Abstract
The transcription factor GATA-1 coordinates multiple events during terminal erythroid cell maturation. GATA-1 participates in the transcription of virtually all erythroid-specific genes, blocks apoptosis of precursor cells, and controls the balance between proliferation and cell cycle arrest. Prior studies suggest that the function of GATA-1 is mediated in part through association with transcriptional cofactors. CREB-binding protein (CBP) and its close relative p300 serve as coactivators for a variety of transcription factors involved in growth control and differentiation. We report here that CBP markedly stimulates GATA-1’s transcriptional activity in transient transfection experiments in nonhematopoietic cells. GATA-1 and CBP also coimmunoprecipitate from nuclear extracts of erythroid cells. Interaction mapping pinpoints contact sites to the zinc finger region of GATA-1 and to the E1A-binding region of CBP. Expression of a conditional form of adenovirus E1A in murine erythroleukemia cells blocks differentiation and expression of endogenous GATA-1 target genes, whereas mutant forms of E1A unable to bind CBP/p300 have no effect. Our findings add GATA-1, and very likely other members of the GATA family, to the growing list of molecules implicated in the complex regulatory network surrounding CBP/p300.
Keywords: transcriptional activation, GATA factors, adenovirus E1A protein, protein–protein interaction
Tissue-specific gene expression is achieved through combinatorial action of cell-type-specific and general transcription factors. During the past decade, efforts to understand the molecular basis of erythroid development and differentiation led to the discovery of several cell-restricted transcription factors, including GATA-1, erythroid Krüppel-like factor (EKLF), and NF-E2 (for review see ref. 1). GATA-1, the founding member of the GATA factor family, is a zinc finger protein whose expression is restricted to hematopoietic cells and Sertoli cells of the testis (for review see ref. 2). Functionally important GATA sites have been identified in the regulatory regions of virtually all erythroid-expressed genes, including the α- and β-globins. Loss of GATA-1 leads to fatal embryonic anemia (3) due a block to cellular maturation and apoptosis of erythroid precursors (3–7).
When assayed in nonhematopoietic cells GATA-1 exhibits potent transcriptional activation that is dependent on its N terminus (8). Surprisingly, a transcriptionally silent GATA-1 mutant lacking this domain partially restores in vitro differentiation of GATA-1-deficient embryonic stem cells (9), and induces normal terminal maturation of GATA-1-deficient erythroid cells (10). Moreover, conversion of myeloid 416B cells to megakaryocytes is induced by GATA-1 variants lacking the N-terminal activation domain (11). Taken together, these findings suggest that GATA-1 regulates target gene expression through its interaction with other factors, rather than through its own activation domain. Recently, a candidate cofactor (friend of GATA-1, FOG) identified by a yeast two-hybrid screen was shown to synergize with GATA-1 in both erythroid and megakaryocytic differentiation (12).
Several observations point to CREB-binding protein (CBP) (refs. 13 and 14; for review see ref. 15) as a potential coactivator for GATA-1. (i) GATA-1’s transcriptional activity is strongly repressed by ligand-activated nuclear hormone receptors (16). CBP and its close relative p300 (17) serve as coactivators for various nuclear hormone receptors (18–22), and it has been proposed that nuclear receptors inhibit certain transcription factors by competing for limiting amounts of CBP/p300 (19). This suggests that CBP might also act on GATA-1. (ii) GATA-binding sites are present at high density in the β-globin locus control region (23–25), a complex regulatory element that influences chromatin structure of the entire locus (26, 27). CBP/p300 possess intrinsic (28, 29) and associated (30–32) histone acetyltransferase activity. Acetylation of histones is required for triggering and/or maintaining an open chromatin configuration (for review see (33, 34). At the β-globin locus histone acetylation is associated with general DNase I sensitivity and is believed to reflect a state of chromatin “poised” for transcriptional activation (35). GATA-1 bound to the locus control region might recruit CBP/p300 to influence chromatin function. (iii) CBP/p300 are required for the differentiation of various cell types, including hematopoietic cells of the B-cell lineage (36), and inhibition of CBP/p300 function by the viral transforming protein E1A leads to a block in cellular differentiation and tissue-specific gene expression (for review see ref. 37).
We show here that CBP markedly stimulates transcriptional activation by GATA-1, independent of the GATA-1 N terminus. This effect is blocked by E1A, but not by mutant E1A impaired in CBP binding. GATA-1 and CBP associate in vitro and coimmunoprecipitate from nuclear extracts of erythroid cells. Expression of a conditional form of E1A in murine erythroleukemia (MEL) cells blocks cellular maturation and produces a phenotype similar to that observed upon loss of GATA-1 function including a block in differentiation and reduced cell viability. These results suggest that GATA-1 recruits the widely expressed cofactor CBP to direct cell-type specific gene expression and differentiation.
MATERIALS AND METHODS
Cell Culture and Transfections.
NIH 3T3 cells and MEL cells (clone 745) were grown and transfected as described (38).
Plasmids and Constructs.
The GATA-1 expression plasmid, pXMGATA-1, and mutant derivatives, as well as the M1α-GH and EKLF-GH reporter constructs have been described (8, 39). CBP and glutathione S-transferase (GST)-GATA fusion constructs have also been described (40–42).
E1A estrogen receptor (ER) (43), is a kind gift from Didier Picard. E1A constructs including wild-type adenovirus 5 E1A12S, E1AΔ2–36, E1AΔ38–67, E1Apm928 (44), and the conditional E1A-ER were subcloned into pEF1α-neo (45).
In Vitro Binding Studies.
