β-estradiol (E2; 1 × 10−6 M), or (4) E2 + 1-aminobenzotriazole (1 × 10−5 M) and 2, 4, Dinitrocatechol (1 × 10−5 M) to inhibit 17β-estradiol metabolism. The ET-1 concentration in the media sample is expressed as fmol/ml/gram of tissue (n = 5 pigs).
4. Discussion
Endothelin-1 (ET-1) exacerbates coronary artery disease by inducing the proliferation of cells into the lumen of the artery (Dong et al., 2005; Kizawa et al., 2001) and/or by causing a spasm of the artery (Pernow et al., 2000). This transition of arterial smooth muscle cells into a proliferative, synthetic phenotype can be induced using an arterial organ culture model (Alm et al., 2002; Hill et al., 2000; Ozaki and Karaki, 2002; Voisard et al., 1995). We previously found that organ cultured coronary arteries from market-age pigs (6 to 9 months of age) greatly enhanced isometric tension development to ET-1 (Hill et al., 2000). Our current study found that advanced age (3–4 years of age) did not further accentuate the ET-1-induced contraction or synthesis that we previously described using younger market-age pigs. Also, organ culture did not affect 17β-estradiol to attenuate the ET-1-induced contraction, although it reduced the ability of 17β-estradiol to mediate a relaxation response. Therefore, our study describes how organ culture can exacerbate the constrictive properties of the atherosclerotic peptide, ET-1, and reduce the relaxation effects of the cardioprotective sex hormone, 17β-estradiol. This is highly significant because coronary heart disease rates in women after menopause are two to three times those of women before menopause (Koudy and Suparto, 2004).
As similarly demonstrated by others using market-age pigs (6 to 12 months) (Barber et al., 1996; Dubey et al., 2001; Sudhir et al., 1997; Teoh et al., 2000), our study shows that 17β-estradiol can induce the relaxation of a pre-constricted artery. However, the transition of arterial cells to a proliferative phenotype with organ culture (Hill et al., 2001; Hill et al., 2000), attenuated the 17β-estradiol-induced arterial relaxation. In advanced cases of coronary artery disease, White et al. (2005) even found that 17β-estradiol causes arterial constriction. Mendelsohn and Karas (2005) have postulated that the cardioprotective effects of 17β-estradiol are lost in atherosclerotic arteries. Our results corroborate a study by Lopez-Jaramillo et al. (2004) who recently found that 17β-estradiol therapy increases flow-mediated vasodilation only in younger women and not in postmenopausal women.
Our data suggest that the advancement in age from 6–9 months to 3–4 years does not effect the ET-1-induced contraction in freshly used or organ cultured arteries. The 2-fold exacerbation of the ET-1-induced contraction with organ culture is present in both age groups of pigs as evidenced by the maximum tension developed (Tmax) to 3 × 10−7 M ET-1. We previously attributed this enhanced ET-1 response to the functional upregulation of the ETA receptor subtype (Hill et al., 2000). Therefore, this corroborates previous reports suggesting an increase in ETA receptors with arterial injury, such as atherosclerosis and restenosis (Guh et al., 1998; Katwa et al., 1999; Wang et al., 1995). Organ culture has also been shown to enhance the contractile response to the selective ETB receptor agonist, sarafotoxin S6c, while having no effect on the prostaglandin F2α and interleukin-1β contraction in the human temporal artery (White et al., 1999). Cao et al. (2005) has also demonstrated an organ culture induced increase in the contractile response to 5-hydroxytryptamine in the rat mesenteric artery. Previously, using pigs 6–9 months old, we found a Tmax to 3 × 10−7 M ET-1 of 121 ± 8% and 227 ± 20% of the maximum response to 80 mM KCl in fresh and organ cultured rings, respectively (Hill et al., 2000). In this study, using 3–4 year old pigs, we report a Tmax to 3 × 10−7 M ET-1 of 52.11 ± 4.53% and 113.68 ± 6.47% in fresh and organ cultured rings, respectively. Therefore, because the 80 mM KCl solution depolarizes arterial smooth muscle to cause calcium influx, our study suggests that advanced age reduces the ET-1-induced influx of calcium. Hill et al. (2000) and others (Inui et al., 1999; Kasuya et al., 1989) have demonstrated that ET-1-induced tension development is attributed to the sustained influx of calcium.
