Abstract
The microtubule-based motor cytoplasmic dynein/dynactin is a force generator at the kinetochore. It also transports proteins away from kinetochores to spindle poles. Regulation of such diverse functions, however, is poorly understood. We have previously shown that Nudel is critical for dynein-mediated protein transport, whereas mitosin, a kinetochore protein that binds Nudel, is involved in retention of kinetochore dynein/dynactin against microtubule-dependent stripping. Here we demonstrate that Nudel is required for robust localization of dynein/dynactin at the kinetochore. It localizes to kinetochores after nuclear envelope breakdown, depending mostly (∼78%) on mitosin and slightly on dynein/dynactin. Depletion of Nudel by RNA interference (RNAi) or overexpression of its mutant incapable of binding either Lis1 or dynein heavy chain abolishes the kinetochore protein transport and mitotic progression. Similar to mitosin RNAi, Nudel RNAi also leads to increased stripping of kinetochore dynein/dynactin in the presence of microtubules. Taking together, our results suggest a dual role of kinetochore Nudel: it activates dynein-mediated protein transport and, when interacting with both mitosin and dynein, stabilizes kinetochore dynein/dynactin against microtubule-dependent stripping to facilitate the force generation function of the motor.
INTRODUCTION
The cytoplasmic dynein/dynactin complex constitutes a microtubule (MT) minus end–directed motor that functions in a wide variety of cell activities requiring MT-based motility (Hirokawa, 1998; Dujardin and Vallee, 2002; Cleveland et al., 2003; Vallee et al., 2004). Dynein contains two heavy chains (DHC), three to four intermediate chains (DIC), four light intermediate chains, and several light chains (Hirokawa, 1998). Dynactin is an accessory complex critical for processivity of dynein (King and Schroer, 2000). It contains an actin-like minifilament backbone for association with other cellular structures. The backbone is linked through the p50 subunit (dynamitin) to a flexible projecting sidearm for interaction with dynein (Hirokawa, 1998). Overexpression of p50 disrupts the integrity of dynactin and has thus been widely used to inactivate dynein (Echeverri et al., 1996; Burkhardt et al., 1997).
Dynein plays multiple roles in mitosis. A portion of dynein/dynactin is located to the kinetochore, a three-layer proteinous organelle on chromosome responsible for MT attachment and chromosome segregation. Kinetochore-associated dynein drives poleward chromosome movement and contributes to tension generation across sister kinetochores (Cleveland et al., 2003; Maiato et al., 2004). Recently dynein has been shown to transport outer kinetochore proteins including Mad2, BubR1, Bub1, and mitosin (also named CENP-F) to spindle poles along MTs (Howell et al., 2001; Yan et al., 2003; Yang et al., 2003). Because spindle checkpoint proteins controlling the timing of anaphase onset such as Mad2 and BubR1 are also removed from kinetochores, the transport contributes to inactivation of the checkpoint (Howell et al., 2001; Cleveland et al., 2003). In addition, dynein also mediates transport of NuMA to spindle poles for proper spindle organization (Merdes et al., 2000).
Mammalian NudE/NudE-like (Nudel) and Lis1 are originally proposed to regulate dynein in neuronal migration (Wynshaw-Boris and Gambello, 2001) but seem widely involved in dynein functions. Nudel is essential for dynein-mediated transport of both proteins and membrane cargos. The interactions of Nudel with both Lis1 and dynein appear critical for dynein activity (Yan et al., 2003; Liang et al., 2004; Guo et al., 2006). Lis1 is kinetochore-associated in M phase. Interrupting Lis1 or NudE function causes spindle and mitotic defects presumably by interfering with dynein (Faulkner et al., 2000; Feng and Walsh, 2004). Dynein, Lis1, and Nudel are essential for cell viability (Wynshaw-Boris and Gambello, 2001; Sasaki et al., 2005), whereas NudE knockout mice are viable (Feng and Walsh, 2004).
Molecular connections between dynein and other kinetochore proteins are intricate. The Rod/ZW10/Zwilch complex anchors dynactin to the kinetochore through ZW10-p50 interaction (Karess, 2005). Dynactin in turn recruits dynein through the interaction between its p150glued subunit and DIC (Hirokawa, 1998). Lis1 interacts with p50 as well as several dynein subunits. It is targeted to the kinetochore in a dynein/dynactin-dependent manner and contributes to kinetochore localization of CLIP-170, an MT plus end–binding protein (Coquelle et al., 2002; Tai et al., 2002). Lis1 competes with p150glued for binding CLIP-170 (Lansbergen et al., 2004). In the absence of kinetochore-bound Lis1, localization of CLIP-170 is probably mediated through p150glued (Tai et al., 2002). We have previously shown that overexpression of a Lis1-binding–defective Nudel impairs dynein-mediated kinetochore protein transport (Yan et al., 2003). Nevertheless, whether Nudel affects dynein function at the kinetochore and what is its connection with aforementioned proteins there remain unclear.
Kinetochore dynein/dynactin is expected to undergo complicated regulations. To transport proteins away, the motor must dissociate from the kinetochore upon MT attachment. Protein cargos to be transported must bind dynein/dynactin and dissociate from kinetochores as well. In fact, many outer kinetochore proteins, including dynein/dynactin, are subjected to MT-dependent stripping (King et al., 2000; Hoffman et al., 2001), a poorly investigated phenomenon probably reflecting collective effects of protein deprivation by both the poleward transport and occupation of binding sites by MTs. On the other hand, to drive poleward chromosome movement and contribute to tension generation (Cleveland et al., 2003), it must exhibit sufficient affinity to the kinetochore. Nevertheless, little is known about mechanisms balancing both types of functions.
Our previous results suggest that mitosin can prevent dynein/dynactin from premature MT-dependent stripping (Yang et al., 2005). In mitosin-depleted cells, kinetochore localization of dynein/dynactin is markedly reduced only in the presence of MTs (Yang et al., 2005). Consistently, mitosin-depleted kinetochores exhibit reduced tension (Bomont et al., 2005; Holt et al., 2005; Yang et al., 2005). Nudel in our hands was isolated as a mitosin-associated protein in yeast two-hybrid screen (Ma et al., 2006). In this report, we show that Nudel is a kinetochore protein important for dynein function and localization at the site. Moreover, it is recruited to kinetochores by mitosin and thus links mitosin to dynein motor. Our results provide initial insights into regulation of dynein dynamics at the kinetochore.