[35S]Methionine-labeled GATA and CBP proteins were generated by using the coupled reticulocyte lysate system (TNT, Promega). GST fusion proteins were prepared as described (46). Equal amounts of GST fusion proteins were incubated with in vitro translated, [35S]-methionine-labeled proteins in 150 mM NaCl, 50 mM Tris⋅HCl (pH 7.5), 0.1% Nonidet P-40, 0.5 mM DTT, 0.1 mM ZnCl2, 0.5 mM phenylmethylsulfonyl fluoride, 0.5 μg of leupeptin per ml, 2 μg of aprotinin per ml for 1–2 hr, followed by five washes in the same buffer or buffer containing 350 mM NaCl. Bound protein was analyzed by SDS/PAGE and autoradiography.
Immunoprecipitation Experiments.
Nuclear extracts were prepared by lysing cells in hypotonic buffer containing 10 mM Hepes-KOH (pH 8.0), 1.5 mM MgCl2, 10 mM KCl, 0.1 mM ZnCl2, 0.5 mM DTT, 0.5 mM phenylmethylsulfonyl fluoride, 0.5 μg leupeptin/ml, 2 μg aprotinin/ml. After 20 min of swelling on ice, cells were vortexed and spun at 1500 rpm for 5 min. Nuclei were extracted with high salt buffer containing 20 mM Hepes-KOH (pH 8.0), 25% glycerol, 420 mM NaCl, 1.5 mM MgCl2, 0.1 mM ZnCl2, 0.5 mM DTT, 0.5 mM phenylmethylsulfonyl fluoride, 0.5 μg leupeptin/ml, 2 μg aprotinin/ml, for 20 min on ice. After centrifugation, supernatant was diluted to reduce the NaCl concentration to 150 mM. Immunoprecipitations were performed with anti-CBP antibody raised against the Kix domain (40) or nonimmune serum. GATA-1 was immunoprecipitated with the N6 antibody (Santa Cruz Biotechnology) or, as control, isotype matched irrelevant antibody (anti-PECAM, Santa Cruz Biotechnology). Immune complexes recovered with protein A Sepharose (for rabbit IgG) or protein G Sepharose (for rat IgG, Pharmacia) and separated by SDS/PAGE followed by Western blot analysis. Under the conditions described we noticed a strong propensity of GATA-1 to bind nonspecifically to protein A or G Sepharose beads that was reduced after multiple washes at 350 mM NaCl. This “stickiness” of GATA-1 was only observed when mAbs were used for precipitation, but not in the presence of rabbit antiserum. We believe that at lower antibody concentrations, the beads are not saturated with Igs allowing for more nonspecific binding.
Anti-CBP (1–100) (40) and N6 (Santa Cruz Biotechnology) antibodies were used for Western blot analysis. Ponceau-S staining of Western blots confirmed the presence of equal amounts of precipitating antibody in each sample. Antibodies were detected by the enhanced chemiluminescence method (Amersham).
Benzidine Stains.
Following cytocentrifugation cells were stained with benzidine reagent and counterstained with May-Grunwald as described (10).
RESULTS
CBP Augments GATA-1-Dependent Transcription.
The N-terminal 63-amino acid transcriptional activation domain of GATA-1 is dispensable in various functional assays (9–11), suggesting that GATA-1 regulates gene expression through interaction with other factors. To test the potential of CBP to serve as a cofactor, we determined the effect of expressed CBP on the activity of wild-type (wtGATA-1) and N-terminally truncated GATA-1 (GATA-1Δ63).
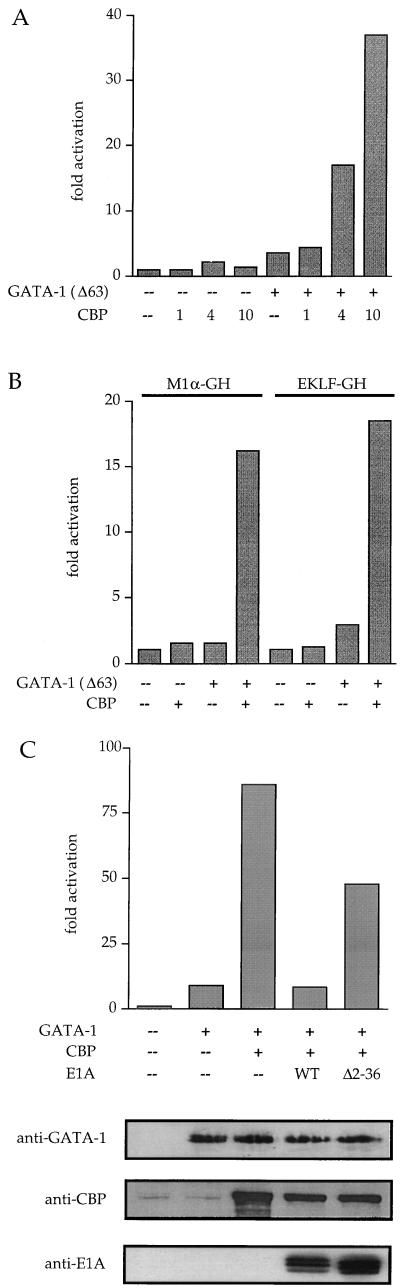
In transiently transfected NIH 3T3 cells, increasing the amount of CBP in the presence of a fixed amount of GATA-1Δ63 led to a dose-dependent activation of synthetic (M1αGH) and natural (EKLF-GH, see ref. 39) GATA-1-dependent promoters, peaking at levels more than ten-fold above that obtained with GATA-1Δ63 alone (Fig. 1 A and B). CBP alone had little or no effect on the basal activity of the reporter genes (Fig. 1 A and B). Cotransfection of CBP also resulted in stimulation of transcription by wtGATA-1 that was most pronounced when low amounts of transfected wtGATA-1 were used (Fig. 1C) Transfection of saturating quantities of wtGATA-1 led to near maximal transcriptional activation of the reporter gene resulting in a blunted enhancement by cotransfected CBP (data not shown). Control Western blots of transfected cells showed that CBP stimulated transcription without affecting the amount of expressed GATA-1 (Fig. 1C). We also observed stimulation of transcription by GATA-2, GATA-3, and GATA-4 in the presence of CBP (data not shown).
