Abstract
The conserved modular complex TRAPP is a guanine nucleotide exchanger (GEF) for the yeast Golgi Ypt-GTPase gatekeepers. TRAPP I and TRAPP II share seven subunits and act as GEFs for Ypt1 and Ypt31/32, respectively, which in turn regulate transport into and out of the Golgi. Trs65/Kre11 is one of three TRAPP II-specific subunits. Unlike the other two subunits, Trs120 and Trs130, Trs65 is not essential for viability, is conserved only among some fungi, and its contribution to TRAPP II function is unclear. Here, we provide genetic, biochemical, and cellular evidence for the role of Trs65 in TRAPP II function. First, like Trs130, Trs65 localizes to the trans-Golgi. Second, TRS65 interacts genetically with TRS120 and TRS130. Third, Trs65 interacts physically with Trs120 and Trs130. Finally, trs65 mutant cells have low levels of Trs130 protein, and they are defective in the GEF activity of TRAPP II and the intracellular distribution of Ypt1 and Ypt31/32. Together, these results show that Trs65 plays a role in the Ypt GEF activity of TRAPP II in concert with the two other TRAPP II-specific subunits. Elucidation of the role played by Trs65 in intracellular trafficking is important for understanding how this process is coordinated with two other processes in which Trs65 is implicated: cell wall biogenesis and stress response.
INTRODUCTION
Intracellular protein trafficking is required for the proper functioning of all eukaryotic cells. The molecular switches Ypt/Rab GTPases are key regulators of the different steps of this process. These GTPases cycle between the GTP-on and GDP-off states, and this cycling is regulated by specific factors: guanine nucleotide exchange factors (GEFs) for stimulation and GTPase activation proteins for down-regulation. When in the GTP-on state, Ypt/Rabs interact with effectors that mediate all the known aspects of vesicular transport, from vesicle formation and motility to their targeting and fusion (Segev, 2001b, a).
In yeast, Ypt1 and Ypt31/32 regulate transport into and out of the Golgi, respectively (Segev et al., 1988; Jedd et al., 1997). TRAPP is a conserved multisubunit complex that comes in two configurations: TRAPPI is required for endoplasmic reticulum-to-Golgi transport, whereas TRAPP II functions in late Golgi (Sacher et al., 1998, 2001). We have identified the TRAPP I and TRAPP II complexes as GEFs for Ypt1 and Ypt31/32, respectively (Jones et al., 2000; Morozova et al., 2006). The two TRAPP complexes share seven subunits, and TRAPP II contains three additional subunits, Trs120, Trs130, and Trs65 (Sacher et al., 2000, 2001). Although the two essential TRAPP II-specific subunits, Trs120 and Trs130, are conserved from yeast to humans, the third nonessential subunit, Trs65, is conserved only among some fungi (Cox et al., 2007). Recently, we showed that Trs120 and Trs130 are required for the specificity switch of TRAPP from Ypt1 GEF to Ypt31 GEF (Morozova et al., 2006). However, the role of Trs65 in the GEF function of TRAPP II is not yet clear.
The documented defect of TRS65/KRE11 loss-of-function is in cell wall biogenesis (Brown et al., 1993), and here we point to an additional role for Trs65 in stress response. As for a role in intracellular trafficking, although Trs65 coprecipitates with the TRAPP complex (Sacher et al., 2001; Gavin et al., 2002), its functional connection with this complex until now has been limited to one genetic interaction with a TRAPP I subunit. Specifically, deletion of two nonessential TRAPP subunits, Trs33 (TRAPP I/II) and Trs65 (TRAPP II), results in a synthetic lethal growth phenotype (Tong et al., 2004), and this double mutant can be rescued by overexpression of Ypt31 (Sciorra et al., 2005). This genetic interaction supports a role for Trs65 with the TRAPP complex.
Here, we provide genetic, biochemical, and cellular evidence for the role of Trs65 in the Ypt31/32 GEF activity of TRAPP II. Specifically, Trs65 localizes to the trans-Golgi, interacts genetically and physically with Trs120 and Trs130, and affects TRAPP II function.
MATERIALS AND METHODS
Strains, Plasmids, and Reagents
Yeast strains and plasmids used in this study are summarized in Supplemental Table S1. The antibodies used in this study are mouse monoclonal anti-hemagglutinin (HA) (clone12CA5; Roche Diagnostics, Indianapolis, IN), mouse monoclonal anti-Myc (clone 9E10; Santa Cruz Biotechnology, Santa Cruz, CA), affinity-purified rabbit anti-Ypt31 (Jedd et al., 1997), affinity-purified rabbit anti-Ypt1 (Segev et al., 1988), rabbit anti-glucose-6-phosphate dehydrogenase (G-6-PDH) (A-9521; Sigma-Aldrich, St. Louis, MO), rabbit anti-glutathione S-transferase (GST) (immunoglobulin [Ig]G fraction; Invitrogen, Carlsbad, CA), horseradish peroxidase-linked anti-rabbit and anti-mouse IgG (GE Healthcare, Little Chalfont, Buckinghamshire, United Kingdom), and Texas Red dye-conjugated anti-rabbit IgG (Jackson ImmunoResearch Laboratories, West Grove, PA). All chemical reagents were purchased from Sigma-Aldrich, unless otherwise noted.
Culture Conditions
Yeast cells were grown in rich (YPD) media, or minimal (SC) media, supplemented with the appropriate auxotrophic requirements (Rose et al., 1988). Carbon sources were added to 2% (wt/vol). Yeast cells expressing GST-Bet5 or GST under the CUP1 promoter were induced with 0.5 mM CuSO4 for 2 h at 26°C, unless otherwise noted. Yeast transformations were performed by the overnight lithium acetate method (Gietz et al., 1992).
Preparation of Cell Lysates and Protein Analyses
Yeast cell extracts were prepared as described previously (Chen et al., 2005). Cell breakage buffers were supplemented with an EDTA-free protease-inhibitors cocktail (Roche Diagnostics). Protein concentrations were determined by a Bio-Rad protein assay (Bio-Rad, Hercules, CA). Ten micrograms of yeast whole cell lysates were loaded on 10% SDS-polyacrylamide gel electrophoresis (PAGE). Gels were run, and proteins were transferred to polyvinylidene difluoride membranes and subjected to immunoblot analysis. Quantification of protein bands was done using the AlphaEase FC and Alpha-Imager (Alpha Innotech, San Leonardo, CA).
Purification of GST Fusion Proteins
Ypt1 and Ypt31 proteins expressed in bacteria were purified as described previously (Jones et al., 1995). GST-tagged proteins expressed in yeast were purified as described previously (Morozova et al., 2006). The total protein concentration of the eluted fractions ranged between 0.05 and 0.4 mg/ml. GST-associated complexes (0.2 μg) were tested by immunoblot analysis as described above for lysates.
GDP Release Assays
GDP release assays were performed as described previously (Morozova et al., 2006). GST–Bet5- or GST-associated complexes were purified from yeast and added to the reaction as a source of GEF.
