Summary
It is clear from both clinical observations of women, and research in laboratory animals, that gonadal hormones exert a profound influence on neuronal excitability, seizures, and epilepsy. These studies have led to a focus on two of the primary ovarian steroid hormones, estrogen and progesterone, to clarify how gonadal hormones influence seizures in women with epilepsy. The prevailing view is that estrogen is proconvulsant, whereas progesterone is anticonvulsant. However, estrogen and progesterone may not be the only reproductive hormones to consider in evaluating excitability, seizures, or epilepsy in the female. It seems unlikely that estrogen and progesterone would exert single, uniform actions given our current understanding of their complex pharmacological and physiological relationships. Their modulatory effects are likely to depend on endocrine state, relative concentration, metabolism, and many other factors. Despite the challenges these issues raise to future research, some recent advances have helped clarify past confusion in the literature. In addition, testable hypotheses have developed for complex clinical problems such as “catamenial epilepsy.” Clinical and animal research, designed with the relevant endocrinological and neurobiological issues in mind, will help advance this field in the future.
Keywords: Neuroendocrinology, Estrogen, Progesterone, Allopregnanolone, Catamenial epilepsy, Menstrual cycle, Estrous cycle, Estradiol, Progestin, Ovulation, Progesterone withdrawal, Area CA1, Brain-derived neurotrophic factor (BDNF), Neuropeptide Y (NPY), Osmolarity, Tonic inhibition
Since the Greek era, it has been clear that the seizures of women with epilepsy are influenced by reproductive state (1). Following the clinical observations of Gowers and others (2–4), Hans Selye began a new era when he demonstrated a profound central effect of a reproductive steroid hormone (5). Selye’s work showed that progesterone was anticonvulsant in laboratory rats. Since that time, our understanding of the potent neurobiological actions of each of the major gonadal hormones—estrogen, progesterone, and testosterone—has rapidly advanced. In parallel, numerous studies have defined the effects of these hormones on seizure threshold, epileptogenesis, and epilepsy.
As a result of this wealth of information, a general consensus has developed that estrogen is “proconvulsant,” and that progesterone is “anticonvulsant.” The origins of these ideas can be traced to some of the first clinical observations of women with epilepsy. It appeared that there was often a rise in either the frequency or severity of seizures at the time of menses (2–4; Fig. 1). Additional clinical research suggested that when progesterone levels were high—e.g., during the mid-luteal phase of the menstrual cycle (Fig. 1)—seizures were relatively infrequent (6–9). Because serum progesterone falls just before the onset of menses, it was suggested that progesterone was an endogenous anticonvulsant, and this idea was supported further by experiments in animals which showed that progesterone administration decreased seizures that were induced by convulsants (5,10,11).
FIG. 1.

Comparison of the ovarian cycle of women (the menstrual cycle) and the rat (the estrous cycle). A. A diagram of the menstrual cycle of the normal adult woman (from 183). The follicular phase is the first half of the 28-day cycle. Ovulation occurs at mid-cycle, and is followed by the luteal phase, which is the second half of the cycle. Menstruation (menses) begins at the end of the luteal phase. LH, luteinizing hormone; FSH, follicle-stimulating hormone. B. A diagram of the 4-day estrous cycle of the normal adult Sprague-Dawley rat (modified from 184). The estradiol surge that precedes ovulation occurs during the morning of proestrus. Progesterone begins to rise in the afternoon of proestrus and has fallen by the morning of the next day, which is called estrus. The day “estrus” is distinct from “behavioral estrus”; the latter refers to the evening of proestrus, when sexual behavior peaks. Following proestrus and estrus is a 2-day period termed diestrus: the first day is diestrus 1 (also called metestrus), and it is followed by diestrus 2. Dark bars on the X axis indicate night, dashed lines denote midnight. Days 1–4 are numbered arbitrarily on the X axis for clarification of the timing of events.
In parallel, studies of estrogen provided evidence that it facilitates seizure activity, although this was not necessarily the case for all experimental subjects. Logothetis and colleagues (12) administered estrogen to women with epilepsy, and found EEG changes that were consistent with increased cortical activity; seizures were induced by estrogen administration in 4 of 16 individuals. Logothetis and Harner (13) then showed that afterdischarge thresholds in rabbits decreased after cortical or systemic administration of Premarin, which is commonly used for estrogen replacement therapy after menopause. Other animal studies also showed that estrogens applied to cortex could increase cortical electrographic activity and/or elicit seizures, either in normal animals or those with a preexisting cortical focus (14–16). Subsequent studies that examined stimulus-evoked seizure threshold in rats supported the idea that estrogen decreased seizure threshold or afterdischarge threshold (17,18).
These studies have formed the basis for the current common conception that estrogen is proconvulsant and progesterone has the opposite effect. However, as mentioned above and also reinforced by more recent experiments, there are exceptions to this “rule.” The exceptions have led to some reservations, and caution has increased with our greater understanding of estrogen, progesterone, and hormone action. For example, it is now recognized that Premarin is composed of various equine estrogens, as well as other substances (see below), which are likely to have actions that are distinct from physiological forms of estrogen in women. There have also been advances in our understanding of the mechanisms underlying seizures, epileptogenesis, and epilepsy. These new insights have suggested alternative hypotheses to the common conception that estrogen and progesterone levels are entirely responsible for the changes in seizure frequency during the menstrual cycle in women with epilepsy.
Therefore, it seems timely to summarize the current understanding of estrogen and progesterone as potential modulators of neuronal excitability and seizures in the female brain. This overview illustrates some of the reasons why generalities (e.g., that estrogen is proconvulsant) may not be the best way to describe the relationship between gonadal hormones and epilepsy. In fact, past studies of gonadal hormones on seizures have tended to focus on limited aspects of hormone action (to facilitate design of experimental studies), without taking into account all of the potential complexities of hormonal responses or neuronal excitability in the CNS. By reviewing the actions of gonadal hormones, particularly as they relate to neuronal excitability, seizures, and epilepsy, some of the apparent conflicts may be better understood, if not entirely resolved. Such a discussion can also clarify the optimal approaches needed for future studies.
GONADAL HORMONES IN THE FEMALE BRAIN
An important issue to consider at the outset is which gonadal hormones are relevant to a discussion of seizures in the female brain. The obvious candidates are estrogen and progesterone. Other gonadal hormones are present, however, and they are known to influence excitability, seizures, and epilepsy. Perhaps the best example is testosterone. Testosterone is commonly thought of as a “male” hormone because it represents the primary steroidal product of the testes. However, testosterone is not only abundant in the ovary, but is also essential, because it represents the precursor of estradiol. Testosterone is synthesized in the thecal interstitial cells of the ovary, and then converted to estradiol in the granulosa cells of the primordial follicle. From there, estradiol is secreted into the bloodstream to act on target tissues. Classic endocrinological studies have shown that testosterone cycles in the female (19), just as estradiol and progesterone do. Moreover, testosterone is always more abundant in the circulation than estradiol. However, we have rather limited understanding about the actions of testosterone, or its metabolites, on excitability in the female CNS. It is also worth noting that there are other reproductive hormones that are relevant to seizures which are not necessarily “gonadal.” These hormones have effects on excitability, either directly or indirectly (Table 1). Finally, it is commonly assumed that the ovary is the only source of estrogen and progesterone in women. However, the adrenal gland, liver, subcutaneous fat, and bone are sites of synthesis of steroid hormones. This point is important because adrenal and liver function may be altered in the epileptic patient; furthermore, anticonvulsant drugs can cause osteoporosis, potentially changing steroid synthesis or release.
TABLE 1.
Nonsteroidal reproductive hormones that influence the brain
| Hormone | Proposed neurobiological effect(s) | References |
|---|---|---|
| Hypothalamic releasing hormones | ||
| LHRH (GnRH) | ↑ Glutamatergic transmission | (170) |
| ↑ Intrinsic excitability | (171–173) | |
| Hypothalamic hormones | ||
| Luteinizing hormone (LH) | ↑ Amyloid | (174) |
| ↑ Maze performance | (174) | |
| Follicle-stimulating hormone (FSH) | ↓ Visuospatial skill | (175) |
| ↑ Language fluency | (175) | |
| Prolactin | ↑ Neurogenesis | (176) |
| ↑ JAK-STAT signaling | (177) | |
| ↑ Firing of supraoptic neurons | (177) | |
| Hormones of pregnancy | ||
| Human chorionic gonadotropin | ↑ Regeneration | (178) |
| Change in sleep | (179) | |
| ↑ Prostaglandin synthesis | (179) | |
| Oxytocin | ↑ Inhibition | (180) |
| ↓ Synaptic transmission | (181) | |
| ↑ Release intracellular calcium | (181) | |
| ↑ Nonspecific cation channel | (181) | |
Hormones associated with reproductive function, which also have potential effects on neuronal excitability and seizures, are listed. An increase in an effect is denoted by an arrow pointing upward, and a decrease in an effect is indicated by an arrow pointing downward. A combination of an arrow pointing up and down reflects mixed effects or unclear effects. JAK-STAT, Janus-activating kinase-signal transducer and activator of transcription.
Perhaps even more important, from the perspective of seizure control, are the CNS sites of estrogen and progesterone synthesis. Brain synthesis is frequently overlooked in studies that use the serum level of hormones to predict brain effects, and there can be a poor correlation under some conditions (20,21). The brain has the capacity to synthesize both estrogen and progesterone from cholesterol, which is the precursor for all steroid hormones. The synthesizing enzymes have been shown in tissue resected from patients with intractable temporal lobe epilepsy (22), although whether they are altered relative to the normal brain has not yet been studied. However it is likely that they are, because aromatase is increased in reactive glia within hippocampus after kainic acid-induced seizures in the rat (23), an effect that would potentially lead to increased conversion of testosterone to estradiol.
In the discussion below we will consider primarily estrogen and progesterone, because more experimental information is available for these hormones than any others, and there is no question that they play an important role in epilepsy. For simplicity, they are considered consecutively. However, one of the most important considerations in assessing their effects on excitability is that their actions differ depending on their relative concentrations. Thus, progesterone sensitivity is highly dependent on prior or concurrent estrogen exposure, at least in part because estrogens induce progesterone receptor synthesis, in the brain as well as in many other tissues (24,25). Conversely, progesterone has been shown to antagonize estrogen effects on sexual behavior and uterine weight (26–28). Given this perspective, it seems obvious that estrogen could exert different effects on seizure activity if examined in a female at different times of the ovarian cycle (Fig. 1), or in an intact versus gonadectomized female. This point is illustrated by the interaction of estrogen and progesterone during the estrous cycle in rat. Prior to ovulation, the proestrous rise in estrogen is followed within a few hours by a rise in progesterone, which—at that point in the cycle—appears to potentiate many of the neuroendocrine and behavioral effects of estrogen (29,30). However, at other times of the estrous cycle, when progesterone is elevated but estrogen is not, progesterone antagonizes the behavioral effects of estrogen (26).
Estrogen
Types of estrogens: estrone, estradiol, and estriol
One of the most common misconceptions about estrogen in the female is that there is only one ligand for estrogen receptors, estradiol. Actually, there are many other estrogens, and each can bind estrogen receptors. The three principal circulating estrogens are estrone (E1), estradiol (E2), and estriol (E3; Fig. 2). Estrone and estriol are formed either by aromatization of androstenedione (a major androgen), the further metabolism of estradiol, or via placental aromatization of fetal steroids. Estrone is the primary estrogen after menopause, and its main source is subcutaneous fat, where it is produced by aromatization of androstenedione. Estrone may be important to consider in women with epilepsy after menopause, especially if they are severely overweight. Estriol may be important in pregnancy because it is synthesized in large quantities via placental aromatization of fetal androgen, and is also made in the liver by hydroxylation of estrone.
FIG. 2.
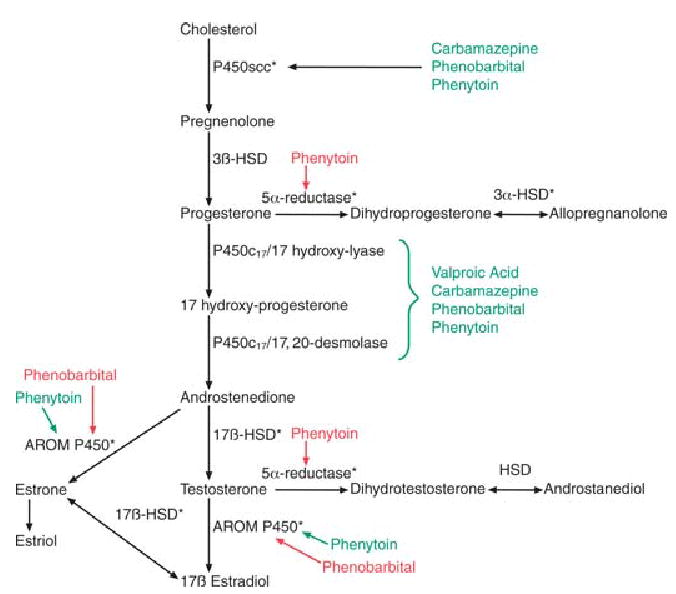
Estrogen and progesterone biosynthesis and the influence of anticonvulsant drugs. Major steps in the synthesis of progesterone and estrogen are shown. Anticonvulsants that induce enzymes are shown in green, and those that inhibit enzyme activity are shown in red. Regarding the effects of anticonvulsants, most information has been derived from studies of hepatic enzymes, nonhuman species, and reduced preparations, using varied concentrations and times of exposure to drugs. Therefore, generalizations (i.e., to patients with epilepsy) should be made with caution. Asterisks indicate enzymes that have been shown to be present in human tissue from patients with epilepsy (22). HSD, hydroxysteroid dehydrogenase; AROM P450, aromatase P450.