MATERIALS AND METHODS
Plasmid Constructs
Plasmids for GFP or FLAG-tagged Nudel, its mutants, NudE, ZW10, and mitosin were described previously (Yan et al., 2003; Liang et al., 2004; Wang et al., 2004; Zhou et al., 2005). To express RFP-tagged Nudel or mutant, the GFP coding region was replaced with mRFP cDNA provided by Dr. E. Fuchs (Rockefeller University). To express H2B-GFP, the full-length histone 2B1 cDNA (a gift from Dr. W.-M. Yang, National Chung Hsing University, Taiwan, China) was cloned into pEGFP-N1 (Clontech, Palo Alto, CA). pEGFP-p50 was made from pUHD30F-p50 (Liang et al., 2004).
To construct plasmids for RNA interference (RNAi), oligos containing complementary hairpin sequences were synthesized and cloned into pTER vector as suggested (van de Wetering et al., 2003). One resultant plasmid, pTER-Nudi, contained the sequence 5¢-GGATGAAGCAAGAGATTTA-3¢ from human Nudel cDNA (Guo et al., 2006). The second one, pTER-ZWi, contained a sequence that has been successfully used for knocking down ZW10 (Kops et al., 2005). The third one, pTER-Luci, contained a sequence from firefly luciferase cDNA (Elbashir et al., 2001) and served as a control. pBS/U6/Mi-1, an RNAi construct for mitosin, was described previously (Yang et al., 2005). Another construct, psiRNA-CENP-F, was kindly provided by Dr. D. W. Cleveland (University of California at San Diego; Bomont et al., 2005).
Cell Culture and Transfection
HEK293T cells were cultured as described (Liang et al., 2004). For protein overexpression, cells were transfected for 48 h using the calcium phosphate method. Transfection for RNAi was carried out in 35-mm dishes using Lipofectamine 2000 (Invitrogen, Carlsbad, CA). Depletion of mitosin by RNAi was as described (Yang et al., 2005). To test specificity of RNAi constructs, 4 μg of pTER-derived plasmid was cotransfected with 0.4 μg of pEGFP-Nudel or pEGFP-NudE per dish for 48 h. To repress endogenous proteins, cells were transfected with 4 μg of an RNAi construct for 72 h. For the second round of transfection in the case of Nudel RNAi, cells transfected for 72 h were transferred into 60-mm dishes for 24 h and transfected again with 8 μg plasmid for an additional 72 h. For live cell microscopy, 0.04 μg of pEGFP-H2B were cotransfected at the second round.
Antibodies and Immunoblotting
Antibodies to DIC and α-tubulin were purchased from Sigma-Aldrich (St. Louis, MO). Anti-p150glued, mitosin, and BubR1 mAbs were from BD Transduction Laboratories (San Diego, CA). Rabbit anti-Nudel and Lis1 antibodies were kindly provided by Dr. L.-H. Tsai (Harvard Medical School). Rabbit anti-NuMA antibody was a gift from Dr. D. A. Compton (Dartmouth Medical School). Chicken anti-Nudel IgY and anti-NudE IgY were prepared by using bacterially expressed Nudel and NudE as antigens. Polyclonal antibodies to CENP-E, CLIP-170, and the CREST antigen were gifts from Drs. T. Yen (Fox Chase Cancer Center), N. Galjart (Erasmus University, The Netherlands), and K. H. Choo (Royal Children's Hospital, Australia), respectively. Antibodies against human ZW10 and Rod were provided by Dr. G. Chan (University of Alberta, Canada). All the antibodies were verified by immunoblotting before use (data not shown). Secondary antibodies labeled with Alexa 488, 546, and 647 were purchased from Invitrogen (Carlsbad, CA).
Coimmunoprecipitation was performed as described (Liang et al., 2004). Immunoblots were visualized with Western Lightning Chemiluminescence Reagent Plus (PerkinElmer Life and Analytical Sciences, Boston, MA) and exposed to x-ray films (Eastman Kodak, Rochester, NY).
Fluorescence Staining and Microscopy
Kinetochore staining was performed as described (Howell et al., 2001) with minor modifications. Briefly, HEK293T cells grown on polylysine-coated coverslips were extracted with 0.5% Triton X-100 for 40 s and immersed in 3.7% fresh formaldehyde in PHEM buffer for 15 min at room temperature. To disassemble MTs, cells were treated with nocodazole (10 μg/ml) for 1 h before fixation. Proper antibody combinations were used for multicolor staining. All primary and secondary antibody incubations were performed at 37°C for 30 min. Green fluorescent protein (GFP) or red fluorescent protein (RFP) fusion proteins were visualized directly through the autofluorescence. Nuclear DNA was stained with 4,6-diamidino-2-phenylindole (DAPI). For chromosome spread preparation, cells were treated with nocodazole (5 μg/ml) for 3 h and then incubated in 75 mM KCl for 15 min, followed by centrifugation (Zhu, 1999) and fixation.
Confocal microscopy was performed with the Leica SP2 system using a Leica HCX PL APO 63×/1.4 objective (Deerfield, IL). Images were acquired using four-line mean averaging protocol. Each Z-series typically contained 10–16 slices of ∼0.3 μm thick for a total stack depth of ∼5 μm. Z-stack images were formed by maximum intensity projections. Other images were captured using a cooled CCD camera (SPOT II, Diagnostic Instruments, Sterling Heights, MI) on Olympus BX51 microscope (Melville, NY). Grayscale images were converted to color ones using Confocal Assistant (Bio-Rad, CA) or Adobe Photoshop (San Jose, CA). Illustrations were organized using Adobe Photoshop.
Quantitation for kinetochore fluorescence intensities was done as described (Hoffman et al., 2001). To reduce influences of quenching and different spatial distribution, only kinetochores showing brighter staining than most of the remaining ones were quantitated in each cell. The average intensity from control cells was set at 10, whereas that of experimental cells was made proportional to this value to obtain the relative intensity. Statistic data were obtained in a blind manner whenever possible and presented as mean ± SEM.