Fluorescence Microscopy
Immunofluorescence microscopy was performed as described previously, using affinity-purified anti-Ypt1 and anti-Ypt31/32 antibodies (Jedd et al., 1997). TRS65 was tagged on the chromosome with yellow fluorescent protein (YFP) at the N terminus in wild-type (BY4741/NSY825) and Sec7-DsRed (NSY986; Chen et al., 2005) strains as described previously (Prein et al., 2000) to provide NSY1179 and NSY1180, respectively. Briefly, YFP was amplified by polymerase chain reaction (PCR) from pDH22 (pNS610) and then transformed into yeast cells. Correct targeting was verified by diagnostic PCR and PCR-product sequencing. The strain expressing both YFP-Trs65 and Cop1-red fluorescent protein (RFP) was constructed by mating and dissection of NSY1179 with NSY862. DsRed-FYVE plasmid (pNS716) was transformed into NSY1178 for testing the colocalization of YFP-TRS65 with this endosomal marker. Cells expressing Sec7-DsRed and Cop1-RFP were in YPD to mid-log phase, whereas cells expressing DsRed-FYVE were grown in SD-Leu to mid-log phase and then switched to SD-Leu-Met for 2 h. Live cell deconvolution microscopy was performed as described previously (Chen et al., 2005). Briefly, a series of 5–10 Z stacks, 275 nm each, were collected for each field using a 63× objective, and deconvolved using regularized inverse filter and Axiovision 4.3 software (Carl Zeiss, Thornwood, NY).
General Secretion Assay
Yeast cells were grown at 26°C to log phase. Ten OD6oo units of cells were spun at 3000 rpm for 5 min. The cell pellet was washed twice with 5 ml of SD-Cys-Met, and then it was resuspended in 1 ml of this medium. Cells were preincubated at 37°C for 20 min and then pulsed with 10 μCi/OD Trans35S label for 1.5 h at 37°C. Media proteins were analyzed after trichloroacetic acid (TCA) precipitation as described previously (Gaynor and Emr, 1997).
Electron Microscopy
Yeast cells were grown at 26°C to early log phase (0.5 OD600). Half the culture was left at 26°C, and the other half was shifted to 37°C for 1.5 h. Cells were fixed in 0.2 M sodium cacodylate buffer, pH 6.7, and 4% glutaraldehyde (both reagents are from Ted Pella, Redding, CA), and they were processed for electron microscopy as described previously (Byers and Goetsch, 1975).
RESULTS
Trs65, Like Trs130, Localizes to Late Golgi
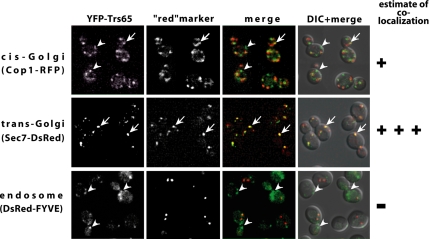
Trs65 coprecipitates with the TRAPP II complex (Sacher et al., 2001). To determine whether the intracellular localization of Trs65 is similar to that of TRAPP II, we compared the localization pattern of Trs65 to that of Trs130. The colocalization of Trs130-GFP with the late Golgi marker Sec7, and not with the endosomal marker FYVE domain, was determined previously by direct fluorescence microscopy (Cai et al., 2005). Trs65 was tagged on the chromosome at its N terminus with YFP. YFP-Trs65 is functional because it does not confer a cell wall defect, similar to that displayed by trs65Δ mutant cells (Supplemental Figure S1). The intracellular localization of YFP-Trs65 was determined by its colocalization with known compartmental markers labeled with a red fluorescent tag, by using live cell deconvolution microscopy. Figure 1 demonstrates that Trs65 shows strong colocalization with the trans-Golgi marker Sec7 and partial colocalization with the early Golgi marker Cop1. In addition, as has been reported previously for Trs130, Trs65 does not colocalize with the endosomal marker FYVE domain. In summary, Trs65, like another TRAPP II GEF subunit, Trs130, and the substrate of this GEF, Ypt31 GTPase (Chen et al., 2005), localizes primarily to the trans-Golgi. These results indicate that Trs65 not only coprecipitates with TRAPP II but also colocalizes with it mainly to the trans-Golgi.
Figure 1.
Trs65 localizes mainly to the trans-Golgi. YFP-Trs65 colocalizes with trans-Golgi marker Sec7-DsRed (middle) and to a lesser extent with the cis-Golgi marker Cop1-RFP (top), but not with the endosome marker DsRed-FYVE domain (bottom). TRS65 on the chromosome was tagged with YFP at the N terminus in cells expressing Sec7-DsRed from the chromosome (NSY1180), in cells expressing Cop1-RFP also from the chromosome (NSY1182), or in wild-type cells (NSY1178) expressing DsRed-FYVE domain from a plasmid (pNS716). Cells were grown to mid-log phase, and examined by deconvolution microscopy. Panels show from left to right: YFP-Trs65, the red-labeled compartmental marker, merge, and merge + differential interference contrast (DIC). Arrows point to regions of colocalization, whereas arrowheads point to regions of Trs65 (green) that do not colocalize with the compartmental markers (red). Results shown here are representative of at least two experiments.
Genetic Interaction of TRS65 with TRS120 and TRS130
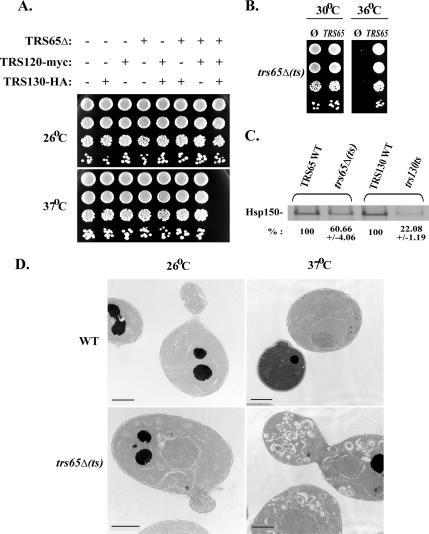
The two TRAPP II-specific subunits Trs120 and Trs130 are essential for yeast cell viability. Tagging endogenous Trs120 and Trs130 at their C terminus with myc and HA, respectively, does not result in temperature-sensitive growth phenotype, indicating that these tagged versions are functional. The third TRAPP II-specific subunit Trs65 is not essential for viability, and its deletion in wild-type cells does not result in any growth phenotype. However, deletion of TRS65 in cells that express Trs120-myc and Trs130-HA confers temperature sensitivity: these cells cannot grow at temperatures >34°C (Figure 2A). We term these cells trs65Δ(ts). The temperature sensitivity of the triple mutant can be complemented by GST-tagged Trs65 expressed from a 2μ plasmid (Figure 2B). This synthetic interaction suggests that the tagged versions of the Trs120 and Trs130 are somewhat impaired for function and that Trs65 functions together with Trs120 and Trs130.
Figure 2.
