Abstract
Ferroportin (Fpn) is the only known iron exporter in vertebrates. Hepcidin, a peptide secreted by the liver in response to iron or inflammation, binds to Fpn, inducing its internalization and degradation. We show that after binding of hepcidin, Fpn is tyrosine phosphorylated at the plasma membrane. Mutants of human Fpn that do not get internalized or that are internalized slowly show either absent or impaired phosphorylation. We identify adjacent tyrosines as the phosphorylation sites and show that mutation of both tyrosines prevents hepcidin-mediated Fpn internalization. Once internalized, Fpn is dephosphorylated and subsequently ubiquitinated. An inability to ubiquitinate Fpn does not prevent hepcidin-induced internalization, but it inhibits the degradation of Fpn. Ubiquitinated Fpn is trafficked through the multivesicular body pathway en route to degradation in the late endosome/lysosome. Depletion of proteins involved in multivesicular body trafficking (Endosome Sorting Complex Required for Transport proteins), by small-interfering RNA, reduces the trafficking of Fpn-green fluorescent to the lysosome.
INTRODUCTION
Iron homeostasis in vertebrates is dominated by the lack of an excretory route for excess iron. Plasma iron level is regulated by the rate of iron entry through the duodenal mucosa, which affects net iron absorption, and by the rate of iron release from macrophages recycling iron from aged or damaged erythrocytes (Hentze et al., 2004; Camaschella, 2005; Donovan et al., 2006). Export of iron from duodenal enterocytes and macrophages into plasma is regulated by the plasma membrane transporter ferroportin (Fpn) (Abboud and Haile, 2000; Donovan et al., 2000; McKie et al., 2000). Fpn is the receptor for hepcidin, a peptide of 25 amino acids synthesized by the liver in response to inflammation and increased iron stores (Ganz and Nemeth, 2006). Binding of hepcidin to Fpn results in the internalization and degradation of Fpn, leading to a decrease in iron export (Nemeth et al., 2004). Decreased plasma iron leads to a reduction in hepcidin synthesis, resulting in increased plasma membrane Fpn and increased iron delivery into plasma.
Inadequate hepcidin production can explain many of the genetic forms of iron overload disease, whereas high levels of hepcidin produced by prolonged inflammatory stimuli can account for hypoferremia and anemia of chronic disease (Weiss and Goodnough, 2005; Pietrangelo, 2006). There is one form of genetic iron overload disease, however, that is not caused by abnormal hepcidin production. This disease, referred to as ferroportin disease or hereditary hemochromatosis type 4, results from mutations in the Fpn gene (Pietrangelo, 2004). Characterization of mutant alleles of Fpn showed that some Fpn mutant proteins are not appropriately targeted to the cell surface, whereas other mutant proteins are appropriately targeted to the plasma membrane, but they have impaired internalization in response to hepcidin (De Domenico et al., 2005; Drakesmith et al., 2005; Liu et al., 2005; Goncalves et al., 2006). To understand the molecular basis of the defect in hepcidin-resistant Fpn mutants, we examined the mechanism of hepcidin-induced internalization and degradation. We show that in response to hepcidin, Fpn is phosphorylated and that phosphorylation is required for internalization. Once internalized, Fpn is ubiquitinated and trafficked through the multivesicular body (MVB) for degradation in the lysosome.
MATERIALS AND METHODS
Cells and Media
Mouse Fpn was expressed in a cytomegalovirus (CMV)-containing vector (pEGFP-N1, Clontech, Mountain View, CA; or pCMV-Tag4 [FLAG], Stratagene, La Jolla, CA) as described previously (De Domenico et al., 2005). Human embryonic kidney (HEK)293T cells were maintained in DMEM with 10% fetal bovine serum and transfected with pFpn-enhanced green fluorescent protein (EGFP)-N1, pFpn(mutations)-EGFP-N1, pCMV-Fpn-FLAG, pDynamin-EGFP, or pDynaminK44A-EGFP by using Nucleofector technology (Amaxa, Gaithersburg, MD), according to the manufacturer's directions. FM3A and ts85 cells were maintained in RPMI 1640 medium with 5% fetal bovine serum and transfected using Nucleofector technology. HEK293T Fpn, a stable cell line in which Fpn-green fluorescent protein (GFP) expression is regulated by the ecdysone promoter, has been described previously (Nemeth et al., 2004; De Domenico et al., 2005).
Generation of Fpn Constructs
All Fpn mutations were generated in pFpn-EGFP-N1 by using the QuikChange site-directed mutagenesis kit (Stratagene), amplified in Escherichia coli, and sequence-verified before transfecting into mammalian cells.
Small Interfering RNA (siRNA) Transfection
siRNA oligonucleotide or siRNA pools matching selected regions of TSG101, LIP5, and CHMP5 were as described previously (Garrus et al., 2001; Ward et al., 2005). siRNA oligonucleotides for CHMP6 and EAP20 were as described in Langelier et al. (2006). siRNA oligonucleotide pools matching selected regions of epsin were obtained from Dharmacon RNA Technologies (Lafayette, CO). HEK293T Fpn-GFP cells were transfected with siRNA oligonucleotides at a final concentration of 50–100 nM by using OligofectAMINE reagent (Invitrogen, Carlsbad, CA). Twenty-four to 48 h after transfection, the cells were trypsinized and plated onto 60-mm plates with or without coverslips. Twenty-four hours after plating, the cells were induced to express Fpn-GFP, incubated in the presence or absence of 1 μg/ml hepcidin, and then processed for fluorescence microscopy or Western analysis.
Other Procedures
Biotinylation of the plasma membrane of HEK293T cells was performed using sulfosuccinimidyl-2-(biotinamido)ethyl-1,3-dithiopropinate (sulfo-NHS-SS-biotin) (Pierce Chemical, Rockford, IL) according to the manufacturer's instructions. Immunoprecipitation of Fpn-GFP or Fpn-FLAG was performed as described previously (De Domenico et al., 2005) using either protein A/G resin (Santa Cruz Biotechnology, Santa Cruz, CA) and rabbit anti-GFP (ab6556l; Abcam, Cambridge, MA), anti-FLAG M2 affinity gel (Sigma-Aldrich, St. Louis, MO), or immobilized streptavidin affinity gel (Pierce Chemical). Immunoprecipitation of phosphotyrosine was performed using mouse anti-phosphotyrosine antibody (1:500) (Calbiochem, San Diego, CA) and protein A/G resin (Santa Cruz Biotechnology). Samples were analyzed by SDS-polyacrylamide gel electrophoresis under nonreducing conditions. Samples were heated at 37°C for 10 min before being applied to the SDS-PAGE. Western analysis was performed using either mouse anti-FLAG antibody (1:10,000; Sigma-Aldrich); rabbit anti-GFP (1:10,000, ab6556; Abcam); goat anti-human actin (1:1000; Santa Cruz Biotechnology); mouse anti-tubulin (1:1000; GeneTex, San Antonio, TX); mouse anti-phosphotyrosine clone 16F4 (1:500; Calbiochem), mouse anti-ubiquitin (1:1000; Covance, Berkeley, CA); mouse anti-TSG101 (1:1000; GeneTex), rabbit anti-LIP5 (1:500), rabbit anti-CHMP5 (1:500), rabbit anti-CHMP6 (1:1000), rabbit anti-EAP20 (1:1000), or rabbit anti-epsin (1:1000; Santa Cruz Biotechnology) followed by either peroxidase-conjugated goat anti-mouse immunoglobulin (Ig)G (1:10,000; Jackson ImmunoResearch Laboratories, West Grove, PA), peroxidase-conjugated goat anti-rabbit IgG (1:10,000; Jackson ImmunoResearch Labs, West Grove, PA) or peroxidase-conjugated donkey anti-goat IgG (1:5000; Santa Cruz Biotechnology). All Westerns were normalized for the total protein concentration using the bicinchoninic acid assay (Pierce Chemical). Immunofluorescence and ferritin analysis were performed as described previously (De Domenico et al., 2005). All experiments were performed a minimum of three times, and error bars represent the SD. Analysis of fluorescent images was performed as described previously (Langelier et al., 2006). The calf intestinal phosphatase was obtained from New England Biolabs (Ipswich, MA), and it was used according to the manufacturer's instructions.
