Abstract
Genetic and cell biological studies have indicated that Indian hedgehog (Ihh) plays an important role in bone development and osteoblast differentiation. However, the molecular mechanism by which Ihh regulates osteoblast differentiation is complex and remains to be fully elucidated. In this study, we investigated the role of Ihh signaling in osteoblast differentiation using mesenchymal cells and primary osteoblasts. We observed that Ihh stimulated alkaline phosphatase (ALP) activity, osteocalcin expression, and calcification. Overexpression of Gli2- but not Gli3-induced ALP, osteocalcin expression, and calcification of these cells. In contrast, dominant-negative Gli2 markedly inhibited Ihh-dependent osteoblast differentiation. Ihh treatment or Gli2 overexpression also up-regulated the expression of Runx2, an essential transcription factor for osteoblastogenesis, and enhanced the transcriptional activity and osteogenic action of Runx2. Coimmunoprecipitation analysis demonstrated a physical interaction between Gli2 and Runx2. Moreover, Ihh or Gli2 overexpression failed to increase ALP activity in Runx2-deficient mesenchymal cells. Collectively, these results suggest that Ihh regulates osteoblast differentiation of mesenchymal cells through up-regulation of the expression and function of Runx2 by Gli2.
INTRODUCTION
Indian hedgehog (Ihh), a member of the hedgehog family, plays an important role in the regulation of tissue patterning, skeletogenesis, and cellular proliferation (Ingham, 1998; Yamaguchi et al., 2000). Ihh, and its receptor components, patched (PTCH) and smoothened (Smo), are expressed in primary osteoblasts and its lineages (Murakami et al., 1997; Jemtland et al., 2003). In addition, Ihh stimulates osteoblast differentiation of the mesenchymal cell line C3H10T1/2 (Pathi et al., 2001). Mice deficient in the Ihh gene show reduced proliferation and maturation of chondrocytes and failure of osteoblast development in endochondral bones (St-Jacques et al., 1999; Hilton et al., 2005). Moreover, blockade of Ihh signaling in perichondrial cells of mice results in impaired development of long bones and osteoblastogenesis (Long et al., 2003). These studies indicate that Ihh regulates osteogenesis by promoting osteoblast differentiation of mesenchymal cells. However, the molecular basis by which Ihh conducts this biological process remains unclear.
Hedgehog family proteins, including Ihh, exert their biological effects through PTCH and Smo (Denef et al., 2000). After binding of Ihh to PTCH, Smo, which is repressed by PTCH, is activated and transduces signals into the cytoplasm though the intracellular signaling molecule, Fused, and the transcription factors, the Gli family members, which contain a zinc finger domain (Murone et al., 2000; Koebernick and Pieler, 2002). Activated Gli family members translocate from the cytoplasm to the nucleus and regulate the transcription of target genes (Murone et al., 2000). In vertebrates, three members of Gli, namely Gli1, Gli2, and Gli3, have been identified. Gli2 and Gli3 function as direct mediators for Ihh signaling, whereas Gli1 appears to indirectly mediate the effects of Ihh, as Gli1 expression is regulated by Gli2 and Gli3 (Dai et al., 1999; Sasaki et al., 1999). Targeted disruption of the Gli2 or Gli3 genes leads to abnormal skeletogenesis (Hui and Joyner, 1993; Mo et al., 1997; Motoyama et al., 1998b), whereas inherited mutations of the Gli3 gene in humans causes Greig cephalopolysyndactyly syndrome (Shin et al., 1999), Pallister-Hall syndrome, or postaxial polydactyly type A, characterized by severe impairment of skeletal development (Kang et al., 1997; Radhakrishna et al., 1997). These studies suggest important roles for Gli2 and Gli3 in bone development; however, the role of Gli2 and Gli3 in Ihh-regulated osteogenesis or osteoblast development is currently unclear.
A hedgehog family member, Sonic hedgehog (Shh), regulates embryo development in cooperation with bone morphogenetic proteins (BMPs; Bitgood and McMahon, 1995), which are known as powerful cytokines that induce bone formation and osteoblastogenesis (Yamaguchi et al., 2000). Consistently, Ihh and BMP2 synergistically stimulate osteoblast differentiation of mesenchymal cells (Nakamura et al., 1997), suggesting the cooperative role of Ihh and BMP2 signaling in osteoblast development. Runx2 (Cbfa1/PEBP2αA/Osf2), an essential transcription factor for bone formation and osteoblast differentiation, has been shown to function as a downstream of BMP2 signaling during osteoblastogenesis (Zhang et al., 2000; Nishimura et al., 2002). Therefore, it is possible that Ihh regulates Runx2 during osteoblast differentiation of mesenchymal cells.
To understand the molecular basis by which Ihh regulates osteoblastogenesis, we have investigated the role of Gli2 and Gli3 in osteoblast differentiation and the relationship between Gli2/Gli3 and Runx2. In the present study, we found that Gli2 but not Gli3 up-regulated Runx2 expression and enhanced the osteogenic action of Runx2 through physical association with Runx2. Thus, Ihh controls osteoblast differentiation of mesenchymal cells through the interaction between Gli2 and Runx2.
MATERIALS AND METHODS
Cells and Reagents
The C3H10T1/2, C2C12, 293, and CHO-K1 cell lines were purchased from the RIKEN cell bank (Tsukuba, Japan), and cultured in α-modified MEM (α-MEM) containing 10% fetal bovine serum (FBS). Recombinant Shh and Wnt3a were purchased from R&D Systems (Minneapolis, MN). Cyclopamine was purchased from Biomol (Plymouth Meeting, PA). Anti-Runx2, anti-β-actin, anti-Myc, anti-Gli3, anti-Gli2, and anti-Flag antibodies were purchased from Santa Cruz Biotechnology (Santa Cruz, CA), Abcam (Cambridge, MA), and Sigma-Aldrich (St. Louis, MO). Recombinant BMP2 and Ihh were obtained from the conditioned media of CHO-K1 cells infected with either BMP2 or Ihh adenovirus as previously reported (Hata et al., 2005). The activity of BMP2 or Ihh was determined by a comparison with human recombinant BMP2 or mouse recombinant Shh, respectively.
Isolation of Primary Osteoblasts and Mesenchymal Cells
The calvariae were isolated from 2- or 3-d-old neonatal mice and digested with 0.1% collagenase and 0.2% dispase for 7 min at 37°C (Ichida et al., 2004). The cells were then collected by centrifugation. These cells contained a small amount of alkaline phosphatase (ALP)-positive cells. The digested calvariae were sequentially digested four times with 0.1% collagenase and 0.2% dispase for 7 min at 37°C. The last three groups of fractionated cells were collected and used as the primary osteoblasts. The cells showed ALP activity. Runx2-deficient mesenchymal cells were isolated from the calvariae of Runx2-deficient embryos as described previously (Ichida et al., 2004).