Figure 1.
(A) CBP stimulates activity of GATA-1(Δ63) in a dose-dependent manner using a synthetic reporter (M1α-GH). Numbers indicate amounts of transfected plasmid in μg. (B) CBP potentiates GATA-1(Δ63)-mediated activation of synthetic (M1α-GH) and natural (EKLF-GH) GATA-1 target promoters by with comparable efficiency. (C) CBP potentiates wtGATA-1 (Upper). wt E1A, but not E1AΔ2–36, inhibit CBP-mediated activation (Upper) without affecting expression of GATA-1 and CBP as determined by Western blot analysis (Lower).
The adenovirus E1A oncoprotein can be used to probe the involvement of its binding proteins in cellular differentiation pathways. Expression of E1A blocks the function of CBP and its close relative p300 in various cellular settings (13, 14, 17, 37). Two regions of E1A are required for CBP/p300 binding: one at the N terminus (amino acids 1–25) and the other in conserved region 1 (amino acids 37–80) (47, 48). Coexpression of wild-type E1A (wtE1A) blocked the effects of CBP on GATA-1-dependent transcription, whereas a mutant form of E1A unable to bind CBP (E1AΔ2–36) had little effect (Fig. 1B). Western blot analysis demonstrated that E1A expression did not perturb the expression of either GATA-1 or CBP (Fig. 1B).
It has been proposed that nuclear hormone receptors and other transcription factors, such as AP-1, may inhibit each other by competing for limiting amounts of CBP (19). GATA-1 has previously been shown to be inhibited by the ligand-bound ER (16) and glucocorticoid receptor (49). Cotransfection of the ER resulted in a ligand-dependent block of CBP-mediated stimulation of GATA-1 activity, and, conversely, overexpression of CBP abolished ER-mediated inhibition of GATA-1 (data not shown), consistent with a model in which GATA-1 and the ER compete for CBP function.
Physical Association of GATA-1 and CBP.
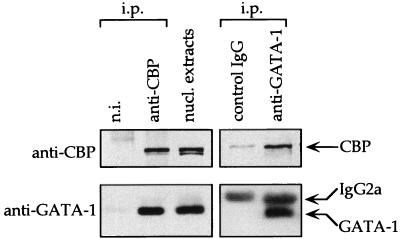
To determine whether GATA-1 and CBP associate in erythroid cells, MEL cell nuclear extracts were subjected to immunoprecipitation followed by Western blot analysis. Fig. 2 shows that anti-CBP antibodies, but not control rabbit serum, coprecipitated ∼15% of total GATA-1 protein, even after extensive washing in buffer containing 350 mM NaCl. The inverse experiment shows that monoclonal anti-GATA-1 antibody, but not an isotype-matched control antibody, coprecipitates CBP (Fig. 2).
Figure 2.
Coimmunoprecipitation of GATA-1 and CBP. CBP was precipitated with rabbit anti-CBP serum or, as control, rabbit nonimmune serum (n.i.) containing equal amounts of total IgG. GATA-1 was precipitated with a rat mAb or an isotype-matched irrelevant control rat mAb. The secondary anti-rat IgG antibody used for Western blot analysis recognized the rat IgG2a used for the GATA-1 immunoprecipitation. i.p.: indicates antibodies used for immunoprecipitation.
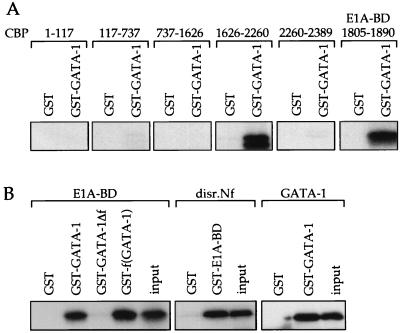
To map the interacting domains of GATA-1 and CBP, GST-pulldown experiments were performed. GST or GST-GATA-1 was immobilized on glutathione-agarose beads and incubated with in vitro-translated portions of CBP, or, as positive control, GATA-1, which self-associates (41). The region spanning amino acids 1626–2260 of CBP, which encompasses the E1A-binding domain (E1A-BD), was the only fragment that bound to GATA-1 (Fig. 3A). Indeed, the E1A-BD alone (amino acids 1805–1890) was sufficient to bind GATA-1 with high avidity (Fig. 3A). These results are consistent with transfection experiments demonstrating that E1A blocks the effects of CBP on GATA-1-dependent transcription.
Figure 3.
GST pulldown experiments demonstrating interaction between the finger domain of GATA-1 and the E1A-binding region (E1A-BD). (A) Mapping of the domain of CBP that interacts with GATA-1. Numbers indicate amino acids present in the in vitro translated CBP. (B) Mapping of the region of GATA-1 that interacts with CBP. Disr.Nf bears a deletion spanning amino acids 200–248. GST-GATA-1Δf lacks both fingers, GST-f(GATA-1) represents the finger region alone. GATA-1 binding to itself served as positive control. Twenty percent of in vitro translated product was analyzed directly (input).
By using immobilized GST-fusion constructs as an affinity matrix, we mapped the domains of GATA-1 required for interaction with the E1A-BD. Deletion of the zinc finger region (GATA-1Δf) abrogated the interaction while the finger region alone f(GATA-1) was sufficient for binding (Fig. 3B). Disruption of the N-terminal zinc finger of GATA-1 in the context of the whole molecule (disr.Nf) did not eliminate binding to GST-E1A-BD (Fig. 3B), suggesting that the C-terminal finger might be sufficient for interaction.