Both estrone and estriol are likely to have complex effects on excitability—in part because they are not as effective activators of estrogen receptor function as estradiol, but also because estrone itself can act as a precursor for estradiol, via the actions of the 17-hydroxysteroid dehydrogenase enzymes. Unfortunately, we know very little about how these estrogens influence excitability, or how the relative concentrations of E1/E2/E3 could influence the changes in seizure frequency of the woman with epilepsy under particular conditions (e.g., during pregnancy). However, seizure frequency could potentially change simply due to altered ratios of E1/E2/E3 because these steroids have distinct biological actions (31,32). Furthermore, seizure frequency may be altered differently if hormone replacement therapy utilizes estradiol primarily, or some other hormone preparation—e.g., Premarin, which is a complex mixture of estrogens extracted from the horse, and includes substantial amounts of androgens and progestins, as well as estrogen (33). A better understanding of the impact of each of the estrogens could help clarify the difficult clinical problem of changes in seizure frequency of women at menopause (34,35).
It is also important to note that there is more to E2 (estradiol) itself than the 17β-estradiol normally considered to represent the physiological ovarian estrogen. Specifically, there are two isoforms of estradiol, 17α-estradiol and 17β-estradiol. Although most consider 17β-estradiol to be the only isoform with bioactivity, 17α-estradiol merits attention because it is synthesized in the brain (36) and has equal or greater bioactivity on a number of CNS endpoints of estrogen action than its 17β isomer (37–39).
Finally, it should be mentioned that some physiological steroids that are typically considered to be androgens (because they are not aromatized and therefore retain the 19-carbon steroid structure typical of androgens) do in fact have considerable estrogenic bioactivity. In particular, the androstanediols, produced via 3α- and 3β-hydroxysteroid dehydrogenase-mediated reduction of dihydrotestosterone (Fig. 2), exert estrogen-like actions (40). As further described below, these steroids may be truly multifunctional hormones, because they also share some of the properties of progesterone metabolites, as allosteric modulators of GABAA receptors in the brain.
Estrogen receptors: ERα, ERβ, and “membrane” receptors
One of the most important discoveries in reproductive medicine was the identification during the 1960s of “nuclear” steroid hormone receptors. The receptors are transcription factors that bind to regulatory elements on genes to modify cellular responses. Thus, reproductive steroids, which readily cross the plasma membrane, gain access to receptors within the cytoplasm and/or cell nucleus, and activate them. In the case of estrogen, there are two known nuclear receptors, ERα and ERβ (and multiple isoforms of ERα and ERβ; (41,42). Importantly, these two receptors appear to have very different ligand binding specificities, so that ERα- and ERβ-mediated responses are differentially sensitive to estrogens. Thus, ERβ is more sensitive than ERα to activation by either flavonoid phytoestrogens (such as those found in soy meal) or androstanediol (40,43). After ligand binding to a receptor, receptor dimerization occurs. Receptors may form homodimers (2 ERα or 2 ERβ) or heterodimers (1 ERα with 1 ERβ). Homo-and heterodimers of ER have distinct actions; for example, ERβ activation appears to inhibit subsequent effects of ERα (42). Steroid coactivator proteins, accessory proteins, and potentially other factors then bind to the receptor complex, and the entire complex binds to hormone response elements of target genes (Fig. 3).
FIG. 3.
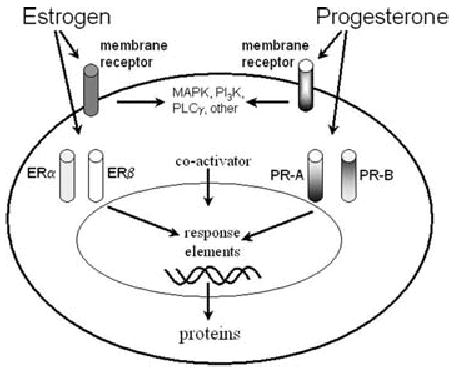
Estrogen and progesterone receptor action. Cellular actions of estrogen and progesterone are depicted schematically for a generic neuron. Both steroids easily cross the plasma membrane to bind to cytoplasmic receptors; after receptor dimerization and activation (which may involve steroid coactivators and other accessory proteins), the receptor complexes bind to steroid response elements on target genes. Dimerization may involve identical or different subunits (homo- or hetero-dimerization). In addition, membrane receptors exist which potentially activate various signal transduction pathways. MAPK, mitogen-activated protein kinase; PI3K, phosphoinositide-3 kinase; PLC γ, phospholipase-C γ.
Studies have demonstrated that many of the cellular effects of estrogens are mediated by nuclear and hence “genomic” actions. More recently, however, alternate types of estrogen receptors have been suggested because actions of estrogen have been identified that cannot easily be explained by genomic actions. These studies have shown that there are cellular responses to estradiol that are not blocked by pharmacological antagonists of the nuclear receptors, and many of these are rapid responses, suggestive of a nongenomic action. These studies have led to the concept of a “membrane receptor” for estrogen (44). Although the nature of membrane receptors remain to be defined, it may be a form of ERα or ERβ (45,46), or an entirely distinct protein (ERX; 47).
Progesterone
Progestins, progestagens, and progesterone
Many of the same misconceptions about the source and synthesis of estrogens also occur for progesterone. To begin, progesterone is not the only molecule that binds to progesterone receptors. There is a family of compounds, commonly referred to as progestins, which include progesterone and progesterone derivatives. Many synthetic steroids have been synthesized that have activity as “progestagens,” by virtue of their ability to induce differentiation of the endometrium (the lining of the uterus) in the same way as progesterone (progestagen: “pro” + “gestation”). However, these synthetic compounds do not necessarily have exactly the same biological activity in the brain as progesterone itself. Conversely, many progesterone metabolites do not exhibit potent activity as progestagens on the endometrium, but still retain considerable biological activity on other systems in the body.
Progesterone, and its immediate 5α-reduced metabolite dihydroprogesterone (Fig. 2), are two natural progestins that bind to nuclear progesterone receptors (see below), although dihydroprogesterone binds with lower affinity. Progesterone actions are therefore complex, because they may be due to progesterone itself or to an indirect action (i.e., after conversion to dihydroprogesterone). Perhaps more importantly, dihydroprogesterone is further metabolized to a neuroactive steroid, allopregnanolone (Fig. 2), which is a potent modulator of GABAA receptor function (105). Therefore, progesterone may also act by conversion to allopregnanolone. While conversion of progesterone to dihydroprogesterone is irreversible, the reduction of dihydroprogesterone to allopregnanolone is reversible. Thus, theoretically, administration of progesterone, dihydroprogesterone, or allopregnanolone may lead to actions mediated by either progestin receptor activation or modulation of GABAA receptor activity. Additional experimental constraints arise because nuclear receptors for progesterone are often labile in the preparations where effects on excitability are typically examined (e.g., tissue slices, dissociated cells). Furthermore, the 5α-reductase enzyme that represents the initial rate-determining step in the pathway is preferentially localized to glial cells (48) and may be altered in epilepsy because of gliosis. Thus, the availability and actions of different progestins may be quite different in normal versus epileptic brain.
Interestingly, some of the metabolic considerations for progesterone (i.e., conversion to other bioactive steroids) also apply to androgens. As already mentioned, testosterone and dihydrotestosterone are converted in the body to androstanediols (e.g., 5α-androstane-3β, 17β-diol). In addition to being a weak ligand for ERβ, this steroid also shares the same property as allopregnanolone: it is an allosteric modulator of GABAA receptor function (49) and is anticonvulsant (50,51).
Progesterone receptors: PR-A, PR-B, and “membrane” receptors
There are two known progesterone receptors, PR-A and PR-B, which are thought to act as nuclear transcription factors, analogous to ERs. There is only one gene for progesterone receptors. Both PRs have similar affinities for progesterone, but are expressed in distinct brain regions. There are also likely to be membrane receptors for progesterone, analogous to the membrane receptors for estradiol.
Fundamental aspects of PR structure, expression, and function have yet to be comprehensively defined. Based on studies conducted to date, PR function presents a number of complexities. One is the fact that PR activation does not necessarily require progesterone as an agonist. Such “ligand-independent activation” has been shown, for example, by dopamine activation of PRs without progesterone (52). Intriguingly, responses mediated via the PR-A and PR-B progestin receptor isoforms appear to be differentially regulated via progesterone and dopamine agonists, suggesting that PR-A and PR-B may utilize distinct intracellular signaling pathways in response to progesterone-mediated activation versus ligand-independent activation (53). Because the ratio of PR-A: PR-B expression is not constant from one region of the brain to another, but is itself subject to regulation by circulating estradiol levels (54), it is easy to see how the balance between progesterone-dependent and ligand-independent PR activation in different regions of the brain could be affected by reproductive state. Therefore, the effects of PR activation on neuronal excitability may vary with brain region and endocrine condition.
THE INFLUENCE OF ESTROGEN AND PROGESTERONE ON SEIZURES
Estrogen
The influence of estradiol on seizures
General considerations
What is the state of our current understanding with respect to estradiol’s influence on seizures? When studies in experimental animals are considered, they initially may appear inconsistent, perhaps because of the diverse effects of estradiol on excitability, even within one region of the brain (see below). However, the apparent inconsistencies across studies may simply be the consequence of diverse experimental designs. More often than not, different conditions and endpoints have been used that are not well-suited for comparisons (Table 2). Choice of comparison groups is a prime example of these differences in experimental design. For example, actions of estradiol on seizures may be inferred by comparing females and males—but there are obviously many more distinctions than estradiol levels between these two experimental groups that could influence the response to a convulsant drug. A second common comparison is between the ovariectomized rat and intact female at diestrus. Both of these conditions would be associated with low levels of serum estradiol, but additional differences also would be present that could be confounding: the ovariectomized and intact female rat have different levels of neurotransmitters, receptors, and modulators in the brain that profoundly alter excitability. Ovariectomy changes GABA levels, the synthetic enzyme for GABA, and the KCC2 transporter that contributes to chloride gradients which are critical for GABAA receptor-mediated actions (55). A third type of comparison involves intact female animals at different times of the estrous cycle. One group of animals presumably has higher estradiol levels at the time of convulsant administration than the comparison group. However, there are many changes that occur during the estrous cycle that may influence seizure sensitivity besides a difference in serum estradiol. Indeed, investigators often underestimate the many changes that occur during the estrous cycle besides those that involve levels of estradiol and progesterone.
TABLE 2.
Effects of estradiol on kainic acid-induced seizures in ovariectomized female rats: a comparison of experimental designs and outcomes
| Experimental design
|
Endpoints
|
|||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Reference | Rat strain | Age at time of Ovx (days) | Weight at time of Ovx (g) | Kainic acid dose(mg/kg, i.p.) | E2 or EB | Estradiol dose & site | Estradiol regimen | Estradiol-to-kainic acid interval | Latency | Severity | Incidence | Mortality | Result Effect of estradiol | Conclusion (effect of estradiol) |
| (182) | Sprague-Dawley | 55 | ? | 10 or 15 | EB | 3 μg s.c. Injection | Once | 48 h | WDS & clonic seizures | No | No | No | Decreased latency | Proconvulsant |
| (60) | Sprague-Dawley | ? | 220–250 | 15 | E2 | 10 μg, s.c. injection | 1/day for 2 days | 48 h | Behavioral seizures | no | Yes (tonic extension) | Yes | Decreased latency | Proconvulsant |
| (63) | Sprague-Dawley | ? | 150–220 | 8 | E2 | 20 μg, s.c. injection | 1/day for 5 days | 24 h? | Behavioral seizures & EEG | ? | Yes (behavioral seizures) | No | No effect | No effect on seizures |
| (62) | Sprague-Dawley | ? | 200–225 | 8.5 | E2 | 0.5–1.0 mg per 21d pellet | Continuous 7 days | ? | N/A | Yes | No | Yes | Decreased mortality | No effect on seizures |
| (61) | Sprague-Dawley | ? | 150–175 | 16 | EB | 2 μg, s.c. injection | 1/day for 2 days | 19 h | Behavioral seizures & status | No | No | Yes | Decreased mortality | No effect on seizures |
A comparison of studies that have tested the effects of estradiol pretreatment on the seizures induced by the convulsant kainic-acid (60–63, 182). Ovx, ovariectomy; E2, 17β-estradiol; EB, estradiol benzoate (a form of estradiol which is more slowly absorbed than 17β-estradiol); WDS, wet dog shakes, N/A, not applicable (because latency was not measured). “Estradiol-to-kainic acid interval” refers to the time between the end of the last injection of estradiol, or the end of the estradiol administration period, to the time of kainic acid administration.
Besides the endocrine state at the time of convulsant administration, a critical issue is the convulsant itself. The choice of convulsant (e.g., kainic acid) is important because estradiol has robust effects on receptors that mediate convulsant actions. For example, estradiol increases KA2, a kainic acid receptor subunit (56), and has been reported to inhibit glutamate uptake (57–59), which would facilitate actions of kainic acid further. Thus, if estradiol is administered before kainic acid, it may upregulate KA2, and kainic acid might then have a more potent effect. Indeed, this possibility is consistent with published reports showing that estradiol decreases the latency to kainic-acid induced seizures, but not to fluorothyl-induced seizures (60,61). While other studies found no effect of estradiol pretreatment on kainic acid-induced seizures, they may have not tested animals at a time after estradiol pretreatment when KA2 had been upregulated (60), or the dose of estradiol could have been too low to influence KA2 (61,62). Another relevant variable is the delay between ovarietomy and convulsant administration (60,63). For example, ovariectomy changes GABA levels, but does so only after 1–2 weeks (55), so seizure threshold could change if experiments were conducted a few days versus several weeks after ovariectomy. In summary, many variables may contribute to the conclusions one can draw about the effect of estradiol on seizures, including choice of convulsant drug, drug doses, and latency from ovariectomy (Table 2). Indeed, estradiol can alter electrolytes (64), vascular permeability (65,66), and blood brain barrier function (67).