RESULTS
Fpn Is Ubiquitinated and Phosphorylated in Response to Hepcidin
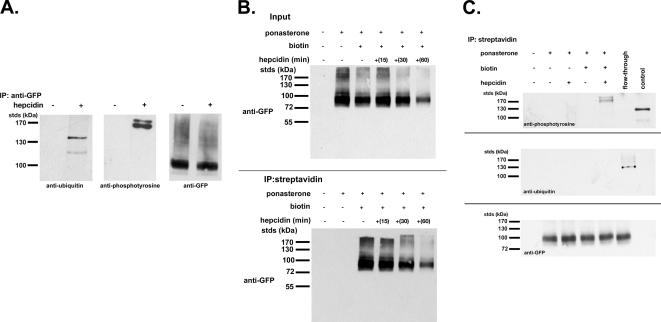
Ubiquitination and phosphorylation are two protein modifications known to signal the internalization of plasma membrane proteins (Bonifacino and Traub, 2003). We examined whether hepcidin binding to Fpn induces either of these modifications. Hepcidin was added to cells, and Fpn-GFP was immunoprecipitated and examined for Fpn modifications by Western blot analysis using specific antibodies. As shown previously, Fpn-GFP is fully active for iron export and is hepcidin responsive (Nemeth et al., 2004). A 15-min incubation of Fpn-GFP–expressing cells with hepcidin led to both ubiquitination and tyrosine phosphorylation of Fpn-GFP (Figure 1A). Both modifications were independent of the epitope tag on Fpn, and tyrosine phosphorylation was detected using four different monoclonal antibodies (data not shown). Both ubiquitination and phosphorylation changed the apparent molecular mass of Fpn-GFP. Although the change in molecular mass of the ubiquitinated species was consistent with addition of multiple ubiquitins, a polyubiquitin “ladder” was not seen. The change in the molecular mass of the phosphorylated species was also unexpectedly large. The samples are run under nonreducing conditions because reduction and heat treatment result in an inability to detect Fpn. In the Western blot, the high-molecular-mass Fpn-GFP species are a small fraction of the total Fpn-GFP, suggesting that phosphorylation and ubiquitination are brief transient events.
Figure 1.
Fpn-GFP is ubiquitinated and phosphorylated. (A) HEK293T cells (stably transfected with an inducible Fpn-GFP) induced to express Fpn-GFP (+ponasterone) were incubated in the presence or absence of 1 μg/ml hepcidin for 15 min. Cells were placed at 0°C and solubilized in 1.0% Triton X-100, 150 mM NaCl, 10 mM EDTA, 10 mM Tris, pH 7.4, protease inhibitor mixture, 50 mM N-ethylmaleimide, and protein phosphatase inhibitor set. Samples were immunoprecipitated with rabbit anti-GFP antibodies as described in Materials and Methods. Immunoprecipitated samples were analyzed by Western blots probing for ubiquitin by using mouse anti-ubiquitin or for phosphotyrosine by using mouse anti-phosphotyrosine followed by a peroxidase-conjugated goat anti-mouse IgG as secondary antibody. Blots were also probed for Fpn-GFP by using rabbit anti-GFP followed by peroxidase-conjugated goat anti-rabbit IgG as secondary. (B) HEK293T cells were induced to express Fpn-GFP with ponasterone for 18 h. Cells were incubated in the presence or absence of 1 μg/ml hepcidin for the indicated times, placed at 0°C, and the cell surface was biotinylated using sulfo-NHS-SS-biotin. After biotinylation, cells were solubilized as described in A, and the biotinylated proteins were affinity purified using streptavidin affinity gel. The affinity-purified samples were analyzed by Western blot using a rabbit anti-GFP followed by peroxidase-conjugated goat anti-rabbit IgG. (C) HEK293T Fpn-GFP cells were induced to express Fpn-GFP as described in B. Cells were incubated in the presence or absence of 1 μg/ml hepcidin for 15 min, placed at 0°C, and the cell surface was biotinylated. Biotinylated samples were purified using streptavidin beads as described in B. Affinity-purified samples were analyzed by Western blot with mouse anti-phosphotyrosine, mouse anti-ubiquitin followed by a peroxidase-conjugated goat anti-mouse IgG as secondary, or Fpn-GFP by using rabbit anti-GFP followed by peroxidase-conjugated goat anti-rabbit IgG as secondary. “Control” in the top panel is a mixture of tyrosine-modified proteins purchased from Calbiochem as a control for the anti-phosphotyrosine antibody. Flow-through is the sample that did not bind the streptavidin affinity gel (nonbiotinylated Fpn-GFP) but that was subsequently immunopurified using rabbit anti-GFP and the immunopurified sample analyzed by Western blot to test for the presence of phosphotyrosine or ubiquitin modifications on Fpn-GFP.
Phosphorylation of Fpn Occurs at the Cell Surface
We next examined whether ubiquitination and phosphorylation were required for internalization. To determine whether these events occur at the cell surface, we used the membrane-impermeable biotinylation reagent sulfo-NHS-SS-biotin to distinguish between cell surface and internalized Fpn. Cells were treated with sulfo-NHS-SS-biotin at specific times after incubation with hepcidin to distinguish cell surface Fpn-GFP from internalized Fpn-GFP. Biotinylated proteins were purified using streptavidin beads, and the affinity-purified samples were examined for Fpn-GFP by Western blot analysis using antibodies against GFP. Treatment of cells with the sulfo-NHS-SS-biotin resulted in biotinylation of Fpn-GFP (Figure 1B, bottom). In the absence of ponasterone A (no Fpn-GFP expression), there was no detectable biotinylated Fpn-GFP. No Fpn-GFP was detected in the absence of sulfo-NHS-SS-biotin. The amount of biotinylated Fpn-GFP decreased upon addition of hepcidin. The disappearance of total cellular Fpn-GFP closely parallels the disappearance of cell surface Fpn-GFP, indicating that internalized Fpn-GFP is rapidly degraded. We note that Fpn-GFP often occurs as a doublet (De Domenico et al., 2005). Biotinylated Fpn-GFP was examined for protein modifications. Cell surface Fpn-GFP was phosphorylated within 15 min of hepcidin addition (Figure 1C, lane 5, top) but not ubiquitinated (Figure 1C, lane 5, middle). In contrast, internalized Fpn-GFP, which was not biotinylated and did not bind to streptavidin beads (“flow-through”), was ubiquitinated (Figure 1C, lane 6, middle), but phosphorylation was no longer detected (Figure 1C, lane 6, top). These results suggest that phosphorylation is the primary event in response to hepcidin and that it occurs on the cell surface followed by ubiquitination, which occurs once Fpn-GFP has been internalized.