Constructs and Transfection
Ihh cDNA was isolated from C3H10T1/2 cells by RT-PCR. Myc-Gli2 and Flag-Gli3 cDNA were kind gifts from Dr. Charles P. Emerson (University of Pennsylvania School of Medicine) and Dr. Bert Vogelstein (Johns Hopkins), respectively. Venus cDNA, a variant of green fluorescent protein (GFP; Nagai et al., 2002), was a kind gift from Dr. Atsushi Miyawaki (RIKEN). The PTCH gene promoter fused firefly luciferase construct was kindly provided by Drs. Rune Toftgard and Marie Argen (Karolinska Institutet). Dominant negative mutants of Gli2 (amino acids 2 through 497) or Runx2 (amino acids 2 through 242) were generated by subcloning of corresponding PCR products into pcDNA3 tagged with Myc-epitope at the N-terminus. Venus-fusion constructs were generated by subcloning of corresponding PCR products into pcDNA3 tagged with Venus at the N-terminus. Wild-type or dominant negative mutant of Runx2 were ligated into pcDNA3 tagged with six repeats of histidine at the N-terminus. The sequences of the Gli2 mutants and Venus fusion constructs were confirmed by DNA sequence analysis. Myc-tagged DKK1 cDNA was kindly provided by Dr. Toshimi Michigami (Osaka Medical Center and Research Institute for Maternal and Child Health). Supernatant of 293T cells transfected DKK1 cDNA was used as recombinant DKK1. Transfection of C3H10T1/2 cells was carried out using FuGENE6 (Roche, Indianapolis, IN) according to the manufacturer's protocol (Ichida et al., 2004).
Generation of Adenovirus
Recombinant adenoviruses carrying a wild-type or mutant form of Ihh, Gli2, or Gli3 was constructed by homologous recombination between the expression cosmid cassette (pAxCAwt) and the parental virus genome in 293 cells as previously described (Ichida et al., 2004) using an adenovirus construction kit (Takara, Tokyo, Japan). Adenoviruses carrying Runx2, Smad6, or Noggin were used as described previously (Nishimura et al., 2002; Nifuji et al., 2004). The viruses showed no proliferative activity due to a lack of E1A-E1B (Nishimura et al., 2002). Titers of the viruses were determined using a modified point assay (Nishimura et al., 2002). Infection of recombinant adenoviruses with C3H10T1/2 cells, C2C12 cells, or primary osteoblasts was performed by incubation with the adenoviruses at a multiplicity of infection (moi) of 40, except where specifically indicated.
Immunoprecipitation and Immunoblotting
The cells were washed four times with ice-cold phosphate-buffered saline (PBS) and solubilized in lysis buffer (Nishimura et al., 2002). The lysates were then centrifuged for 20 min at 4°C at 16,000 × g and incubated with antibodies for 4 h at 4°C, followed by immunoprecipitation with protein A-Sepharose (Zymed, South San Francisco, CA) or protein G-agarose (Roche). Immunoprecipitates were washed five times with lysis buffer and boiled in SDS sample buffer containing 0.5 M β-mercaptoethanol. The supernatants were recovered as immunoprecipitate samples. These samples were separated by SDS-PAGE, transferred to nitrocellulose membranes, immunoblotted with corresponding antibodies, and visualized with horseradish peroxidase coupled to anti-mouse, -rabbit or -goat IgG antibodies (Jackson ImmunoResearch Laboratories, West Grove, PA) with enhancement by electrochemiluminescence (ECL) advanced Western blotting detection kits (Amersham).
Luciferase Assay
The luciferase reporter construct driven by the PTCH or osteocalcin gene promoter was cotransfected with the TK-renilla luciferase construct (Promega, Madison, WI) into C3H10T1/2 cells. Two days after transfection, cells were lysed, and luciferase activity was determined using specific substrates in a luminometer (Promega) according to the manufacturer's protocol. Transfection efficiency was normalized by determining the activity of renilla luciferase.
RT-PCR
Total RNA was isolated from cells using the RNAeasy kit (Qiagen, Chatsworth, CA) and treated with DNase (Wako, Osaka, Japan) for 30 min. After denaturation of total RNA at 70°C for 10 min, cDNA was synthesized using an oligo-dT primer and reverse transcriptase (Invitrogen). PCR amplifications were performed using the specific primers for mouse Gli2 (sense primer: 5′-CATGGTATCCCTAGCTCCTC-3′; anti-sense primer 5′-GATGGCATCAAAGTCAATCT-3′), mouse Gli3 (sense primer: 5′-CATGAACAGCCCTTTAAGAC-3′; anti-sense primer 5′-TGATATGTGAGGTAGCACCA-3′) or mouse osteocalcin (sense primer: 5′-GACAAAGCCTTCATGTCCAAGC-3′; anti-sense primer: 5′-AAAGCCGAGCTGCCAGAGTTTG-3′). PCR products were separated by agarose-gel. After the PCR products were subcloned into the TA-cloning vector, they were verified by DNA sequence analysis.
Determination of ALP Activity
ALP activity was determined as described previously (Nishimura et al., 2002). The cells were washed with PBS and lysed with 0.05% Triton X-100 solution. ALP activity of the lysates was determined using p-nitrophenol-phosphate as a substrate. The protein content of the lysates was measured using the Bradford protein assay reagent (Bio-Rad, Richmond, CA). For cytochemical analysis, cells were washed with PBS, fixed with 3.7% formaldehyde, and stained with a mixture of 330 μg/ml nitro blue tetrazolium, 165 μg/ml bromochoroindoyl phosphate, 100 mM NaCl, 5 mM MgCl2, and 100 mM Tris (pH 9.5).
Determination of Osteocalcin Production
Osteocalcin in the culture media was determined using a mouse osteocalcin EIA kit (Biomedical Technologies, Stoughton, MA) according to the manufacturer's protocol.
Alizarin Red Staining
The calvaria cells were cultured in the presence of ascorbic acid (100 μg/ml) and β-glycerophosphate (5 mM), rinsed twice with PBS, fixed in 10% buffered Formalin, and stained with 1% alizarin red solution for 5 min.
Statistical Analysis
Data were analyzed by analysis of variance followed by a paired t test. Values shown are mean ± SD.