Live Cell Microscopy
HEK293T cells were grown on glass coverslips and cultured in Leibovitz's (L-15)-based medium supplemented with 10% fetal bovine serum (Invitrogen) and 7 mM HEPES (pH 7.2). Time-lapse microscopy was performed at 37°C using a Leica system with HCX PL APO 63×/1.30 GLYC CORR 37°C objective (Yang et al., 2005). Images were recorded using a CCD camera (CoolSNAP HQ, Roper Scientific, Tucson, AZ) at 1.5-min intervals for up to 3 h.
Flow Cytometry
HEK293T cells overexpressing GFP-tagged NudelN20/C36 or NudelC36 were fixed with 1% formaldehyde in PBS for 15 min, resuspended in ice-cold 70% ethanol for 30 min, and then stained with 50 μg/ml propidium iodide in the presence of RNase A (200 μg/ml). Samples were assayed with a fluorescence-activated cell sorter (BD Biosciences, San Jose, CA). Cell cycle profiles of GFP-positive cells were presented.
RESULTS
Nudel Associates with Kinetochores after Nuclear Envelope Breakdown
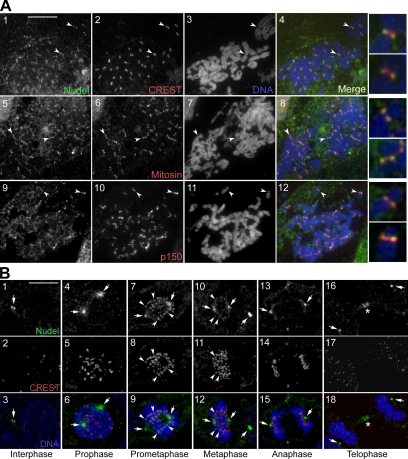
We have previously shown that human Nudel is important for dynein-mediated poleward transport of outer kinetochore proteins and thus speculated its kinetochore localization (Yan et al., 2003). Although cells fixed with methanol failed to show detectable kinetochore staining of Nudel (Yan et al., 2003), formaldehyde fixation, which has been successfully used for detection of many kinetochore proteins (Earnshaw and Rattner, 1991; Chan et al., 2000; Howell et al., 2001), enabled the detection of centromeric staining with a rabbit anti-Nudel antibody (Niethammer et al., 2000) in chromosome spreads of HEK293T and other primate cells (Figure 1A; data not shown). The staining was distal to the CREST antigen but colocalized well with p150glued and mitosin (Figure 1A; Zhu et al., 1995; Echeverri et al., 1996), suggesting a localization at the outer kinetochore domain (Cleveland et al., 2003).
Figure 1.
Subcellular distributions of Nudel in HEK293T cells. (A) Localization of Nudel at the outer kinetochore. Mitotic chromosome spreads were prepared and immunostained to visualize the indicated proteins. Chromosome DNA was stained with DAPI. Arrowheads indicate representative kinetochores that are shown in the 4′ enlargements. (B) Distributions of Nudel in the cell cycle. Typical cells in the indicated phases are shown. Arrowheads point to typical kinetochore staining. Arrows indicate centrosomes or spindle poles. Asterisks mark the midbody. Scale bar, 10 μm.
We then examined patterns of kinetochore-bound Nudel after M phase progression using a chicken anti-Nudel IgY to full-length human Nudel. This antibody is specific for Nudel and hardly cross-reacts with NudE (Guo et al., 2006; also see Supplementary Figure S3A). In intact mitotic cells, kinetochore Nudel staining was mainly observed in prometaphase (Figure 1B, panels 7–9), barely visible in metaphase (panels 10–12) and not seen at other stages (panels 4–6 and 13–18). In contrast, the centrosome/spindle pole localization of Nudel was seen throughout the cell cycle (Figure 1B; Sasaki et al., 2000; Yan et al., 2003). In telophase, a portion of Nudel was located at the midbody (Figure 1B, panels 16–18). Thus, kinetochore Nudel emerges soon after nuclear envelope breakdown (NEBD) and seems sensitive to MT attachment (Figure 1B). The spatiotemporal dynamics of Nudel resembles those of Lis1, dynein, dynactin, and CLIP-170 (Echeverri et al., 1996; Faulkner et al., 2000; King et al., 2000; Coquelle et al., 2002; Tai et al., 2002), suggesting a functional connection among these proteins.
Kinetochore Localization of Nudel Does Not Strongly Depend on Dynein/Dynactin
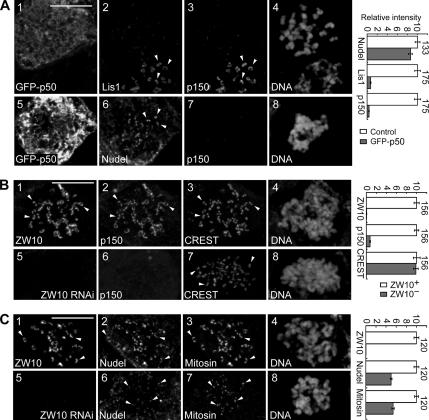
Because kinetochore localizations of dynein, Lis1, and CLIP-170 depend on dynactin (Echeverri et al., 1996; Coquelle et al., 2002; Tai et al., 2002), we examined whether this was also true for Nudel. To eliminate influence of MT-mediated shedding of kinetochore proteins (Hoffman et al., 2001), cells were treated with nocodazole to disassemble MTs so that protein localizations at “naked” kinetochores were compared. Consistent with previous reports (Echeverri et al., 1996; Coquelle et al., 2002; Tai et al., 2002), both p150glued and Lis1 were dislocated from kinetochores upon GFP-p50 overexpression (Figure 2A). Nudel, however, still exhibited strong kinetochore localization (Figure 2A, panels 5–8). The average intensity was only reduced by 14.1 ± 3.9% after quantitative analysis (Figure 2A), suggesting that Nudel is targeted to the kinetochore mainly in a dynactin/dynein-independent manner. To confirm that Lis1 was also dispensable, a Lis1-binding–defective mutant, NudelN20 (Yan et al., 2003), was overexpressed and found to exhibit kinetochore localization (Supplementary Figure S1A). Furthermore, overexpression of GFP-Lis1N, which dislocates endogenous Lis1 from the kinetochore (Tai et al., 2002), had little effect on Nudel (Supplementary Figure S1B).