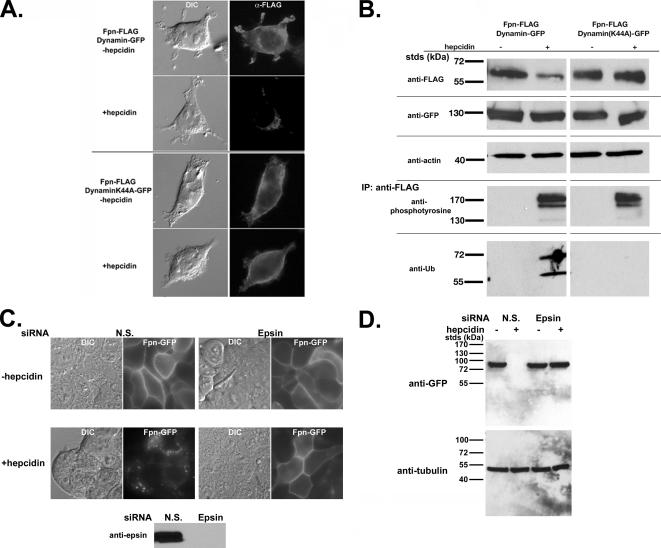
A dominant-negative mutant of dynamin 1, unable to hydrolyze GTP, inhibits both clathrin- and caveolin-mediated internalization (Damke et al., 1994). We took advantage of this dynamin mutant to confirm that phosphorylation is a proximal effect of hepcidin binding to Fpn. Transfection of HEK293T cells with plasmids expressing wild-type dynamin-GFP and Fpn-FLAG did not prevent hepcidin-induced Fpn-FLAG internalization (Figure 2A). In contrast, transfection with a dominant-negative dynamin(K44A)-GFP inhibited hepcidin-induced internalization of Fpn-FLAG. Likewise, Western blot analysis showed that cells transfected with the dynamin(K44A)-GFP and treated with hepcidin had higher levels of Fpn-FLAG compared with hepcidin-treated cells expressing wild-type dynamin-GFP (Figure 2B). Immunoprecipitation of Fpn-FLAG followed by Western blot analysis showed that Fpn was phosphorylated at the plasma membrane; however, it was not ubiquitinated in cells expressing the dynamin mutant (Figure 2B). These results confirm that phosphorylation is an early event that occurs at the cell surface and that ubiquitination occurs after Fpn is internalized. We note that the migration pattern of ubiquitinated Fpn-FLAG does not increase as would be predicted. Ubiquitination of Fpn-FLAG is not detected in the absence of hepcidin, suggesting that this Fpn-FLAG modification is specific to hepcidin incubation. To determine whether Fpn is internalized through clathrin-coated pits, we silenced epsin, a protein required for clathrin-mediated endocytosis (Chen et al., 1998). Cells expressing Fpn-GFP were transfected with nonspecific oligonucleotide pools or pools specific to epsin. Depletion of epsin, as shown by Western blot analysis (Figure 2C), inhibited hepdicin-mediated Fpn-GFP internalization and degradation (Figure 2D). These results indicate that hepcidin-mediated internalization of Fpn-GFP occurs through clathrin-coated pits.
Figure 2.
Fpn is phosphorylated at the plasma membrane. (A) HEK293T cells were transiently transfected with Fpn-FLAG and either Dynamin-GFP or DynaminK44A-GFP. Cells were incubated in the presence or absence of 1 μg/ml hepcidin for 60 min and processed for immunofluorescence using mouse anti-FLAG followed by Alexa 594-conjugated goat anti-mouse IgG (1:750) as secondary antibody. (B) HEK293T cells were transiently transfected as described in A. Cells were incubated in the presence or absence of 1 μg/ml hepcidin as in A, extracts obtained as described in Figure 1 and Western blotted for Fpn-FLAG, Dynamin-GFP, and actin (loading control). Extracts were immunoprecipitated using anti-FLAG resin (M2), and the immunoprecipitates were examined for the presence of tyrosine phosphorylated Fpn-FLAG or ubiquitinated Fpn-FLAG by using mouse anti-phosphotyrosine or mouse anti-ubiquitin antibodies followed by peroxidase-conjugated goat anti-mouse IgG. (C and D) HEK293T Fpn-GFP cells were transfected with nonspecific (NS) or epsin-specific siRNA oligonucleotide pools. After 48 h of incubation, cells were induced to express Fpn-GFP and then 18 h later 1 μg/ml hepcidin was added for 1 h. The efficiency of epsin depletion was assessed by Western blot analysis using antibodies to epsin. The presence of Fpn-GFP was assessed by epifluorescence (C) and Western blot analysis (D).
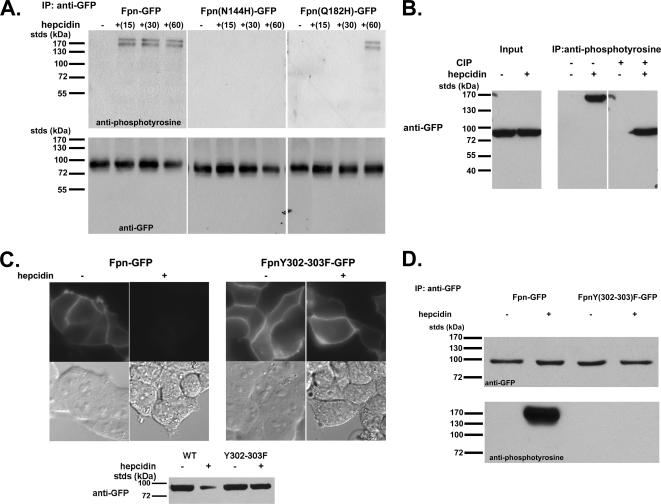
Tyrosine phosphorylation of Fpn is a rapid response to hepcidin binding, occurring within 15 min of hepcidin addition (Figure 3A). Phosphorylation of Fpn-GFP was independent of the mode of Fpn expression (inducible vs. transient/CMV) or epitope. Critically, mutants of Fpn that are expressed at the cell surface at similar levels to wild type bind hepcidin, but they are not internalized [Fpn(N144H)-GFP] or are internalized at a slower rate than wild type [Fpn(Q182H)-GFP] (De Domenico et al., 2005) showed either no phosphorylation or delayed phosphorylation, respectively, supporting the hypothesis that phosphorylation is the signal for internalization. There is a large difference in the mobility of the protein detected by the anti-phosphotyrosine antibody and that of Fpn-GFP, raising the possibility that the phosphoprotein might not be Fpn. Alternatively, phosphorylation might affect Fpn conformation, because the samples of Fpn applied to SDS-PAGE are not boiled nor do they contain reducing agents; boiling or reducing agents results in an inability to detect Fpn. To examine this issue, we immunoprecipitated extracts with anti-phosphotyrosine antibody and then probed the immunoprecipitate for Fpn-GFP. After hepcidin addition, the anti-phosphotyrosine antibody precipitated a high-molecular-mass Fpn-GFP, as shown by Western blotting (Figure 3B). The immunoprecipitate was treated with calf intestinal phosphatase, which resulted in the detection of Fpn-GFP at a lower molecular mass, similar to cells not incubated with hepcidin. Based on these results, we conclude that Fpn-GFP is phosphorylated after hepcidin treatment.
Figure 3.