Other nonendocrinological considerations—experimental and semantic—are also important. For example, some studies use the term “proconvulsant” if the latency to a convulsant-induced seizure decreases, whereas others examine the severity of a convulsant-induced seizure. However, the ability to decrease the latency to a seizure may be due to different mechanisms than those which govern seizure severity. Indeed, it has been suggested that estradiol may preferentially influence some of the steps involved in generalization rather than all (68). Furthermore, the latency of a convulsant-induced seizure may not be controlled by the same mechanisms that control the latency to convulsant-induced status epilepticus. This has been suggested by a study that examined the effects of estradiol pretreatment on kainic acid-induced seizures: the latency to seizures was influenced, but not status (56). Therefore, studies that examine different aspects of seizures do not necessarily imply an inconsistency in estradiol action.
What is the evidence that estradiol is proconvulsant?
As discussed at the outset of this review, clinical and animal studies prior to 1970 led to a perspective that estrogen is proconvulsant. While there were some problems with those studies (e.g., the complex nature of Premarin, the absence of appropriate controls), those studies provided a strong argument that estradiol could increase many aspects of seizure activity, particularly in an experimental paradigm involving focal seizures. Indeed, later studies that involved kindling have also provided strong support for the conclusion that estradiol facilitates seizures in models of focal seizures (68,69). Interestingly, kindling the amygdala, anterior neocortex, or dorsal hippocampus was sensitive to estradiol, but not ventral hippocampus (69–71). Moreover, the ways estradiol altered kindling depended on the site that was chosen for the kindling stimulation (69,70,72). Even when kindling distinct amygdala nuclei was compared, the effects of estradiol on kindling were not the same (73). Therefore, in spite of the general agreement that estradiol facilitates focally induced seizures, the results may only be applicable to certain brain regions. Presumably these regions contain higher concentrations of ER, or ER that have a greater influence on exctiability.
In contrast to focally induced seizures, when systemic methods to induce seizures have been used in experimental animals—typically, peripheral administration of a convulsant in laboratory rats—the effects of estradiol have been much more variable (Table 2). Therefore, it may be that focal seizures (or at least some focal seizures) are indeed more sensitive to estradiol than seizures induced by systemic convulsant administration. What could account for this difference? Focal models depend on fewer brain regions and, as a result, the problem of heterogeneity of estrogen action in the brain is less problematic. However, this explanation is not entirely satisfactory, considering that in the kindling model, seizures only start focally but eventually involve multiple brain regions as seizures generalize. In addition, estradiol appears to have a diverse array of actions even in one area of the brain. In the section below, estradiol’s actions on neuronal excitability are reviewed to clarify the diverse effects of estradiol on seizures.
The influence of estradiol on neuronal excitability
There are numerous studies to date concerning the actions of estradiol in the CNS. The vast majority of studies suggest that estradiol influences neuronal excitability (i.e., action potential generation and/or synaptic function), but not always in the same direction. To discuss in more detail how estradiol may be able to increase or decrease excitability, we will focus on CA1 hippocampal pyramidal cells, because these cells (and the CA1 region) have been studied extensively. In addition, area CA1 is germane to seizure propagation in limbic circuits, and is relevant to temporal lobe epilepsy.
In area CA1 of hippocampus in the rat, estradiol appears to increase excitability in a several ways (Fig. 4), primarily involving synaptic structure and function rather than membrane properties (74). Most of the data are consistent with an ability of estradiol to increase neuronal discharge by enhancing glutamatergic transmission and also by depressing GABAergic inhibition. What are some of these actions of estradiol? One involves structural changes: estradiol increases the number of spine synapses (75), spine density (76), and spine shape (77), effects that are likely to increase glutamatergic synaptic transmission if one assumes that more spine synapses mean more synapses that mediate larger glutamatergic depolarizations (EPSPs)—and in fact this appears to be the case (78–81). Estradiol also can enhance actions of glutamate at ionotropic glutamate receptors; in area CA1, effects are mostly at NMDA receptors (78–84). Estradiol inhibits GABAergic transmission by decreasing the effects of GABA at GABAA receptors of CA1 pyramidal cells, although this effect may be transient, and accompanied by an increase in the duration of inhibitory postsynaptic currents (IPSCs) (which could mask/complicate the predicted disinhibitory effect on pyramidal cells; 81,85). Repolarization of action potentials is also impaired by estradiol because it decreases the slow afterhyperpolarization mediated by calcium-dependent potassium currents (86,87). Together, all of these effects would be expected to increase action potential generation. The type and magnitude of these cellular effects are likely to be regulated by factors such as estradiol dose and the time after estradiol exposure. Therefore, it may not be surprising that these effects have not been consistently reported across laboratories (79,88). In addition to estradiol dose and timing, differences in experimental preparation also may be an important issue. For example, it is difficult to compare results from in vitro experiments with those from intact animals; indeed, investigators have questioned what studies in culture—on neurons taken from embryonic brains—can tell us about estradiol actions in the mature female. Furthermore, many studies of estradiol action use male rats (which have a distinct distribution and composition of ERα and ERβ; 89). The food and housing of animals appears more important than previously anticipated. Thus, it is now clear that the degree of soy phytoestrogen in the diet of female rodents has a large influence on the effects of estrogen on hippocampal-dependent behavior (90,91). It is also clear that there is a substantial interaction of stress and the glucocorticoid system with effects of estrogen and seizures (87). Finally, the relationship of estradiol to excitability may not be a unidirectional: estradiol clearly influences excitability, but the converse may also be true (92). As a result, the type of experimental preparation and history of the animal may lead to very different effects of estradiol.
FIG. 4.

Effects of 17β-estradiol in area CA1 of rat hippocampus. A summary of potential actions of 17β-estradiol in area CA1 of rat hippocampus. Estradiol activates target genes by nuclear hormone receptors that act as transcription factors. Estradiol also acts by nongenomic mechanisms that involve membrane receptors. Its effects are not only on pyramidal cells but also on GABAergic neurons, cholinergic input, glia, and blood vessels. Glutamatergic transmission may be influenced in a number of ways, by pre- and postsynaptic mechanisms. In addition, ion channels on pyramidal cells are modulated by estradiol, influencing neuronal firing behavior. For further description and references, see text.
Besides direct actions, estradiol also has a number of actions that indirectly modify excitability. For example, estradiol has been shown to increase acetylcholine release (93), and it interacts with the cholinergic system to alter NMDA receptor binding in CA1 (94). In hippocampus, the interactions of estradiol and the cholinergic system are largely attributed to septohippocampal projections (95), which express ERα presynaptically, and also express ERα on postsynaptic targets within hippocampus (96). The estradiol-cholinergic relationship appears to have a muscarinic component (94), and possibly a nicotinic component in area CA3 (97). The muscarinic contribution is important to bear in mind when comparing studies of estradiol effects on the seizures induced by the muscarinic convulsant, pilocarpine, with estradiol effects on seizures elicited by other convulsants (see ref. 98 for further discussion). For example, if estradiol and muscarinic cholinergic receptor activation increases NMDA receptor binding, estradiol might facilitate the seizures induced by pilocarpine, not because of direct estradiol effects, but because of its interaction with the particular choice of convulsant.
Other notable effects of estradiol on excitability are mediated by indirect mechanisms, and one example that is especially relevant to seizures is its regulation of the neurotrophin, brain-derived neurotrophic factor (BDNF). Estrogen has a response element on the BDNF gene (99), and BDNF potentiates several of the glutamatergic pathways in hippocampus and other brain regions (100). Using diverse animal models of epilespy, several laboratories have provided evidence that BDNF may be proconvulsant (101). Therefore, the estradiol surge during the periovulatory period may lead to a transient elevation in seizure frequency, particularly limbic seizures, because it induces BDNF (102). Furthermore, BDNF appears to induce neuropeptide Y (NPY;103–105), and NPY has actions which are generally consistent with an anticonvulsant effect, most likely due to its presynaptic actions that depress neurotransmitter release in hippocampus (106). However, like estradiol, the effects of BDNF are also hard to reproduce in vitro, and both BDNF and NPY vary in their effects depending on dose, duration of exposure, and brain region examined.
The effects summarized above support the concept that estradiol can increase excitability, and that it is a proconvulsant agent. However, there is another perspective. Estradiol has been shown to increases the levels of glutamic acid decarboxylase (GAD), the synthetic enzyme for γ-aminobutyric acid (GABA;81,107), and thus increase levels of GABA. ERα is expressed in subsets of GABAergic neurons (108). Estradiol also has other effects consistent with an “anticonvulsant action,” such as the ability to increase the expression of NPY (109,110), as indicated above. Furthermore, estradiol has been shown to be neuroprotective, an effect that most would not find easy to reconcile with a proconvulsant action (110,111).
How can these disparate results be interpreted? It is helpful to consider the studies that have used a nonphysiological endocrine state (such as gonadectomy) and supraphysiological doses of estradiol separately from studies that examine rats under physiological conditions (i.e., examine responses to the normal, physiological rise in estradiol at proestrus). Supraphysiologic doses may be misleading because estrogen-specific responses may actually decrease excitability if dose is high. In addition, supra-physiological concentrations of estradiol bind to the PR (112). However, estradiol levels are not always measured, and estradiol-progesterone interactions are not always considered. Moreover, it is important to remember that different studies use different ages of animals, treat animals at different times after ovariectomy, handle and feed animals inconsistently, and examine them with different endpoints. Under different conditions, it is likely that estradiol has very different—even opposite—effects.
Progesterone
The influence of progestins on seizures
Unlike the relationship between estradiol and seizures, the studies of progesterone on seizures point in a more uniform direction: in general, progesterone administration appears to be anticonvulsant. In laboratory animals, injection of progesterone leads to a decrease in seizure threshold, or delay to the onset of seizures induced by convulsants (113–115). Clinical studies have also shown the efficacy of progesterone to decrease seizure frequency in women with epilepsy (116). However, it is not always clear whether progesterone is responsible for these effects, or the effects are mediated through its metabolites. In animals, it has been argued that allopreganolone is the primary effector, because blockade of 5α-reductase, the enzyme that controls the first step in progesterone metabolism to allopregnanolone (Fig. 2), is able to delay the onset and decrease the incidence of pentylenetetrazol-induced seizures (117,118).
Interestingly, synthetic progestins, such as those used in contraceptives, do not necessarily have an anticonvulsant effect. Indeed, contraceptives can increase seizure frequency (119), although this is not typical (120) and some patients appear to benefit (121). The different effects of contraceptives on seizures relative to “natural” progestins (like progesterone) are likely to be due to their inability to be converted to allopregnanolone, as well as other factors (described in the accompanying review by O’Brien and Guillebaud).
The influence of progesterone and neuronal excitability
Allopregnanolone and the GABAA receptor
Studies described above suggested that allopregnanolone might be responsible for the decreased seizure susceptibility when serum progesterone is elevated, and the anticonvulsant actions of progesterone administration. A series of elegant in vitro studies have shown that allopregnanolone enhances the actions of GABA at GABAA receptors by an allosteric mechanism at a specific “neurosteroid” binding site (122–124). The expression of allopregnanolone-sensitive GABAA receptors seems widespread and robust under many experimental conditions (tissue culture, dissociated cells, brain slices, etc.), which is consistent with the robust anticonvulsant effects of progesterone even when experimental designs are different. However, it is important to point out that other progestins besides allopregnanolone—i.e., progesterone and dihydroprogesterone—may have actions that are relevant to seizure sensitivity. For example, one study suggests that PR activation by progesterone can decrease neuronal excitability in hippocampus, independent of allopregnanolone formation (125). PR- and allopregnanolone-mediated actions are assumed to be independent but this may not always be the case, based on findings in PR knockout mice (126).
PR-mediated effects of progestins are potentially important because they may be enhanced if estradiol rises. Many of the effects of progestin on the brain, particularly those associated with reproductive function, are estrogen-dependent. This interaction would be predicted because of the interdependence of ER and PR synthesis (see above). Indeed, estradiol induces PR synthesis (24,127). Conversely, progestins act via their nuclear receptors to inhibit ER function (128). These findings suggest that the effects of progesterone are dependent on the concurrent levels of estradiol and vice versa. In addition, the temporal sequence of estrogen and progestin exposure may dramatically affect the outcome of exposure to these two hormones. Interestingly, the duration of exposure to estradiol may also alter the actions of estrogen at ERs, independent of progesterone. Exposure to estrogen down-regulates ERα and ERβ expression in the brain over time (129–131), although acute estradiol exposure in vitro can upregulate ERα (132).
The interaction between progesterone and estradiol has implications for the use of progesterone therapy in women with epilepsy. Thus, progesterone administration may exert two effects, actions at PR that may depend on concurrent estradiol levels, and actions at GABAA receptors after conversion to allopregnanolone. Importantly, even the latter could depend on serum estradiol concentration, in part because estradiol alters GABA levels, GABA synthesis, and regulates the reversal potential of the GABAA receptor by changing expression of KCC2, a K+/Cl− co-transporter (as discussed above). If true, progesterone administration during the periovulatory period might not be as effective as a regimen that introduced progesterone at the end of the luteal phase, when serum estradiol is low. These considerations may help explain why progesterone is effective when used at times besides the periovulatory period (133) but not necessarily continuously (134).
Progesterone withdrawal and GABAA receptor subunit composition
Although the concentration of serum progesterone is clearly an important issue, there is also evidence that the fluctuations in its concentration may be important. Indeed, perhaps the most common hypothesis to explain the increase in seizures that occurs at the time of menses in many women with epilepsy is the fact that progesterone has rapidly declined just before that time, a phenomenon that has been described as “progesterone withdrawal” (see Fig. 5; 114). In laboratory animals, decreased serum progesterone during the ovarian cycle appears to have a similar effect (135). Progesterone withdrawal has also been studied in animals with chronic progestin administration followed by abrupt discontinuation of the steroid (136–139). In these animals, there is a decrease in seizure threshold after progesterone administration ends (135,136,139).