Figure 2.
Nudel still binds kinetochores after p50 overexpression or ZW10 depletion. HEK293T cells were transfected with pEGFP-p50 (A), pTER-Luci (B and C, panels 1–4), or pTER-ZWi (B and C, panels 5–8) and treated with nocodazole before fixation. Arrowheads indicate representative kinetochores. Quantitation results are shown on the right. Total n is listed beside each pair of histograms. Scale bar, 10 μm. (A) Typical mitotic cells overexpressing GFP-p50. Panels 1–4 also contain an untransfected cell as a control. (B and C) Typical control (panels 1–4) or ZW10-depleted (panels 5–8) mitotic cells.
Because dynactin is recruited to the kinetochore by ZW10 (Starr et al., 1998; Karess, 2005), we further explored whether silencing ZW10 expression could affect Nudel. The RNAi construct pTER-ZWi repressed exogenous ZW10 by 98.2 ± 0.4% on immunoblot after cotransfection with a GFP-ZW10–expressing plasmid (Wang et al., 2004) for 48 h (Supplementary Figure S2). After its transfection for 72 h, kinetochore-associated ZW10 was reduced by more than 99.5% on average, compared with control cells (Figure 2, B and C). In these ZW10-depleted cells, p150glued was indeed largely diminished (by 92.5%) from the kinetochore (Figure 2B; Starr et al., 1998). Interestingly, kinetochore-associated Nudel was reduced by ∼50% on average (Figure 2C). Mitosin also showed a similar reduction (by 46.3%; Figure 2C). Therefore, ZW10 is important for kinetochore localization of both Nudel and mitosin.
Nudel Is Targeted to Kinetochores Mostly by Mitosin
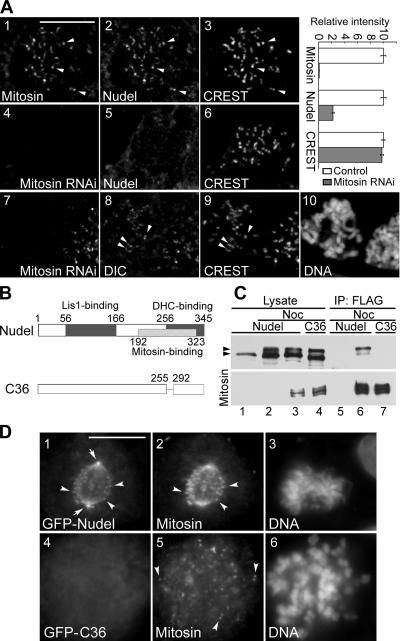
We have previously identified Nudel as a novel mitosin-associated protein in yeast two-hybrid screen (Yan et al., 2003; Ma et al., 2006). Because mitosin (also named CENP-F) is seen at the kinetochore in prophase (Liao et al., 1995; Zhu et al., 1995) when Nudel was still outside the nucleus (Figure 1B; Yan et al., 2003), we reasoned that Nudel might be recruited to kinetochores by mitosin after NEBD at prometaphase. Consistent with this idea, depleting mitosin by RNAi severely inhibited Nudel localization at the kinetochore in nocodazole-treated cells (Figure 3A, panels 4–6). Quantitative analyses indicated that, when kinetochore mitosin was reduced by 99.0 ± 0.3% on average (n = 157) compared with control cells, kinetochore Nudel was reduced by 77.6 ± 1.8%. In contrast, CREST staining was virtually not affected (Figure 3A). We have previously shown that kinetochore localization of dynactin is not affected by mitosin depletion in the presence of nocodazole (Yang et al., 2005). As indicated by DIC staining (Figure 3A, panels 7–10), dynein was not affected, either.
Figure 3.
Mitosin recruits Nudel to the kinetochore. (A) Kinetochore staining of typical cells transfected with pBS/U6 (panels 1–3) or pBS/U6/Mi-1 (panels 4–10). Cells were treated with nocodazole before fixation. Quantitation results for kinetochore intensity are shown on the right. n = 157. (B) Diagrams of Nudel and NudelC36. (C) Coimmunoprecipitation of GFP-tagged Nudel, but not NudelC36, with FLAG-mitosin. Cells overexpressing the indicated proteins (lanes 2–4) were treated with nocodazole (Noc, 0.4 μg/ml) for 16 h to enrich M phase populations and subjected to immunoprecipitation (IP) with anti-FLAG antibody-conjugated resin (lanes 5–7). Proteins were separated with 3–12% gradient SDS-PAGE and immunoblotted with anti-GFP (top panel) or anti-FLAG (bottom panel) antibodies. The top band of GFP-tagged Nudel or NudelC36 seen in nocodazole-treated cells (arrowhead) was a phosphorylated form. (D) Kinetochore localization of GFP-tagged Nudel but not NudelC36. Arrows, spindle poles; arrowheads, typical kinetochores. Scale bar, 10 μm.
To further assess whether kinetochore assembly of Nudel depended on its association with mitosin, we examined localization of Nudel mutant incapable of binding mitosin. We have previously narrowed down the mitosin-binding region of Nudel to residues 192–323 using yeast two-hybrid assays (Ma et al., 2006). We thus reasoned that NudelC36, a mutant lacking residues 256–291 (Liang et al., 2004), might not bind mitosin (Figure 3B). To test this, coimmunoprecipitation was performed. Because both proteins might preferably interact in mitosis, HEK293T cells overexpressing Nudel and mitosin were treated with nocodazole overnight to enrich mitotic populations, marked by the appearance of phosphorylated form (top band) of Nudel or NudelC36 (Figure 3C, lanes 2–4) in lysate (Yan et al., 2003). Coimmunoprecipitation results showed that full-length FLAG-mitosin precipitated GFP-tagged Nudel but not NudelC36 (Figure 3C). Moreover, reproducible enrichment of the phosphorylated Nudel in the immunoprecipitates (Figure 3C, lane 6) suggested a preferential association of such a form with mitosin.