Phosphorylation, location, and function of human Fpn mutations. (A) HEK293T cells were transiently transfected with Fpn-GFP, Fpn(N144H)-GFP, or Fpn(Q182H)-GFP expressed under a CMV promoter. Cells were incubated in the presence or absence of 1 μg/ml hepcidin for indicated times, solubilized, and immunoprecipitated with rabbit anti-GFP antibodies. Immunoprecipitated samples were analyzed on nonreducing 10% SDS-PAGE, and Western blots were probed for either phosphotyrosine by using mouse anti-phosphotyrosine followed by a peroxidase-conjugated goat anti-mouse IgG as secondary or Fpn-GFP by using rabbit anti-GFP followed by peroxidase-conjugated goat anti-rabbit IgG as secondary. (B) HEK293T cells expressing wild-type Fpn-GFP were incubated with and without 1 μg/ml hepcidin for 15 min. Cell extracts were immunoprecipitated with anti-phosphotyrosine antibodies, and the immunoprecipitate was incubated in the presence or absence of calf intestinal phosphatase (CIP) for 60 min at 37°C. The samples were analyzed by SDS-PAGE and Western blot probing for Fpn-GFP. (C) HEK293T cells transfected with Fpn-GFP or Fpn(Y302-303F)-GFP were incubated in the presence of 1 μg/ml hepcidin for up to 24 h. Fpn-GFP localization was examined by fluorescence microscopy after 4 h of hepcidin incubation, and Fpn-GFP levels were measured by Western blot analysis after 24 h of hepcidin incubation. (D) HEK293T cells transfected with Fpn-GFP or Fpn(Y302-303F)-GFP were incubated with hepcidin for 15 min. The cells were solubilized, and Fpn-GFP was immunoprecipitated and analyzed by Western blots as described in A.
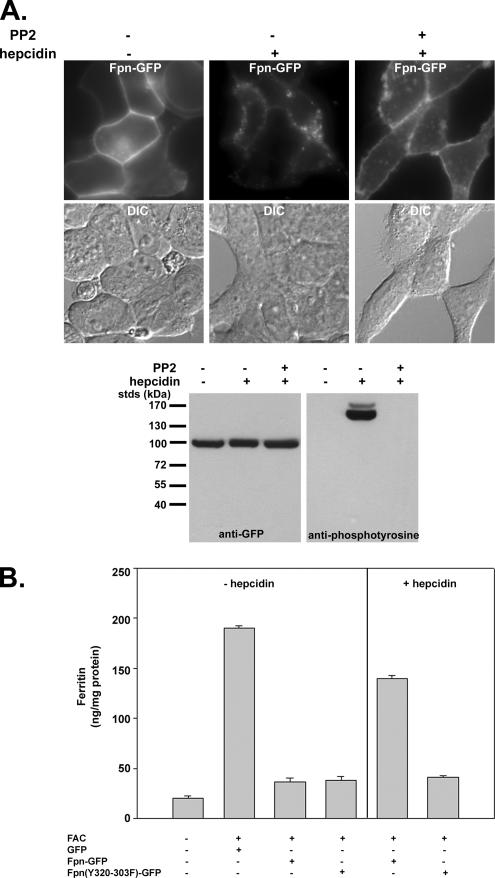
Although the topology of Fpn has not been conclusively resolved, a working model was proposed by Haile and colleagues (Liu et al., 2005). This model suggests that there are four tyrosine residues that face the cytosolic surface. We mutated each of these residues (227, 302, 303, and 537) individually to phenylalanine. The mutated proteins localized to the plasma membrane, and they were internalized similarly to wild-type Fpn in response to hepcidin (data not shown). This result suggests that either the topological model is wrong and other tyrosines are cytosolic or that multiple tyrosines can be phosphorylated. We then mutated multiple tyrosines. We mutated two adjacent tyrosines contained within a putative cytosolic peptide located between transmembrane regions VI and VII. The mutant protein Fpn(Y302-303F)-GFP, expressed in HEK293T cells, was targeted appropriately to the plasma membrane, but it was not internalized in response to hepcidin, even after 24 h (Figure 3C). To determine whether the mutant protein was phosphorylated, Fpn-GFP or Fpn(Y302-303F)-GFP expressed using the CMV promoter was immunoprecipitated from cells after a brief (15-min) exposure to hepcidin. This brief exposure to hepcidin is not long enough to see significant loss of Fpn-GFP. Western blot analysis of the immunoprecipitate confirmed the absence of the high-molecular-weight phosphorylated species (Figure 3D). Sequence analysis of Fpn does not suggest that it is a kinase. A number of potassium and chloride channels are tyrosine phosphorylated by a nonreceptor tyrosine kinase, usually belonging to the src kinase family (Davis et al., 2001). Cells expressing Fpn-GFP were incubated with PP2, a src kinase inhibitor. The inhibitor did not affect the expression of Fpn-GFP, but it prevented hepcidin-mediated tyrosine phosphorylation, internalization, and degradation of Fpn-GFP (Figure 4A). These data indicate that phosphorylation of Fpn is required for its internalization. Fpn(Y302-303F)-GFP is a functional iron exporter, because expression in iron-loaded cells resulted in decreased levels of the iron storage protein ferritin similar to wild-type Fpn-GFP (Figure 4B). Addition of hepcidin does not, however, prevent iron export as shown by the low level of ferritin in Fpn(Y302-303F)-GFP–expressing cells. This result confirms the conclusion based on human Fpn mutants that hepcidin regulates Fpn iron export activity by facilitating Fpn removal.
Figure 4.
Effect of src kinase inhibitor PP2 on hepcidin-induced phosphorylation and internalization of Fpn-GFP. (A) HEK293T Fpn-GFP–expressing cells were incubated with 100 μM PP2 for 15 min. Hepcidin was added for an additional 20 min, and Fpn-GFP localization was examined by fluorescence microscopy, and tyrosine phosphorylation was assayed as on immunoprecipitated Fpn-GFP as described in Figure 3. (B) HEK293T cells, transfected with either pEGP vector, Fpn-GFP, or Fpn(Y302-303F)-GFP, were incubated with or without ferric ammonium citrate (FAC) (10 μM Fe) for 16 h, and the amount of cell-associated ferritin was determined. Iron-loaded cells expressing Fpn-GFP or Fpn(Y302-303F)-GFP were incubated in the presence of 1 μg/ml hepcidin for 24 h, and the amount of cell-associated ferritin was determined.
Ubiquitination of Fpn Is Required for Trafficking and Degradation
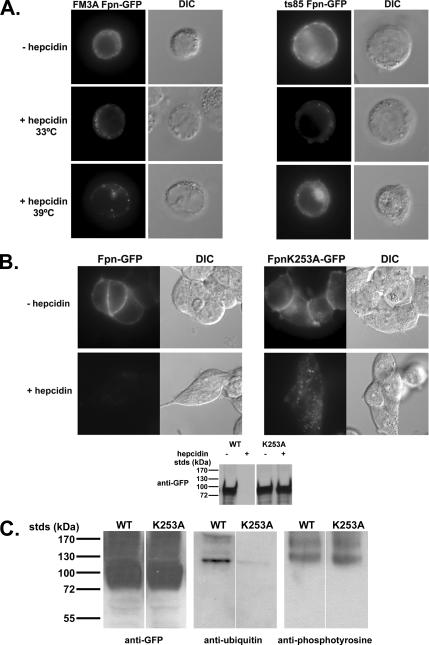
Once internalized, Fpn is targeted to the lysosome where it is degraded (Nemeth et al., 2004). Most membrane proteins degraded in the lysosome are trafficked through the MVB pathway en route to lysosomes, and entry into the MVB typically requires ubiquitination of target proteins (Hurley and Emr, 2006). To determine whether ubiquitination is a requirement for internalized Fpn to enter the MVB, we took advantage of the cell line ts85 that has a temperature-sensitive E1-ubiquitin ligase. This cell line cannot add ubiquitin to target proteins at the restrictive temperature of 39°C (Ikehata et al., 1997). Both wild-type (FM3A) and mutant cells (ts85) were transformed with a plasmid containing a CMV-regulated Fpn-GFP. Fpn-GFP was found on the cell surface at the permissive (Figure 5A) and restrictive temperatures (data not shown). Addition of hepcidin to either wild-type or ts85 cells led to the internalization of Fpn-GFP at both permissive and restrictive temperatures. Fpn was degraded in both cell types at the permissive temperature; however, at the restrictive temperature Fpn-GFP was degraded in wild-type cells, but it was not degraded in ts85 cells. In ts85 cells, at the restrictive temperature, Fpn-GFP accumulated in intracellular vesicles with some present on the plasma membrane.