We hypothesized that the ET-1-induced contraction (due to the sustained ET-1-mediated influx of calcium) and may be inhibited by 17β-estradiol, which can mediate arterial smooth muscle relaxation by inhibiting calcium influx (Crews and Khalil, 1999; Salom et al., 2002). We found that the ET-1-induced constrictive response is unaffected by short-term exposure to physiological (1 × 10−9 M to 1 × 10−8 M) and pharmacological (1 × 10−7 M to 1 × 10−4 M) concentrations of 17β-estradiol in fresh and organ cultured coronary arteries. This contrasts previous studies which demonstrated physiological concentrations of 17β-estradiol reduced the constrictive response to ET-1 in market-age pigs (Sudhir et al., 1997; Teoh et al., 1999). However, Wynne et al. (2004) similarly found that advanced age decreases the inhibitory effect of 17β-estradiol on calcium influx.
In vascular smooth muscle cells, ET-1 mediates an initial, transient intracellular calcium increase followed by a sustained influx of calcium into the cell (Bowles et al., 1995; Hill et al., 2000). The transient intracellular calcium increase may mediate the mitogenic properties of ET-1 and cause neointimal thickening during vascular injury (Assender et al., 1996; Douglas et al., 1994; Hill et al., 2000). Because this transient calcium response is not responsible for the generation of contractile force we wanted to determine if 17β-estradiol could attenuate ET-1 synthesis. Results from our study suggest that 17β-estradiol does not inhibit ET-1 synthesis. This contradicts previous studies by Dubey et al. (2001) and Morey et al. (1998) who used endothelial cell cultures to demonstrate that ET-1 synthesis was inhibited by 17β-estradiol. We predict that these differences may be due to our use of intact coronary artery segments in contrast to a cultured endothelial cell line.
5. Conclusions
We used 3–4 year old pigs to contrast our previous study, which used 6–9 month old market-age pigs (Hill et al., 2000). The organ culture-induced exacerbation of the ET-1 contraction was present in both age groups of pigs; however, it appears that advanced age reduces the ET-1-induced contraction. Our results agree with Barber et al. (1996) who found that the strength of ET-1-induced contractions declines with a drop in 17β-estradiol levels in sexually mature female pigs. In addition, even though 17β-estradiol mediated a direct relaxation effect, it did not affect the synthesis of ET-1 or its resultant contraction. The 17β-estradiol-induced relaxation was further attenuated with the induction of a proliferative cell phenotype (with is present with vascular disease) using organ culture.
Acknowledgments
This study was made possible by a grant from the University of Central Arkansas Research Council, and also partially by Grant Number P20 RR-16460 from the National Center for Research Resources (NCRR), a component of the National Institutes of Health (NIH). Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the NCRR or NIH. We also appreciate Odoms Tennesse Pride Sausage plant and its staff (Little Rock, AR) for their donation of the pig hearts.
Abbreviations
- ET-1
endothelin-1
- PSS
physiological saline solution (Kreb’s media)
- EC50
-log of the effective concentration to generate a 50% response
- Tmax
maximum tension developed
- Cyto-P450
cytochrome P450
- COMT
catechol-O-methyltransferase
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alm R, Edvinsson L, Malmsjo M. Organ culture: a new model for vascular endothelium dysfunction. BMCCardiovascDisord. 2002;2:8. doi: 10.1186/1471-2261-2-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Assender JW, Irenius E, Fredholm BB. Endothelin-1 causes a prolonged protein kinase C activation and acts as a co-mitogen in vascular smooth muscle cells. Acta Physiologica Scandinavica. 1996;157:451. doi: 10.1046/j.1365-201X.1996.511285000.x. [DOI] [PubMed] [Google Scholar]