RESULTS
Ihh Promotes Osteoblast Differentiation though Activation of Gli2
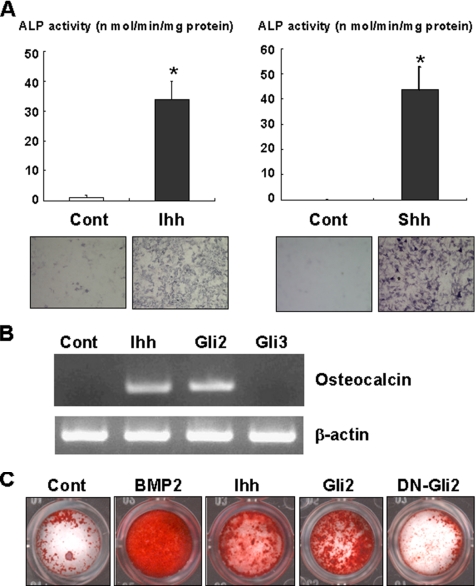
To verify the osteogenic action of Ihh, we first determined the effects of Ihh on C3H10T1/2 cells and primary osteoblasts isolated from mouse calvariae. Consistent with a previous report (Nakamura et al., 1997), Ihh dramatically increased ALP activity of C3H10T1/2 cells as well as Shh (Figure 1A). Ihh also induced osteocalcin expression (Figure 1B) and markedly stimulated calcification in primary osteoblasts (Figure 1C). These data indicate that Ihh stimulates osteoblast differentiation and exhibits osteogenic action.
Figure 1.
Promotion of osteoblast differentiation by Ihh. (A) C3H10T1/2 cells were cultured with or without Indian hedgehog (Ihh; 500 ng/ml) or Sonic hedgehog (Shh; 500 ng/ml) for 7 d. The cells were determined by ALP activity and staining. Data represent mean ± SD. *p < 0.01. Similar results were obtained from five independent experiments. (B) C3H10T1/2 cells were infected with control, Gli2 or Gli3 adenovirus as indicated, and cultured for 7 d in the absence or presence of Ihh (500 ng/ml). The total RNA isolated from the cells was subjected to RT-PCR analysis using the specific primes for osteocalcin (top) or β-actin (bottom). Similar results were obtained from three independent experiments. (C) Primary mouse osteoblasts infected with control adenovirus were cultured in the presence or absence of either BMP2 (300 ng/ml) or Ihh (500 ng/ml). Primary mouse osteoblasts infected with Myc-tagged wild-type or dominant-negative Gli2 adenovirus (DN-Gli2) were incubated without BMP2 and Ihh. After a 14-d incubation, the cells were examined by Alizarin red staining. Similar results were obtained from three independent experiments.
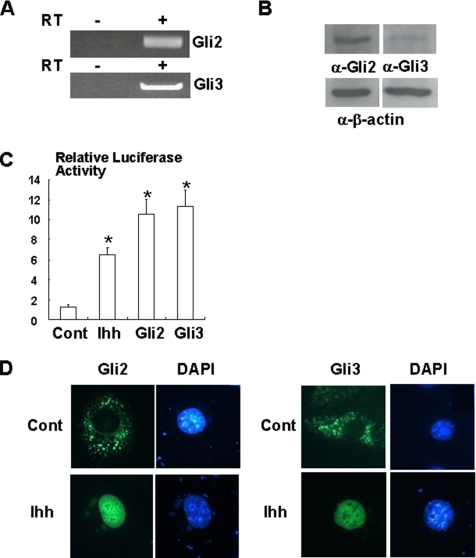
We next evaluated whether Ihh mediates osteogenic activity through Gli2 or Gli3 in undifferentiated mesenchymal cells. To address this, we first determined the expression and function of Gli2 and Gli3 in C3H10T1/2 cells. RT-PCR and Western blotting analysis indicated that C3H10T1/2 cells constitutively expresses Gli2 and Gli3 (Figure 2, A and B). Treatment with Ihh clearly stimulated PTCH promoter activity (Figure 2C), and overexpression of Gli2 or Gli3 also increased PTCH promoter activity (Figure 2C). Consistent with these results, Ihh induced the translocation of Gli2 or Gli3 into the nucleus (Figure 2D). These results indicate that Ihh/Gli signaling is functional in C3H10T1/2 cells.
Figure 2.
Activation of Ihh/Gli2 signaling in C3H10T1/2 cells. (A) Total RNA was isolated from C3H10T1/2 cells and analyzed by RT-PCR analysis (RT) for Gli2. Total RNA untreated with reversed-transcriptase (RT, −) was used as the negative controls. Similar results were obtained from three independent experiments. (B) Lysates of C3H10T1/2 cells were determined by immunoblotting with anti-Gli2 or Gli3 antibody (top). β-actin expression was determined as loading controls (bottom). Similar results were obtained from four independent experiments. (C) PTCH-luciferase and TK-renilla reporter constructs were transfected into C3H10T1/2 cells together with pcDNA3 (control), Gli2 or Gli3 expression vector and incubated with Ihh. At the end of culture, cells were lysed and the luciferase activity measured and normalized by determining renilla luciferase activity as described in the text. Data represent mean ± SD. *p < 0.05 (vs. control). Similar results were obtained from six independent experiments. (D) C3H10T1/2 cells were transfected with Venus-Gli2 or Venus-Gli3 expression vector and stimulated with or without Ihh (500 ng/ml) for 30 min. The cells were stained with DAPI and examined under a fluorescent microscope. Similar results were obtained from three independent experiments.