Disruption of CBP/p300 Function in Erythroid Cells.
If CBP is a necessary cofactor for GATA-1-dependent transcription, interference with CBP action would be expected to mimic a GATA-1 loss of function mutation, leading to an erythroid cell maturation arrest, reduction of GATA-1 target gene expression, and decreased cell viability (3–5, 38). In an effort to disrupt CBP/p300 function in intact cells we stably transfected MEL cells with plasmids expressing wtE1A or the mutants E1AΔ2–36, E1AΔ38–67, and pm928. E1AΔ2–36 lacks the N-terminal CBP/p300 interaction domain, E1AΔ38–67 lacks most of conserved region 1 (CR1) and pm928 carries a point mutation in conserved region 2 (CR2). The extreme N terminus and CR1 are required for CBP/p300 binding, whereas CR1 and CR2 mediate interaction with p105 RB, p107, and p130 (ref. 47, for review see ref. 37).
Western blot analysis with antibody to E1A revealed that the majority of MEL clones stably transfected with CBP/p300 binding-deficient constructs E1AΔ2–36 and E1AΔ38–67 expressed large amounts of protein. These clones grew normally and differentiated well in the presence of dimethyl sulfoxide (DMSO) (see below). We were unable, however, to obtain clones expressing detectable amounts of wtE1A or pm928, both of which bind and inhibit CBP/p300. This suggested that interference with CBP/p300 function might be incompatible with cell growth and/or survival.
To circumvent problems associated with constitutive wtE1A expression, we employed a conditional, estradiol-responsive form of E1A (E1A-ER) constructed by fusing the N-terminal 150 amino acids of E1A to the ligand-binding domain of the estrogen receptor (43). MEL cells stably expressing E1A-ER were cultured in the presence or absence of estradiol and assayed for DMSO-induced differentiation by benzidine staining. Upon prolonged treatment with estradiol we observed a reduction in cell number when compared with controls, which might be attributed to the induction of cell death. In an attempt to increase cell survival following estradiol treatment, we forced expression of the Bcl-2 family member Bcl-XL in two E1A-ER lines without effect (data not shown). Furthermore, treatment of cells with the broad range caspase inhibitor ZVAD-FMK, which blocks apoptosis induced by various stimuli (for review see ref. 50), did not augment cell number detectably. Therefore, differentiation assays were performed at a time point following estradiol treatment at which substantial cell loss was not observed.
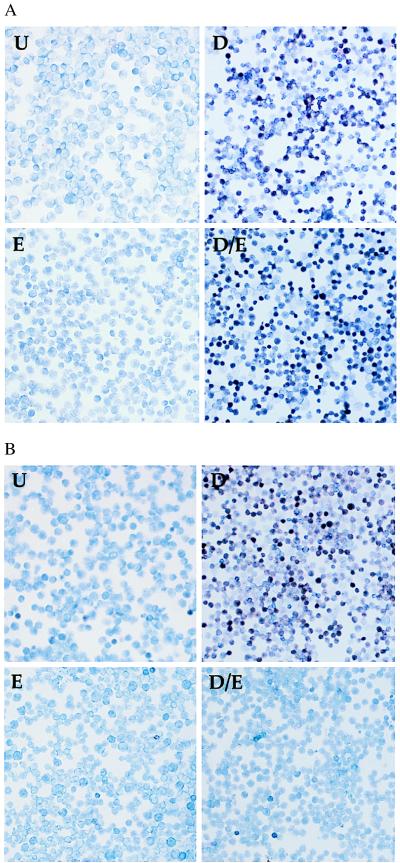
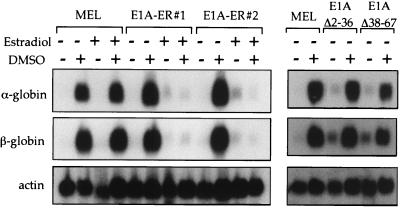
E1A-ER expressing lines and control MEL lines were treated with DMSO, estradiol, or both, for 3 days prior to benzidine staining. Differentiation was strongly reduced in five estradiol-treated E1A-ER-expressing lines, one of which is shown in Fig. 4B. Estradiol had no effect on parental MEL cells (Fig. 4A) or control lines transfected with an empty vector (data not shown). Furthermore, lines constitutively expressing high levels of E1AΔ2–36 or E1AΔ38–67, mutants defective for CBP-binding, differentiated normally (see also below). These findings were corroborated by assay of globin mRNAs. Upon estradiol treatment, cells expressing E1A-ER, but not parental MEL cells, showed a block in induction of α- and β-globin transcripts (Fig. 5). Expression levels of other putative GATA-1 target genes such as EKLF and GATA-1 itself, which are not increased upon DMSO treatment, were modestly reduced (2- to 3-fold) upon estradiol treatment (data not shown). Globin gene expression was normally induced in cells constitutively expressing E1AΔ2–36 and E1AΔ38–67 (Fig. 5) and, as expected, estradiol treatment had no effect (data not shown).
Figure 4.
Activated E1A-ER blocks MEL differentiation. (A) Control MEL cells untreated (U) or treated with estradiol (E), DMSO (D), or both (D/E). Cells were stained with benzidine and May-Grunwald. (B) A MEL line expressing E1A-ER. Note the marked reduction of benzidine positive cells in the sample treated with DMSO and estradiol.
Figure 5.
Activated E1A-ER causes a block in α- and β-globin gene induction. Northern analysis of control MEL cells, two E1A-ER expressing lines, and two lines constitutively expressing high levels of E1AΔ2–36 and E1AΔ38–67 treated with DMSO or estradiol. Blots were probed with a labeled β-globin fragment, stripped, and successively reprobed with α-globin and actin cDNA probes.