- Barber DA, Sieck GC, Fitzpatrick LA, Miller VM. Endothelin receptors are modulated in association with endogenous fluctuations in estrogen. AmJPhysiol. 1996;271:H1999. doi: 10.1152/ajpheart.1996.271.5.H1999. [DOI] [PubMed] [Google Scholar]
- Bowles DK, Laughlin MH, Sturek M. Exercise training alters the Ca 2+ and contractile responses of coronary arteries to endothelin. Journal of Applied Physiology. 1995;78:1079. doi: 10.1152/jappl.1995.78.3.1079. [DOI] [PubMed] [Google Scholar]
- Brandes RP, Fleming I, Busse R. Endothelial aging. Cardiovasc Res. 2005;66:286. doi: 10.1016/j.cardiores.2004.12.027. [DOI] [PubMed] [Google Scholar]
- Cao YX, He LC, Xu CB, Luo GG, Edvinsson L. Enhanced transcription of contractile 5-hydroxytryptamine 2A receptors via extracellular signal-regulated kinase 1/2 after organ culture of rat mesenteric artery. Basic ClinPharmacolToxicol. 2005;96:282. doi: 10.1111/j.1742-7843.2005.pto960402.x. [DOI] [PubMed] [Google Scholar]
- Chatrath R, Ronningen KL, Severson SR, LaBreche P, Jayachandran M, Bracamonte MP, Miller VM. Endothelium-dependent responses in coronary arteries are changed with puberty in male pigs. AmJPhysiol Heart CircPhysiol. 2003;285:H1168. doi: 10.1152/ajpheart.00029.2003. [DOI] [PubMed] [Google Scholar]
- Crews JK, Khalil RA. Antagonistic effects of 17 beta-estradiol, progesterone, and testosterone on Ca2+ entry mechanisms of coronary vasoconstriction, Arteriosclerosis, Thrombosis. and Vascular Biology. 1999;19:1034. doi: 10.1161/01.atv.19.4.1034. [DOI] [PubMed] [Google Scholar]
- Deedwania PC. Endothelin, the bad actor in the play: A marker or mediator of cardiovascular disease. Journal of the American College of Cardiology. 1999;33:939. doi: 10.1016/s0735-1097(98)00662-7. [DOI] [PubMed] [Google Scholar]
- Dong F, Zhang X, Wold LE, Ren Q, Zhang Z, Ren J. Endothelin-1 enhances oxidative stress, cell proliferation and reduces apoptosis in human umbilical vein endothelial cells: role of ETB receptor, NADPH oxidase and caveolin-1. British Journal of Pharmacology. 2005;145:323. doi: 10.1038/sj.bjp.0706193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douglas SA, Louden C, Vickery-Clark LM, Storer BL, Hart T, Feuerstein GZ, Elliot JD, Ohlstein EH. A role for endogenous endothelin-1 in neointimal formation after rat arotid artery balloon angioplasty. Circulation Research. 1994;75:190. doi: 10.1161/01.res.75.1.190. [DOI] [PubMed] [Google Scholar]
- Dubey RK, Gillespie DG, Zacharia LC, Barchiesi F, Imthurn B, Jackson EK. CYP450- and COMT-Derived Estradiol Metabolites Inhibit Activity of Human Coronary Artery SMCs. Hypertension. 2003;41:807. doi: 10.1161/01.HYP.0000048862.28501.72. [DOI] [PubMed] [Google Scholar]
- Dubey RK, Jackson EK. Estrogen-induced cardiorenal protection: potential cellular, biochemical, and molecular mechanisms. AmJPhysiol Renal Physiol. 2001;280:F365. doi: 10.1152/ajprenal.2001.280.3.F365. [DOI] [PubMed] [Google Scholar]
- Dubey RK, Jackson EK, Keller PJ, Imthurn B, Rosselli M. Estradiol metabolites inhibit endothelin synthesis by an estrogen receptor-independent mechanism. Hypertension. 2001;37:640. doi: 10.1161/01.hyp.37.2.640. [DOI] [PubMed] [Google Scholar]
- Dubey RK, Tofovic SP, Jackson EK. Cardiovascular pharmacology of estradiol metabolites. Journal of Pharmacology and Experimental Therapeutics. 2004;308:403. doi: 10.1124/jpet.103.058057. [DOI] [PubMed] [Google Scholar]
- Guh JH, Chueh SC, Hwang TL, Chen J, Teng CM. Cell proliferation in human prostatic smooth muscle cells involves the modulation of protein kinase C isozymes. European Journal of Pharmacology. 1998;359:281. doi: 10.1016/s0014-2999(98)00683-9. [DOI] [PubMed] [Google Scholar]
- Hill BJF, Dixon JL, Sturek M. Effect of atorvastatin on intracellular calcium uptake in coronary smooth muscle cells from diabetic pigs fed an atherogenic diet. Atherosclerosis. 2001;159:117. doi: 10.1016/s0021-9150(01)00501-9. [DOI] [PubMed] [Google Scholar]