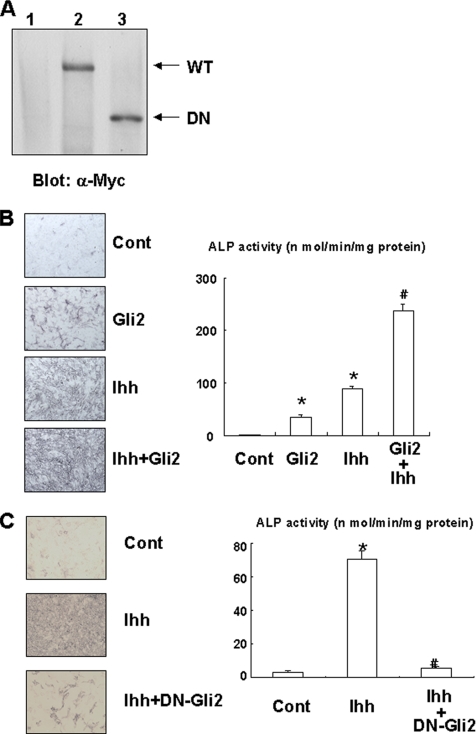
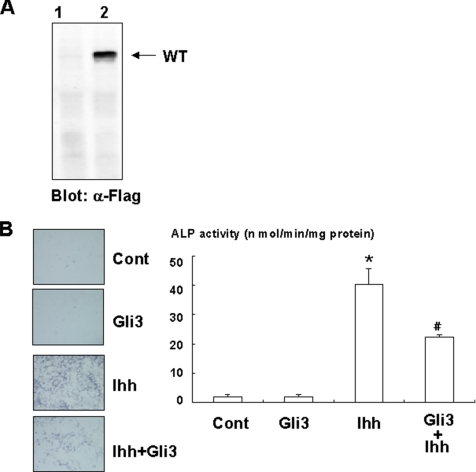
To examine whether Gli2 and/or Gli3 is involved in the osteogenic action of Ihh, we determined the effects of Gli2 and Gli3 overexpression on osteoblast differentiation in C3H10T1/2 cells. Gli2 overexpression, induced using an adenovirus system, induced ALP activity and osteocalcin expression in C3H10T1/2 cells (Figures 1B and 3, A and B). This effect of Gli2 is enhanced by Ihh treatment. In addition, Gli2 overexpression promotes calcification in primary osteoblasts (Figure 1C). As expected, a dominant-negative mutant of Gli2 failed to stimulate calcification in primary osteoblasts (Figure 1C). Consistently, dominant-negative Gli2 markedly inhibited the effect of Ihh on ALP activity (Figure 3, A and C). In contrast, overexpression of Gli3 had no effect on ALP activity and suppressed Ihh-induced ALP activity (Figure 4A and B). These results indicate that Gli2 but not Gli3 mediates the osteogenic action of Ihh.
Figure 3.
Regulation of osteoblast differentiation by Gli2. (A) C3H10T1/2 cells infected with control (lane 1), Myc-tagged wild-type Gli2 (lane 2), or dominant-negative Gli2 adenovirus (lane 3) were determined by immunoblotting with anti-Myc antibody. (WT, wild-type; DN, dominant-negative). Similar results were obtained from five independent experiments. (B) C3H10T1/2 cells were infected with control or Myc-tagged Gli2 adenovirus in the presence or absence of Ihh (500 ng/ml) and cultured for 7 d. The cells were then analyzed for ALP staining or activity. Data represent mean ± SD. *p < 0.01 (vs. control); #p < 0.01 (vs. Gli2 or Ihh). Similar results were obtained from three independent experiments. (C) C3H10T1/2 cells were infected with control or Myc-tagged dominant-negative Gli2 adenovirus in the presence or absence of Ihh (500 ng/ml), and cultured for 7 d. The cells were then analyzed for ALP staining or activity. Data represent mean ± SD. *p < 0.01 (vs. control); #p < 0.01 (vs. Gli2). Similar results were obtained from three independent experiments.
Figure 4.
Inhibition of Ihh-dependent osteoblast differentiation by Gli3. (A) C3H10T1/2 cells infected with control (lane 1), or Flag-tagged Gli3 (lane 2) adenovirus were determined by immunoblotting with anti-Flag antibody. Similar results were obtained from five independent experiments. (B) C3H10T1/2 cells were infected with control or Flag-tagged Gli3 adenovirus in the presence or absence of Ihh (500 ng/ml) and cultured for 7 d. The cells were then analyzed for ALP staining or activity. Data represent mean ± SD. *p < 0.05 (vs. control); #p < 0.05 (vs. Ihh). Similar results were obtained from three independent experiments.
Ihh/Gli2 Signaling Stimulates BMP2-induced Osteoblast Differentiation
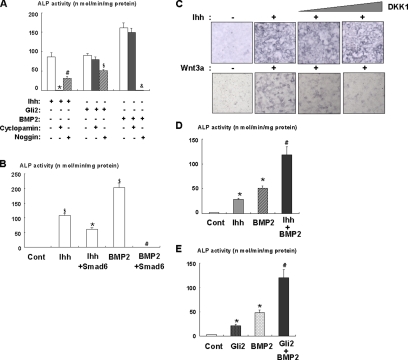
Because hedgehog has been shown to function as an upstream of BMP in several systems (Garrett et al., 2003; Zhao et al., 2006), we examined the effect of BMP antagonist, Noggin, in C3H10T1/2 cells. Noggin effectively abolished BMP2-induced ALP activity, but only partially suppressed Ihh-induced ALP activity in C3H10T/2 cells (Figure 5A). In contrast, a hedgehog inhibitor, cyclopamine, completely blocked Ihh-induced ALP activity (Figure 5A). Consistent with these results, Noggin did not completely inhibit ALP activity induced by Gli2 overexpression. Furthermore, Smad6 partially inhibited Ihh-induced ALP activity (Figure 5B). These results suggest that the Ihh/Gli2 pathway regulates osteoblast differentiation of mesenchymal cells though BMP-dependent and -independent mechanisms. In contrast, we did not observe the effect of DKK1, which is a selective inhibitor for Wnt (Kawano et al., 2003), on ALP activity induced by Ihh (Figure 5C). This result indicate that Wnt is not implicated in Ihh-mediated osteoblastogenesis. To confirm the functional relationship between Ihh and BMP2 signaling, we determined the effects of Ihh and Gli2 on ALP activity in the presence of BMP2. As previously reported, Ihh and BMP2 cooperatively increased ALP activity (Figure 5D). We also found that overexpression of Gli2 enhanced BMP2-induced ALP activity in C3H10T1/2 cells (Figure 5E). These results suggest a functional relationship between Ihh/Gli2 and BMP2 signaling during osteoblastogenesis.
Figure 5.
Cooperative effects of Ihh/Gli2 and BMP2 signaling on osteoblast differentiation. (A) C3H10T1/2 cells were infected with control, Noggin, or Myc-tagged Gli2 adenovirus and then incubated in the presence or absence of Cyclopamine, Ihh (500 ng/ml), or BMP2 (300 ng/ml) for 7 d as indicated. The cells were then analyzed for ALP activity. Data represent mean ± SD. *p < 0.01 (vs. Ihh alone); #p < 0.05 (vs. Ihh alone, Ihh+Cyclopamine); $p < 0.05 (vs. Gli2 alone); &p < 0.01 (vs. BMP2 alone). Similar results were obtained from three independent experiments. (B) C3H10T1/2 cells were infected with control or Smad6 adenovirus and then incubated in the presence or absence of Ihh (500 ng/ml) or BMP2 (300 ng/ml) for 7 d as indicated. The cells were then analyzed for ALP activity. Data represent mean ± SD. $p < 0.01 (vs. control); *p < 0.01 (vs. control, Ihh alone); #p < 0.01 (vs. BMP2 alone). Similar results were obtained from three independent experiments. (C) C3H10T1/2 cells were incubated in the presence or absence of Ihh (500 ng/ml) or Wnt3a (100 ng/ml) with DKK1 for 7 d as indicated. The cells were then analyzed for ALP staining. Similar results were obtained from three independent experiments. (D) C3H10T1/2 cells were cultured with Ihh (500 ng/ml), BMP2 (300 ng/ml), or both for 7 d. The cells were then analyzed for ALP activity. Data represent mean ± SD. *p < 0.01 (vs. control), #p < 0.05 (vs. BMP2, Ihh). Similar results were obtained from three independent experiments. (E) C3H10T1/2 cells were infected with control or Myc-tagged Gli2 adenovirus in the presence or absence of BMP2 (300 ng/ml) and cultured for 7 d. The cells were then analyzed for ALP activity. Data represent mean ± SD. *p < 0.05 (vs. control), #p < 0.05 (vs. BMP2, Ihh). Similar results were obtained from three independent experiments.