FIG. 5.
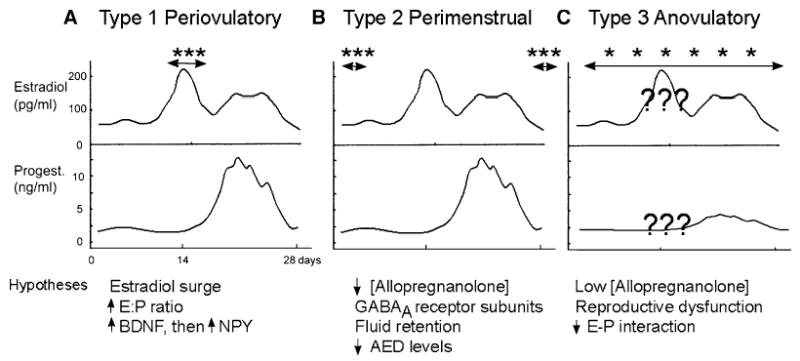
Catamenial epilepsy subtypes and associated mechanistic hypotheses. The subtypes of catamenial epilepsy proposed by Herzog and colleagues (149) are shown schematically, using a diagram of the fluctuations in estradiol and progesterone during the menstrual cycle of a normal adult woman (modified from 184). Note that the normal menstrual cycle is assumed to occur in women with epilepsy. However, irregular fluctuations in serum levels of estrogen and progesterone have been reported (148), so the assumption may not be true in all cases. Double-headed arrows with adjacent asterisks are placed over the schematic to reflect the time of the menstrual cycle when seizures increase (in frequency or severity) for each subtype. It has also been reported that seizures may change in nature (7). Hypotheses that have been suggested to explain the seizure patterns are listed below the diagram of each subtype (see text).
Animal studies have determined that the likely reason for the increase in excitability following sudden loss of progesterone is due to altered expression of GABAA receptor subunits. GABAA receptors normally are heteromeric complexes of various subunits, and subunit combination confers specificity for modulators such as allopregnanolone and benzodiazepines (124). It also appears that different GABAA receptor combinations subserve distinct functions. GABAA receptors with δ subunits, sub-serve “tonic” inhibition. Tonic inhibition is primarily mediated by ambient GABA release at extrasynaptic GABAA receptors, creating an inhibitory “tone” that has been suggested to reduce excitability in a widespread and continuous manner. Indeed, transgenic animals lacking δ subunits, or normal mice treated with antisense mRNA to the δ subunit, demonstrated decreased latency to seizures induced by kainic acid, and longer periods of seizures (135).
Studies in laboratory mice have determined that there are changes in the expression of GABAA receptor subunits associated with tonic inhibition during the normal ovarian cycle, and these changes in turn alter the sensitivity of GABAA receptors to modulators like allogpregnanolone, as well as the degree of tonic inhibition (135). In the mouse, δ subunit expression is relatively high during the period of the cycle associated with elevated serum progesterone, and low levels of the δ subunit were found at a time of the cycle when serum progesterone was low. In the same mice, tonic inhibition and seizure susceptibility correlated with the levels of δ subunits. Interestingly, γ2 subunit expression also differed between the two times of the cycle that were examined (135), so there may be a complex series of GABA receptor alterations that do not solely involve δ subunits. The importance of other types of subunits is underscored by the fact that tonic inhibition is not only dependent on δ subunits. Another subunit that appears to contribute to tonic inhibition is the α4 subunit, and indeed this appears to be upregulated in an animal model of progesterone withdrawal (138). While these data provide new insight into the mechanisms that underlie changes in seizures during the ovarian cycle, they also suggest that more information will be necessary before a comprehensive understanding is possible. Some of the issues that need to be addressed include the results that are model-specific, area-specific, or related to differences in species, because data from cycling mice and the progesterone-withdrawal model in rats do not agree completely (135,138).
EPILEPSY
Hormone-sensitive epilepsy
It has been clear since the time of Gowers (2) that there are effects of reproductive hormones on seizures of women with epilepsy. Why, then, are some women with epilepsy more sensitive to hormonal fluctuations than others? Epidemiological data have not provided compelling evidence that hormone sensitivity is more common in one syndrome versus another in the few studies that have made comparisons (140). One possibility is that only specific types of epilepsy may be sensitive to reproductive steroids, depending on the underlying cause and associated pathology. For example, if a focus develops in a brain area with strong connections to hypothalamic structures, e.g., the amygdala, one might predict that hormones in the circulation would modulate activity in the focus. Indeed, this possibility may explain why kindled seizures are regulated by estradiol if the site of kindling is in the amygdala, but not necessarily elsewhere (68–73). Alternatively, if neuronal loss occurs in the epileptic brain, there could be compensatory changes in local steroid production (e.g., increased estradiol synthesis in reactive glia, as described above), leading to enhanced sensitivity of that brain area to circulating steroids. If a genetic cause exists, and the defect involves a protein normally modulated by estradiol or progesterone, the defective gene could confer increased, or altered, responses to gonadal hormones.
Although it is not clear the fraction of women with epilepsy who have seizures that are susceptible to fluctuations in reproductive hormone levels, it is likely many do—and that there could be diverse reasons. Unfortunately, these patients are poorly characterized to date. Below we focus on the subset of women with hormone-sensitive seizures who appear to follow a pattern: those with catamenial epilepsy. It is acknowledged that this subpopulation is difficult to define (141–143). Therefore, our understanding of this subset of patients remains somewhat unclear.
Catamenial epilepsy
“Catamenial epilepsy” is a phrase derived from the Greek word catamenia, which refers to seizures occurring at menstruation. However, it has become clear that other times of the menstrual cycle, such as ovulation, can be associated with changes in seizures. In addition, there can be increases in seizure frequency when there is an anovulatory cycle. Therefore, “catamenial epilepsy” is now used to refer to changes in seizures—frequency, severity, or type—if they appear to be associated with hormonal fluctuations during the menstrual cycle. In using this phrase, we tend to assume that that these hormonal fluctuations contribute to, or cause, the changes in seizures. However, as will be discussed further below, the causes of catamenial epilepsy have not been definitively identified, and there may be many factors that are relevant besides serum estradiol and progesterone levels.
As suggested above, the prevalence of catamenial epilepsy in women with epilepsy is subject to debate. Some individuals predict prevalence is greater than 30%, but others are more cautious (141,144–146). The difficulty in estimating prevalence may be inherent in the criteria used to define these patients: some studies may mandate precise cycles with exactly the same pattern for each cycle, while other studies are not so strict about the definition. The problem with the stricter approach is that there could be variability from cycle to cycle. Indeed, many normal women have different cycle lengths or variation in the time of ovulation during a cycle, reflecting variability in hormonal fluctuations in the normal condition. Thus, one would predict the same might be the case for women with epilepsy. Indeed, women with epilepsy appear to have irregular fluctuations in estrogen and progesterone, possibly exacerbated by recurrent seizures (147,148). The strict criteria are useful for a definition of a clinical syndrome, but they can obviously lead to exclusion of patients who, in the case of catamenial epilepsy, should be included. Another problem is the primary tool used to define the catamenial epilepsy—self-reporting of seizures from the patients themselves. Patient self-reports, diaries, or other “uncontrolled” accounts have inherent inaccuracy. Indeed, some patients report an increase in seizures at menses, but this could not be demonstrated in the same people when they were examined in a clinical study (146).
Nevertheless, there have been many studies of catamenial seizure patterns in women with epilepsy to try to define the fundamental causes. Herzog and colleagues have proposed that there are three primary types of catamenial epilepsy, and they provide a useful framework to evaluate current hypotheses. Fig. 5 illustrates the three types of catamenial epilepsy schematically (149). Prevailing and alternative hypotheses are listed in Fig. 5, and discussed below.
Periovulatory catamenial epilepsy (type I): estradiol, the E:P ratio, and BDNF/NPY
The first type of catamenial epilepsy recognized by Herzog and colleagues (149) is characterized by a rise in seizure frequency during the so-called periovulatory period. This is the time when seizures rise (in frequency or severity) as estradiol surges, just before ovulation. Subsequently, both estradiol levels, and seizures, fall (Figs. 1 and 5). Given the coincident increase in seizures and serum estradiol levels, one might suspect that serum estradiol contributes to the increase in seizures at this time. However, there are several reasons to question such a linear relationship between estradiol and seizures. First, as discussed above, there is limited evidence that the dose–response relationship for estradiol is linear. Second, clinical studies of women with catamenial epilepsy that have tried to correlate serum estradiol with seizure frequency have failed to find a significant correlation (7). Some investigators even find it difficult to detect a periovulatory rise in seizure frequency at all (150,151). Finally, some women with catamenial epilepsy have an increase in seizures at the time of ovulation, as well as an increase at menses (144,149). The latter is difficult to explain purely on the basis of serum estradiol levels.
It has therefore been suggested that the serum level of estradiol and progesterone, considered together, can explain the periovulatory rise in seizures. This relationship, the ratio of serum estradiol to serum progesterone (the E:P ratio), significantly correlated with seizure patterns in a study of 7 patients (7). It explains seizures at ovulation (high E and low P, a high E:P ratio), but is less able to explain the seizures at menses (low E and low P, not a high E:P ratio). Interestingly, even in the studies of individuals by Bäckstrom (7), the E:P ratio did not provide a clear explanation for each case. The variability from patient to patient was striking (7), possibly due to factors outside the scope of the study, such as the different causes/syndromes, patient history, different anticonvulsant drugs, etc.
Are there alternative hypotheses besides the E:P ratio? Many changes in the brain occur during the periovulatory period, and some are not directly related to estradiol or progesterone. For example, there is a rise in glucocorticoid levels during the LH-FSH surge at ovulation (152). Other hormones that are associated with the estradiol surge such as LH and FSH, would be predicted to have effects on seizure threshold (Table 1).
Some of the target genes of estrogen may help explain periovulatory seizures. One example is the BDNF gene. As described above, sequential induction of BDNF followed by NPY could explain the transient rise in seizure frequency (due to BDNF) and fall (due to NPY) during the periovulatory period. Alternatively, the beginning of the luteal phase, and elevation in serum progesterone, could terminate the change in BDNF, because progesterone administration to female rats leads to a decline in BDNF levels (153). Therefore, BDNF or NPY levels may predict seizures better than the E:P ratio; E only indirectly leads to changes in excitability, while BDNF and NPY may be the primary effectors.
Perimenstrual catamenial epilepsy (type II): allopregnanolone, progesterone withdrawal, water retention, and anticonvulsant drug levels
The second type of catamenial epilepsy described by Herzog and colleagues (149) is associated with increased seizures during the perimenstrual period (Fig. 5). The perimenstrual period includes the days when menses occurs, but some investigators also include a few days before and after this time. The association of this time with increased seizure frequency has perhaps the widest clinical acceptance because it was the first pattern that was recognized clinically, and has remained the most commonly observed (1,2,4,149).
One of the explanations for the rise in seizures during the perimenstrual period is the decrease in progesterone during the second half of the menstrual cycle, which remains low during menses (Fig. 1). Indeed, seizure frequency does appear to correlate inversely with progesterone levels (7). The fall in serum progesterone has been suggested to be important because it would lead to a decline in its metabolite, allopregnanolone. The prediction is that GABAergic inhibition would weaken, because allopregnanolone normally facilitates the action of GABA at GABAA receptors. This hypothesis is supported by the studies in rodents described above. In addition, changes in GABAA receptor composition upon “progesterone withdrawal,” discussed above, could contribute to perimenstrual seizures.
A potentially important issue that was identified even in the earliest clinical studies was the possibility that water retention at the end of the luteal phase could play a role in the rise in seizures at menses (6). This hypothesis is attractive given recent studies showing that neuronal swelling increases excitability in vitro and seizures in vivo (154). Indeed, this mechanism has been used to explain the success of diuretic treatments in some patients with epilepsy (although diuretics are not widely used as monotherapies; 115). The diuretic acetazolamide has efficacy in women with catamenial epilepsy (156), but it is currently unclear whether the effectiveness of acetazolamide in these patients is related specifically to catamenial epilepsy (i.e., diuretics may suppress seizures in any patient with epilepsy).
Another factor to consider is related to steroid metabolism. Antiepileptic drugs such as phenytoin influence steroid biosynthesis, leading to increased synthesis of androgen (and potentially estradiol) because of induction of hepatic microsomal enzymes (Fig. 2). The end result would be a decrease in progesterone levels in favor of downstream androgen and estradiol. This is complicated by the fact that phenytoin levels have been reported to decline at menses, particularly in catamenial epileptics (151,157,158).
Anovulatory catamenial epilepsy (type III): anovulatory cycles and the problem of reproductive dysfunction
The third type of catamenial epilepsy defined by Herzog and colleagues (149) are seizures which increase during anovulatory cycles. In these women, serum progesterone is decreased during the luteal phase, reflecting a failure to ovulate. The most common hypothesis to explain seizures associated with anovulatory cycles is that serum progesterone and allopregnanolone are insufficient to retain the necessary activity of brain GABAA receptors.
In actuality, the situation may be much more complex, not only because GABAergic regulation of excitability is complicated, but also because a deficit in progesterone levels may not be the only problem during anovulatory cycles. For example, there may be alterations in many aspects of neuroendocrine function if ovulation does not occur. Indeed, in many of the studies of women with recurrent seizures, there is often a concurrent level of reproductive dysfunction. Recurrent seizures may induce changes in reproductive function (159) and the consequences may lead to changes which influence seizure threshold (147). An example is the woman who has polycystic ovarian syndrome (PCOS), which leads to high androgen levels (160). High serum androgen would be likely to act on brain androgen receptors to alter neuronal structure and function. Women with PCOS also are characterized by persistent elevation in serum estradiol, which may have effects on brain ER similar to chronic estradiol administration. Importantly, even women who do not have PCOS may still have alterations in the hypothalamic-pituitary-adrenal (HPA) axis, which could influence brain excitability.