- Hill BJF, Katwa LC, Wamhoff BR, Sturek M. Enhanced endothelin A receptor-mediated calcium mobilization and contraction in organ cultured porcine coronary arteries. Journal of Pharmacology and Experimental Therapeutics. 2000;295:484. [PubMed] [Google Scholar]
- Inui D, Yoshizumi M, Okishima N, Houchi H, Tsuchiya K, Kido H, Tamaki T. Mechanism of endothelin-1-(1-31)-induced calcium signaling in human coronary artery smooth muscle cells. American Journal of Physiology: Endocrinology and Metabolism. 1999;276:E1067. doi: 10.1152/ajpendo.1999.276.6.E1067. [DOI] [PubMed] [Google Scholar]
- Kadziolka A, Gasior W. Coronary arterial disease and atheromatosis in breeding sows. Acta Physiol Pol. 1983;34:29. [PubMed] [Google Scholar]
- Kasuya Y, Ishikawa T, Yanagisawa M, Kimura S, Goto K, Masaki T. Mechanism of contraction to endothelin in isolated porcine coronary artery. American Journal of Physiology. 1989;257:H1828. doi: 10.1152/ajpheart.1989.257.6.H1828. [DOI] [PubMed] [Google Scholar]
- Katwa L, Campbell SE, Tanner MA, Myers PR. The upregulation of endothelin and its receptors during restenosis in porcine coronary arteries in a double ballon injury model of restenosis. Basic ResCardiol. 1999;94:445. doi: 10.1007/s003950050160. [DOI] [PubMed] [Google Scholar]
- Kizawa Y, Ohuchi N, Saito K, Kusama T, Murakami H. Effects of endothelin-1 and nitric oxide on proliferation of cultured guinea pig bronchial smooth muscle cells. Comp BiochemPhysiol CToxicolPharmacol. 2001;128:495. doi: 10.1016/s1532-0456(01)00172-7. [DOI] [PubMed] [Google Scholar]
- Koketsu Y, Takahashi H, Akachi K. Longevity, lifetime pig production and productivity, and age at first conception in a cohort of gilts observed over six years on commercial farms. JVetMedSci. 1999;61:1001. doi: 10.1292/jvms.61.1001. [DOI] [PubMed] [Google Scholar]
- Koudy WJ, Suparto I. Hormone replacement therapy and cardiovascular disease: lessons from a monkey model of postmenopausal women. ILAR J. 2004;45:139. doi: 10.1093/ilar.45.2.139. [DOI] [PubMed] [Google Scholar]
- Lopez-Jaramillo P, Diaz LA, Pardo A, Parra G, Jaimes H, Chaudhuri G. Estrogen therapy increases plasma concentrations of nitric oxide metabolites in postmenopausal women but increases flow-mediated vasodilation only in younger women. FertilSteril. 2004;82:1550. doi: 10.1016/j.fertnstert.2004.05.083. [DOI] [PubMed] [Google Scholar]
- Mendelsohn ME, Karas RH. Molecular and cellular basis of cardiovascular gender differences. Science. 2005;308:1583. doi: 10.1126/science.1112062. [DOI] [PubMed] [Google Scholar]
- Miyauchi T, Masaki T. Pathophysiology of endothelin in the cardiovascular system. Annual Review of Physiology. 1999;61:391. doi: 10.1146/annurev.physiol.61.1.391. [DOI] [PubMed] [Google Scholar]
- Morey AK, Razandi M, Pedram A, Hu RM, Prins BA, Levin ER. Oestrogen and progesterone inhibit the stimulated production of endothelin-1. BiochemJ. 1998;330(Pt 3):1097. doi: 10.1042/bj3301097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikkari ST, Koistinaho J, Jaakkola O. Changes in the composition of cytoskeletal and cytocontractile proteins of rat aortic smooth muscle cells during aging. Differentiation. 1990;44:216. doi: 10.1111/j.1432-0436.1990.tb00620.x. [DOI] [PubMed] [Google Scholar]
- Ozaki H, Karaki H. Organ culture as a useful method for studying the biology of blood vessels and other smooth muscle tissues. JpnJPharmacol. 2002;89:93. doi: 10.1254/jjp.89.93. [DOI] [PubMed] [Google Scholar]
- Pernow J, Bohm F, Johansson BL, Hedin U, Ryden L. Enhanced vasoconstrictor response to endothelin-B-receptor stimulation in patients with atherosclerosis. Journal of Cardiovascular Pharmacology. 2000;36:S418. doi: 10.1097/00005344-200036051-00122. [DOI] [PubMed] [Google Scholar]