We then examined localization of GFP-NudelC36 in M phase. GFP-Nudel exhibited kinetochore localization in mitotic cells, whereas GFP-NudelC36 did not (Figure 3D). Although NudelC36 fails to bind both mitosin (Figure 3C) and dynein (Liang et al., 2004), dynein is not important for kinetochore localization of Nudel (Figure 2). We thus conclude that Nudel binds kinetochores through mitosin. We also noticed that C36-positive cells were frequently seen in a prometaphase-like stage with rather dispersed chromosomes (Figure 3D, panels 4–6), suggesting defects in chromosome congression.
Nudel Stabilizes Kinetochore Association of Dynactin
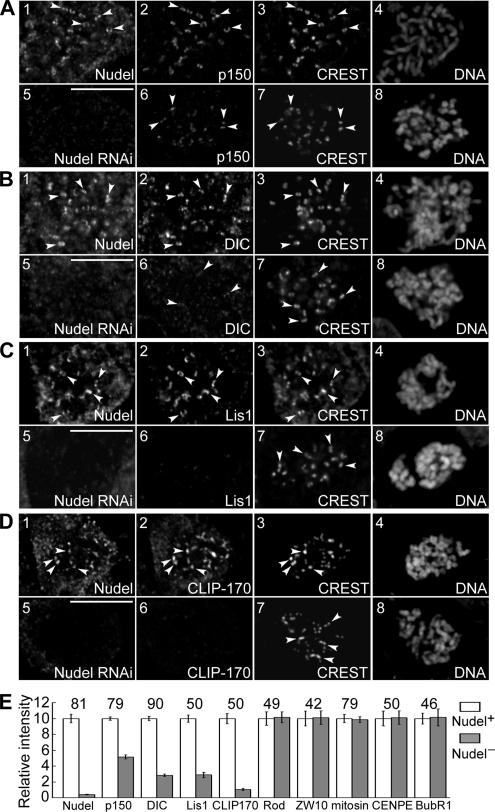
We have previously shown that mitosin stabilizes kinetochore dynein/dynactin against MT-dependent stripping and speculated that Nudel may link mitosin to dynein (Yang et al., 2005). If so, depletion of Nudel would increase the stripping of dynein/dynactin. To test this, vector-based RNAi was used to knock down Nudel expression. The RNAi construct, pTER-Nudi, is highly specific for Nudel upon transfection, with little influence on NudE (Supplementary Figure S3B; Guo et al., 2006). Immunoblotting showed that a single transfection with pTER-Nudi typically repressed endogenous Nudel in HEK293T cells by 72% at ∼72 h, whereas a second round of transfection (see Materials and Methods) reduced Nudel by 96% without influencing NudE, DIC, p150glued, p50dynamitin, Lis1, or CLIP-170 (Supplementary Figure S3B).
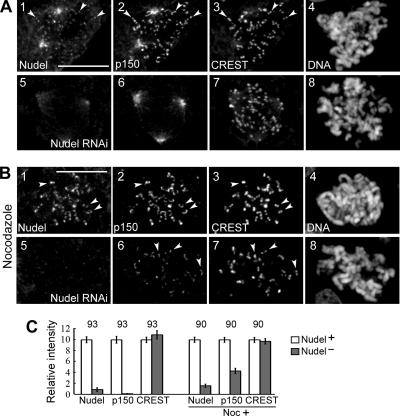
We then compared kinetochore localization of dynactin in HEK293T cells transfected with either pTER-Nudi or pTER for 72 h. We found that, compared with control cells, kinetochore p150glued was almost undetectable in early prometaphase upon Nudel depletion (Figure 4, A and C). Nevertheless, it became obvious after nocodazole treatment for 1 h, despite a reduction by 57% on average compared with control cells (Figure 4, B and C). Therefore, although Nudel RNAi reduces kinetochore dynactin in the absence of MTs, the presence of MTs indeed induces a further reduction.
Figure 4.
Effects of Nudel depletion on kinetochore dynactin. HEK293T cells were transfected with either pTER (panels 1–4) or pTER-Nudi (panels 5–8) for 72 h. Typical cells either in early prometaphase (A) or after nocodazole treatment (B) are shown. Arrowheads indicate representative kinetochores. Scale bar, 10 μm. (C) Relative kinetochore intensities of the indicated proteins. Total n is shown over each pair of histograms.
Kinetochore Localizations of Dynein, Lis1, and CLIP-170 Are Diminished upon Nudel RNAi
The reduced localization of dynactin in nocodazole-treated cells lacking Nudel (Figure 4, B and C) suggests involvement of Nudel in kinetochore assembly of dynein/dynactin. To test whether the partial reduction was due to incomplete Nudel depletion, we further examined cells subjected to two rounds of pTER-Nudi transfection for better knockdown of Nudel (Supplementary Figure S3B). Kinetochore p150glued in such cells was reduced by 49% on average (Figure 5, A and E), indicating that Nudel is important but not essential for the localization of dynactin. Dynein, Lis1, and CLIP-170 were also progressively reduced at the kinetochore (Figure 5, B–E). ZW10, Rod, CENP-E, BubR1, and mitosin, however, remained unchanged (Figure 5E). Similar results were obtained upon GFP-NudelC36 overexpression (Supplementary Figure S4), possibly due to its dominant-negative effect on endogenous Nudel.
Figure 5.
Nudel depletion reduces kinetochore-bound dynein, dynactin, Lis1, and CLIP-170. (A–D) Immunostaining of typical nocodazole-treated cells transfected twice with pTER (panels 1–4) or pTER-Nudi (panels 5–8). Arrowheads, representative kinetochores. Scale bar, 10 μm. (E) Relative kinetochore intensities of the indicated proteins. Total n is shown over each pair of histograms.