Gli2 Regulates the Expression and Function of Runx2 during Ihh-induced Osteoblast Differentiation
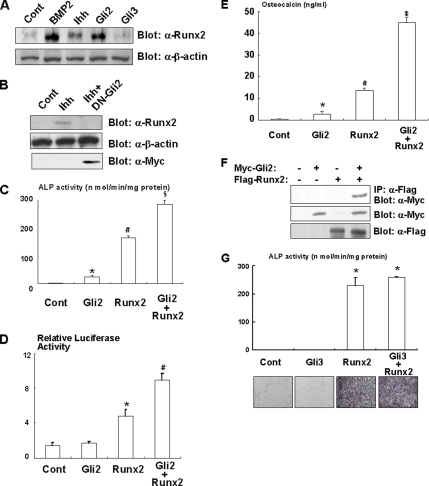
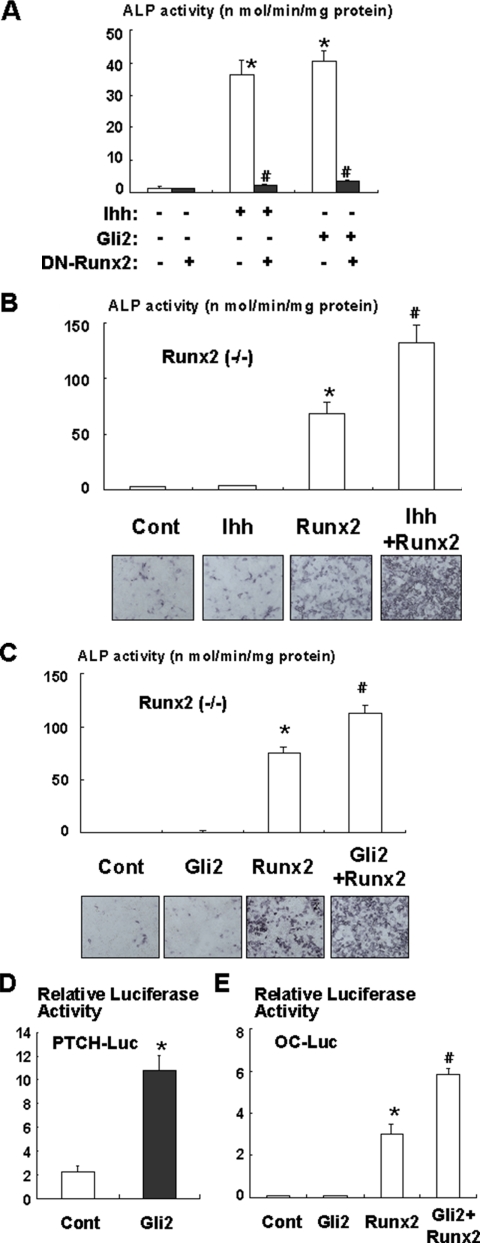
To further understand the relationship between Ihh/Gli2 and BMP2 signaling during osteoblast differentiation, we examined the effects of Ihh and Gli2 on the expression and function of Runx2, which functions as a downstream of BMP2 signaling during osteoblast differentiation (Zhang et al., 2000; Nishimura et al., 2002). We found that Ihh induced expression of Runx2 in C3H10T1/2 cells (Figure 6A). In addition, overexpression of Gli2 also induced Runx2 expression (Figure 6A). In contrast, dominant-negative Gli2 inhibited Ihh-induced Runx2 expression (Figure 6B). These data suggest that Ihh regulates osteoblast differentiation, at least in part, by stimulating Runx2 expression. We next examined the effect of Gli2 on the osteogenic function of Runx2. As shown in Figure 6, C and D, overexpression of Gli2 increased Runx2-dependent ALP activity and osteocalcin promoter activity. In addition, Gli2 stimulated Runx2-induced osteocalcin production (Figure 6E). Furthermore, coimmunoprecipitation analysis demonstrated a physical interaction between Gli2 and Runx2 (Figure 6F). In contrast, Gli3 had no effect on the osteoblastogenic activity of Runx2 (Figure 6G). Collectively, these results suggest that Gli2 functionally up-regulates the osteoblastogenic activity of Runx2. To verify the importance of Runx2 in Ihh-dependent osteoblast differentiation, we performed experiments using a dominant-negative mutant of Runx2. Dominant-negative Runx2 markedly inhibited Ihh or Gli2-induced ALP activity (Figure 7A). Consistently, Ihh or Gli2 overexpression failed to induce ALP activity in mesenchymal cells isolated from Runx2 null mice (Figure 7B and C). Furthermore, Gli2 increased PTCH promoter activity but not osteocalcin promoter activity in Runx2-deficient cells (Figure 7, D and E). Interestingly, Ihh or Gli2 overexpression increased ALP activity and osteocalcin promoter activity in the presence of exogenous Runx2 in mesenchymal cells isolated from Runx2 null mice (Figure 7, B, C, and E). These results support the notion that Gli2 and Runx2 physically and functionally regulate osteoblast differentiation in mesenchymal cells.
Figure 6.