TRS65 interacts genetically with TRS120 and TRS130: characterization of the trs65Δ(ts) triple mutant. (A) Deletion of TRS65 in cells expressing Trs120-myc and Trs130-HA results in a temperature-sensitive growth phenotype. Cells containing all the different combinations of the three TRAPP II subunits mentioned above (as detailed at the top) were plated on YPD plates (in 10-fold serial dilutions from top to bottom) and incubated at the indicated temperatures (26 or 37°C). Cells that contain only one or two of the alleles grow at all temperatures. Only trs65Δ cells that also express the tagged versions of Trs120 and Trs130 are temperature sensitive for growth. Hereafter, this triple mutation strain is referred to as trs65Δ(ts), whereas the strain without the TRS65 deletion but with tagged Trs120 and Trs130 is referred as its corresponding wild type (WT). (B) TRS65 can rescue the temperature-sensitive growth phenotype of trs65Δ(ts). GST-tagged Trs65 was overexpressed in trs65Δ(ts) cells from a plasmid (2μ; URA3), and the empty GST vector served as a negative control. Cells were plated on SD-Ura plates to select for the plasmid (10-fold serial dilutions from top to bottom) and incubated at the indicated temperatures. GTS-Trs65, but not GST, can rescue the temperature-sensitive growth phenotype of trs65Δ(ts) mutant cells. (C) General secretion is defective in trs65Δ(ts) triple mutant cells. Equal amount of wild-type (TRS65 WT) and trs65Δ(ts) cells at log phase was shifted to 37°C for 20 min and labeled with Trans35S for 1.5 h at 37°C. Proteins secreted to the medium were precipitated with TCA and analyzed by SDS-PAGE. The major band at ∼150 kDa, which corresponds to Hsp150 (Gaynor and Emr, 1997), is shown. This band was quantified, and the percentage of Hsp150 secreted by mutant versus wild-type cells is indicated at the bottom. For comparison, we included in this assay the trs130ts mutant, with its corresponding wild type (TRS130 WT). Results are representative of two independent experiments, and error bars are indicated. (D) Aberrant Golgi membranes accumulate in trs65Δ(ts) mutant cells at their restrictive temperature (37°C). Wild type and mutant cells were incubated at the indicated temperatures for 90 min, and then they were fixed and processed for electron microscopy analysis. Representative cells are shown. Bar, 1 μm.
Until now the only documented defect of trs65Δ cells has been in cell wall biogenesis (Brown et al., 1993). We used the trs65Δ(ts) mutant cells to search for protein-trafficking defects. First, we tested the ability of trs65Δ(ts) mutant cells to secrete Hsp150 to the medium (Gaynor and Emr, 1997). At the nonpermissive temperature, trs65Δ(ts) mutant cells exhibit a reduction in secretion of Hsp150. This defect is less severe than the secretory defect of trs130ts mutant cells in the same assay, but it was significant (Figure 2C). Second, mutants defective in exit from the Golgi, such as sec7, trs130, and ypt31/32, exhibit accumulation of abnormally large Golgi, termed Berkeley bodies (Novick et al., 1981; Jedd et al., 1997; Sacher et al., 2001). The trs65Δ(ts) mutant cells, at their nonpermissive temperature, show accumulation of Berkeley bodies (Figure 2D). Together, these results place Trs65 with the other two TRAPP II-specific subunits Trs120 and Trs130, and they suggest that it functions in the exit from the trans-Golgi.
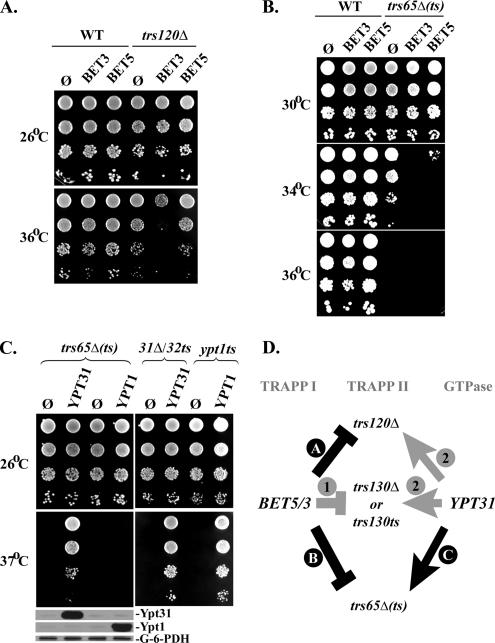
If Trs65 functions in TRAPP II together with Trs120 and Trs130, we expect that trs65Δ(ts) would exhibit genetic interactions similar to those conferred by the trs120 and trs130 loss-of-function mutations. First, we tested the trs65Δ(ts) triple mutation for interaction with genes encoding the TRAPP I/II subunits Bet3 and Bet5. We have reported previously that overexpression of these two subunits, which are shared by TRAPP I and TRAPP II, enhance the growth phenotype of trs130Δ and trs130ts mutant cells (Morozova et al., 2006). Here, we show that overexpression of Bet3, but not Bet5, enhances the growth phenotype of trs120Δ mutant cells (Figure 3A). We also show that overexpression of Bet3 and Bet5 enhances the growth phenotype of trs65Δ(ts) mutant cells (Figure 3B). Second, it has been shown previously that overexpression of Ypt31/32, but not Ypt1, rescues the growth phenotype of trs120Δ, trs130Δ, and trs130ts mutant cells (Yamamoto and Jigami, 2002; Zhang et al., 2002; Sciorra et al., 2005). Here, we show a similar genetic interaction with the trs65Δ(ts) triple mutation. Specifically, overexpression of Ypt31, but not Ypt1, rescues the growth defect of trs65Δ(ts) mutant cells at 37°C (Figure 3C). Together, the similarity of genetic interactions of the trs65Δ(ts) triple mutation to those of trs120 and trs130 (summarized in Figure 3D) further supports a common role for these TRAPP II-specific subunits.
Figure 3.
Similar genetic interactions of trs65Δ(ts), trs120 and trs130 mutant cells. (A) Overexpression of BET3, but not BET5, enhances the growth defect of trs120Δ mutant cells. Plasmids (2μ; URA3) expressing GST-tagged Bet3 (pNS795) or Bet5 (pNS424) were transformed into wild-type (NSY1187) and trs120Δ mutant cells (NSY1046). Transformants were plated on SD-Ura-Leu plates (in 10-fold serial dilutions from top to bottom), and the plates were incubated at the indicated temperatures. (B) Overexpression of BET3, and to a lesser extent BET5, enhances the growth defect of trs65Δ(ts) triple mutant cells. The experiment was done as described in A, except that wild-type (NSY1176) and trs65Δ(ts) mutant cells (NSY1177) were used and cells were plated on SD-Ura plates. (C) Overexpression of YPT31, but not YPT1, rescues the temperature-sensitive phenotype of trs65Δ(ts). Left, experiment was done as described in A, except that plasmids overexpressing Ypt31 (pNS781) or Ypt1 (pNS993) were used. Left, bottom, the overexpression of Ypt31 and Ypt1 proteins was confirmed using immunoblot analysis and anti-Ypt31 and anti-Ypt1 antibodies; G-6-PDH was used as a loading control. Right, the functionality of Ypt31 and Ypt1 plasmids was confirmed by their ability to rescue their respective temperature-sensitive mutants ypt31Δ/32ts (NSY340) and ypt1ts (NSY1082). (D) Summary of genetic interactions of genes encoding TRAPP II-specific subunits with TRAPP I/II subunits and Ypt31/32. Arrows depict suppression of mutant growth phenotype, whereas flat arrows represent enhancement of the mutant phenotype. Black arrows indicate interactions reported in this study (A–C), whereas gray arrows indicate observations from previous studies: Morozova et al. (2006) (1) and Yamamoto and Jigami, 2002, Zhang et al. (2002), and Sciorra et al. (2005) (2). Results shown here are representative of two independent experiments.