Nudel Is Required for Dynein-mediated Kinetochore Protein Transport
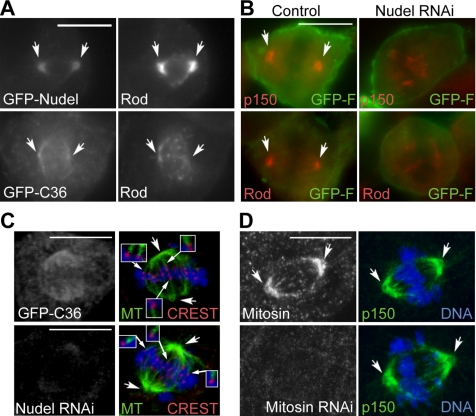
We have previously shown that overexpression of NudelN20, a Lis1-binding–defective mutant, suppresses dynein-dependent transport of Bub1 and mitosin from kinetochores to spindle poles (Yan et al., 2003; Yang et al., 2003). Importance of the Nudel-DHC interaction, however, was not assessed. Whether the suppression of transport was due to a collective effect on both endogenous Nudel and NudE was not clarified. We thus examined the influence of GFP-NudelC36 overexpression or Nudel depletion on kinetochore protein transport using ATP inhibitor assay, in which dissociations of dynein and its cargos from the spindle poles are blocked upon azide treatment so that proteins transported to poles are accumulated there over time (Howell et al., 2001). According to studies in Drosophila, poleward transport of the Rod/ZW10 complex is mediated by dynein as well (Williams et al., 1996; Wojcik et al., 2001; Karess, 2005). Consistently, both GFP-Nudel and Rod became accumulated at poles (Figure 6A) after azide treatment. In C36-positive cells, however, the polar accumulation was not obvious (Figure 6A). ZW10, Mad2, and CENP-E also failed to accumulate at poles (data not shown). Similarly, poleward transport of p150glued and Rod was repressed in Nudel-depleted cells (Figure 6B). For convenience, a membrane-localized GFP, GFP-F (Jiang and Hunter, 1998), was used as a transfection marker in the RNAi experiments (Figure 6B).
Figure 6.
Nudel is crucial for poleward transport of kinetochore proteins. (A and B) NudelC36 overexpression or Nudel depletion represses poleward kinetochore protein transport. Mitotic HEK293T cells were subjected to ATP inhibitor assays (Howell et al., 2001) and immunostained for Rod or p150glued. For convenience, GFP-F was used as a transfection marker for mock-depleted (control) or Nudel-depleted (Nudel RNAi) cells (Liang et al., 2004). A typical late prometaphase cell is shown. (C) Existence of MT-kinetochore attachment in late prometaphase cells overexpressing NudelC36 or lacking Nudel. A representative optical section is shown. Insets are 2′ enlargements in which MT staining is enhanced digitally for better views of kinetochore fibers. (D) Mitosin-depletion does not inhibit poleward kinetochore protein transport. Cells were immunostained for mitosin and p150glued after ATP inhibitor assays. Concaved arrows, spindle poles. Scale bar, 10 μm.
To clarify whether the defect in poleward transport of kinetochore proteins was due to disruption of the MT-kinetochore interaction or the spindle organization, cells were labeled for MTs and the CREST antigen. When cells in late prometaphase containing bipolar spindles were examined, MT-kinetochore associations were seen regardless of NudelC36 overexpression or Nudel depletion (Figure 6C). Therefore, Nudel is indeed essential for dynein-mediated transport of proteins from kinetochores to spindle poles.
Because mitosin depletion markedly reduced kinetochore-associated Nudel (Figure 3A), we also examined whether this led to inactivation of dynein. As shown in Figure 6D, in mitosin-depleted cells, p150glued still accumulated to poles in a way indistinguishable from control cells. Similar results were seen for BubR1 and CENP-E (data not shown). Therefore, unlike Nudel depletion, mitosin depletion does not abolish dynein-mediated kinetochore protein transport.
Nudel Is Critical for M Phase Progression
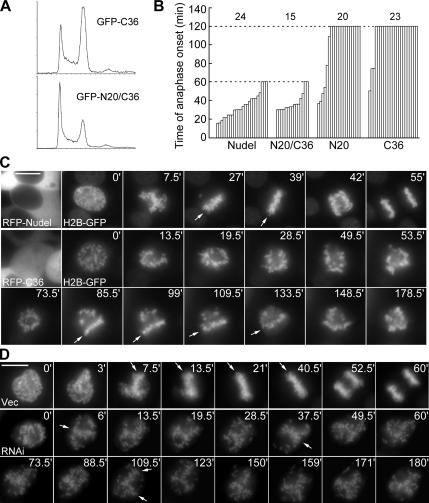
We finally investigated whether interruption of Nudel function affected M phase progression. Flow cytometry indicated that, compared with GFP-NudelN20/C36, a double mutant with little effect on dynein in membrane trafficking (Liang et al., 2004), overexpression of GFP-NudelC36 induced a marked G2/M accumulation (Figure 7A). Time-lapse microscopy further revealed a mitotic block before metaphase upon overexpression of either GFP-tagged NudelN20 or NudelC36 (Figure 7B). When randomly picked and imaged, most mitotic cells overexpressing Nudel (21/24) or NudelN20/C36 (13/15) initiated anaphase in 60 min (Figure 7B). In contrast, anaphase onset was not seen in most cells overexpressing NudelN20 (14/20) or NudelC36 (20/23) when recording was terminated at 120 min (Figure 7B).
Figure 7.
Effects of Nudel on M phase progression. (A) Representative cell cycle profiles. HEK293T cells expressing GFP-NudelC36 or GFP-NudelN20/C36 were analyzed by flow cytometry. (B) Summary of time-lapse studies on M phase progression. Mitotic cells were randomly picked and imaged till anaphase onset or for 60 min (Nudel or N20/C36-overexpressing cells)/120 min (N20- or C36-overexpressing cells). Total n is shown over each group. (C) Representative time-lapse images. Early mitotic cells coexpressing H2B-GFP and RFP-Nudel or NudelC36 were recorded at 1.5-min intervals for up to 3 h. Arrows indicate metaphase plate. Scale bar, 10 μm. Also see Supplementary Videos 1 and 2. (D) Effect of Nudel depletion on mitosis. Cells were transfected twice with either vector (Vec) or pTER-Nudi (RNAi) as described in Materials and Methods. H2B-GFP was transiently expressed as both transfection and chromosome markers. Cells in early M phase were recorded at 1.5-min intervals for up to 3 h. Also see Supplementary Videos 3 and 4.
For a clear view of chromosome behavior in live cells, H2B-GFP was used as a chromosome marker (Kanda et al., 1998). Similar to the results in Figure 7B, early mitotic cells overexpressing RFP-Nudel showed normal congression and started anaphase within 60 min (n = 12; Figure 7C and Supplementary Video 1). In contrast, RFP-NudelC36–positive cells (10/11) usually failed to progress from prometaphase to metaphase in 3 h (Figure 7C and Supplementary Video 2), though transient, partial congression was observed (Figure 7C, arrows). Therefore, the mitotic block was attributed to defects that hindered stable chromosome alignment.