These results demonstrate that interfering with CBP/p300 function through activation of a conditional form of E1A blocks erythroid cell differentiation.
DISCUSSION
Cell type-specific gene expression is established through the combinatorial action of restricted and more widely expressed transcription factors. The architecture of a regulatory element directs the assembly of these factors into higher order structures that ultimately determine the transcriptional readout. The ubiquitously expressed transcriptional coactivators CBP and p300 serve multiple functions depending on cellular and promoter contexts (for review see ref. 15). Our findings add GATA-1 to the increasing set of factors whose function is regulated by CBP/p300. We showed that CBP markedly augments GATA-1-dependent transcription of both synthetic and natural target promoters. Stimulation is independent of the N-terminal activation domain of GATA-1, which also appears dispensable for GATA-1 function in differentiating hematopoietic cells (9–11). Coexpression of wtE1A, but not mutant E1A, abrogates the effects of CBP on GATA-1 function.
GATA-1 and CBP coimmunoprecipitate from nuclear extracts of erythroid cells. In vitro binding studies identify the E1A-binding domain of CBP and the finger region of GATA-1 as the respective sites of interaction. The C-terminal finger of GATA-1 appears sufficient for interaction in solution, although it remains possible that either finger may associate with CBP. In other studies, the multitype finger protein FOG has been identified as a candidate cofactor for GATA-1 (12). FOG, which associates specifically with the N-finger of GATA-1, is coexpressed with GATA-1 and synergizes with it to promote both erythroid and megakaryocyte differentiation. How FOG association with GATA-1 leads to enhanced GATA-1 dependent transcription is unknown. An obvious question is whether it is sterically possible for FOG and CBP to interact simultaneously with the finger region of GATA-1. If so, they could represent components of a large complex assembled on GATA-1. Alternatively, it is possible that GATA-1 assembles different combinations of cofactors depending on cell and promoter context.
While the zinc finger domain of GATA-1 is necessary and sufficient for interaction with CBP in vitro, regions flanking the finger domain are required for full activation by CBP in transient transfection experiments (data not shown). Hence, the mechanism by which CBP stimulates GATA-1 activity remains to be defined, but it is plausible that CBP induces a conformational change involving these regions. In addition, it cannot be ruled out that other important factors interact with GATA-1 outside the finger region.
Consistent with the association of CBP with the zinc-finger region of GATA-1, we observed that CBP stimulates transcription dependent on GATA-2, GATA-3, and GATA-4 (data not shown). Thus, our findings may be extended beyond GATA-1 to other members of the GATA-protein family. GATA-1 and GATA-2 are expressed in distinct but overlapping patterns (for review see ref. 2). Levels of GATA-1 are low in hematopoietic progenitors and increase with erythroid maturation (51, 52). Conversely, GATA-2 levels are initially high and decline in a GATA-1-dependent manner (5, 51, 52). While GATA-2 is required for expansion of early pluripotent hematopoietic progenitor cells (53), GATA-1 appears to control later stages of erythroid maturation (3, 5–7) including cell cycle arrest (10). This raises the interesting speculation that GATA-1 and GATA-2 compete for CBP activity during the transition from proliferation to terminal maturation.
To establish a role for CBP/p300 in erythroid cells we introduced a conditional form of E1A stably into MEL cells. Activation of E1A-ER with estradiol resulted in a block in DMSO-induced differentiation and greatly reduced α- and β-globin induction. We also observed a modest (≈2–3-fold), but consistent, reduction of GATA-1 mRNA and protein levels following estradiol treatment (data not shown) that might reflect impaired positive autoregulation (54–57). As GATA-1 mRNA levels do not rise appreciably during DMSO-induced differentiation, inhibition by E1A-ER would not be expected to lead to a pronounced decrease in steady-state GATA-1 mRNA levels. In the absence of further transcription, mRNA stability would determine the rate of fall of GATA-1 mRNA. The finding of appreciable levels of GATA-1 after E1A-ER activation is consistent with a mechanism by which E1A blocks the function of GATA-1 at target gene promoters, rather than primarily influencing GATA-1 expression.
Upon binding of estradiol, the ligand-binding domain of the ER binds to several coactivators including CBP/p300 (18–22). Therefore, the ER portion of E1A-ER might contribute to impaired differentiation by binding to, or squelching, CBP/p300. However, we found that treatment with 4-hydroxytamoxifen also resulted in a block to differentiation equivalent to that obtained upon estradiol treatment. 4-Hydroxytamoxifen binds to the ligand binding domain and activates E1A-ER but, in contrast to estradiol, fails to induce binding to coactivators (22, 58). This indicates that the activity of the E1A-ER construct derives from the E1A moiety.
Our results obtained with a panel of E1A mutants strongly suggest that CBP/p300 is the relevant target of the E1A-induced differentiation block as constitutive expression of mutant alleles of E1A, which lack CBP/p300 binding but retain the potential to interact with RB-related proteins, had no effect on cell growth and differentiation. Activated E1A induces a phenotype in cells similar to that anticipated for loss of GATA-1 function, i.e., a maturation block, reduced globin gene expression and decreased cell survival. This supports a model by which CBP or p300 mediate at least some of the functions of GATA-1.