Figure 5.
Ubiquitination is required for Fpn-GFP trafficking to the lysosome. (A) Wild-type (FM3A) or mutant cells (ts85) were transfected with Fpn-GFP. Cells were then incubated at the permissive (33°C) or restrictive (39°C) temperature in the presence or absence of 1 μg/ml hepcidin for 60 min. Cells were examined by epifluorescence microscopy for the localization of Fpn-GFP. (B) HEK293T cells were transiently transfected with Fpn-GFP or Fpn(K253A)-GFP and grown for 12 h. Cells were incubated in the presence or absence of 1 μg/ml hepcidin for 60 min at 37°C, and then they were examined for the localization of Fpn-GFP, and protein levels were assessed by Western blot. (C) Cells expressing wild-type or Fpn(K253A)-GFP were incubated in the presence of 1 μg/ml hepcidin for 15 min. The cells were solubilized, and Fpn-GFP was immunoprecipitated as in Figure 1. Immunoprecipitates were analyzed by SDS-PAGE and Western blot for the presence of Fpn-GFP by using rabbit anti-GFP followed by peroxidase-conjugated goat anti-rabbit IgG or for ubiquitinated or tyrosine-phosphorylated Fpn-GFP by using mouse anti-ubiquitin or mouse anti-phosphotyrosine antibodies followed by peroxidase-conjugated goat anti-mouse IgG.
Haile's model of Fpn topology (Liu et al., 2005) suggests that there are 11 lysine residues on the cytosolic surface of Fpn that may be sites for ubiquitin addition. We focused our attention on the lysines present in the large cytoplasmic loop that is between two putative transmembrane (TM) domains (TM-VI and –VII), because this loop also contains tyrosines 302 and 303. Mutation of lysine residues 253, 258, or 269 to alanine had no effect on the plasma membrane localization of Fpn-GFP or on the ability of the mutated Fpn to be internalized and degraded in response to hepcidin (data not shown). Mutation of lysine residue 253 resulted in a protein that targeted to the cell surface and could be internalized by hepcidin (Figure 5B). The mutant protein Fpn(K253A)-GFP was degraded at a much slower rate than wild-type protein and was found in intracellular vesicles. Fpn(K253A)-GFP was phosphorylated in response to hepcidin, but it was no longer ubiquitinated, suggesting that residue 253 is a site for ubiquitination (Figure 5C). These results show that ubiquitination is not required for internalization but that it is required for degradation. There was a difference in the appearance of internalized Fpn-GFP in ts85 cells and in Fpn(K253A)-GFP in HEK293T cells. We feel that this difference can be ascribed to the difference in morphology between the nonadherent ts85 cells and the adherent HEK293T cells.
Fpn Traffics through the MVB En Route to the Lysosome
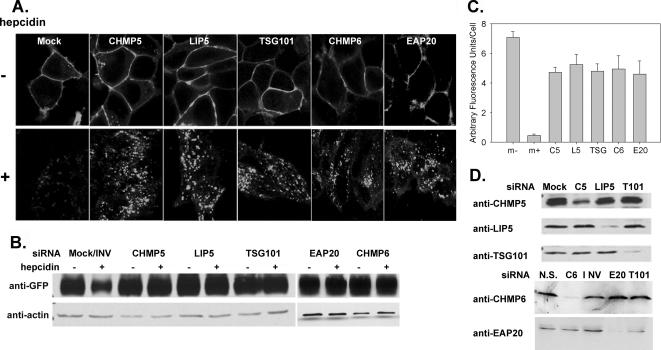
Trafficking of plasma membrane proteins to the lysosome is dependent on sorting through the MVB (Raiborg et al., 2003; Hurley and Emr, 2006). Many proteins involved in sorting through the MVB have been identified, and most are associated with three complexes that are sequentially recruited to the endosomal membrane during MVB formation, endosome sorting complex required for transport (ESCRT) I–III (Babst et al., 2002a,b; Katzmann et al., 2002). We used RNA interference oligonucleotide pools specific to different proteins in the MVB pathway to determine whether degradation of Fpn requires trafficking through the MVB. TSG101(Vps23p) is a component of the ESCRT-I complex, which is required for recognition of ubiquitinated cargos destined for the MVB (Garrus et al., 2001; Katzmann et al., 2001). Depletion of TSG101 in mammalian cells results in the accumulation of epidermal growth factor-epidermal growth factor receptor (EGFR) in endocytic compartments (Bishop et al., 2002), and deletion of the yeast homologue results in the missorting of the vacuolar peptidase carboxypeptidase S (CPS) and the formation of a vesicle termed the “class E compartment” (Babst et al., 2000). EAP20(Vps25p) is a component of ESCRT-II, and ESCRT-II has been shown to bridge ESCRT-I and ESCRT-III (Babst et al., 2002b; Teo et al., 2004, 2006; Yorikawa et al., 2005; Langelier et al., 2006). The absence of ESCRT-II components in yeast results in the accumulation of CPS and a class E or enlarged MVB compartment (Babst et al., 2002b). CHMP6(Vps20p) binds EAP20 and is part of the ESCRT-III complex (Teo et al., 2004; Langelier et al., 2006). The absence of Vps20p also results in the missorting and accumulation of CPS in a class E compartment (Babst et al., 2002a). CHMP5(Vps60p) is thought to be a part of the ESCRT-III complex, and it has been shown to be involved in trafficking EGFR through the MVB/lysosomal pathway (Ward et al., 2005). Finally, LIP5(Vta1p) is thought to act after ESCRT-III, binding and activating the MVB ATPase Vps4 (Scott et al., 2005; Azmi et al., 2006; Lottridge et al., 2006). LIP5 is involved in trafficking EGFR through the MVB/lysosomal pathway (Fujita et al., 2004). siRNA transfection by using oligonucleotide pools directed against specific ESCRT proteins did not affect the cell surface localization of Fpn-GFP nor hepcidin-mediated internalization (Figure 6A). Depletion of ESCRT-I, ESCRT-II, and ESCRT-III proteins or ESCRT III accessory proteins (CHMP5 or LIP5), however, did lead to a delay in the degradation of Fpn-GFP, as assessed by Western blot analysis (Figure 6B) with Fpn-GFP accumulation in endocytic vesicles (Figure 6A). The relative amounts of Fpn-GFP after hepcidin addition was quantified (Figure 6C). Protein depletion by specific siRNA oligonucleotide pools was assessed by Western analysis using protein-specific antibodies (Figure 6D). These data demonstrate that upon internalization, Fpn-GFP is trafficked through the MVB in route to the lysosomes for degradation and that efficient degradation requires all three ESCRT complexes as well as late-acting accessory factors.
Figure 6.