Physical Interaction of Trs65 with TRAPP II Subunits
Physical interaction of Trs65 with TRAPP I/II subunits Bet3, Trs20, and Trs33 has been shown previously using affinity capture (Sacher et al., 2001; Gavin et al., 2002). Here, we used the yeast two-hybrid assay to test for physical interaction of Trs65 with the TRAPP II-specific subunits Trs120 and Trs130. Cells expressing Trs65 fusion with a binding domain were mated with cells expressing Trs120 or Trs130 fusion with an activation-domain. Growth on plates without histidine shows interaction of Trs65 with both Trs120 and Trs130 (Figure 4). These interactions are also detectable on plates without histidine in the presence of 5 mM 3-amino-1,2,4-triazole and under the most restrictive selection for interaction: media without adenine. We know that these interactions are specific, because controls using empty vectors and the TRAPP I/II subunit Trs23 in this yeast two-hybrid assay do not show any growth on the selective medium. Similar negative results were obtained with three other TRAPP I/II subunits: Bet3, Bet5, and Trs31 (data not shown). These negative results indicate that the physical interaction of Trs65 with the other two TRAPP II-specific subunits in the yeast two-hybrid assay is not due to an indirect interaction with the whole TRAPPI/II complex, as is the case with the affinity-capture interactions.
Figure 4.
Trs65 interacts with TRAPP II subunits in the yeast two-hybrid system. Trs65 specifically interacts with the TRAPP II-specific subunits TRS120 and TRS130 but not with the TRAPP I/II subunit Trs23. MATa cells expressing Trs120, Trs130, or Trs23 from the pACT2 (GAL4-AD, LEU2) vector were mated with MATα cells expressing Trs65 from pGBDU-C2 (GAL4-BD, URA3) vector. Diploids were selected on SD-Ura-Leu medium. The growth control of the diploids is shown on the left (-Ura-Leu), and interaction is shown at the right (-Ura-Leu-His). Cells were plated in 10-fold serial dilutions from top to bottom and were incubated at 30°C. Empty vector (ø) for both plasmids are shown as negative controls. Results shown in this figure are representative of at least two independent experiments.
Loss of Trs65 Confers Lower Trs130 Protein Levels in Cell Lysates and Purified TRAPP Complexes
We have previously shown that Trs120 and Trs130 are important for the Ypt GEF activity of TRAPP II (Morozova et al., 2006). Because we found here that Trs65 interacts with these two TRAPP II-specific subunits genetically and physically (see above), we wanted to determine whether Trs65 affects TRAPP II complex composition. The protein levels of the tagged versions of two essential TRAPP II-specific subunits, Trs120 and Trs130, in trs65Δ cell lysates were determined by immunoblot analysis and compared with those present in wild-type cell lysates. We found that the level of Trs130-HA protein in trs65Δ cell lysates is lower than its level in wild-type lysates (∼50% at 37°C). The level of Trs130-HA, but not Trs120-myc, is also lower in trs65Δ(ts) triple mutant cell lysates even at 26°C, and this effect is more severe at 37°C (Figure 5A). These results show that Trs65 is important for the normal cellular level of the Trs130 protein.
Figure 5.
Effect of trs65 loss-of-function mutations on the protein level of Trs120 and Trs130 in cell lysates and purified TRAPP complexes. (A) The level of Trs130-HA, but not Trs120-myc, is lower in trs65Δ and trs65Δ(ts) mutant cell lysates, especially at 37°C. In trs65Δ(ts) mutant cells overexpressing GST-Bet5, the level of both Trs120 and Trs130 is lower. Top, trs65Δ mutant cells expressing Trs130-HA (NSY1110) and their corresponding wild type (NSY991). Middle, trs65Δ(ts) mutant cells (NSY1177) and their corresponding wild type (NSY1176). Bottom, trs65Δ(ts) mutant cells and their corresponding wild type from above, overexpressing GST-Bet5. Lysates (10 μg), prepared from cells grown to mid-log phase at 26°C (left), or shifted to 37°C for 1.5 h (right), were analyzed by immunoblot analysis by using anti-HA or anti-myc antibodies. G-6-PDH serves as a loading control. (B) The protein level of Trs130-HA and Trs120-myc is lower in GST–Bet5-associated complexes purified from trs65Δ mutant cells. GST–Bet5- and GST-associated complexes were purified from cell lysates of trs65Δ (NSY1111; top left), trs65Δ Trs130-HA (NSY1110; top right), or trs65Δ(ts) (NSY1177; bottom: 26°C, left; 37°C, right), and their corresponding wild-type cells (NSY825, NSY991, and NSY1176, respectively). Lysates were prepared as described in A except that GST-Bet5 or GST were overexpressed from a plasmid, and GST-associated complexes were purified using glutathione agarose-beads. Equal amounts (as determined by Bradford assay) of purified GST complexes (∼0.2 μg), were subjected to immunoblot analyses using the following antibodies: anti-HA for Trs130-HA, anti-myc for Trs120-myc, and anti-GST for GST and GST-Bet5. Results shown here are representative of at least two independent experiments.
A lower cellular Trs130 level could reflect a lower level of Trs130 protein in TRAPP II complexes. To determine whether this is the case, the protein level of tagged Trs120 and Trs130 was examined in TRAPP complexes purified by GST-Bet5 pull-down. In TRAPP complexes purified from trs65Δ mutant cells expressing only Trs130-HA, there was a lower level of the tagged Trs130 than in TRAPP complexes purified from wild-type cells. Lysates and TRAPP complexes purified from trs65Δ(ts) mutant cells overexpressing GTS-Bet5 have lower levels of both tagged Trs130 and Trs120, when compared with wild-type lysates and TRAPP complexes (Figure 5). This effect could be due to the negative synthetic growth defect of overexpressing Bet5 in trs65Δ(ts) mutant cells described above (Figure 3B). Together, these results show that deletion of TRS65 confers in a lower cellular level of Trs130, as well as a lower Trs130 level in TRAPP complexes.