Similar mitotic defect was also observed in Nudel-depleted cells cotransfected to express H2B-GFP. Although most control cells in early M phase (24/27) initiated normal anaphase within 1 h (Figure 7D and Supplementary Video 3), most mitotic cells transfected with pTER-Nudi (21/23) failed to progress into metaphase in 3 h (Figure 7D and Supplementary Video 4). Transient, partial congressions were also seen (Figure 7D, arrows). Moreover, 52.2% of cells (12/23) showed chromosome deformation typical of cell death during recording (data not shown). Flow cytometry also showed G2/M accumulation and cell death (data not shown). Cell death is not unexpected because Nudel is essential for cell viability (Sasaki et al., 2005).
Although we mainly focused on the kinetochore, inactivation of dynein has been shown to cause aberrant spindle organization as well (Echeverri et al., 1996). We then tested whether spindle organization was affected by GFP-NudelC36; 38.6 ± 1.9% of C36-positive cells (n = 204) exhibited multipolar spindles and 17.7% of them showed monopolar or asymmetrical bipolar spindles (Supplementary Figure S5A), compared with 8.5 ± 1.5 and 6.6%, respectively, for GFP-Nudel–positive ones (n = 123). The phenotypes resemble those of p50 overexpression (Echeverri et al., 1996). Moreover, NuMA was no longer solely restricted to poles in most C36-positive cells: bright speckles were seen, mostly along the spindle (Supplementary Figure S5A), further indicating inactivation of dynein (Merdes et al., 2000). In addition, 67.7 ± 3.7% of Nudel-depleted mitotic cells (n = 300) exhibited multipolar spindles (Supplementary Figure S5B). Therefore, Nudel affects multiple aspects of the mitotic apparatus. The mitotic delay (Figure 7) is thus a collective result of multiple abnormalities.
DISCUSSION
Nudel Is Mainly Recruited to the Kinetochore by Mitosin after NEBD
Our results indicate that Nudel is a transient kinetochore protein in M phase. Its kinetochore localization was readily observed with a rabbit antibody (Niethammer et al., 2000; Figure 1A) and a chicken antibody (Figure 1B, 5), and was further proved through the autofluorescence of GFP-Nudel (Figure 3D and Supplementary Figure S4A). Kinetochore Nudel emerged after NEBD and declined from metaphase (Figure 1B). Nudel colocalized better with dynactin and mitosin than with the CREST antigen (Figure 1A), suggesting its localization at the outer kinetochore region (Maiato et al., 2004).
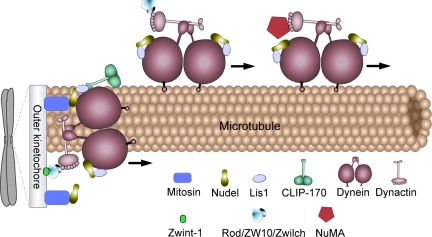
We identified mitosin as the major kinetochore protein that recruits Nudel (Figure 8). The Nudel-mitosin connection was initially found in a yeast two-hybrid screen (Ma et al., 2006). Coimmunoprecipitation further suggests an interaction in vivo (Figure 3C). Depletion of mitosin indeed dramatically diminished kinetochore localization of Nudel, but not vice versa (Figures 3A and 5E). Knocking down mitosin with another RNAi construct (Kops et al., 2005) had similar results (data not shown), thus excluding possible off-target effect. These observations are further supported by lack of GFP-NudelC36 at the kinetochore (Figure 3D). Mitosin is seen at kinetochores in prophase (Liao et al., 1995; Zhu et al., 1995), thus able to recruit Nudel after NEBD. The Rod/ZW10/Zwilch complex also recruit dynein/dynactin to kinetochores at the same stage (Karess, 2005).
Figure 8.
A model for Nudel functions in M phase. Dynein/dynactin binds the kinetochore through the Rod/ZW10/Zwilch complex. Nudel is mainly recruited by mitosin (CENP-F), whereas a portion of it also binds dynein and Lis1 independent of mitosin. Nudel associated with both dynein, and Lis1 activates dynein/dynactin-mediated poleward transport of outer kinetochore proteins, such as ZW10, Rod, and Mad2 (not shown), to facilitate inactivation of the spindle checkpoint. It also activates NuMA transport for spindle assembly. On the other hand, when interacting with both mitosin and dynein, Nudel stabilizes dynein/dynactin against MT-dependent stripping to facilitate the motor's force generation function for chromosome movement and tension. See text for detailed discussion.
How mitosin is tethered to the kinetochore is unclear. Mitosin requires Bub1, Sgt1, CENP-I, RanBP2, and Zwint-1 for its kinetochore localization (Ma et al., 2006; Varis et al., 2006). Interestingly, Zwint-1 is documented as the kinetochore anchor for the Rod/ZW10/Zwilch complex (Figure 8; Wang et al., 2004; Kops et al., 2005). The mitosin/Nudel linkage and the Rod/ZW10/Zwilch linkage are therefore both downstream of Zwint-1. Nevertheless, mitosin may not directly bind Zwint-1 because they are not copurified (Kops et al., 2005). Rather, its partial dependence on ZW10 (Figure 2C) implies that mitosin may be anchored by two or more proteins. This is supported by the finding that kinetochore intensity of mitosin is affected by multiple sequence regions (Zhu, 1999).
Nudel Also Binds Kinetochore Dynein/Dynactin
Mitosin is not the only protein responsible for kinetochore localization of Nudel because some Nudel (∼22% of control cells) was still present at kinetochores in mitotic cells almost completely (99%) lacking mitosin (Figure 3A). Moreover, depletion of mitosin did not disrupt movement of dynein/dynactin from kinetochores to spindle poles (Figure 6D; Howell et al., 2001). Because such motility requires Nudel (Figure 6; Yan et al., 2003), we conclude that the small amount of kinetochore Nudel in mitosin-depleted cells (Figure 3A) is associated with dynein/dynactin. Consistently, in mitotic cells overexpressing GFP-p50, kinetochore-bound Nudel was reduced by ∼14% (Figure 2A). We therefore speculate that, in intact cells, kinetochore Nudel may bind only DHC, only mitosin, or both (Figure 8).