- Rossi R, Grimaldi T, Origliani G, Fantini G, Coppi F, Modena MG. Menopause and cardiovascular risk. PathophysiolHaemostThromb. 2002;32:325. doi: 10.1159/000073591. [DOI] [PubMed] [Google Scholar]
- Salom JB, Burguete MC, Perez-Asensio FJ, Centeno JM, Torregrosa G, Alborch E. Acute relaxant effects of 17-beta-estradiol through non-genomic mechanisms in rabbit carotid artery. Steroids. 2002;67:339. doi: 10.1016/s0039-128x(01)00185-4. [DOI] [PubMed] [Google Scholar]
- Schiffrin EL, Touyz RM. Vascular biology of endothelin. Journal of Cardiovascular Pharmacology. 1998;32:S2. [PubMed] [Google Scholar]
- Sell DR, Lane MA, Johnson WA, Masoro EJ, Mock OB, Reiser KM, Fogarty JF, Cutler RG, Ingram DK, Roth GS, Monnier VM. Longevity and the genetic determination of collagen glycoxidation kinetics in mammalian senescence. ProcNatlAcadSciUSA. 1996;93:485. doi: 10.1073/pnas.93.1.485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shyu JJ, Cheng CH, Erlandson RA, Lin JH, Liu SK. Ultrastructure of intramural coronary arteries in pigs with hypertrophic cardiomyopathy. CardiovascPathol. 2002;11:104. doi: 10.1016/s1054-8807(01)00101-6. [DOI] [PubMed] [Google Scholar]
- Sudhir K, Ko E, Zellner C, Wong HE, Hutchison SJ, Chou TM, Chatterjee K. Physiological concentrations of estradiol attenuate endothelin 1-induced coronary vasoconstriction in vivo. Circulation. 1997;96:3626. doi: 10.1161/01.cir.96.10.3626. [DOI] [PubMed] [Google Scholar]
- Teoh H, Leung SW, Man RY. Short-term exposure to physiological levels of 17 beta-estradiol enhances endothelium-independent relaxation in porcine coronary artery. CardiovascRes. 1999;42:224. doi: 10.1016/s0008-6363(98)00265-x. [DOI] [PubMed] [Google Scholar]
- Teoh H, Quan A, Leung SW, Man RY. Differential effects of 17beta-estradiol and testosterone on the contractile responses of porcine coronary arteries. British Journal of Pharmacology. 2000;129:1301. doi: 10.1038/sj.bjp.0703164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas L, Dial GD, March WE. Lifetime reproductive performance in female pigs having distinct reasons for removal. Livestock Production Sci. 1999;63:213. [Google Scholar]
- Voisard R, Jensch V, Baur R, Hîher M, Hombach V. A coronary porcine organ culture system for studies of postangioplasty cell proliferation. Coronary Artery Dis. 1995;6:657. doi: 10.1097/00019501-199508000-00011. [DOI] [PubMed] [Google Scholar]
- Wang QD, Uriuda Y, Pernow J, Hemsen A, Sjoquist PO, Ryden L. Myocardial release of endothelin (ET) and enhanced ET A receptor-mediated coronary vasoconstriction after coronary thrombosis and thrombolysis in pigs. Journal of Cardiovascular Pharmacology. 1995;26:770. doi: 10.1097/00005344-199511000-00014. [DOI] [PubMed] [Google Scholar]
- White LR, Leseth KH, Moller S, Juul R, Adner M, Cappelen J, Bovim G, Aasly J, Edvinsson L. Interleukin-1beta potentiates endothelin ET(B) receptor-mediated contraction in cultured segments of human temporal artery. RegulPept. 1999;81:89. doi: 10.1016/s0167-0115(99)00030-0. [DOI] [PubMed] [Google Scholar]
- White RE, Han G, Dimitropoulou C, Zhu S, Miyake K, Fulton D, Dave S, Barman SA. Estrogen-induced contraction of coronary arteries is mediated by superoxide generated in vascular smooth muscle. AmJPhysiol Heart CircPhysiol. 2005;289:H1468. doi: 10.1152/ajpheart.01173.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wynne FL, Payne JA, Cain AE, Reckelhoff JF, Khalil RA. Age-related reduction in estrogen receptor-mediated mechanisms of vascular relaxation in female spontaneously hypertensive rats. Hypertension. 2004;43:405. doi: 10.1161/01.HYP.0000111833.82664.0c. [DOI] [PubMed] [Google Scholar]
- Zhao Y, Fukumoto Y, Mohri M, Shimokawa H, Takeshita A. Impact of sex and age on coronary basal tone. InternMed. 2005;44:354. doi: 10.2169/internalmedicine.44.354. [DOI] [PubMed] [Google Scholar]