Regulation of Runx2 by Gli2 during osteoblast differentiation. (A) C3H10T1/2 cells infected with control, Myc-tagged Gli2, or Flag-tagged Gli3 adenovirus were incubated in the presence or absence of Ihh (500 ng/ml) or BMP2 (300 ng/ml) for 7 d. The lysates of these cells were analyzed by immunoblotting with anti-Runx2 (top) or β-actin (bottom) antibody. Similar results were obtained from three independent experiments. (B) C3H10T1/2 cells were infected with control or Myc-tagged dominant-negative Gli2 adenovirus (DN-Gli2) and incubated in the presence or absence of Ihh (500 ng/ml) for 7 d. The lysates of these cells were analyzed by immunoblotting with anti-Runx2 (top), β-actin (middle), or Myc (bottom) antibody. Similar results were obtained from three independent experiments. (C) C3H10T1/2 cells were infected with control, Myc-tagged Gli2, or Runx2 adenovirus and cultured for 7 d. The cells were then analyzed for ALP activity. Data represent mean ± SD. *p < 0.05 (vs. control); #p < 0.01 (vs. control); $p < 0.05 (vs. Runx2, Gli2). Similar results were obtained from three independent experiments. (D) C3H10T1/2 cells were transfected with osteocalcin luciferase and TK-renilla reporter constructs together with pcDNA3 (Cont), Myc-tagged Gli2 expression vector, Runx2 expression vector, or both for 2 d. Luciferase activity in the lysates was measured. Data represent mean ± SD. *p < 0.05 (vs. control); #p < 0.05 (vs. Runx2, Gli2). Similar results were obtained from three independent experiments. (E) C3H10T1/2 cells were infected with Gli2 and/or Runx2 adenovirus and incubated for 7 d. The amount of osteocalcin in the conditioned medium was determined using a mouse osteocalcin EIA kit. *p < 0.05 (vs. control); #p < 0.01 (vs. control); $p < 0.01 (vs. Runx2, Gli2). Similar results were obtained from three independent experiments. (F) C3H10T1/2 cells were transfected with Myc-tagged Gli2 expression vector, Flag-tagged Runx2 expression vector, or both and incubated for 2 d. The lysates of these cells were immunoprecipitated with anti-Flag antibody and immunoblotted with anti-Myc antibody (top). The expression levels of Myc-tagged Gli2 or Flag-tagged Runx2 were monitored by immunoblotting with anti-Myc (middle) or anti-Flag (bottom) antibody. Similar results were obtained from three independent experiments. (G) C3H10T1/2 cells were infected with control, Flag-tagged Gli3, or Runx2 adenovirus and cultured for 7 d. The cells were then analyzed for ALP activity. Data represent mean ± SD. *p < 0.05 (vs. control). Similar results were obtained from three independent experiments.
Figure 7.
Requirement of Runx2 for Ihh/Gli2 dependent osteoblast differentiation. (A) C3H10T1/2 cells were infected with control, Myc-tagged Gli2, or dominant-negative Runx2 adenovirus (DN-Runx2) and incubated in the presence or absence of Ihh (500 ng/ml) for 7 d. The cells were then analyzed for ALP activity. Data represent mean ± SD. *p < 0.01 (vs. control); #p < 0.01 (vs. Ihh or Gli2). Similar results were obtained from three independent experiments. (B) Runx2-deficient mesenchymal cells infected with control or Runx2 adenovirus were incubated in the presence or absence of Ihh (500 ng/ml) for 7 d. The cells were then analyzed for ALP activity. Data represent mean ± SD. *p < 0.01 (vs. control); #p < 0.05 (vs. Runx2). Similar results were obtained from three independent experiments. (C) Runx2-deficient mesenchymal cells infected with control, Myc-tagged Gli2 adenovirus, Runx2 adenovirus, or both were incubated for 7 d. The cells were then analyzed for ALP activity. Data represent mean ± SD. *p < 0.01 (vs. control); #p < 0.05 (vs. Runx2). Similar results were obtained from three independent experiments. (D) Runx2-deficient mesenchymal cells transfected with PTCH-luciferase and TK-renilla reporter constructs together with pcDNA3 (Cont) or Myc-tagged Gli2 expression construct and incubated for 2 d. The luciferase activities in the lysates were then measured. Data represent mean ± SD. *p < 0.01 (vs. control). Similar results were obtained from three independent experiments. (E) Runx2-deficient mesenchymal cells transfected with osteocalcin promoter-luciferase and TK-renilla reporter constructs together with pcDNA3 (Cont), Myc-tagged Gli2 expression vector, Runx2 expression vector, or both expression vectors and incubated for 2 d. The luciferase activities in the lysates were then measured. Data represent mean ± SD. *p < 0.01 (vs. control); #p < 0.05 (vs. Runx2). Similar results were obtained from three independent experiments.
DISCUSSION
The molecular mechanism by which Ihh stimulates osteoblast differentiation of mesenchymal cells is currently unclear. Because hedgehog family members up-regulate the expression of BMPs in several tissues and cells, it is likely that Ihh exhibits its osteogenic action through up-regulation of BMPs expression. Zhao et al. (2006) recently showed that Shh and Gli2 stimulate BMP2 expression. Consistently, we observed that treatment with Noggin or Smad6 overexpression suppressed the effect of Ihh on osteoblastogenesis. However, we found that the inhibitory effect of Noggin or Smad6 overexpression on Ihh-mediated osteoblastogenesis is partial, suggesting that Ihh stimulates osteoblast differentiation in BMP-dependent and -independent mechanisms. Consistent with a previous report (Nakamura et al., 1997), we showed that Ihh and BMP2 cooperatively promote osteoblast differentiation. This observation also suggests a direct effect of Ihh on osteoblastogenesis as well as interaction between Ihh and BMP signaling. To support this, we demonstrated that Ihh/Gli2 signaling promotes osteoblast differentiation of mesenchymal cells by up-regulating Runx2 expression and stimulating the osteoblastogenic function of Runx2. In contrast, we did not observe any cooperative effects of Ihh/Gli2 and Osterix, an essential transcription factor for bone formation (data not shown). Together with the biochemical result of the physical interaction between Gli2 and Ruxn2, these findings indicate that Gli2 plays an important role in Ihh-mediated osteoblastogenesis by regulating Runx2. Interestingly, dominant-negative Runx2 overexpression inhibited Ihh or Gli2-induced osteoblast differentiation. Furthermore, both Ihh and Gli2 failed to promote osteoblast differentiation in mesenchymal cells deficient in the Runx2 gene. Thus, our results indicate that Ihh temporally and spatially regulates Runx2 through Gli2 and consequently stimulates osteoblast differentiation.