While various transcription factors are regulated by CBP/p300, GATA-1 is likely to be a key CBP/p300 target in erythroid cells. A surprisingly small set of hematopoietic-restricted factors is thought to play a role in globin gene expression. In addition to GATA-1, these include NF-E2, a heterodimer of a erythroid cell-restricted subunit p45 and a ubiquitously expressed subunit p18, and EKLF. By GST-pulldown experiments, Cheng et al. observed an interaction between CBP and p45 (59). It remains to be determined, however, whether this interaction occurs in vivo and whether CBP stimulates transactivation of the p45/p18 complex. Another candidate factor for interaction with CBP is EKLF. Disruption of the EKLF gene results in a selective reduction in adult β-globin gene expression, while levels of other globin gene transcripts are largely unaffected (60–63). In our experiments, activated E1A efficiently blocked both α- and β-globin gene induction. Therefore, even if E1A targeted EKLF function, it would not be the sole factor through which CBP participates in globin gene expression.
In addition to having its own intrinsic histone acetyltransferase activity, CBP associates with several molecules that possess histone acetyltransferase activity (28–32). This raises the obvious possibility that GATA-1 regulates transcription through recruitment of a histone acetyltransferase complex, thereby altering chromatin structure. Consistent with this notion is the density of GATA-binding sites in the β-globin locus control region, which is thought to contribute to establishing or maintaining an open chromatin configuration of the locus (26, 27). Interestingly, a correlation has been observed between core histone acetylation and DNase I sensitivity of the chicken β-globin locus (35). In this context it is also worth noting that NF-E2-binding sites are required for remodeling of chromatin in the locus control region (64) and that the NF-E2 protein disrupts in vitro assembled chromatin structure in an ATP-dependent manner (65). This suggests multiple mechanisms by which chromatin structure is regulated. Finally, CBP might also serve as a link between GATA-1 and components of the basal promoter machinery, because CBP is found in complexes containing RNA polymerase II (66–68) and TBP (69–72).
Acknowledgments
We thank Didier Picard for the E1A-ER construct, Margaret Chou for critical reading of the manuscript, and Jason Lau for expert technical assistance. This work was supported in part by the Florence R.C. Murray Award (G.A.B.). S.H.O. is an Investigator at the Howard Hughes Medical Institute.
ABBREVIATIONS
- CREB
cAMP response element-binding protein
- CBP
CREB-binding protein
- EKLF
erythroid Krüppel-like factor
- FOG
friend of GATA-1
- MEL
murine erythroleukemia
- GST
glutathione S-transferase
- Cr1 and -2
conserved region 1 and 2
- DMSO
dimethyl sulfoxide
- BD
binding domain
- ER
estrogen receptor
References
- 1.Orkin S H. Curr Opin Cell Biol. 1995;7:870–877. doi: 10.1016/0955-0674(95)80072-7. [DOI] [PubMed] [Google Scholar]
- 2.Weiss M J, Orkin S H. Exp Hematol. 1995;23:99–107. [PubMed] [Google Scholar]
- 3.Fujiwara Y, Browne C P, Cunniff K, Goff S C, Orkin S H. Proc Natl Acad Sci USA. 1996;93:12355–12358. doi: 10.1073/pnas.93.22.12355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Weiss M J, Orkin S H. Proc Natl Acad Sci USA. 1995;92:9623–9627. doi: 10.1073/pnas.92.21.9623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Weiss M J, Keller G, Orkin S H. Genes Dev. 1994;8:1184–1197. doi: 10.1101/gad.8.10.1184. [DOI] [PubMed] [Google Scholar]
- 6.Pevny L, Simon M C, Robertson E, Klein W H, Tsai S-F, D’Agati V, Orkin S H, Costantini F. Nature (London) 1991;349:257–260. doi: 10.1038/349257a0. [DOI] [PubMed] [Google Scholar]
- 7.Pevny L, Lin C S, D’Agati V, Simon M C, Orkin S H, Costantini F. Development (Cambridge, UK) 1995;121:163–172. doi: 10.1242/dev.121.1.163. [DOI] [PubMed] [Google Scholar]
- 8.Martin D I K, Orkin S H. Genes Dev. 1990;4:1886–1898. doi: 10.1101/gad.4.11.1886. [DOI] [PubMed] [Google Scholar]
- 9.Blobel G A, Simon M C, Orkin S H. Mol Cell Biol. 1995;15:626–633. doi: 10.1128/mcb.15.2.626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Weiss M J, Yu C, Orkin S H. Mol Cell Biol. 1997;17:1642–1651. doi: 10.1128/mcb.17.3.1642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Visvader J E, Crossley M, Hill J, Orkin S H. Mol Cell Biol. 1995;15:634–641. doi: 10.1128/mcb.15.2.634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tsang A P, E, V J, A, T C, Fujiwara Y, Yu C, Weiss M J, Crossley M, Orkin S H. Cell. 1997;90:109–119. doi: 10.1016/s0092-8674(00)80318-9. [DOI] [PubMed] [Google Scholar]