Animal models of catamenial epilepsy
The discussion above provides an overview of the many hypotheses and questions that currently are associated with the subject of catamenial epilepsy. Although clinical studies address many of the issues, there are considerable obstacles to the kinds of clinical research that would be most helpful. For example, how can one conduct long-term studies on patients with regular hormonal and seizure evaluation? How can one tease apart the effects of recurrent seizures (i.e., epilepsy per se) versus the effects of anticonvulsant drugs?
These difficulties make it advantageous to consider an animal model of catamenial epilepsy. Efforts to evaluate such models have been valuable, but there are significant limitations. One major concern is the substantial endocrinological differences between animals commonly used for such studies, (i.e., rats or mice) and women. For example, rodents have a 4–7-day estrous cycle and a woman has a cycle that lasts approximately 28 days. Although there are many parallels (Fig. 1), there are numerous endocrinological differences (161,162).
One of the striking aspects of the animal literature is that few studies have been undertaken even to examine a female rat with spontaneous recurrent seizures, let alone a female rat with seizures that fit a catamenial pattern. Instead, many conclusions seem based on studies that have administered steroid hormones to normal rats. It would seem that the most useful approach would be to study female rats with epilepsy, and to specifically follow the changes in seizures (i.e, frequency, duration, severity) as a function of serum levels of reproductive steroids. Perhaps surprisingly, this type of study has not been carried out—although for a very good reason. It is difficult to induce epileptogenesis in female rats and at the same time maintain their normal ovarian cyclicity. Amado and colleagues induced spontaneous recurrent seizures using pilocarpine-induced status epilepticus as an initial stimulus (163), or intracerebral kainic acid (164), but most female rats stopped cycling regularly. Although the reasons are unclear, it appears that status epilepticus or recurrent seizures lead to damage in the areas of the brain that are important to maintaining cyclicity (i.e., the hypothalamus; 165,166). Therefore, better animal models will be important to model women with catamenial epilepsy.
Still, there are many issues relevant to women with epilepsy that can be studied in laboratory rats. For example, a PCOS-like condition also develops after kindling (167) or pilocarpine-induced status epilepticus and recurrent seizures (168). Studies of such models may help to resolve clinical issues that are difficult to address in patients. Indeed, it has been difficult to determine whether anticonvulsants or recurrent seizures cause PCOS in a clinical setting, because medication cannot be withheld from patients. This difficulty has led to considerable debate about the cause of cystic ovaries in women with epilepsy. Animal studies can be advantageous by allowing the effects of distinct variables to be defined. For example, experiments in rats have shown that valproate can alter ovarian morphology independent of seizures (169). In addition, repeated seizures in the absence of anticonvulsants can lead to a significantly higher incidence of cystic ovaries relative to age-matched controls (167,168).
SUMMARY AND OUTLOOK
The influence of gonadal hormones on neuronal excitablity, seizures, and epilepsy in the female is a complicated topic. Advances in our understanding of hormone action and the neurobiology of epilepsy have provided greater insight into the interaction between gonadal hormones and epilepsy. They also provide insight into the types of future studies that merit consideration to advance our understanding.
Evidence accumulated to date does not offer a strong scientific basis for the dogma that estradiol is proconvulsant. The data do show, however, that estradiol has potent effects on neuronal excitability and no doubt influences seizure activity in women with epilepsy. The multitude of effects of estradiol may be best interpreted in light of the new appreciation that estradiol is a hormone which changes its actions depending on context. This view is consistent with the idea that estradiol, and its associated receptors, evolved to optimize the ability of the female to function in many different capacities. However, it is inconsistent with a singular action of estradiol on seizures and epilepsy.
In contrast, there is relatively consistent evidence to support the view that progesterone, at least its metabolite allopregnanolone, is anticonvulsant. However, there remain many unanswered questions. In short, progestins simply have not been studied enough to date to allow one to generalize the animal data to the clinic.
Given the current uncertainties, what would be the best directions for future efforts? Experiments should be designed to have the greatest relevance to the clinical population of interest. Thus, an animal that is gonadectomized early in life may not be relevant to the postmenopausal woman who loses ovarian function at age 50. An important direction is to carefully examine the effects of steroids, or steroid fluctuations, in animals with chronic seizures rather than seizure induction in normal rats.
Acknowledgments
We acknowledge the support from NIH grant NS 37562. We thank Philip Schwartzkroin for his constructive comments.
References
- 1.Temkin O. The falling sickness. Baltimore: The Johns Hopkins University Press; 1971. [Google Scholar]
- 2.Gowers WR. Epilepsy and other chronic convulsive diseases. London: J. A. Churchill; 1881. [Google Scholar]
- 3.Gordon A. Epilepsy in its relation to menstrual periods. NY Med J. 1909;XC:733–5. [Google Scholar]
- 4.Turner WA. Epilepsy: study of idiopathic disease. New York: Macmillan Co; 1907. [Google Scholar]
- 5.Selye H. The antagonism between anesthetic steroid hormones and pentamethylenetetrazol (Metrazol) J Lab Clin Med. 1942;27:1052–3. [Google Scholar]
- 6.Ansell B, Clarke E. Epilepsy and menstruation: the role of water retention. Lancet. 1956;2:1232–5. doi: 10.1016/s0140-6736(56)90002-2. [DOI] [PubMed] [Google Scholar]
- 7.Bäckstrom T. Epileptic seizures in women in relation to variations of plasma estrogen and progesterone during the menstrual cycle. Acta Psych Scand. 1976;54:321–47. doi: 10.1111/j.1600-0404.1976.tb04363.x. [DOI] [PubMed] [Google Scholar]
- 8.Laidlaw J. Catamenial epilepsy. Lancet. 1956;2:1235–7. doi: 10.1016/s0140-6736(56)90003-4. [DOI] [PubMed] [Google Scholar]
- 9.Mattson RH, Lerner E, Dix G. Precipitating and inhibiting factors in epilepsy: a statistical study. Epilepsia. 1974;15:271–2. [Google Scholar]
- 10.Spiegel E, Wycis H. Anticonvulsant effects of steroids. J Lab Clin Med. 1945;30:947–53. [Google Scholar]
- 11.Costa PJ, Bonnycastle DD. The effect of DCA compound E testosterone, progesterone, and ACTH in modifying gene-induced convulsions in dogs. Arch Int Pharmacodyn Ther. 1952;91:330–8. [PubMed] [Google Scholar]
- 12.Logothetis I, Harner R, Morell F, et al. The role of estrogens in catamenial exacerbation of epilepsy. Neurology. 1959;9:352–60. doi: 10.1212/wnl.9.5.352. [DOI] [PubMed] [Google Scholar]
- 13.Logothetis J, Harner R. Electrocortical activation by estrogens. Arch Neurol. 1960;3:290–7. doi: 10.1001/archneur.1960.00450030068007. [DOI] [PubMed] [Google Scholar]
- 14.Marcus EM, Watson CW, Goldman PL. Effects of steroids on cerebral electrical activity. Epileptogenic effects of conjugated estrogens and related compounds in the cat and rabbit. Arch Neurol. 1966;15:521–32. doi: 10.1001/archneur.1966.00470170075008. [DOI] [PubMed] [Google Scholar]
- 15.McQueen JK, Woodbury DM. Attempts to produce spike-and-wave complexes in the electrocorticogram of the rat. Epilepsia. 1975;16:295–300. doi: 10.1111/j.1528-1157.1975.tb06060.x. [DOI] [PubMed] [Google Scholar]
- 16.Julien RM, Fowler GW, Danielson MG. The effects of antiepileptic drugs on estrogen-induced electrographic spike-wave discharge. J Pharmacol Exp Ther. 1975;193:647–56. [PubMed] [Google Scholar]
- 17.Woolley DE, Timiras PS. The gonad-brain relationship: effects of female sex hormones on electroshock convulsions in the rat. Endrocrinology. 1962;70:196–209. doi: 10.1210/endo-70-2-196. [DOI] [PubMed] [Google Scholar]
- 18.Stitt SL, Kinnard WJ. The effects of certain progestins and estrogen on the threshold of electrically induced seizure patterns. Neurology. 1968;18:213–6. doi: 10.1212/wnl.18.3.213. [DOI] [PubMed] [Google Scholar]
- 19.Rush ME, Blake CA. Serum testosterone concentrations during the 4-day estrous cycle in normal and adrenalectomized rats. Proc Soc Exp Biol Med. 1982;169:216–21. doi: 10.3181/00379727-169-41334. [DOI] [PubMed] [Google Scholar]
- 20.Bernardi F, Salvestroni C, Casarosa E, et al. Aging is associated with changes in allopregnanolone concentrations in brain, endocrine glands and serum in male rats. Eur J Endocrinol. 1998;38:316–21. doi: 10.1530/eje.0.1380316. [DOI] [PubMed] [Google Scholar]
- 21.Paul SM, Purdy RH. Neuroactive steroids. FASEB J. 1992;6:2311–22. [PubMed] [Google Scholar]
- 22.Beyenburg S, Stoffel-Wagner B, Bauer J, et al. Neuroactive steroids and seizure susceptibility. Epilepsy Res. 2001;44:141–53. doi: 10.1016/s0920-1211(01)00194-2. [DOI] [PubMed] [Google Scholar]
- 23.Garcia-Segura LM, Wozniak A, Azcoitia I, et al. Aromatase expression by astrocytes after brain injury: implications for local estrogen formation in brain repair. Neuroscience. 1999;89:567–78. doi: 10.1016/s0306-4522(98)00340-6. [DOI] [PubMed] [Google Scholar]
- 24.Parsons B, Rainbow TC, MacLusky NJ, et al. Progestin receptor levels in rat hypothalamic and limbic nuclei. J Neurosci. 1982;2:1446–52. doi: 10.1523/JNEUROSCI.02-10-01446.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.MacLusky NJ, McEwen BS. Progesterone receptors in rat brain: distribution and properties of cytoplasmic progestin binding sites. Endocrinology. 1980;106:192–202. doi: 10.1210/endo-106-1-192. [DOI] [PubMed] [Google Scholar]
- 26.Feder HH, Marrone BL. Progesterone: its role in the central nervous system as a facilitator and inhibitor of sexual behavior and gonadotropin release. Ann N Y Acad Sci. 1977;286:331–54. doi: 10.1111/j.1749-6632.1977.tb29428.x. [DOI] [PubMed] [Google Scholar]
- 27.Clark JH, Hsueh AJ, Peck EJ., Jr Regulation of estrogen receptor replenishment by progesterone. Ann N Y Acad Sci. 1977;286:161–79. doi: 10.1111/j.1749-6632.1977.tb29414.x. [DOI] [PubMed] [Google Scholar]
- 28.Okulicz WC, Evans RW, Leavitt WW. Progesterone regulation of estrogen receptor in the rat uterus: a primary inhibitory influence on the nuclear fraction. Steroids. 1981;37:463–71. doi: 10.1016/0039-128x(81)90047-7. [DOI] [PubMed] [Google Scholar]
- 29.Edwards DA, Pfeifle JK. Hormonal control of receptivity, proceptivity and sexual motivation. Physiol Behav. 1983;30:437–43. doi: 10.1016/0031-9384(83)90150-6. [DOI] [PubMed] [Google Scholar]
- 30.Etgen AM. Progestin receptors and the activation of female reproductive behavior: a critical review. Behav Brain Res. 1984;15:93–103. doi: 10.1016/0018-506x(84)90027-8. [DOI] [PubMed] [Google Scholar]
- 31.Margeat E, Bourdoncle A, Margueron R, et al. Ligands differentially modulate the protein interactions of the human estrogen receptors α and β. J Mol Biol. 2003;326:77–92. doi: 10.1016/s0022-2836(02)01355-4. [DOI] [PubMed] [Google Scholar]
- 32.Clark JH, Paszko Z, Peck EJ., Jr Nuclear binding and retention of the receptor estrogen complex: relation to the agonistic and antagonistic properties of estriol. Endocrinology. 1977;100:91–96. doi: 10.1210/endo-100-1-91. [DOI] [PubMed] [Google Scholar]