Several lines of evidence indicate that Gli2 and Gli3 exhibit diverse and redundant biological roles in different tissues and cell types. Gli2 and Gli3 appear to compensate for each other during the development of lung and neural tube and skeletal muscle formation (Motoyama et al., 1998a; Sasaki et al., 1999; McDermott et al., 2005). On the other hand, Gli2 and Gli3 show distinct or opposite functions in ventral cell fate specification and motor neuron differentiation (Motoyama et al., 1998a; Ruiz i Altaba, 1998). In the present study, we found that Gli3 inhibits Ihh-dependent osteoblastogenesis in contrast to Gli2. Notably, the opposite role of Gli2 and Gli3 in osteoblastogenesis is also consistent with the distinctive skeletal phenotype of Gli2- and Gli3-deficient mice (Mo et al., 1997; Motoyama et al., 1998a). To understand the mechanism of the inhibitory role of Gli3 in osteoblastogenesis, we examined whether Gli3 affected Runx2, which cooperatively stimulates osteoblastogenesis with Gli2. However, we found that Gli3 did not affect either the expression or function of Runx2. In addition, we could not detect physical interaction between Gli3 and Runx2 (data not shown). These results suggest that Gli3 negatively regulates osteoblastogenesis independently of Runx2. Because it has been shown that c-Ski associates with Gli3, forms a histone deacetylase complex and functions as a repressor for the induction of Gli1 (Dai et al., 1999), Gli3 may inhibit osteoblastogenesis by recruiting corepressors such as c-Ski.
In the present study, we showed that Ihh stimulates both Gli2 and Gli3 in the undifferentiated mesenchymal cell line C3H10T1/2. We demonstrated that Gli2 and Gli3 have contrary effects on osteoblastogenesis, however, Ihh promotes osteoblast differentiation of C3H10T1/2 cells. It remains a mysterious why the osteoblastogenic activity of Gli2 is predominant over the inhibitory effect of Gli3. One possibility is that expression of Gli2 would be higher than that of Gli3 in undifferentiated mesenchymal cells. Indeed, we observed that Gli2 expression appeared to be moderately higher than Gli3 expression in C3H10T1/2 cells. Gli3 is ubiquitinated by SCF ubiquitin ligase complex, and subsequently Gli3 is degraded or cleaved into a suppressive form that works as a dominant-negative Gli3 (Garrett et al., 2003). Although the involvement of Ihh signaling in Gli3 ubiquitination is unknown at present, it might be possible that Ihh dynamically alters the expression and protein processing of Gli3. The development of antibodies that enable us to evaluate the kinetics of both Gli2 and Gli3 expression may allow further dissection of the functional network between Gli2 and Gli3 during osteoblast differentiation.
In conclusion, we demonstrated that Gli2 is an important mediator for Ihh-dependent osteoblast differentiation. We also demonstrated that Gli2 controls the expression and function of Runx2 during osteoblastogenesis. Thus, our findings contribute to the understanding of the molecular basis by which Ihh promotes osteoblast differentiation of mesenchymal cells.
ACKNOWLEDGMENTS
We thank Drs. Charles P. Emerson, Bert Vogelstein, and Toshimi Michigami for the Gli2, Gli3, and DKK1 cDNA, respectively. This work was supported in part by Ministry of Education, Science, Sports, and Culture Grants-in-Aid for Scientific Research A 11307041 (T.Y.) and B 15390560 (R.N.), a Grant-in-Aid for Scientific Research on Priority Areas B 12137205 (T.Y.), and The 21st Century COE Program (T.Y., R.N.).
Footnotes
This article was published online ahead of print in MBC in Press (http://www.molbiolcell.org/cgi/doi/10.1091/mbc.E06-08-0743) on April 18, 2007.
REFERENCES
- Bitgood M. J., McMahon A. P. Hedgehog and Bmp genes are coexpressed at many diverse sites of cell-cell interaction in the mouse embryo. Dev. Biol. 1995;172:126–138. doi: 10.1006/dbio.1995.0010. [DOI] [PubMed] [Google Scholar]
- Dai P., Akimaru H., Tanaka Y., Maekawa T., Nakafuku M., Ishii S. Sonic Hedgehog-induced activation of the Gli1 promoter is mediated by GLI3. J. Biol. Chem. 1999;274:8143–8152. doi: 10.1074/jbc.274.12.8143. [DOI] [PubMed] [Google Scholar]
- Denef N., Neubüser D., Perez L., Cohen S. M. Hedgehog induces opposite changes in turnover and subcellular localization of patched and smoothened. Cell. 2000;102:521–531. doi: 10.1016/s0092-8674(00)00056-8. [DOI] [PubMed] [Google Scholar]
- Garrett I. R., et al. Selective inhibitors of the osteoblast proteasome stimulate bone formation in vivo and in vitro. J. Clin. Invest. 2003;111:1771–1782. doi: 10.1172/JCI16198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hata K., Nishimura R., Ueda M., Ikeda F., Matsubara T., Ichida F., Hisada K., Nokubi T., Yamaguchi A., Yoneda T. A CCAAT/enhancer binding protein beta isoform, liver-enriched inhibitory protein, regulates commitment of osteoblasts and adipocytes. Mol. Cell. Biol. 2005;25:1971–1979. doi: 10.1128/MCB.25.5.1971-1979.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hilton M. J., Tu X., Cook J., Hu H., Long F. Ihh controls cartilage development by antagonizing Gli3, but requires additional effectors to regulate osteoblast and vascular development. Development. 2005;132:4339–4351. doi: 10.1242/dev.02025. [DOI] [PubMed] [Google Scholar]
- Hui C. C., Joyner A. L. A. mouse model of greig cephalopolysyndactyly syndrome: the extra-toesJ mutation contains an intragenic deletion of the Gli3 gene. Nat. Genet. 1993;3:241–246. doi: 10.1038/ng0393-241. [DOI] [PubMed] [Google Scholar]
- Ichida F., et al. Reciprocal roles of MSX2 in regulation of osteoblast and adipocyte differentiation. J. Biol. Chem. 2004;279:34015–34022. doi: 10.1074/jbc.M403621200. [DOI] [PubMed] [Google Scholar]
- Ingham P. W. Transducing Hedgehog: the story so far. EMBO J. 1998;17:3505–3511. doi: 10.1093/emboj/17.13.3505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jemtland R., Divieti P., Lee K., Segre G. V. Hedgehog promotes primary osteoblast differentiation and increases PTHrP mRNA expression and iPTHrP secretion. Bone. 2003;130:611–620. doi: 10.1016/s8756-3282(03)00092-9. [DOI] [PubMed] [Google Scholar]