- 13.Kwok R P, Lunblad J R, Chrivia J C, Richards J P, Bachinger H P, Brennan R G, Roberts S G, Green M R, Goodman R H. Nature (London) 1994;370:223–226. doi: 10.1038/370223a0. [DOI] [PubMed] [Google Scholar]
- 14.Chrivia J C, Kwok R P S, Lamb N, Hagiwara M, Montminy M R, Goodman R G. Nature (London) 1993;365:855–859. doi: 10.1038/365855a0. [DOI] [PubMed] [Google Scholar]
- 15.Shikama N, Lyon J, La Thangue N B. Trends Cell Biol. 1997;7:230–236. doi: 10.1016/S0962-8924(97)01149-5. [DOI] [PubMed] [Google Scholar]
- 16.Blobel G A, Sieff C A, Orkin S H. Mol Cell Biol. 1995;15:3147–3153. doi: 10.1128/mcb.15.6.3147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Eckner R, Ewen M E, Newsome D, Gerdes M, DeCaprio J A, Lawrence J B, Livingston D M. Genes Dev. 1994;8:869–884. doi: 10.1101/gad.8.8.869. [DOI] [PubMed] [Google Scholar]
- 18.Heery D M, Kalkhoven E, Hoare S, Parker M G. Nature (London) 1997;387:733–736. doi: 10.1038/42750. [DOI] [PubMed] [Google Scholar]
- 19.Kamei Y, Xu L, Heinzel T, Torchia J, Kurokawa R, Gloss B, Lin S, Heyman R A, Rose D, Glass C K, Rosenfeld M G. Cell. 1996;85:403–414. doi: 10.1016/s0092-8674(00)81118-6. [DOI] [PubMed] [Google Scholar]
- 20.Yao T-P, Ku G, Zhou N, Scully R L, Livingston D M. Proc Natl Acad Sci USA. 1996;93:10626–10631. doi: 10.1073/pnas.93.20.10626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chakravarti D, LaMorte V J, Nelson M C, Nakajima T, Schulman I G, Juguilon H, Montminy M, Evans R M. Nature (London) 1996;383:99–103. doi: 10.1038/383099a0. [DOI] [PubMed] [Google Scholar]
- 22.Hanstein B, Eckner R, DiRenzo J, Halachmi S, Liu H, Searcy B, Kurokawa R, Brown M. Proc Natl Acad Sci USA. 1996;93:11540–11545. doi: 10.1073/pnas.93.21.11540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ellis J, Talbot D, Dillon N, Grosveld F. EMBO J. 1993;12:127–134. doi: 10.1002/j.1460-2075.1993.tb05638.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Philipsen S, Pruzina S, Grosveld F. EMBO J. 1993;12:1077–1085. doi: 10.1002/j.1460-2075.1993.tb05749.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Stamatoyannopoulos J A, Goodwin A, Joyce T, Lowrey C H. EMBO J. 1995;14:106–116. doi: 10.1002/j.1460-2075.1995.tb06980.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Forrester W C, Epner E, Driscoll M C, Brice M, Papayannopoulou T, Groudine M. Genes Dev. 1990;4:1637–1649. doi: 10.1101/gad.4.10.1637. [DOI] [PubMed] [Google Scholar]
- 27.Kioussis D, Vanin E, deLange T, Flavell R A, Grosveld F G. Nature (London) 1983;306:662–666. doi: 10.1038/306662a0. [DOI] [PubMed] [Google Scholar]
- 28.Bannister A J, Kouzarides T. Nature (London) 1996;384:641–643. doi: 10.1038/384641a0. [DOI] [PubMed] [Google Scholar]
- 29.Ogryzko V V, Schiltz L R, Russanova V, Howard B H, Nakatani Y. Cell. 1996;87:953–959. doi: 10.1016/s0092-8674(00)82001-2. [DOI] [PubMed] [Google Scholar]
- 30.Chen H, Lin R J, Schiltz R L, Chakravarti D, Nash A, Nagy L, Privalsky M L, Nakatani Y, Evans R M. Cell. 1997;90:569–580. doi: 10.1016/s0092-8674(00)80516-4. [DOI] [PubMed] [Google Scholar]
- 31.Yang X-J, Ogryzko V V, Nishikawa J, Howard B H, Nakatani Y. Nature (London) 1996;382:319–324. doi: 10.1038/382319a0. [DOI] [PubMed] [Google Scholar]
- 32.Spencer T E, Jenster G, Burcin M M, Allis C D, Zhou J, Mizzen C A, McKenna N J, Onate S A, Tsai S Y, Tsai M J, O’Malley B W. Nature (London) 1997;389:194–198. doi: 10.1038/38304. [DOI] [PubMed] [Google Scholar]
- 33.Roth, S. Y. & Allis, C. D. (1996) Cell 5–8. [DOI] [PubMed]
- 34.Pazin M J, Kadonaga J T. Cell. 1997;89:325–328. doi: 10.1016/s0092-8674(00)80211-1. [DOI] [PubMed] [Google Scholar]
- 35.Hebbes T R, Clayton A L, Thorne A W, Crane-Robinson C. EMBO J. 1994;13:1823–1830. doi: 10.1002/j.1460-2075.1994.tb06451.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Eckner R, Yao T-P, Oldread E, Livingston D M. Genes Dev. 1996;10:2478–2490. doi: 10.1101/gad.10.19.2478. [DOI] [PubMed] [Google Scholar]
- 37.Moran E. Curr Opin Genet Dev. 1993;3:63–70. doi: 10.1016/s0959-437x(05)80342-9. [DOI] [PubMed] [Google Scholar]
- 38.Blobel G A, Orkin S H. Mol Cell Biol. 1996;16:1687–1694. doi: 10.1128/mcb.16.4.1687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Crossley M, Tsang A, Bieker J J, Orkin S H. J Biol Chem. 1994;269:15440–15444. [PubMed] [Google Scholar]
- 40.Nakajima T, Fukamizu A, Takahashi J, Gage F H, Fisher T, Blenis J, Montminy M R. Cell. 1996;86:465–474. doi: 10.1016/s0092-8674(00)80119-1. [DOI] [PubMed] [Google Scholar]