Depletion of MVB proteins affects the trafficking and degradation of Fpn-GFP. (A) HEK293T Fpn-GFP cells were transfected with siRNA oligonucleotide pools specific for TSG101 (ESCRT I), EAP20 (ESCRT II), CHMP6 (ESCRT III), CHMP5 (ESCRT III), LIP5, nonspecific (N.S.), or inverted TSG101 (INV) by using OligofectAMINE. Forty-eight hours later, cells were induced to express Fpn-GFP, and after 18 h, the cells were incubated in the presence or absence of hepcidin. Cells were examined by confocal microscopy, and the localization of Fpn-GFP was determined. (B) Cells treated as described in A were solubilized as in Figure 1, analyzed on SDS-PAGE under nonreducing conditions, and Western blotted for either Fpn-GFP or actin. (C) Images from A were analyzed using National Institutes of Health ImageJ, and the amount of relative fluorescence per cell was determined. The data are expressed as arbitrary fluorescence units per cell, and error bars represent the analysis of greater than five fields per sample. (D) To assess silencing, samples from A were applied to SDS-PAGE under reducing conditions and analyzed by Western blot by using mouse or rabbit antibodies against TSG101 (T101), EAP20 (E20), CHMP6 (C6), CHMP5 (C5), or LIP5 followed by peroxidase-conjugated goat anti-mouse/rabbit IgG.
DISCUSSION
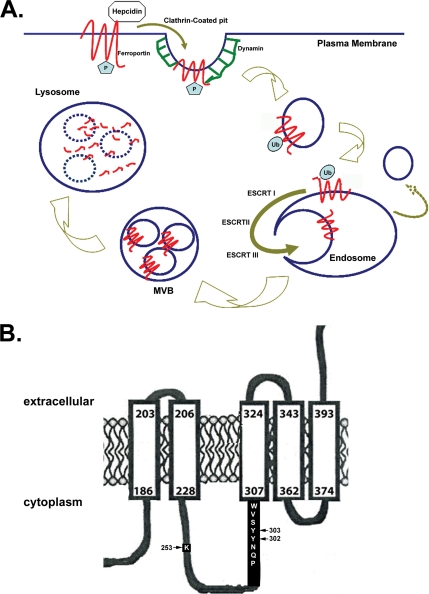
The relationship between hepcidin and Fpn provides insight into mammalian iron homeostasis and its dysregulation. Hepcidin-mediated internalization of Fpn results in reduced cellular iron export and provides an explanation for the regulation of iron absorption and for malregulation of iron absorption leading to both iron deficiency and excessive iron accumulation (Ganz and Nemeth, 2006). In this study, we examined the initial events after the binding of hepcidin to Fpn. Our results show that hepcidin induces Fpn phosphorylation, which is critical for internalization (Figure 7A). The data that support this conclusion are as follows: 1) Fpn tyrosine phosphorylation occurs at the cell surface; 2) Fpn tyrosine phosphorylation is not blocked by inhibitors of endocytosis; 3) Fpn tyrosine phosphorylation is defective in human Fpn mutants N144H and Q182H that are not internalized or that are internalized slowly; 4) mutation of Fpn Y302 and Y303 prevents phosphorylation, and thus Fpn endocytosis; and 5) inhibitors of src kinases prevent Fpn-GFP phosphorylation and also prevent Fpn-GFP internalization.
Figure 7.
Model for Fpn internalization and degradation. (A) Hepcidin binds Fpn at the plasma membrane where Fpn is tyrosine phosphorylated. Once Fpn is internalized, the phosphates are removed, and Fpn is ubiquitinated, which targets it to the MVB for degradation in the lysosomes. (B) Topology of Fpn showing the potential transmembrane domain containing the phosphorylation (Y302 and Y303) and ubiquitination (K253) sites.
These results show that phosphorylation of either Fpn Y302 or Fpn Y303 is an early event after hepcidin binding and that it is required for internalization. Phosphorylation of Fpn can occur on either of these adjacent tyrosines, because mutation of either tyrosine alone has little effect on internalization. This result suggests that only one of the two tyrosines needs to be phosphorylated for Fpn internalization. We note that phosphorylation of Fpn leads to a decreased mobility on SDS-PAGE that is discrepant with the additional mass of either one or two phosphates. We feel confident that it is Fpn that is phosphorylated and hypothesize that the nonboiled protein retains some conformation that is affected by phosphorylation. We cannot, however, rule out the possibility that the migration on SDS-PAGE is due to a protein that binds to the phosphorylated residues. The Y302 and Y303 residues are adjacent to a potential transmembrane segment (Figure 7B), and it is possible that phosphorylation results in a significant structural alteration in the protein–lipid interaction. Furthermore, sequence analysis (ExPASy-prosite database) suggests that the amino acids surrounding the two tyrosines 302-303 may be a src homology 2 domain, indicating that the phosphorylated protein could interact with a variety of adaptor proteins to effect internalization. There is a precedent for tyrosine phosphorylation of membrane transporters/channels leading to either internalization or modification of channel function (for review, see Davis et al., 2001). Our data suggest that Fpn, like other ion transporters such as potassium and chloride channels, is tyrosine phosphorylated by a src kinase. It remains to be determined what conformational features in Fpn are altered upon binding of hepcidin and how these features recruit a nonreceptor tyrosine kinase. Similarly, the nature of the structural changes resulting from N144H and Q182H, which are predicted to be on a cytoplasmic loop but not the loop containing the phosphorylation site are also unknown. Fpn is a dimer, and these mutations might affect the conformation of the multimeric protein (De Domenico et al., 2007; Zohn et al., 2007).
Our data show that ubiquitination of Fpn occurs subsequently to internalization (Figure 7A). Inhibition of ubiquitination, through the use of cells with a temperature-sensitive E1 ligase or through site-directed mutagenesis of lysine residue K253, leads to the accumulation of Fpn-GFP in intracellular vesicles as well as at the plasma membrane. The presence of Fpn-GFP at the plasma membrane may reflect a decrease in the internalization of Fpn-GFP, or it may represent the recycling of Fpn-GFP when it is not ubiquitinated. The recycling of membrane proteins back to the plasma membrane, if not ubiquitinated, has been demonstrated for the EGFR (Grovdal et al., 2004), and it may indicate specific sorting of membrane proteins that have been internalized but not ubiquitinated. It is of interest that the ubiquitination site is on the same large cytosolic loop as the phosphorylation sites (Figure 7B), suggesting that a ubiquitin ligase may respond to alterations in Fpn structure or binding partners induced by phosphorylation.
Targeting of most membrane proteins to the lysosome is accomplished by their entry into the MVB. Data suggest, however, that not all ESCRT subunits are required for trafficking through the mammalian MVB. Depletion of HRS by siRNA prevents the agonist induced lysosomal degradation of δ opiod receptors, whereas depletion of TSG101/ESCRT-I does not prevent δ opioid receptor lysosomal degradation (Hislop et al., 2004). Similarly, TSG101/ESCRT I depletion inhibits MHC class I degradation, whereas EAP20/ESCRT-II is not required for major histocompatibility complex (MHC)-I receptor down-regulation (Bowers et al., 2006), although this complex is required for efficient degradation of internalized epidermal growth factor-receptors (Langelier et al., 2006). Our data demonstrate that trafficking of Fpn-GFP to the lysosome goes through the MVB pathway and requires participation of all of the ESCRT complexes. Depletion of members of ESCRT I, ESCRT II, or ESCRT III complexes leads to the accumulation of Fpn-GFP in intracellular vesicles and reduced degradation. We note that the block on degradation is not absolute, as Fpn-GFP levels do decrease with time. Whether the lack of a complete block is due to incomplete silencing or to redundancy in ESCRT proteins is unresolved. Nevertheless, our studies demonstrate a role for the MVB pathway in hepcidin-mediated degradation of Fpn.