- Kang S., Graham J. M., Olney A. H., Biesecker L. G. GLI3 frameshift mutations cause autosomal dominant Pallister-Hall syndrome. Nat. Genet. 1997;15:266–268. doi: 10.1038/ng0397-266. [DOI] [PubMed] [Google Scholar]
- Kawano Y., Kypta R. Secreted antagonists of the Wnt signalling pathway. J. Cell Sci. 2003;116:2627–2634. doi: 10.1242/jcs.00623. [DOI] [PubMed] [Google Scholar]
- Koebernick K., Pieler T. Gli-type zinc finger proteins as bipotential transducers of Hedgehog signaling. Differentiation. 2002;70:69–76. doi: 10.1046/j.1432-0436.2002.700201.x. [DOI] [PubMed] [Google Scholar]
- Long F., Chung U. I., Ohba S., McMahon J., Kronenberg H. M., McMahon A. P. Ihh signaling is directly required for the osteoblast lineage in the endochondral skeleton. Development. 2003;33:1309–1318. doi: 10.1242/dev.01006. [DOI] [PubMed] [Google Scholar]
- McDermott A., Gustafsson M., Elsam T., Hui C. C., Emerson C. P., Borycki A. G. Gli2 and Gli3 have redundant and context-dependent function in skeletal muscle formation. Development. 2005;132:345–357. doi: 10.1242/dev.01537. [DOI] [PubMed] [Google Scholar]
- Mo R., et al. Specific and redundant functions of Gli2 and Gli3 zinc finger genes in skeletal patterning and development. Development. 1997;124:113–123. doi: 10.1242/dev.124.1.113. [DOI] [PubMed] [Google Scholar]
- Motoyama J., Liu J., Mo R., Ding Q., Post M., Hui C. C. Essential function of Gli2 and Gli3 in the formation of lung, trachea and oesophagus. Nat. Genet. 1998a;20:54–57. doi: 10.1038/1711. [DOI] [PubMed] [Google Scholar]
- Motoyama J., Milenkovic L., Iwama M., Shikata Y., Scott M. P., Hui C. C. Differential requirement for Gli2 and Gli3 in ventral neural cell fate specification. Dev. Biol. 1998b;20:150–161. doi: 10.1016/s0012-1606(03)00159-3. [DOI] [PubMed] [Google Scholar]
- Murakami S., Nifuji A., Noda M. Expression of Indian hedgehog in osteoblasts and its posttranscriptional regulation by transforming growth factor-beta. Endocrinology. 1997;138:1972–1978. doi: 10.1210/endo.138.5.5140. [DOI] [PubMed] [Google Scholar]
- Murone M., Luoh S. M., Stone D., Li W., Gurney A., Armanini M., Grey C., Rosenthal A., de Sauvage F. J. Gli regulation by the opposing activities of fused and suppressor of fused. Nat. Cell Biol. 2000;2:310–312. doi: 10.1038/35010610. [DOI] [PubMed] [Google Scholar]
- Nagai T., Ibata K., Park E. S., Kubota M., Mikoshiba K., Miyawaki A. A variant of yellow fluorescent protein with fast and efficient maturation for cell-biological applications. Nat. Biotechnol. 2002;20:87–90. doi: 10.1038/nbt0102-87. [DOI] [PubMed] [Google Scholar]
- Nakamura T., et al. Induction of osteogenic differentiation by hedgehog proteins. Biochem. Biophys. Res. Commun. 1997;237:465–469. doi: 10.1006/bbrc.1997.7156. [DOI] [PubMed] [Google Scholar]
- Nifuji A., Kellermann O., Noda M. Noggin inhibits chondrogenic but not osteogenic differentiation in mesodermal stem cell line C1 and skeletal cells. Endocrinology. 2004;145:3434–3442. doi: 10.1210/en.2003-0685. [DOI] [PubMed] [Google Scholar]
- Nishimura R., Hata K., Harris S. E., Ikeda F., Yoneda T. Core-binding factor alpha 1 (Cbfa1) induces osteoblastic differentiation of C2C12 cells without interactions with Smad1 and Smad5. Bone. 2002;31:303–312. doi: 10.1016/s8756-3282(02)00826-8. [DOI] [PubMed] [Google Scholar]
- Pathi S., Pagan-Westphal S., Baker D. P., Garber E. A., Rayhorn P., Bumcrot D, Tabin C. J., Blake Pepinsky R., Williams K. P. Comparative biological responses to human Sonic, Indian, and Desert hedgehog. Mech. Dev. 2001;265:107–117. doi: 10.1016/s0925-4773(01)00427-0. [DOI] [PubMed] [Google Scholar]
- Radhakrishna U., Blouin J. L., Mehenni H., Patel U. C., Patel M. N., Solanki J. V., Antonarakis S. E. Mapping one form of autosomal dominant postaxial polydactyly type A to chromosome 7p15–q11.23 by linkage analysis. Am. J. Hum. Genet. 1997;60:597–604. [PMC free article] [PubMed] [Google Scholar]
- Ruiz i Altaba A. Combinatorial Gli gene function in floor plate and neuronal inductions by Sonic hedgehog. Development. 1998;125:2203–2212. doi: 10.1242/dev.125.12.2203. [DOI] [PubMed] [Google Scholar]
- Sasaki H., Nishizaki Y., Hui C., Nakafuku M., Kondoh H. Regulation of Gli2 and Gli3 activities by an amino-terminal repression domain: implication of Gli2 and Gli3 as primary mediators of Shh signaling. Development. 1999;126:3915–3924. doi: 10.1242/dev.126.17.3915. [DOI] [PubMed] [Google Scholar]
- Shin S. H., Kogerman P., Lindström E., Toftgárd R., Biesecker L. G. G.L.I3 mutations in human disorders mimic Drosophila cubitus interruptus protein functions and localization. Proc. Natl. Acad. Sci. USA. 1999;96:2880–2884. doi: 10.1073/pnas.96.6.2880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- St-Jacques B., Hammerschmidt M., McMahon A. P. Indian hedgehog signaling regulates proliferation and differentiation of chondrocytes and is essential for bone formation. Genes Dev. 1999;80:2072–2086. doi: 10.1101/gad.13.16.2072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi A., Komori T., Suda T. Regulation of osteoblast differentiation mediated by bone morphogenetic proteins, hedgehogs, and Cbfa1. Endocr. Rev. 2000;21:393–411. doi: 10.1210/edrv.21.4.0403. [DOI] [PubMed] [Google Scholar]
- Zhang Y. W., Yasui N., Ito K., Huang G., Fujii M., Hanai J., Nogami H., Ochi T., Miyazono K., Ito Y. A RUNX2/PEBP2alpha A/CBFA1 mutation displaying impaired transactivation and Smad interaction in cleidocranial dysplasia. Proc. Natl. Acad. Sci. USA. 2000;97:10549–10554. doi: 10.1073/pnas.180309597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao M., Qiao M., Harris S. E., Chen D., Oyajobi B. O., Mundy G. R. The zinc finger transcription factor Gli2 mediates bone morphogenetic protein 2 expression in osteoblasts in response to hedgehog signaling. Mol. Cell. Biol. 2006;26:6197–6208. doi: 10.1128/MCB.02214-05. [DOI] [PMC free article] [PubMed] [Google Scholar]