- 33.Dey M, Lyttle CR, Pickar JH. Recent insights into the varying activity of estrogens. Maturitas. 2000;34(suppl 2):S25–33. doi: 10.1016/s0378-5122(00)00110-9. [DOI] [PubMed] [Google Scholar]
- 34.Harden CL, Koppel BS, Herzog AG, et al. Seizure frequency is associated with age at menopause in women with epilepsy. Neurology. 2003;61(6 suppl 2):S16–22. doi: 10.1212/01.wnl.0000081228.48016.44. [DOI] [PubMed] [Google Scholar]
- 35.Harden CL, Pulver MC, Ravdin L, et al. The effect of menopause and perimenopause on the course of epilepsy. Epilepsia. 1999;40:1402–7. doi: 10.1111/j.1528-1157.1999.tb02012.x. [DOI] [PubMed] [Google Scholar]
- 36.Toran-Allerand CD, Tinnikov AA, Singh RJ, et al. 17α-estradiol: a brain-active estrogen? Endocrinology. 2005;146:3842–50. doi: 10.1210/en.2004-1616. [DOI] [PubMed] [Google Scholar]
- 37.Toran-Allerand CD. Novel sites and mechanisms of oestrogen action in the brain. Novartis Found Symp. 2000;230:56–69. doi: 10.1002/0470870818.ch6. [DOI] [PubMed] [Google Scholar]
- 38.MacLusky NJ, Luine VN, Hajszan T, et al. The 17α and 17β isomers of estradiol both induce rapid spine synapse formation in the CA1 hippocampal subfield of ovariectomized female rats. Endocrinology. 2005;146:287–93. doi: 10.1210/en.2004-0730. [DOI] [PubMed] [Google Scholar]
- 39.Luine VN, Jacome LF, Maclusky NJ. Rapid enhancement of visual and place memory by estrogens in rats. Endocrinology. 2003;144:2836–44. doi: 10.1210/en.2003-0004. [DOI] [PubMed] [Google Scholar]
- 40.Pak TR, Chung WC, Lund TD, et al. The androgen metabolite, 5α-androstane-3β, 17β-diol, is a potent modulator of estrogen receptor-β1-mediated gene transcription in neuronal cells. Endocrinology. 2005;146:147–55. doi: 10.1210/en.2004-0871. [DOI] [PubMed] [Google Scholar]
- 41.Lewandowski SKK, Kaczmarek L. Estrogen receptor. Potential functional significance of a variety of mRNA isoforms. FEBS Lett. 2002;524:1–5. doi: 10.1016/s0014-5793(02)03015-6. [DOI] [PubMed] [Google Scholar]
- 42.Matthews J, Gustafsson JA. Estrogen signaling: a subtle balance between ER α and ER α. Mol Interv. 2004;3:281–92. doi: 10.1124/mi.3.5.281. [DOI] [PubMed] [Google Scholar]
- 43.Setchell KD, Clerici C, Lephart ED, et al. S-equol, a potent ligand for estrogen receptor β, is the exclusive enantiomeric form of the soy isoflavone metabolite produced by human intestinal bacterial flora. Am J Clin Nutr. 2005;81:1072–9. doi: 10.1093/ajcn/81.5.1072. [DOI] [PubMed] [Google Scholar]
- 44.Ramirez VD, Zheng J. Membrane sex-steroid receptors in the brain. Front Neuroendocrinol. 1996;17:402–39. doi: 10.1006/frne.1996.0011. [DOI] [PubMed] [Google Scholar]
- 45.Wang Z, Zhang X, Shen P, et al. A variant of estrogen receptor-{α}, hER-{α}36: transduction of estrogen- and antiestrogen-dependent membrane-initiated mitogenic signaling. Proc Natl Acad Sci U S A. 2006;103:9063–8. doi: 10.1073/pnas.0603339103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wade CB, Robinson S, Shapiro RA, et al. Estrogen receptor (ER)α and ERβ exhibit unique pharmacologic properties when coupled to activation of the mitogen-activated protein kinase pathway. Endocrinology. 2001;142:2336–2342. doi: 10.1210/endo.142.6.8071. [DOI] [PubMed] [Google Scholar]
- 47.Toran-Allerand CD, Guan X, Maclusky NJ, et al. ER-X: a novel, plasma membrane-associated, putative estrogen receptor that is regulated during development and after ischemic brain injury. J Neurosci. 2002;22:8391–401. doi: 10.1523/JNEUROSCI.22-19-08391.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Melcangi RC, Magnaghi V, Martini L. Steroid metabolism and effects in central and peripheral glial cells. J Neurobiol. 1999;40:471–83. [PubMed] [Google Scholar]
- 49.Frye CA, Duncan JE, Basham M, et al. Behavioral effects of 3α-androstanediol. II: hypothalamic and preoptic area actions via a GABAergic mechanism. Behav Brain Res. 1996;79:119–30. doi: 10.1016/0166-4328(96)00005-8. [DOI] [PubMed] [Google Scholar]
- 50.Kaminski RM, Marini H, Kim WJ, et al. Anticonvulsant activity of androsterone and etiocholanolone. Epilepsia. 2005;46:819–27. doi: 10.1111/j.1528-1167.2005.00705.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Reddy D. Anticonvulsant activity of the testosterone-derived neurosteroid 3α-androstanediol. Neuroreport. 2004;15:515–8. doi: 10.1097/00001756-200403010-00026. [DOI] [PubMed] [Google Scholar]
- 52.Mani SK, Allen JM, Clark JH, et al. Convergent pathways for steroid hormone- and neurotransmitter-induced rat sexual behavior. Science. 1994;265:1246–9. doi: 10.1126/science.7915049. [DOI] [PubMed] [Google Scholar]
- 53.Mani SK, Reyna AM, Chen JZ, et al. Differential response of progesterone receptor isoforms in hormone-dependent and -independent facilitation of female sexual receptivity. Mol Endocrinol. 2006;20:1322–32. doi: 10.1210/me.2005-0466. [DOI] [PubMed] [Google Scholar]
- 54.Camacho-Arroyo I, Guerra-Araiza C, Cerbon MA. Progesterone receptor isoforms are differentially regulated by sex steroids in the rat forebrain. Neuroreport. 1998;9:3993–6. doi: 10.1097/00001756-199812210-00001. [DOI] [PubMed] [Google Scholar]
- 55.Nakamura NH, Rosell DR, Akama KT, et al. Estrogen and ovariectomy regulate mRNA and protein of glutamic acid decarboxylases and cation-chloride cotransporters in the adult rat hippocampus. Neuroendocrinology. 2004;80:308–23. doi: 10.1159/000083657. [DOI] [PubMed] [Google Scholar]
- 56.Eyigor O, Jennes L. Kainate receptor subunit-positive gonadotropin-releasing hormone neurons express c-fos during the steroid-induced luteinizing hormone surge in the female rat. Endocrinology. 2000;141:779–86. doi: 10.1210/endo.141.2.7299. [DOI] [PubMed] [Google Scholar]
- 57.Koenig JI. Estrogen interactions with glutamate and dopamine neurotransmitter systems. Neuropsychopharmacology. 2000;23:S93. [Google Scholar]
- 58.Sato K, Matsuki N, Ohno Y, et al. Estrogens inhibit l-glutamate uptake activity of astrocytes via membrane estrogen receptor. J Neurochem. 2003;86:1498–505. doi: 10.1046/j.1471-4159.2003.01953.x. [DOI] [PubMed] [Google Scholar]
- 59.Pawlak J, Brito V, Kuppers E, et al. Regulation of glutamate transporter GLAST and GLT-1 expression in astrocytes by estrogen. Mol Brain Res. 2005;138:1–7. doi: 10.1016/j.molbrainres.2004.10.043. [DOI] [PubMed] [Google Scholar]
- 60.Woolley CS. Estradiol facilitates kainic acid-induced, but not flurothyl-induced, behavioral seizure activity in adult female rats. Epilepsia. 2000;41:510–5. doi: 10.1111/j.1528-1157.2000.tb00203.x. [DOI] [PubMed] [Google Scholar]
- 61.Veliskova J, Velisek L, Galanopoulou AS, et al. Neuroprotective effects of estrogens on hippocampal cells in adult female rats after status epilepticus. Epilepsia. 2000;41:S30–5. doi: 10.1111/j.1528-1157.2000.tb01553.x. [DOI] [PubMed] [Google Scholar]
- 62.Hoffman GE, Moore N, Fiskum G, et al. Ovarian steroid modulation of seizure severity and hippocampal cell death after kainic acid treatment. Exp Neurol. 2003;183:124–34. doi: 10.1016/s0014-4886(03)00104-3. [DOI] [PubMed] [Google Scholar]
- 63.Reibel S, Andre V, Chassagnon S, et al. Neuroprotective effects of chronic estradiol benzoate treatment on hippocampal cell loss induced by status epilepticus in the female rat. Neuroscience. 2000;281:79–82. doi: 10.1016/s0304-3940(00)00784-9. [DOI] [PubMed] [Google Scholar]
- 64.Woolley DE, Timiras PS. Water and electrolyte alterations in plasma, brain and liver of rats after castration and sex hormone administration. Acta Endocrinol (Copenh) 1964;46:12–24. doi: 10.1530/acta.0.0460012. [DOI] [PubMed] [Google Scholar]
- 65.Herve MA, Meduri G, Petit FG, et al. Regulation of the vascular endothelial growth factor (VEGF) receptor Flk-1/KDR by estradiol through VEGF in uterus. J Endocrinol. 2006;188:91–9. doi: 10.1677/joe.1.06184. [DOI] [PubMed] [Google Scholar]
- 66.Hyder SM, Huang JC, Nawaz Z, et al. Regulation of vascular endothelial growth factor expression by estrogens and progestins. Environ Health Perspect. 2000;108(suppl 5):785–90. doi: 10.1289/ehp.00108s5785. [DOI] [PubMed] [Google Scholar]
- 67.Oztas B, Camurcu S, Kaya M. Influence of sex on the blood brain barrier permeability during bicuculline-induced seizures. Int J Neurosci. 1992;65:131–9. doi: 10.3109/00207459209003284. [DOI] [PubMed] [Google Scholar]
- 68.Edwards H, Burnham WM, Mendonca A. Steroid hormones affect limbic afterdischarge thresholds and kindling rates in adult female rats. Brain Res. 1999;838:136–50. doi: 10.1016/s0006-8993(99)01619-4. [DOI] [PubMed] [Google Scholar]
- 69.Hom AC, Buterbaugh GG. Estrogen alters the acquisition of seizures kindled by repeated amygdala stimulation or pentylenetetrazol administration in ovariectomized female rats. Epilepsia. 1986;27:103–08. doi: 10.1111/j.1528-1157.1986.tb03510.x. [DOI] [PubMed] [Google Scholar]
- 70.Buterbaugh GG, Hudson GM. Estradiol replacement to female rats facilitates dorsal hippocampal but not ventral hippocampal kindled seizure acquisition. Exp Neurol. 1991;111:55–64. doi: 10.1016/0014-4886(91)90050-m. [DOI] [PubMed] [Google Scholar]
- 71.Buterbaugh GG. Postictal events in amygdala-kindled female rats with and without estradiol replacement. Exp Neurol. 1987;95:697–713. doi: 10.1016/0014-4886(87)90310-4. [DOI] [PubMed] [Google Scholar]
- 72.Buterbaugh GG. Estradiol replacement facilitates the acquisition of seizures kindled from the anterior neocortex in female rats. Epilepsy Res. 1989;4:207–15. doi: 10.1016/0920-1211(89)90005-3. [DOI] [PubMed] [Google Scholar]
- 73.Buterbaugh GG. Acquisition of amygdala-kindled seizures in female rats: relationship between the effect of estradiol and intra-amygdaloid electrode location. Pharmacol Biochem Behav. 1987;28:291–7. doi: 10.1016/0091-3057(87)90227-9. [DOI] [PubMed] [Google Scholar]
- 74.Joels M, Karst H. Effects of estradiol and progesterone on voltage-gated calcium and potassium conductances in rat CA1 hippocampal neurons. J Neurosci. 1995;15:4289–97. doi: 10.1523/JNEUROSCI.15-06-04289.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Maclusky NJ, Luine V, Hajszan T, et al. The 17α and 17β isomers of estradiol both induce rapid spine synapse formation in the CA1 hippocampal subfield of ovariectomized female rats. Endocrinology. 2005;146:287–93. doi: 10.1210/en.2004-0730. [DOI] [PubMed] [Google Scholar]
- 76.Pozzo-Miller LD, Inoue T, Murphy DD. Estradiol increases spine density and NMDA-dependent Ca2++ transients in spines of CA1 pyramidal neurons from hippocampal slices. J Neurophysiol. 1999;81:1404–11. doi: 10.1152/jn.1999.81.3.1404. [DOI] [PubMed] [Google Scholar]
- 77.Li C, Brake WG, Romeo RD, et al. Estrogen alters hippocampal dendritic spine shape and enhances synaptic protein immunoreactivity and spatial memory in female mice. Proc Natl Acad Sci U S A. 2004;101:2185–90. doi: 10.1073/pnas.0307313101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Zamani MR, Levy WB, Desmond NL. Estradiol increases delayed, N-methyl-D-aspartate receptor-mediated excitation in the hippocampal CA1 region. Neuroscience. 2004;129:243–54. doi: 10.1016/j.neuroscience.2004.06.082. [DOI] [PubMed] [Google Scholar]
- 79.Foy MR, Xu J, Xie X, et al. 17β-estradiol enhances NMDA receptor-mediated EPSPs and long-term potentiation. J Neurophysiol. 1999;81:925–9. doi: 10.1152/jn.1999.81.2.925. [DOI] [PubMed] [Google Scholar]
- 80.Woolley CS, Weiland NG, McEwen BS, et al. Estradiol increases the sensitivity of hippocampal CA1 pyramidal cells to NMDA receptor-mediated synaptic input: correlation with dendritic spine density. J Neurosci. 1997;17:1848–59. doi: 10.1523/JNEUROSCI.17-05-01848.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Rudick CN, Woolley CS. Estrogen regulates functional inhibition of hippocampal CA1 pyramidal cells in the adult female rat. J Neurosci. 2001;21:6532–43. doi: 10.1523/JNEUROSCI.21-17-06532.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Cyr M, Ghribi O, Di Paolo T. Regional and selective effects of oestradiol and progesterone on NMDA and AMPA receptors in the rat brain. J Neuroendocrinol. 2000;12:445–52. doi: 10.1046/j.1365-2826.2000.00471.x. [DOI] [PubMed] [Google Scholar]