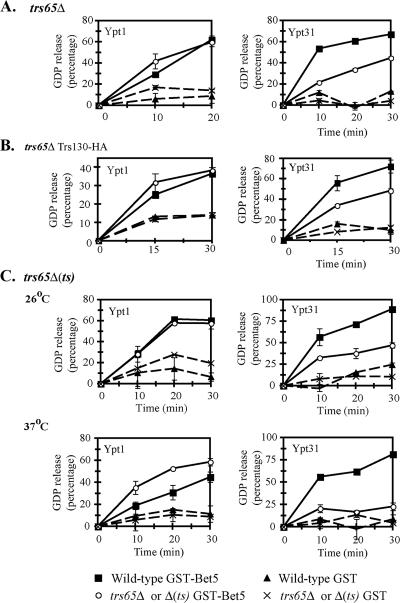
Loss of Trs65 Affects the Ypt GEF Activity of TRAPP
We have previously shown that loss of Trs120 and Trs130 function results in reduced Ypt31/32 GEF activity of TRAPP. In addition, TRAPP complexes purified from trs120 or trs130 mutant cells possess higher Ypt1 GEF activity (Morozova et al., 2006). Because TRAPP complexes purified from trs65Δ mutant cells have low levels of Trs130, we expected that these complexes would be defective in their Ypt GEF activity. To test this possibility, TRAPP complexes purified from trs65Δ mutant cells were tested for stimulation of GDP release from Ypt31 and Ypt1 proteins. GST–Bet5-purified complexes from trs65Δ mutant cells, in which neither Trs130 nor Trs120 were tagged, have lower Ypt31 GEF activity compared with wild-type complexes. However, the Ypt1 GEF activity remained unaffected (Figure 6A). Moreover, complexes purified from trs65Δ mutant cells in which only Trs130 is tagged show the same result (Figure 6B), suggesting that tagging Trs130 alone in trs65Δ mutant cells does not affect the Ypt1 GEF function of TRAPP. Finally, TRAPP complexes purified from trs65Δ(ts) mutant cells (in which both Trs120 and Trs130 are tagged) grown at their permissive temperature (26°C) also showed similar results. Only when the trs65Δ(ts) mutant cells were grown at their nonpermissive temperature (37°C), the Ypt31 GEF activity of TRAPP was abolished, and the Ypt1 GEF activity was higher than that of wild-type TRAPP (Figure 6C). In experiments using Ypt32 as a substrate, we got results similar to those of Ypt31 (data not shown). Thus, when the level of Trs130 is reduced in trs65Δ mutant cells, only the Ypt31/32 GEF activity is affected. However, when the level of all three TRAPP II-specific subunits is reduced, the GEF specificity switch of TRAPP from Ypt1 to Ypt31/32 is also affected. These results suggest that Trs65 contributes to the Ypt GEF activity of TRAPP, probably through its effect on the TRAPP II complex assembly or stability.
Figure 6.
Effect of trs65 loss-of-function mutations on the Ypt-GEF activity of TRAPP. (A) The Ypt31 GEF activity of TRAPP complexes purified from trs65Δ mutant cells is lower than that of wild-type complexes, whereas the Ypt1 GEF activity is unaffected. (B) The Ypt31 GEF activity of TRAPP complexes purified from trs65Δ expressing Trs130-HA mutant cells is lower than that of wild-type complexes, whereas the Ypt1 GEF activity is unaffected. (C) The Ypt31 GEF activity of TRAPP complexes purified from trs65Δ(ts) mutant cells is lower than that of wild-type complexes. This defect is more severe for TRAPP complexes purified from mutant cells grown at the restrictive temperature (37°C). The Ypt1 GEF activity of trs65Δ(ts) mutant complexes purified from cells grown at 37°C (bottom), but not 26°C (top), is higher than that of wild-type complexes. GST–Bet5- and GST-associated complexes were purified as described in Figure 5 legend. Equal amounts (1.6 μg) of complexes from wild-type and mutant cells were used in a GDP-release assay for Ypt1 (left) or Ypt31 (right). Legend for A–C is shown at the bottom. Results shown in this figure are the average of duplicate measurements, and they are representative of at least two experiments. Error bars represent SEM.
Loss of Trs65 Affects the Intracellular Localization of the Golgi Ypt GTPases
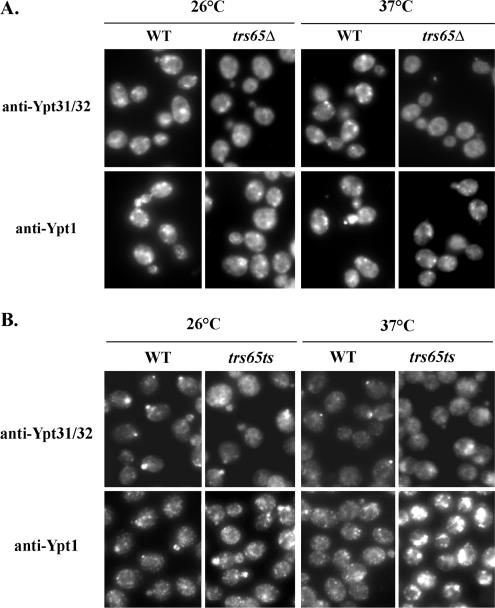
We have shown previously that in trs120 and trs130 mutant cells, the intracellular localization of Ypt1 and Ypt31/32 is affected in opposite ways: Ypt31/32 exhibit a diffuse localization pattern, whereas Ypt1 puncta are brighter than in wild-type cells (Morozova et al., 2006). To determine the effect of the trs65Δ mutation on the localization of the Golgi Ypts, the localization of Ypt1 and Ypt31/32 in wild-type and mutant cells was compared by immunofluorescence microscopy. In trs65Δ mutant cells in which Trs120 and Trs130 are not tagged, the localization of Ypt31 is more diffuse than in wild-type cells, especially at 37°C, but there is no effect on the Ypt1 punctate staining pattern in these cells (Figure 7A). In trs65Δ(ts) mutant cells at the nonpermissive temperature, the localization of both Ypt31 and Ypt1 are affected in opposite ways: the Ypt31/32 localization is diffuse, whereas the Ypt1 staining is brighter (Figure 7B). Thus, the Ypt localization results are in agreement with those of the Ypt GEF activity: the trs65Δ mutation, which affects only the Ypt31/32 GEF activity, affects only the localization of this GTPase pair. In contrast, the more severe trs65Δ(ts) triple mutation, which affects the GEF activities for Ypt1 and Ypt31/32 in opposite ways, also affects the localization of these GTPases in opposite ways. Membrane attachment of Ypt1 and Ypt31/32 in trs130 mutant cells, as determined by cell fractionation analysis, was found to be similar to that of wild-type cells (our unpublished data). We therefore expect that trs65Δ will also not have an effect on the Ypts distribution between membrane and cytoplasm. We suggest that TRAPP II is not required for membrane attachment of the Ypts, but it is required for their proper localization to the right cellular compartment.
Figure 7.