- 41.Crossley M, Merika M, Orkin S H. Mol Cell Biol. 1995;15:2448–2456. doi: 10.1128/mcb.15.5.2448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Merika M, Orkin S H. Mol Cell Biol. 1995;15:2437–2447. doi: 10.1128/mcb.15.5.2437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Spitkovsky D, Steiner P, Lukas J, Lees E, Pagano M, Schulze A, Joswig S, Picard D, Tommasino M, Eilers M, et al. J Virol. 1993;68:2206–2214. doi: 10.1128/jvi.68.4.2206-2214.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kraus V B, Moran E, Nevins J R. Mol Cell Biol. 1992;12:4391–4399. doi: 10.1128/mcb.12.10.4391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kotkow K, Orkin S H. Mol Cell Biol. 1995;15:4640–4647. doi: 10.1128/mcb.15.8.4640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Smith D B, Johnson K S. Gene. 1988;67:31–40. doi: 10.1016/0378-1119(88)90005-4. [DOI] [PubMed] [Google Scholar]
- 47.Whyte P, Williamson N M, Harlow E. Cell. 1989;56:67–75. doi: 10.1016/0092-8674(89)90984-7. [DOI] [PubMed] [Google Scholar]
- 48.Wang H G, Rikitake Y, Carter M C, Yaciuck P, Abraham S E. J Virol. 1993;67:476–488. doi: 10.1128/jvi.67.1.476-488.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chang T-J, Scher B M, Waxman S, Scher W. Mol Endocrinol. 1993;7:528–542. doi: 10.1210/mend.7.4.8502237. [DOI] [PubMed] [Google Scholar]
- 50.Salvesen G S, Dixit V M. Cell. 1997;91:443–446. doi: 10.1016/s0092-8674(00)80430-4. [DOI] [PubMed] [Google Scholar]
- 51.Sposi N M, Zon L I, Care A, Valtieri M, Testa U, Gabbianelli M, Mariani G, Bottero L, Mather C, Orkin S H, Peschle C. Proc Natl Acad Sci USA. 1992;89:6353–6357. doi: 10.1073/pnas.89.14.6353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Leonard M, Brice M, Engel J D, Papayannopoulou T. Blood. 1993;82:1071–1079. [PubMed] [Google Scholar]
- 53.Tsai F-Y, Keller G, Kuo F C, Weiss M J, Chen J, Rosenblatt M, Alt F W, Orkin S H. Nature (London) 1994;371:221–226. doi: 10.1038/371221a0. [DOI] [PubMed] [Google Scholar]
- 54.Tsai S-F, Strauss E, Orkin S H. Genes Dev. 1991;5:919–931. doi: 10.1101/gad.5.6.919. [DOI] [PubMed] [Google Scholar]
- 55.Trainor C D, Omichinski J G, Vandergon T L, Gronenborn A M, Clore G M, Felsenfeld G. Mol Cell Biol. 1996;16:2238–2247. doi: 10.1128/mcb.16.5.2238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Nicolis S, Bertini C, Ronchi A, Crotta S, Lanfranco L, Moroni E, Giglioni B, Ottolenghi S. Nucleic Acids Res. 1991;19:5285–5291. doi: 10.1093/nar/19.19.5285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hannon R, Evans T, Felsenfeld G, Gould H. Proc Natl Acad Sci USA. 1991;88:3004–3008. doi: 10.1073/pnas.88.8.3004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Halachmi S, Marden E, Martin G, MacKay H, Abbondanza C, Brown M. Science. 1994;264:1455–1458. doi: 10.1126/science.8197458. [DOI] [PubMed] [Google Scholar]
- 59.Cheng X, Reginato M J, Andrews N C, Lazar M A. Mol Cell Biol. 1997;1:1407–1416. doi: 10.1128/mcb.17.3.1407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Perkins A C, Sharpe A H, Orkin S H. Nature (London) 1995;375:318–322. doi: 10.1038/375318a0. [DOI] [PubMed] [Google Scholar]
- 61.Perkins A C, Gaensler K M, Orkin S H. Proc Natl Acad Sci USA. 1996;93:12267–12271. doi: 10.1073/pnas.93.22.12267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Nuez B, Michalovich D, Bygrave A, Ploemacher R, Grosveld F. Nature (London) 1995;375:316–318. doi: 10.1038/375316a0. [DOI] [PubMed] [Google Scholar]
- 63.Wijgerde M, Gribnau J, Trimborn T, Nuez B, Philipsen S, Grosveld F, Fraser P. Genes Dev. 1996;10:2894–2902. doi: 10.1101/gad.10.22.2894. [DOI] [PubMed] [Google Scholar]
- 64.Gong Q, McDowell J C, Dean A. Mol Cell Biol. 1996;16:6055–6064. doi: 10.1128/mcb.16.11.6055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Armstrong J A, Emerson B M. Mol Cell Biol. 1996;16:5634–5644. doi: 10.1128/mcb.16.10.5634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kee B, Arias J, Montminy M. J Biol Chem. 1996;271:2373–2375. doi: 10.1074/jbc.271.5.2373. [DOI] [PubMed] [Google Scholar]
- 67.Nakajima T, Uchida C, Anderson S F, Parvin J D, Montminy M. Genes Dev. 1997;11:738–747. doi: 10.1101/gad.11.6.738. [DOI] [PubMed] [Google Scholar]
- 68.Nakajima T, Uchida C, Anderson S F, Lee C-G, Hurwitz J, Parvin J D, Montminy M. Cell. 1997;90:1107–1112. doi: 10.1016/s0092-8674(00)80376-1. [DOI] [PubMed] [Google Scholar]
- 69.Abraham S E, Lobo S, Yaciuk P, Wang H G, Moran E. Oncogene. 1993;8:1639–47. [PubMed] [Google Scholar]
- 70.Swope D L, Mueller C L, Chrivia J C. J Biol Chem. 1996;271:28138–28145. doi: 10.1074/jbc.271.45.28138. [DOI] [PubMed] [Google Scholar]
- 71.Dallas P B, Yaciuk P, Moran E. J Virol. 1997;71:1726–1731. doi: 10.1128/jvi.71.2.1726-1731.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Sang N, Avantaggiati M L, Giordano A. J Cell Biochem. 1997;66:277–85. doi: 10.1002/(sici)1097-4644(19970901)66:3<277::aid-jcb1>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]