Many mutations in Fpn cause iron overload disease, which has two distinct presentations (Pietrangelo, 2006). One form of the disease results from Fpn mutants that are unable to reach the plasma membrane (De Domenico et al., 2005; Schimanski et al., 2005). Cells expressing these Fpn mutations are compromised in their ability to export iron and show excessive iron accumulation. A second form of the disease results from Fpn mutants that are targeted to the plasma membrane but do not bind hepcidin or bind hepcidin and are not internalized (De Domenico et al., 2005; Drakesmith et al., 2005). These results confirm that hepcidin regulates Fpn-mediated iron export by regulating the concentration of Fpn at the plasma membrane and not by affecting the transport activity of Fpn.
ACKNOWLEDGMENTS
We express our appreciation to Dr. Martin Rechsteiner (University of Utah) for the cell lines FM3A and ts85, to Dr. Matt Mulvey (University of Utah) for dynamin constructs, and to the Kaplan laboratory for critically reading this manuscript. Images for confocal microscopy were obtained at the University of Utah Imaging Core under the direction of Dr. Chris Rodesch. This work was supported by National Institutes of Health grants DK-070947 and HL-26922 (to J.K.) and AI-051174 (to W.I.S.).
Abbreviations used:
- ESCRT
endosome sorting complex required for transport
- Fpn
ferroportin
- GFP
green fluorescent protein
- MVB
multivesicular body.
Footnotes
This article was published online ahead of print in MBC in Press (http://www.molbiolcell.org/cgi/doi/10.1091/mbc.E07-01-0060) on May 2, 2007.
REFERENCES
- Abboud S., Haile D. J. A novel mammalian iron-regulated protein involved in intracellular iron metabolism. J. Biol. Chem. 2000;275:19906–19912. doi: 10.1074/jbc.M000713200. [DOI] [PubMed] [Google Scholar]
- Azmi I., Davies B., Dimaano C., Payne J., Eckert D., Babst M., Katzmann D. J. Recycling of ESCRTs by the AAA-ATPase Vps4 is regulated by a conserved VSL region in Vta1. J. Cell Biol. 2006;172:705–717. doi: 10.1083/jcb.200508166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babst M., Katzmann D. J., Estepa-Sabal E. J., Meerloo T., Emr S. D. Escrt-III: an endosome-associated heterooligomeric protein complex required for mvb sorting. Dev. Cell. 2002a;3:271–282. doi: 10.1016/s1534-5807(02)00220-4. [DOI] [PubMed] [Google Scholar]
- Babst M., Katzmann D. J., Snyder W. B., Wendland B., Emr S. D. Endosome-associated complex, ESCRT-II, recruits transport machinery for protein sorting at the multivesicular body. Dev. Cell. 2002b;3:283–289. doi: 10.1016/s1534-5807(02)00219-8. [DOI] [PubMed] [Google Scholar]
- Babst M., Odorizzi G., Estepa E. J., Emr S. D. Mammalian tumor susceptibility gene 101 (TSG101) and the yeast homologue, Vps23p, both function in late endosomal trafficking. Traffic. 2000;1:248–258. doi: 10.1034/j.1600-0854.2000.010307.x. [DOI] [PubMed] [Google Scholar]
- Bishop N., Horman A., Woodman P. Mammalian class E vps proteins recognize ubiquitin and act in the removal of endosomal protein-ubiquitin conjugates. J. Cell Biol. 2002;157:91–101. doi: 10.1083/jcb.200112080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonifacino J. S., Traub L. M. Signals for sorting of transmembrane proteins to endosomes and lysosomes. Annu. Rev. Biochem. 2003;72:395–447. doi: 10.1146/annurev.biochem.72.121801.161800. [DOI] [PubMed] [Google Scholar]
- Bowers K., Piper S. C., Edeling M. A., Gray S. R., Owen D. J., Lehner P. J., Luzio J. P. Degradation of endocytosed epidermal growth factor and virally ubiquitinated major histocompatibility complex class I is independent of mammalian ESCRTII. J. Biol. Chem. 2006;281:5094–5105. doi: 10.1074/jbc.M508632200. [DOI] [PubMed] [Google Scholar]
- Camaschella C. Understanding iron homeostasis through genetic analysis of hemochromatosis and related disorders. Blood. 2005;106:3710–3717. doi: 10.1182/blood-2005-05-1857. [DOI] [PubMed] [Google Scholar]
- Chen H., Fre S., Slepnev V. I., Capua M. R., Takei K., Butler M. H., Di Fiore P. P., De Camilli P. Epsin is an EH-domain-binding protein implicated in clathrin-mediated endocytosis. Nature. 1998;394:793–797. doi: 10.1038/29555. [DOI] [PubMed] [Google Scholar]
- Damke H., Baba T., Warnock D. E., Schmid S. L. Induction of mutant dynamin specifically blocks endocytic coated vesicle formation. J. Cell Biol. 1994;127:915–934. doi: 10.1083/jcb.127.4.915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis M. J., Wu X., Nurkiewicz T. R., Kawasaki J., Gui P., Hill M. A., Wilson E. Regulation of ion channels by protein tyrosine phosphorylation. Am. J. Physiol. 2001;281:H1835–H1862. doi: 10.1152/ajpheart.2001.281.5.H1835. [DOI] [PubMed] [Google Scholar]
- De Domenico I., Ward D. M., Musci G., Kaplan J. Evidence for the multimeric structure of ferroportin. Blood. 2007;109:2205–2209. doi: 10.1182/blood-2006-06-032516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Domenico I., Ward D. M., Nemeth E., Vaughn M. B., Musci G., Ganz T., Kaplan J. The molecular basis of ferroportin-linked hemochromatosis. Proc. Natl. Acad. Sci. USA. 2005;102:8955–8960. doi: 10.1073/pnas.0503804102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donovan A., et al. Positional cloning of zebrafish ferroportin1 identifies a conserved vertebrate iron exporter. Nature. 2000;403:776–781. doi: 10.1038/35001596. [DOI] [PubMed] [Google Scholar]
- Donovan A., Roy C. N., Andrews N. C. The ins and outs of iron homeostasis. Physiology. 2006;21:115–123. doi: 10.1152/physiol.00052.2005. [DOI] [PubMed] [Google Scholar]
- Drakesmith H., et al. Resistance to hepcidin is conferred by hemochromatosis-associated mutations of ferroportin. Blood. 2005;106:1092–1097. doi: 10.1182/blood-2005-02-0561. [DOI] [PubMed] [Google Scholar]
- Fujita H., et al. Mammalian class E Vps proteins, SBP1 and mVps2/CHMP2A, interact with and regulate the function of an AAA-ATPase SKD1/Vps4B. J. Cell Sci. 2004;117:2997–3009. doi: 10.1242/jcs.01170. [DOI] [PubMed] [Google Scholar]
- Ganz T., Nemeth E. Iron imports. IV. Hepcidin and regulation of body iron metabolism. Am. J. Physiol. 2006;290:G199–G203. doi: 10.1152/ajpgi.00412.2005. [DOI] [PubMed] [Google Scholar]
- Garrus J. E., et al. Tsg101 and the vacuolar protein sorting pathway are essential for HIV-1 budding. Cell. 2001;107:55–65. doi: 10.1016/s0092-8674(01)00506-2. [DOI] [PubMed] [Google Scholar]