- 83.Weiland NG. Estradiol selectively regulates agonist binding sites on the N-methyl-D-aspartate receptor complex in the CA1 region of the hippocampus. Endocrinology. 1992;131:662–8. doi: 10.1210/endo.131.2.1353442. [DOI] [PubMed] [Google Scholar]
- 84.Smith CC, McMahon LL. Estrogen-induced increase in the magnitude of long-term potentiation occurs only when the ratio of NMDA transmission to AMPA transmission is increased. J Neurosci. 2005;25:7780–91. doi: 10.1523/JNEUROSCI.0762-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Segal M, Murphy D. Estradiol induces formation of dendritic spines in hippocampal neurons: functional correlates. Horm Behav. 2001;40:156–9. doi: 10.1006/hbeh.2001.1688. [DOI] [PubMed] [Google Scholar]
- 86.Carrer HF, Araque A, Buno W. Estradiol regulates the slow Ca2++-activated K++ current in hippocampal pyramidal neurons. J Neurosci. 2003;23:6338–44. doi: 10.1523/JNEUROSCI.23-15-06338.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Kumar A, Foster TC. 17β-estradiol benzoate decreases the AHP amplitude in CA1 pyramidal neurons. J Neurophysiol. 2002;88:621–6. doi: 10.1152/jn.2002.88.2.621. [DOI] [PubMed] [Google Scholar]
- 88.Barraclough DJ, Ingram CD, Brown MW. Chronic treatment with oestradiol does not alter in vitro LTP in subfield CA1 of the female rat hippocampus. Neuropharmacology. 1999;38:65–71. doi: 10.1016/s0028-3908(98)00157-9. [DOI] [PubMed] [Google Scholar]
- 89.Romeo RD, McCarthy JB, Wang A, et al. Sex differences in hippocampal estradiol-induced N-methyl-D-aspartic acid binding and ultrastructural localization of estrogen receptor-α. Neuroendocrinology. 2005;81:391–9. doi: 10.1159/000089557. [DOI] [PubMed] [Google Scholar]
- 90.Lee YB, Lee HJ, Won MH, et al. Soy isoflavones improve spatial delayed matching-to-place performance and reduce cholinergic neuron loss in elderly male rats. J Nutr. 2004;134:1827–31. doi: 10.1093/jn/134.7.1827. [DOI] [PubMed] [Google Scholar]
- 91.Hwang IK, Lee YB, Yoo KY, et al. Soybean isoflavones alter parvalbumin in hippocampus of mid-aged normal female, ovariectomized female, and normal male rats. Acta Pharmacol Sin. 2006;27:59–65. doi: 10.1111/j.1745-7254.2006.00258.x. [DOI] [PubMed] [Google Scholar]
- 92.Rhodes ME, Harney JP, Frye CA. Gonadal, adrenal, and neuroactive steroids’ role in ictal activity. Brain Res. 2004;1000:8–18. doi: 10.1016/j.brainres.2003.12.023. [DOI] [PubMed] [Google Scholar]
- 93.Marriott LK, Korol DL. Short-term estrogen treatment in ovariectomized rats augments hippocampal acetylcholine release during place learning. Neurobiol Learn Mem. 2003;80:315–22. doi: 10.1016/j.nlm.2003.08.003. [DOI] [PubMed] [Google Scholar]
- 94.Daniel JM, Dohanich GP. Acetylcholine mediates the estrogen-induced increase in NMDA receptor binding in CA1 of the hippocampus and the associated improvement in working memory. J Neurosci. 2001;21:6949–56. doi: 10.1523/JNEUROSCI.21-17-06949.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Gibbs RB. Basal forebrain cholinergic neurons are necessary for estrogen to enhance acquisition of a delayed matching-to-position T-maze task. Horm Behav. 2002;42:245–57. doi: 10.1006/hbeh.2002.1825. [DOI] [PubMed] [Google Scholar]
- 96.Towart LA, Alves SE, Znamensky V, et al. Subcellular relationships between cholinergic terminals and estrogen receptor-α in the dorsal hippocampus. J Comp Neurol. 2003;463:390–401. doi: 10.1002/cne.10753. [DOI] [PubMed] [Google Scholar]
- 97.Scharfman HE, Mercurio TC, Goodman JH, et al. Hippocam-pal excitability increases during the estrous cycle in the rat: a potential role for brain-derived neurotrophic factor. J Neurosci. 2003;23:11641–52. doi: 10.1523/JNEUROSCI.23-37-11641.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Scharfman HE, Goodman JH, Rigoulot M-A, et al. Seizure susceptibility in intact and ovariectomized female rats treated with the convulsant pilocarpine. Exp Neurol. 2005;196:73–86. doi: 10.1016/j.expneurol.2005.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Sohrabji F, Miranda RC, Toran-Allerand CD. Identification of a putative estrogen response element in the gene encoding brain-derived neurotrophic factor. Proc Natl Acad Sci USA. 1995;92:11110–4. doi: 10.1073/pnas.92.24.11110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Lu B, Gottschalk W. Modulation of hippocampal synaptic transmission and plasticity by neurotrophins. Prog Brain Res. 2000;128:231–41. doi: 10.1016/S0079-6123(00)28020-5. [DOI] [PubMed] [Google Scholar]
- 101.Scharfman HE. BDNF and epilepsy: a missing link? Epilepsy Currents. 2005;5:83–8. doi: 10.1111/j.1535-7511.2005.05312.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Scharfman HE, MacLusky NJ. Similarities between BDNF and estrogen in hippocampus: coincidence or clue? Trends Neurosci. 2005;28:79–85. doi: 10.1016/j.tins.2004.12.005. [DOI] [PubMed] [Google Scholar]
- 103.Barnea A, Roberts J. Induction of functional and morphological expression of neuropeptide Y (NPY) in cortical cultures by brain-derived neurotrophic factor (BDNF): evidence for a requirement for extracellular-regulated kinase (ERK)-dependent and ERK-independent mechanisms. Brain Res. 2001;919:57–69. doi: 10.1016/s0006-8993(01)02999-7. [DOI] [PubMed] [Google Scholar]
- 104.Williams AG, Hargreaves AC, Gunn-Moore FJ, et al. Stimulation of neuropeptide Y gene expression by brain-derived neurotrophic factor requires both the phospholipase Cγ and Shc binding sites on its receptor, TrkB. Biochem J. 1998;333:505–9. doi: 10.1042/bj3330505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Vezzani A, Ravizza T, Moneta D, et al. Brain-derived neurotrophic factor immunoreactivity in the limbic system of rats after acute seizures and during spontaneous convulsions: temporal evolution of changes as compared to neuropeptide Y. Neuroscience. 1999;90:1445–61. doi: 10.1016/s0306-4522(98)00553-3. [DOI] [PubMed] [Google Scholar]
- 106.Vezzani A, Sperk G, Colmers W. Neuropeptide Y: emerging evidence for a functional role in seizure modulation. Trends Neurosci. 1999;22:25–30. doi: 10.1016/s0166-2236(98)01284-3. [DOI] [PubMed] [Google Scholar]
- 107.Weiland NG. Glutamic acid decarboxylase messenger ribonucleic acid is regulated by estradiol and progesterone in the hippocampus. Endocrinology. 1992;131:2697–702. doi: 10.1210/endo.131.6.1446611. [DOI] [PubMed] [Google Scholar]
- 108.Hart SA, Patton JD, Woolley CS. Quantitative analysis of ER α and GAD colocalization in the hippocampus of the adult female rat. J Comp Neurol. 2001;440:144–55. doi: 10.1002/cne.1376. [DOI] [PubMed] [Google Scholar]
- 109.Titolo D, Cai F, Belsham DD. Coordinate regulation of neuropeptide Y and agouti-related peptide gene expression by estrogen depends on the ratio of ER{α} to ER{β} in clonal hypothalamic neurons. Mol Endocrinol. 2006 doi: 10.1210/me.2006-0027. in press. [DOI] [PubMed] [Google Scholar]
- 110.Veliskova J. The role of estrogens in seizures and epilepsy: the bad guys or the good guys? Neuroscience. 2006;138:837–44. doi: 10.1016/j.neuroscience.2005.07.005. [DOI] [PubMed] [Google Scholar]
- 111.Wise PM. Estradiol: a protective factor in the adult brain. J Pediatr Endocrinol Metab. 2000;13:1425–9. doi: 10.1515/jpem-2000-s617. [DOI] [PubMed] [Google Scholar]
- 112.Parsons B, Rainbow TC, Snyder L, et al. Progesterone-like effects of estradiol on reproductive behavior and hypothalamic progestin receptors in the female rat. Neuroendocrinology. 1984;39:25–30. doi: 10.1159/000123950. [DOI] [PubMed] [Google Scholar]
- 113.Frye CA, Bayon L. Seizure activity is increased in endocrine states characterized by decline in endogenous levels of the neurosteroid 3,5-THP. Neuroendocrinology. 1998;68:272–80. doi: 10.1159/000054375. [DOI] [PubMed] [Google Scholar]
- 114.Rogawski MA. Progesterone, neurosteroids, and the hormonal basis of catamenial epilepsy. Ann Neurol. 2003;53:288–91. doi: 10.1002/ana.10534. [DOI] [PubMed] [Google Scholar]
- 115.Belelli D, Bolger MB, Gee KW. Anticonvulsant profile of the progesterone metabolite 5-pregnan-3-ol-20-one. Eur J Pharmacol. 1989;166:325–9. doi: 10.1016/0014-2999(89)90077-0. [DOI] [PubMed] [Google Scholar]
- 116.Herzog A. Progesterone therapy in women with epilepsy: a 3-year follow-up. Neurology. 1999;52:1917–8. doi: 10.1212/wnl.52.9.1917-a. [DOI] [PubMed] [Google Scholar]
- 117.Kokate TG, Banks MK, Magee T, et al. Finasteride, a 5α-reductase inhibitor, blocks the anticonvulsant activity of progesterone in mice. J Pharmacol Exp Ther. 1999;288:679–84. [PubMed] [Google Scholar]
- 118.Frye CA, Rhodes ME, Walf A, et al. Progesterone reduces pentylenetetrazol-induced ictal activity of wild-type mice but not those deficient in type I 5α-reductase. Epilepsia. 2002;43(suppl 5):14–17. doi: 10.1046/j.1528-1157.43.s.5.19.x. [DOI] [PubMed] [Google Scholar]
- 119.Bickerstaff E. Neurological complications of oral contraceptives. Oxford: Clarendon Press; 1975. [Google Scholar]
- 120.Mattson RH, Cramer JA, Darney PD, et al. Use of oral contraceptives by women with epilepsy. JAMA. 1986;256:238–40. [PubMed] [Google Scholar]
- 121.Mattson RH, Cramer JA, Caldwell BV, et al. Treatment of seizures with medroxyprogesterone acetate: preliminary report. Neurology. 1984;34:1255–8. doi: 10.1212/wnl.34.9.1255. [DOI] [PubMed] [Google Scholar]
- 122.Majewska MD, Harrison NL, Schwartz RD, et al. Steroid hormone metabolites are barbiturate-like modulators of the GABA receptor. Science. 1986;232:1004–7. doi: 10.1126/science.2422758. [DOI] [PubMed] [Google Scholar]
- 123.Kokate TG, Svensson BE, Rogawski MA. Anticonvulsant activity of neurosteroids: correlation with γ-aminobutyric acid-evoked chloride current potentiation. J Pharmacol Exp Ther. 1994;270:1223–9. [PubMed] [Google Scholar]
- 124.Belelli D, Lambert JL. Neurosteroids: endogenous regulators of the GABA (A) receptor. Nature Rev Neurosci. 2005;6:565–75. doi: 10.1038/nrn1703. [DOI] [PubMed] [Google Scholar]
- 125.Edwards HE, Epps T, Carlen PL, et al. Progestin receptors mediate progesterone suppression of epileptiform activity in tetanized hippocampal slices in vitro. Neuroscience. 2001;101:895–906. doi: 10.1016/s0306-4522(00)00439-5. [DOI] [PubMed] [Google Scholar]
- 126.Reddy DS, Castaneda DC, O’Malley BW, et al. Anticonvulsant activity of progesterone and neurosteroids in progesterone receptor knockout mice. J Pharmacol Exp Ther. 2004;310:230–9. doi: 10.1124/jpet.104.065268. [DOI] [PubMed] [Google Scholar]
- 127.Brown TJ, Clark AS, MacLusky NJ. Regional sex differences in progestin receptor induction in the rat hypothalamus: effects of various doses of estradiol benzoate. J Neuroscience. 1987;7:2529–36. [PMC free article] [PubMed] [Google Scholar]
- 128.Leavitt WW, Cobb AD, Takeda A. Progesterone-modulation of estrogen action: rapid down regulation of nuclear acceptor sites for the estrogen receptor. Adv Exp Med Biol. 1987;230:49–78. doi: 10.1007/978-1-4684-1297-0_4. [DOI] [PubMed] [Google Scholar]
- 129.Zhou Y, Shughrue PJ, Dorsa DM. Estrogen receptor protein is differentially regulated in the preoptic area of the brain and in the uterus during the rat estrous cycle. Neuroendocrinology. 1995;61:276–83. doi: 10.1159/000126849. [DOI] [PubMed] [Google Scholar]
- 130.Brown TJ, Scherz B, Hochberg RB, et al. Regulation of estrogen receptor concentrations in the rat brain: effects of sustained androgen and estrogen exposure. Neuroendocrinology. 1996;63:53–60. doi: 10.1159/000126935. [DOI] [PubMed] [Google Scholar]