Effect of trs65 loss-of-function mutations on the intracellular localization of Ypt31/32 and Ypt1. (A) The intracellular localization of Ypt31/32, but not Ypt1, is affected in trs65Δ mutant cells. Wild-type (NSY825) and trs65Δ mutant (NSY1111) cells grown to mid-log phase at 26°C (left), or they were shifted to 37°C for 1.5 h (right). Cells were fixed and processed for immunofluorescence microscopy (IF) by using affinity-purified anti-Ypt31/32 (top) or anti-Ypt1 (bottom) antibodies. The Ypt31/32 staining pattern is more diffuse in trs65Δ mutant cells, especially at 37°C, compared with wild type cell, whereas there is no change in the Ypt1 pattern. (B) The trs65Δ(ts) triple mutation affects the Ypt31/32 and Ypt1 intracellular localization in opposite ways. Wild-type (NSY1176) and trs65Δ(ts) mutant (NSY1177) cells were grown, fixed, and processed for IF as described in A. In trs65Δ(ts) mutant cells, the Ypt31/32 staining is more diffuse than in wild-type cells, whereas the Ypt1 staining is more intense. This opposite effect is more severe at 37 than at 26°C. Results shown in this panel are representative of at least three independent experiments.
Trs65/Kre11 Plays a Role in Three Different Cellular Processes
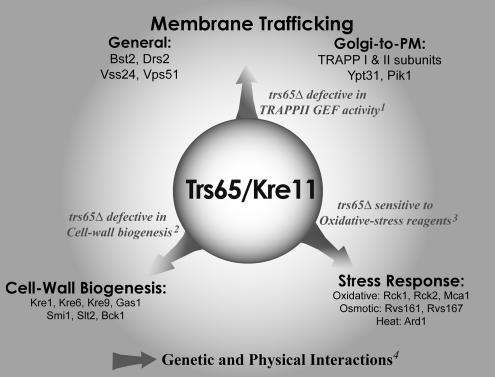
Affinity-capture (Sacher et al., 2001; Gavin et al., 2002) and functional analysis presented here suggest a role for Trs65 in intracellular trafficking. However, TRS65/KRE11 was first identified as a gene important for cell wall biogenesis based on its mutant phenotype (Brown et al., 1993). We wanted to determine whether Trs65 is a multifunctional protein and compare it with the other nonessential TRAPP subunits. For that, we analyzed the Saccharomyces Genome Database (SGD; Ball et al., 2000; as of February 25, 2007) for all the genetic and physical interactions as well as mutant phenotypes reported for TRS65/KRE11, including those obtained from genome-wide studies. Genetic and physical interactions detailed in BioGrid (Stark et al., 2006) support a role for Trs65 in three different cellular processes: cell wall biogenesis (only genetic interactions), stress response, and membrane trafficking (summarized in Supplemental Table S2). Mutant phenotypes support a role for Trs65 in two of these processes: cell wall biogenesis (Brown et al., 1993) and stress response (Supplemental Table S3). The latter is suggested by the sensitivity of trs65Δ mutant cells to three agents that induce oxidative stress: dithiothreitol, diamide, and paraquit (Prophecy; Fernandez-Ricaud et al., 2007). Here, we demonstrate that trs65Δ mutant cells exhibit a phenotype also in the third cellular process: TRAPP II function in intracellular trafficking. Together, the interactions and mutant phenotypes show that Trs65/Kre11 plays a role in three different cellular processes (Figure 8).
Figure 8.
Interaction and mutant phenotype analyses support a role for Trs65/Kre11 in three different cellular processes. Mutant phenotypes together with genetic and physical interactions suggest a role for Trs65/Kre11 in intracellular trafficking, cell wall biogenesis, and stress response. References for trs65Δ mutant phenotypes are 1) TRAPP function (this study); 2) cell wall biogenesis (Brown et al., 1993); and 3) oxidative-stress response, Prophecy (Fernandez-Ricaud et al., 2007) (Supplemental Table S3). 4) Genetic and physical interactions for Trs65 are detailed in this study or in SGD (Ball et al., 2000) and BioGrid (Stark et al., 2006), and they are summarized in Table 1 and Supplemental Table S2.
For comparison, we looked at the mutant phenotypes and interactions for the two nonessential TRAPP I/II subunits Trs33 and Trs85/Gsg1. These two subunits show ample genetic and physical interactions with protein trafficking regulators (Table 1 and Supplemental Tables S4 and S5), more than those shown by Trs65. For cell wall biogenesis, we show here that deletion of TRS33, but not TRS85, results in a cell wall defect similar to that of trs65Δ (Supplemental Figure S1 and Table 2). However, the interaction of Trs33 and Trs85 with cell wall biogenesis regulators is limited to one case: both have a weak genetic interaction with GAS1 (synthetic growth defect, as opposed to the synthetic lethality exhibited with TRS65). For stress response, deletion of TRS33, but not TRS85, results in sensitivity to two of the three agents that induce oxidative stress response (Table 2). However, only Trs85, but not Trs33, shows one physical interaction with one protein that plays a role in stress response (Table 1 and Supplemental Tables S3–S5). Thus, only Trs65 shows both mutant phenotypes and multiple genetic and physical interactions that connect it with cell wall biogenesis and stress response. This survey suggests that not all regulators of protein trafficking play an equal role in the two other cellular processes for which Trs65 is important.
Table 1.
Summary of physical and genetic interactions of the three nonessential TRAPP complex subunits
| TRAPP subunit | No. of interactionsa with proteins that function in |
||||
|---|---|---|---|---|---|
| Membrane trafficking | Cell wall biogenesis | Stress response | Other | Total | |
| Trs65 | 13 (G + P) | 7 (G) | (6 (G + P) | 6 (G + P) | 44 |
| Trs33 | 23 (G + P) | 1 (G) | 0 | 4 (G + P) | 28 |
| Trs85 | 40 (G + P) | 1 (G) | 1 (P) | 31 (G + P) | 63 |
G, genetic interactions; P, physical interactions.
a Interactions from BioGrid (Stark et al., 2006; as of February 25, 2007) are detailed in Supplemental Tables S2, S4, and S5.
Table 2.
Summary of mutant phenotypes of the three nonessential TRAPP complex subunits
a Sensitivity to the lytic enzyme Zymolyase is shown in Supplemental Figure S1.
b Sensitivity to three stress response agents is from Prophecy (Fernandez-Ricaud et al., 2007), and is detailed in Supplemental Table S3.
DISCUSSION
Interaction and mutant phenotype analyses suggest a role for Trs65 in three cellular processes (Figure 8). The documented role of Trs65 is in cell wall biogenesis and both mutant phenotype (Brown et al., 1993) and genetic interactions reviewed here support this role (Supplemental Table S2). A role for Trs65 in stress response is suggested here based on mutant phenotypes and genetic and physical interactions (Figure 8, Tables 1 and 2, and Supplemental Tables S2 and S3). Last, a role for Trs65 in protein trafficking has been suggested previously, based on its physical interaction with the TRAPP complex and its genetic interaction with the nonessential TRAPP I/II subunit Trs33 (Tong et al., 2004; Sciorra et al., 2005). Here, we provide the first functional evidence that connects Trs65 to the TRAPP II complex.