- Goncalves A. S., Muzeau F., Blaybel R., Hetet G., Driss F., Delaby C., Canonne-Hergaux F., Beaumont C. Wild-type and mutant ferroportins do not form oligomers in transfected cells. Biochem. J. 2006;396:265–275. doi: 10.1042/BJ20051682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grovdal L. M., Stang E., Sorkin A., Madshus I. H. Direct interaction of Cbl with pTyr 1045 of the EGF receptor (EGFR) is required to sort the EGFR to lysosomes for degradation. Exp. Cell Res. 2004;300:388–395. doi: 10.1016/j.yexcr.2004.07.003. [DOI] [PubMed] [Google Scholar]
- Hentze M. W., Muckenthaler M. U., Andrews N. C. Balancing acts: molecular control of mammalian iron metabolism. Cell. 2004;117:285–297. doi: 10.1016/s0092-8674(04)00343-5. [DOI] [PubMed] [Google Scholar]
- Hislop J. N., Marley A., Von Zastrow M. Role of mammalian vacuolar protein-sorting proteins in endocytic trafficking of a non-ubiquitinated G protein-coupled receptor to lysosomes. J. Biol. Chem. 2004;279:22522–22531. doi: 10.1074/jbc.M311062200. [DOI] [PubMed] [Google Scholar]
- Hurley J. H., Emr S. D. The ESCRT complexes: structure and mechanism of a membrane-trafficking network. Annu. Rev. Biophys. Biomol. Struct. 2006;35:277–298. doi: 10.1146/annurev.biophys.35.040405.102126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikehata H., Kaneda S., Yamao F., Seno T., Ono T., Hanaoka F. Incubation at the nonpermissive temperature induces deficiencies in UV resistance and mutagenesis in mouse mutant cells expressing a temperature-sensitive ubiquitin-activating enzyme (E1) Mol. Cell. Biol. 1997;17:1484–1489. doi: 10.1128/mcb.17.3.1484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katzmann D. J., Babst M., Emr S. D. Ubiquitin-dependent sorting into the multivesicular body pathway requires the function of a conserved endosomal protein sorting complex, ESCRT-I. Cell. 2001;106:145–155. doi: 10.1016/s0092-8674(01)00434-2. [DOI] [PubMed] [Google Scholar]
- Katzmann D. J., Odorizzi G., Emr S. D. Receptor downregulation and multivesicular-body sorting. Nat. Rev. Mol. Cell Biol. 2002;3:893–905. doi: 10.1038/nrm973. [DOI] [PubMed] [Google Scholar]
- Langelier C., von Schwedler U. K., Fisher R. D., De Domenico I., White P. L., Hill C. P., Kaplan J., Ward D., Sundquist W. I. Human ESCRT-II complex and its role in human immunodeficiency virus type 1 release. J. Virol. 2006;80:9465–9480. doi: 10.1128/JVI.01049-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X. B., Yang F., Haile D. J. Functional consequences of ferroportin 1 mutations. Blood Cells Mol. Dis. 2005;35:33–46. doi: 10.1016/j.bcmd.2005.04.005. [DOI] [PubMed] [Google Scholar]
- Lottridge J. M., Flannery A. R., Vincelli J. L., Stevens T. H. Vta1p and Vps46p regulate the membrane association and ATPase activity of Vps4p at the yeast multivesicular body. Proc. Natl. Acad. Sci. USA. 2006;103:6202–6207. doi: 10.1073/pnas.0601712103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKie A. T., et al. A novel duodenal iron-regulated transporter, IREG1, implicated in the basolateral transfer of iron to the circulation. Mol. Cell. 2000;5:299–309. doi: 10.1016/s1097-2765(00)80425-6. [DOI] [PubMed] [Google Scholar]
- Nemeth E., Tuttle M. S., Powelson J., Vaughn M. B., Donovan A., Ward D. M., Ganz T., Kaplan J. Hepcidin regulates cellular iron efflux by binding to ferroportin and inducing its internalization. Science. 2004;306:2090–2093. doi: 10.1126/science.1104742. [DOI] [PubMed] [Google Scholar]
- Pietrangelo A. The ferroportin disease. Blood Cells Mol. Dis. 2004;32:131–138. doi: 10.1016/j.bcmd.2003.08.003. [DOI] [PubMed] [Google Scholar]
- Pietrangelo A. Hereditary hemochromatosis. Biochim. Biophys. Acta. 2006;1763:700–710. doi: 10.1016/j.bbamcr.2006.05.013. [DOI] [PubMed] [Google Scholar]
- Raiborg C., Rusten T. E., Stenmark H. Protein sorting into multivesicular endosomes. Curr. Opin. Cell Biol. 2003;15:446–455. doi: 10.1016/s0955-0674(03)00080-2. [DOI] [PubMed] [Google Scholar]
- Schimanski L. M., et al. In vitro functional analysis of human ferroportin (FPN) and hemochromatosis-associated FPN mutations. Blood. 2005;105:4096–4102. doi: 10.1182/blood-2004-11-4502. [DOI] [PubMed] [Google Scholar]
- Scott A., Gaspar J., Stuchell-Brereton M. D., Alam S. L., Skalicky J. J., Sundquist W. I. Structure and ESCRT-III protein interactions of the MIT domain of human VPS4A. Proc. Natl. Acad. Sci. USA. 2005;102:13813–13818. doi: 10.1073/pnas.0502165102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teo H., Gill D. J., Sun J., Perisic O., Veprintsev D. B., Vallis Y., Emr S. D., Williams R. L. ESCRT-I core and ESCRT-II GLUE domain structures reveal role for GLUE in linking to ESCRT-I and membranes. Cell. 2006;125:99–111. doi: 10.1016/j.cell.2006.01.047. [DOI] [PubMed] [Google Scholar]
- Teo H., Perisic O., Gonzalez B., Williams R. L. ESCRT-II, an endosome-associated complex required for protein sorting: crystal structure and interactions with ESCRT-III and membranes. Dev. Cell. 2004;7:559–569. doi: 10.1016/j.devcel.2004.09.003. [DOI] [PubMed] [Google Scholar]
- Ward D. M., Vaughn M. B., Shiflett S. L., White P. L., Pollock A. L., Hill J., Schnegelberger R., Sundquist W. I., Kaplan J. The role of LIP5 and CHMP5 in multivesicular body formation and HIV-1 budding in mammalian cells. J. Biol. Chem. 2005;280:10548–10555. doi: 10.1074/jbc.M413734200. [DOI] [PubMed] [Google Scholar]
- Weiss G., Goodnough L. T. Anemia of chronic disease. N. Engl. J. Med. 2005;352:1011–1023. doi: 10.1056/NEJMra041809. [DOI] [PubMed] [Google Scholar]
- Yorikawa C., Shibata H., Waguri S., Hatta K., Horii M., Katoh K., Kobayashi T., Uchiyama Y., Maki M. Human CHMP6, a myristoylated ESCRT-III protein, interacts directly with an ESCRT-II component EAP20 and regulates endosomal cargo sorting. Biochem. J. 2005;387:17–26. doi: 10.1042/BJ20041227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zohn I. E., De Domenico I., Pollock A., Ward D. M., Goodman J. F., Liang X., Sanchez A. J., Niswander L., Kaplan J. The flatiron mutation in mouse ferroportin acts as a dominant negative to cause ferroportin disease. Blood. 2007 doi: 10.1182/blood-2007-01-066068. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]