- 131.Orikasa C, Sakuma Y. Sex and region-specific regulation of oestrogen receptor β in the rat hypothalamus. J Neuroendocrinol. 2004;16:964–9. doi: 10.1111/j.1365-2826.2004.01254.x. [DOI] [PubMed] [Google Scholar]
- 132.Rune GM, Wehrenberg U, Prange-Kiel J, et al. Estrogen up-regulates estrogen receptor α and synaptophysin in slice cultures of rat hippocampus. Neuroscience. 2002;113:167–75. doi: 10.1016/s0306-4522(02)00152-5. [DOI] [PubMed] [Google Scholar]
- 133.Herzog AG. Intermittent progesterone therapy and frequency of complex partial seizures in women with menstrual disorders. Neurology. 1986;36:1607–10. doi: 10.1212/wnl.36.12.1607. [DOI] [PubMed] [Google Scholar]
- 134.Herzog AG. Progesterone therapy in women with complex partial and secondary generalized seizures. Neurology. 1995;45:1660–2. doi: 10.1212/wnl.45.9.1660. [DOI] [PubMed] [Google Scholar]
- 135.Maguire JL, Stell BM, Rafizadeh M, et al. Ovarian cycle-linked changes in GABA(A) receptors mediating tonic inhibition alter seizure susceptibility and anxiety. Nat Neurosci. 2005;8:797–804. doi: 10.1038/nn1469. [DOI] [PubMed] [Google Scholar]
- 136.Moran MH, Smith SS. Progesterone withdrawal I: proconvulsant effects. Brain Res. 1998;807:84–90. doi: 10.1016/s0006-8993(98)00782-3. [DOI] [PubMed] [Google Scholar]
- 137.Reddy DS, Kim HY, Rogawski MA. Neurosteroid withdrawal model of perimenstrual catamenial epilepsy. Epilepsia. 2001;42:328–36. doi: 10.1046/j.1528-1157.2001.10100.x. [DOI] [PubMed] [Google Scholar]
- 138.Smith SS, Gong QH, Hsu FC, et al. GABA(A) receptor α4 subunit suppression prevents withdrawal properties of an endogenous steroid. Nature. 1998;392:926–30. doi: 10.1038/31948. [DOI] [PubMed] [Google Scholar]
- 139.Reddy DS, Rogawski MA. Enhanced anticonvulsant activity of neuroactive steroids in a rat model of catamenial epilepsy. Epilepsia. 2001;42:337–44. doi: 10.1046/j.1528-1157.2001.10200.x. [DOI] [PubMed] [Google Scholar]
- 140.Bazan AC, Montenegro MA, Cendes F, et al. Menstrual cycle worsening of epileptic seizures in women with symptomatic focal epilepsy. Arq Neuropsiquiatr. 2005;63:751–756. doi: 10.1590/s0004-282x2005000500006. [DOI] [PubMed] [Google Scholar]
- 141.French JA. Catamenial epilepsy: the elusive condition. Epilepsy Currents. 2005;5:113–4. doi: 10.1111/j.1535-7511.2005.05301.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Newmark ME. Catamenial epilepsy: a neurologic myth persisting for more than 100 years. Epilepsia. 1989;30:704. [Google Scholar]
- 143.Weatherford KJ. Catamenial epilepsy: in search of a clinical entitiy and its prevalence. J Neurosci Nurs. 1999;31:328–31. doi: 10.1097/01376517-199912000-00002. [DOI] [PubMed] [Google Scholar]
- 144.Herzog AG, Harden CL, Liporace J, et al. Frequency of catamenial seizure exacerbation in women with localization-related epilepsy. Ann Neurol. 2004;56:431–4. doi: 10.1002/ana.20214. [DOI] [PubMed] [Google Scholar]
- 145.Foldvary-Schaeer N, Falcone T. Catamenial epilepsy: pathophysiology, diagnosis, and management. Neurology. 2003;61(6 suppl 2):S2–15. doi: 10.1212/wnl.61.6_suppl_2.s2. [DOI] [PubMed] [Google Scholar]
- 146.Duncan S, Read CL, Brodie MJ. How common is catamenial epilepsy? Epilepsia. 1993;34:827–31. doi: 10.1111/j.1528-1157.1993.tb02097.x. [DOI] [PubMed] [Google Scholar]
- 147.Herzog AG. A hypothesis to integrate partial seizures of temporal lobe origin and reproductive endocrine disorders. Epilepsy Res. 1989;3:151–9. doi: 10.1016/0920-1211(89)90043-0. [DOI] [PubMed] [Google Scholar]
- 148.Bonuccelli U, Melis GB, Paoletti AM, et al. Unbalanced progesterone and estradiol secretion in catamenial epilepsy. Epilepsy Res. 1989;3:100–6. doi: 10.1016/0920-1211(89)90037-5. [DOI] [PubMed] [Google Scholar]
- 149.Herzog AG, Klein P, Ransil BJ. Three patterns of catamenial epilepsy. Epilepsia. 1997;38:1082–8. doi: 10.1111/j.1528-1157.1997.tb01197.x. [DOI] [PubMed] [Google Scholar]
- 150.Jacono JJ, Robertson JMD. The effects of estrogen, progesterone, and ionized calcium on seizures during the menstrual cycle of epileptic women. Epilepsia. 1987;28:571–7. doi: 10.1111/j.1528-1157.1987.tb03690.x. [DOI] [PubMed] [Google Scholar]
- 151.Rosciszewska D, Buntner B, Guz I, et al. Ovarian hormones, anticonvulsant drugs, and seizures during the menstrual cycle in women with epilepsy. J Neurol Neurosurg Psychiatry. 1986;49:47–51. doi: 10.1136/jnnp.49.1.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Genazzani AR, Lemarchand-Beraud T, Aubert ML, et al. Pattern of plasma ACTH, hGH, and cortisol during menstrual cycle. J Clin Endocrinol Metab. 1975;41:431–7. doi: 10.1210/jcem-41-3-431. [DOI] [PubMed] [Google Scholar]
- 153.Frye CA, Rhodes ME. Estrogen-priming can enhance progesterone’s anti-seizure effects in part by increasing hippocampal levels of allopregnanolone. Pharmacol Biochem Behav. 2005;81:907–16. doi: 10.1016/j.pbb.2005.06.016. [DOI] [PubMed] [Google Scholar]
- 154.Schwartzkroin PA, Baraban SC, Hochman DW. Osmolarity, ionic flux, and changes in brain excitability. Epilepsy Res. 1998;32:275–85. doi: 10.1016/s0920-1211(98)00058-8. [DOI] [PubMed] [Google Scholar]
- 155.Reiss WG, Oles KS. Acetazolamide in the treatment of seizures. Ann Pharmacother. 1996;30:514–9. doi: 10.1177/106002809603000515. [DOI] [PubMed] [Google Scholar]
- 156.Lim LL, Foldvary N, Mascha E, et al. Acetazolamide in women with catamenial epilepsy. Epilepsia. 2001;42:746–9. doi: 10.1046/j.1528-1157.2001.33600.x. [DOI] [PubMed] [Google Scholar]
- 157.Kumar N, Behari M, Aruja GK, et al. Phenytoin levels in catamenial epilepsy. Epilepsia. 1988;29:155–8. doi: 10.1111/j.1528-1157.1988.tb04412.x. [DOI] [PubMed] [Google Scholar]
- 158.Shavit G, Lerman P, Korczyn AD, et al. Phenytoin pharmacokinetics in catamenial epilepsy. Neurology. 1984;34:959–61. doi: 10.1212/wnl.34.7.959. [DOI] [PubMed] [Google Scholar]
- 159.Svalheim S, Tauboll E, Bjornenak T, et al. Do women with epilepsy have increased frequency of menstrual disturbances? Seizure. 2003;12:529–33. doi: 10.1016/s1059-1311(03)00195-x. [DOI] [PubMed] [Google Scholar]
- 160.Isojarvi JI, Laatikainen TJ, Pakarinen AJ, et al. Polycyctic ovaries and hyperandrogenism in women taking valproate for epilepsy. N Engl J Med. 1993;329:1383–8. doi: 10.1056/NEJM199311043291904. [DOI] [PubMed] [Google Scholar]
- 161.Knobil E, Neill JD. The physiology of reproduction. New York: Raven; 1994. [Google Scholar]
- 162.Pfaff DJ, Arnold AP, Etgen AM, et al. Hormones, brain and behavior. New York: Academic Press; 2002. [Google Scholar]
- 163.Amado D, Cavalheiro EA. Hormonal and gestational parameters in female rats submitted to the pilocarpine model of epilepsy. Epilepsy Res. 1998;32:266–74. doi: 10.1016/s0920-1211(98)00057-6. [DOI] [PubMed] [Google Scholar]
- 164.Amado D, Verreschi ITN, Berzaghi MPP, et al. Effects of intrahippocampal injection of kainic acid on estrous cycle in rats. Brazilian J Med Biol Res. 1987;20:829–32. [PubMed] [Google Scholar]
- 165.Amado D, Cavalheiro EA, Bentivoglio M. Epilepsy and hormonal regulation: the patterns of GnRH and galanin immunoreactivity in the hypothalamus of epileptic female rats. Epilepsy Res. 1993;14:149–59. doi: 10.1016/0920-1211(93)90019-4. [DOI] [PubMed] [Google Scholar]
- 166.Friedman MN, Geula C, Holmes GL, et al. GnRH-immunoreactive fiber changes with unilateral amygdala-kindled seizures. Epilepsy Res. 2002;54:73–7. doi: 10.1016/s0920-1211(02)00196-1. [DOI] [PubMed] [Google Scholar]
- 167.Edwards HE, Burnham WM, Ng MM, et al. Limbic seizures alter reproductive function in the female rat. Epilepsia. 1999;40:1370–7. doi: 10.1111/j.1528-1157.1999.tb02007.x. [DOI] [PubMed] [Google Scholar]
- 168.Scharfman HE, Goodman JH, Berger RE, et al. An animal model of limbic epilepsy in female rats. Epilepsia. 2003;44(suppl 9):215–6. [Google Scholar]
- 169.Tauboll E, Isojarvi JI, Harbo HF, et al. Long-term valproate treatment induces changes in ovarian morphology and serum sex steroid hormone levels in female Wistar rats. Seizure. 1999;8:490–3. doi: 10.1053/seiz.1999.0342. [DOI] [PubMed] [Google Scholar]
- 170.Yang SN, Lu F, Wu JN, et al. Activation of gonadotropin-releasing hormone receptors induces a long-term enhancement of excitatory postsynaptic currents mediated by ionotropic glutamate receptors in the rat hippocampus. Neurosci Lett. 1999;260:33–6. doi: 10.1016/s0304-3940(98)00939-2. [DOI] [PubMed] [Google Scholar]
- 171.Osada T, Kimura F. LHRH effects on hippocampal neurons are modulated by estrogen in rats. Endocr J. 1995;42:251–7. doi: 10.1507/endocrj.42.251. [DOI] [PubMed] [Google Scholar]
- 172.Palovcik RA, Phillips MI. A biphasic excitatory response of hippocampal neurons to gonadotropin-releasing hormone. Neuroendocrinology. 1986;44:137–41. doi: 10.1159/000124636. [DOI] [PubMed] [Google Scholar]
- 173.Wong M, Eaton MJ, Moss RL. Electrophysiological actions of luteinizing hormone-releasing hormone: intracellular studies in the rat hippocampal slice preparation. Synapse. 1990;5:65–70. doi: 10.1002/syn.890050106. [DOI] [PubMed] [Google Scholar]
- 174.Casadesus G, Webber KM, Atwood CS, et al. Luteinizing hormone modulates cognition and amyloid-β deposition in Alzheimer APP transgenic mice. Biochim Biophys Acta. 2006;1762:447–52. doi: 10.1016/j.bbadis.2006.01.008. [DOI] [PubMed] [Google Scholar]
- 175.Gordon HW, Lee PA. A relationship between gonadotropins and visuospatial function. Neuropsychologia. 1986;24:563–76. doi: 10.1016/0028-3932(86)90100-4. [DOI] [PubMed] [Google Scholar]
- 176.Shingo T, Gregg C, Enwere E, et al. Pregnancy-stimulated neurogenesis in the adult female forebrain mediated by prolactin. Science. 2003;299:117–20. doi: 10.1126/science.1076647. [DOI] [PubMed] [Google Scholar]
- 177.Townsend J, Cave BJ, Norman MR, et al. Effects of prolactin on hypothalamic supraoptic neurones: evidence for modulation of STAT5 expression and electrical activity. Neuro Endocrinol Lett. 2005;26:125–30. [PubMed] [Google Scholar]
- 178.Lei ZM, Rao CV. Neural actions of luteinizing hormone and human chorionic gonadotropin. Sem Repro Med. 2001;19:103–9. doi: 10.1055/s-2001-13917. [DOI] [PubMed] [Google Scholar]
- 179.Toth P, Lukacs H, Hiatt ES, et al. Administration of human chorionic gonadotropin affects sleep-wake phases and other associated behaviors in cycling female rats. Brain Res. 1994;654:181–90. doi: 10.1016/0006-8993(94)90478-2. [DOI] [PubMed] [Google Scholar]
- 180.Zaninetti M, Raggenbass M. Oxytocin receptor agonists enhance inhibitory synaptic transmission in the rat hippocampus by activating interneurons in stratum pyramidale. Eur J Neurosci. 2000;12:3975–84. doi: 10.1046/j.1460-9568.2000.00290.x. [DOI] [PubMed] [Google Scholar]
- 181.Raggenbass M, Alberi S, Zaninetti M, et al. Vasopressin and oxytocin action in the brain: cellular neurophysiological studies. Prog Brain Res. 1998;119:263–73. doi: 10.1016/s0079-6123(08)61574-5. [DOI] [PubMed] [Google Scholar]
- 182.Slamberova R, Vathy I. Estrogen differentially alters NMDA-and kainate-induced seizures in prenatally morphine- and saline-exposed adult female rats. Pharmacol Biochem Behav. 2000;67:501–5. doi: 10.1016/s0091-3057(00)00375-0. [DOI] [PubMed] [Google Scholar]
- 183.Hotchkiss J, Knobil E. The menstrual cycle and its neuroendocrine control. In: Knobil E, Neill JD, editors. The physiology of reproduction. New York: Raven; 1994. pp. 711–49. [Google Scholar]
- 184.Smith MS, Freeman ME, Neill JD. The control of progesterone secretion during the estrous cycle and early pseudopregnancy in the rat: prolactin, gonadotropin and steroid levels associates with rescue of the corpus luteum of pseudopregnancy. Endocrinology. 1975;96:219–26. doi: 10.1210/endo-96-1-219. [DOI] [PubMed] [Google Scholar]