The idea that Trs65 functions as part of the TRAPP II complex is based on the following cellular, genetic, and biochemical evidence. First, Trs65 localizes to the Golgi, like Trs130 and Ypt31. Second, TRS65 interacts genetically with TRS120 and TRS130 to yield the trs65Δ(ts) triple mutation. At their nonpermissive temperature, trs65Δ(ts) mutant cells confer a partial defect in the secretion of Hsp150 to the medium, accumulate aberrant Golgi structures similar to those accumulated in trs130ts and ypt31Δ/32ts mutant cells (Jedd et al., 1997; Sacher et al., 2001), and exhibit genetic interactions similar to those of trs120 and trs130 mutant cells. Third, Trs65 interacts physically with both Trs120 and Trs130 in the yeast two-hybrid assay. Last, loss of Trs65 function results in a lower level of Trs130 in cell lysates and in TRAPP complexes. The level of both Trs120 and Trs130 is reduced in TRAPP complexes purified from trs65Δ(ts) mutant cells overexpressing GST-Bet5. Loss of Trs65 function also affects the Ypt GEF activity of TRAPP and the localization of the Golgi Ypts.
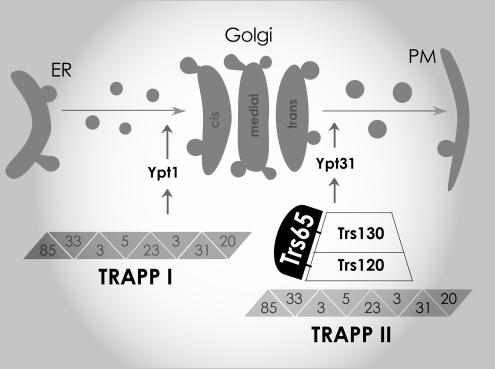
Recently, we suggested a role for Trs120 and Trs130 in switching the GEF specificity of TRAPP II from Ypt1 to Ypt31/32. This idea was based on the findings that trs120 and trs130 mutations result in opposite effects on the GEF activity and localization of Ypt1 and Ypt31/32 (Morozova et al., 2006). We also suggested that Trs120 is required for the stability of Trs130 or to its attachment to the TRAPP complex, based on the finding that the protein level of Trs130 depends on Trs120, but not vice versa (Morozova et al., 2006). The simplest explanation for results presented here is that Trs65 also contributes to the interaction of Trs130 with the TRAPP complex and that uncomplexed TRAPP II-specific subunits are more susceptible to degradation. Furthermore, Trs65 exerts its effect on the Ypt GEF activity of TRAPP II and the localization of the Golgi Ypts through its interactions with the other two essential subunits of this complex, Trs120 and Trs130. In summary, we propose that Trs65 contributes to the assembly and/or stability of TRAPP II and thereby to the GEF activity of this complex (Figure 9).
Figure 9.
Model for the role of Trs65 in TRAPP II function. Findings presented here show a role for Trs65, together with Trs120 and Trs130, in the Ypt–GEF function of the TRAPP II complex. We propose that Trs65 interacts with both Trs120 and Trs130 and contributes to their stability and/or assembly with the TRAPP II complex, thereby regulating the switch of the TRAPP complex GEF activity from Ypt1 to Ypt31/32. This switch serves to coordinate entry into and exit from the Golgi (Morozova et al., 2006).
This idea is based on the correlation between the severity of the trs65Δ effect on Trs130 and Trs120 levels, and the effect on GEF activity and localization of Ypts. In trs65Δ mutant cells, when only Trs130 level is reduced, only the Ypt31/32 GEF activity and localization is affected. In trs65Δ(ts) mutant cells, when the level of both Trs120 and Trs130 is reduced, Ypt1 GEF activity and localization are affected as well. This idea also explains the genetic interaction between trs65Δ and the tagged TRS120 and TRS130. If Trs65 helps stabilizing and/or localizing Trs120 and Trs130 to TRAPP II, and the interactions of tagged Trs120 and Trs130 are somewhat compromised, this might result in increased dependency on Trs65 for their stability in trs65Δ(ts) triple mutant cells.
The mechanisms and regulation of intracellular trafficking have been extensively studied in the last decade. In contrast, our understanding of the coordination of this process with other cellular processes is still vague. In Saccharomyces cerevisiae, Trs65 is important for three different cellular processes; therefore, it is a candidate for their coordination. One possibility is that the three processes in which Trs65 plays a role are dependent on each other, e.g., cell wall biogenesis is dependent on intracellular trafficking (Ortiz and Novick, 2006), and the stress response is dependent on the integrity of the cell wall (Valdivia and Schekman, 2003). In such a case, we expect all intracellular trafficking regulators to have connections with the other two processes similar to those of Trs65. However, our survey of the two other nonessential TRAPP subunits suggests this is not the case (Tables 1 and 2). An alternative possibility is that not all regulators of intracellular trafficking affect the other two cellular processes and that Trs65 is a multifunctional regulator.
Interestingly, our phylogenetic analyses of the TRAPP II- specific subunits showed that unlike the conserved and essential subunits, Trs65 is only present in some fungi (Cox et al., 2007). One explanation for the lack of Trs65 conservation is that higher eukaryotes have a functional homologue for Trs65, which has lost the sequence similarity. Alternatively, Trs120 and Trs130 in higher cells do not need the Trs65 function, because Trs65 serves to connect TRAPP with fungi-specific functions, e.g., cell wall biogenesis. Regardless, it is important to investigate the possible role of Trs65 in coordination of the three cellular processes in which it plays a role: intracellular trafficking, cell wall biogenesis, and stress response.
Supplementary Material
ACKNOWLEDGMENTS
We thank S. Emr (Cornell University) and B. Glick (University of Chicago) for strains and plasmids, Z. Lipatova and S. Chen for helpful discussions and advice, Z. Lipatova for critical reading of the manuscript, and E. Segev for help in editing the manuscript. This research was supported by grant GM-45444 from NIH to N. Segev.
Footnotes
This article was published online ahead of print in MBC in Press (http://www.molbiolcell.org/cgi/doi/10.1091/mbc.E07-03-0221) on May 2, 2007.
 The online version of this article contains supplemental material at MBC Online (http://www.molbiolcell.org).
The online version of this article contains supplemental material at MBC Online (http://www.molbiolcell.org).
REFERENCES
- Ball C. A., et al. Integrating functional genomic information into the Saccharomyces genome database. Nucleic Acids Res. 2000;28:77–80. doi: 10.1093/nar/28.1.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown J. L., Kossaczka Z., Jiang B., Bussey H. A mutational analysis of killer toxin resistance in Saccharomyces cerevisiae identifies new genes involved in cell wall (1→6)-beta-glucan synthesis. Genetics. 1993;133:837–849. doi: 10.1093/genetics/133.4.837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byers B., Goetsch L. Behavior of spindles and spindle plaques in the cell cycle and conjugation of Saccharomyces cerevisiae. J. Bacteriol. 1975;124:511–523. doi: 10.1128/jb.124.1.511-523.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai H., Zhang Y., Pypaert M., Walker L., Ferro-Novick S. Mutants in trs120 disrupt traffic from the early endosome to the late Golgi. J. Cell Biol. 2005;171:823–833. doi: 10.1083/jcb.200505145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen S. H., Chen S., Tokarev A. A., Liu F., Jedd G., Segev N. Ypt31/32 GTPases and their novel F-box effector protein Rcy1 regulate protein recycling. Mol. Biol. Cell. 2005;16:178–192. doi: 10.1091/mbc.E04-03-0258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox R., Chen S. H., Yoo E., Segev N. Conservation of the TRAPP II-specific subunits of a Ypt/Rab exchanger complex. BMC Evol. Biol. 2007;7:12. doi: 10.1186/1471-2148-7-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez-Ricaud L., Warringer J., Ericson E., Glaab K., Davidsson P., Nilsson F., Kemp G. J., Nerman O., Blomberg A. PROPHECY–a yeast phenome database, update 2006. Nucleic Acids Res. 2007;35:D463–D467. doi: 10.1093/nar/gkl1029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gavin A. C., et al. Functional organization of the yeast proteome by systematic analysis of protein complexes. Nature. 2002;415:141–147. doi: 10.1038/415141a. [DOI] [PubMed] [Google Scholar]
- Gaynor E. C., Emr S. D. COPI-independent anterograde transport: cargo-selective ER to Golgi protein transport in yeast COPI mutants. J. Cell Biol. 1997;136:789–802. doi: 10.1083/jcb.136.4.789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gietz D., St. Jean A., Woods R., Schiestl R. Improved method for high efficiency transformation of intact yeast cells. Nucleic Acids Res. 1992;20:1625. doi: 10.1093/nar/20.6.1425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jedd G., Mulholland J., Segev N. Two new Ypt GTPases are required for exit from the yeast trans-Golgi compartment. J. Cell Biol. 1997;137:563–580. doi: 10.1083/jcb.137.3.563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones S., Litt R. J., Richardson C. J., Segev N. Requirement of nucleotide exchange factor for Ypt1 GTPase mediated protein transport. J. Cell Biol. 1995;130:1051–1061. doi: 10.1083/jcb.130.5.1051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones S., Newman C., Liu F., Segev N. The TRAPP complex is a nucleotide exchanger for Ypt1 and Ypt31/32. Mol. Biol. Cell. 2000;11:4403–4411. doi: 10.1091/mbc.11.12.4403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morozova N., Liang Y., Tokarev A. A., Chen S. H., Cox R., Andrejic J., Lipatova Z., Sciorra V. A., Emr S. D., Segev N. TRAPPII subunits are required for the specificity switch of a Ypt-Rab GEF. Nat. Cell Biol. 2006;8:1263–1269. doi: 10.1038/ncb1489. [DOI] [PubMed] [Google Scholar]
- Novick P., Ferro S., Schekman R. Order of events in the yeast secretory pathway. Cell. 1981;25:461–469. doi: 10.1016/0092-8674(81)90064-7. [DOI] [PubMed] [Google Scholar]
- Ortiz D., Novick P. J. Ypt32p regulates the translocation of Chs3p from an internal pool to the plasma membrane. Eur. J. Cell Biol. 2006;85:107–116. doi: 10.1016/j.ejcb.2005.10.006. [DOI] [PubMed] [Google Scholar]
- Prein B., Natter K., Kohlwein S. D. A novel strategy for constructing N-terminal chromosomal fusions to green fluorescent protein in the yeast Saccharomyces cerevisiae. FEBS Lett. 2000;485:29–34. doi: 10.1016/s0014-5793(00)02179-7. [DOI] [PubMed] [Google Scholar]
- Rose M., Winston F., Heiter P. Cold Spring Harbor, NY: Cold Spring Laboratory Press; 1988. Methods in Yeast Genetics. [Google Scholar]
- Sacher M., Barrowman J., Schieltz D., Yates J. R., 3rd, Ferro-Novick S. Identification and characterization of five new subunits of TRAPP. Eur. J. Cell Biol. 2000;79:71–80. doi: 10.1078/S0171-9335(04)70009-6. [DOI] [PubMed] [Google Scholar]
- Sacher M., Barrowman J., Wang W., Horecka J., Zhang Y., Pypaert M., Ferro-Novick S. TRAPP I implicated in the specificity of tethering in ER-to-Golgi transport. Mol. Cell. 2001;7:433–442. doi: 10.1016/s1097-2765(01)00190-3. [DOI] [PubMed] [Google Scholar]
- Sacher M., Jiang Y., Barrowman J., Scarpa A., Burston J., Zhang L., Schieltz D., Yates J. R., 3rd, Abeliovich H., Ferro-Novick S. TRAPP, a highly conserved novel complex on the cis-Golgi that mediates vesicle docking and fusion. EMBO J. 1998;17:2494–2503. doi: 10.1093/emboj/17.9.2494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sciorra V. A., Audhya A., Parsons A. B., Segev N., Boone C., Emr S. D. Synthetic genetic array analysis of the PtdIns 4-kinase Pik1p identifies components in a Golgi-specific Ypt31/rab-GTPase signaling pathway. Mol. Biol. Cell. 2005;16:776–793. doi: 10.1091/mbc.E04-08-0700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segev N. Ypt and Rab GTPases: insight into functions through novel interactions. Curr. Opin. Cell Biol. 2001a;13:500–511. doi: 10.1016/s0955-0674(00)00242-8. [DOI] [PubMed] [Google Scholar]
- Segev N. Ypt/Rab/GTPases: regulators of protein trafficking. Sci. STKE. 2001b;2001:RE11. doi: 10.1126/stke.2001.100.re11. [DOI] [PubMed] [Google Scholar]
- Segev N., Mulholland J., Botstein D. The yeast GTP-binding YPT1 protein and a mammalian counterpart are associated with the secretion machinery. Cell. 1988;52:915–924. doi: 10.1016/0092-8674(88)90433-3. [DOI] [PubMed] [Google Scholar]
- Stark C., Breitkreutz B. J., Reguly T., Boucher L., Breitkreutz A., Tyers M. BioGRID: a general repository for interaction datasets. Nucleic Acids Res. 2006;34:D535–539. doi: 10.1093/nar/gkj109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tong A. H., et al. Global mapping of the yeast genetic interaction network. Science. 2004;303:808–813. doi: 10.1126/science.1091317. [DOI] [PubMed] [Google Scholar]
- Valdivia R. H., Schekman R. The yeasts Rho1p and Pkc1p regulate the transport of chitin synthase III (Chs3p) from internal stores to the plasma membrane. Proc. Natl. Acad. Sci. USA. 2003;100:10287–10292. doi: 10.1073/pnas.1834246100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto K., Jigami Y. Mutation of TRS130, which encodes a component of the TRAPP II complex, activates transcription of OCH1 in Saccharomyces cerevisiae. Curr. Genet. 2002;42:85–93. doi: 10.1007/s00294-002-0336-5. [DOI] [PubMed] [Google Scholar]
- Zhang C. J., Bowzard J. B., Greene M., Anido A., Stearns K., Kahn R. A. Genetic interactions link ARF1, YPT31/32 and TRS130. Yeast. 2002;19:1075–1086. doi: 10.1002/yea.903. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.