Although Nudel also interacts with Lis1 (Sasaki et al., 2000), its kinetochore localization does not require Lis1. Overexpression of exogenous p50 or Lis1N significantly dislocated kinetochore Lis1 (Coquelle et al., 2002; Tai et al., 2002) without affecting Nudel (Figure 2A and Supplementary Figure S1). Moreover, the Lis1-binding–defective mutant NudelN20 (Yan et al., 2003) still exhibited kinetochore localization (Supplementary Figure S1).
Nudel Modulates Kinetochore Localization of Dynein/Dynactin
Nudel depletion significantly reduced kinetochore localization of dynein/dynactin (Figures 4 and 5). Consistent with their dependences on dynein/dynactin for kinetochore targeting (Coquelle et al., 2002; Tai et al., 2002), Lis1 and CLIP-170 were also affected (Figure 5). Excessive NudelC36 seemed to titrate endogenous Nudel through dimer formation (Sasaki et al., 2000; Liang et al., 2004), thus resulting in similar phenotypes as Nudel depletion (Supplementary Figure S4).
Our data suggest a dual effect of Nudel on kinetochore localization of dynactin/dynein. First, Nudel is important for the ability of dynein/dynactin to bind naked kinetochores (Figures 4 and 5). Interestingly, such a role relies more on the presence of Nudel in cells than at the kinetochore, because elimination of most kinetochore Nudel through depletion of mitosin failed to influence kinetochore dynein/dynactin in nocodazole-treated cells (Figure 3A; Yang et al., 2005). How cytoplasmic Nudel plays such a role, however, is not clear. Nudel has been reported to possess oligopeptidase activity (Hayashi et al., 2005). It might affect kinetochore targeting of dynein/dynactin by degrading or modifying certain protein(s) in the cytoplasm.
Second, Nudel helps to stabilize kinetochore dynein/dynactin against MT-dependent stripping. Such an effect requires kinetochore localization of Nudel as well as its interactions with both mitosin and dynein, because depletion of either Nudel or mitosin is sufficient to markedly decrease kinetochore-bound dynactin in the presence of MTs (Figure 4; Yang et al., 2005).
The Nudel-Mitosin Interaction May Switch Dynein Functions at the Kinetochore
The Nudel-mitosin interaction may provide a way to regulate the force generation and protein transport roles of kinetochore dynein (Figure 8). Our results imply that kinetochore dynein may bind 1) no Nudel, 2) Nudel free of mitosin, or 3) Nudel associated with mitosin (Figures 3–5 and 8). Dynein motor exhibits stronger resistance against MT-dependent stripping in the third situation and thus contributes to chromosome movement and tension generation (Figure 8). In the second situation dynein tends to be stripped and exhibits poleward movement as well as protein transport activity (Figures 6D and 8; Yang et al., 2005). Possibly mitosin that fails to bind dynein through Nudel is transported to poles with other kinetochore proteins in form of cargos (Howell et al., 2001; Yang et al., 2003). In the first situation, dynein is inactivated (Figure 6, A and B; Yan et al., 2003) but still prone to stripping (Figure 4), suggesting that it is stripped passively, probably as a result of increasing occupancy of MTs at the kinetochore. In addition, each dynein contains two DHC subunits (Hirokawa, 1998). If each DHC binds one Nudel molecule (Figure 8), six kinds of dynein may emerge from different combinations of the three situations, thus further expanding the complexity and versatility of the motor.
Nudel Regulates Dynein Activity and Mitotic Progression
Our previous and current data suggest that both the Nudel-Lis1 interaction and the Nudel-DHC interaction are crucial for dynein activity in poleward transport of kinetochore proteins and mitotic progression (Figures 6A and 7; Yan et al., 2003; Yang et al., 2003). The RNAi experiments (Figures 6B and 7) further strengthened importance of Nudel per se in these processes.
The mitotic block resulted from perturbation of Nudel functions (Figure 7) is likely a collective effect of multiple defects. In addition to kinetochore protein transport, Nudel is also involved in poleward transport of NuMA (Supplementary Figure S5 and Figure 8) by dynein (Merdes et al., 2000). Failure of such a transport in cells overexpressing NudelC36 is apparently correlated with aberrant spindle organization (Supplementary Figure S5; Merdes et al., 2000). Spindle defect (Supplementary Figure S5) in turn hinder chromosome congression (Figure 7). Nudel is not essential for MT-kinetochore attachment (Figure 6), though whether it affects efficiency of the attachment remains unclear. Further studies will thus be interesting to understand its detailed roles in M phase progression.
Supplementary Material
ACKNOWLEDGMENTS
The authors thank Lirong Liu and Wei Bian for technical assistance and Dr. Yixian Zheng (Carnegie Institution of Washington) for critical reading of the manuscript. We are grateful to Drs. G. Chan for kindly providing anti-ZW10 and Rod, K. H. Choo for anti-CREST antigen, N. Galjart for anti-CLIP-170, L.-H. Tsai for anti-Nudel and Lis1, T. Yen for anti-CENP-E, and D. A. Compton for anti-NuMA antibodies. We also thank Drs. E. Fuchs for mRFP construct, D. W. Cleveland for psiRNA-CENP-F, and W.-M. Yang for histone 2B1 cDNA. This work was supported by Grants 30330330, 30421005, and 30623003 from the National Science Foundation of China; 2005CB522703 (X.Z.) and 2002CB713700 (X.Y.) from Ministry of Science and Technology of China; S048014317, 06DZ22032, and 058014578 from the Shanghai Municipal Council for Science and Technology; and CXTD-S2005-3 from Chinese Academy of Sciences.
Abbreviations used:
- DHC
dynein heavy chain
- DIC
dynein intermediate chain
- GFP
green fluorescence protein
- MT
microtubule
- NEBD
nuclear envelope breakdown
- RFP
red fluorescence protein
- RNAi
RNA interference.
Footnotes
This article was published online ahead of print in MBC in Press (http://www.molbiolcell.org/cgi/doi/10.1091/mbc.E06-04-0345) on May 9, 2007.

 The online version of this article contains supplemental material at MBC Online (http://www.molbiolcell.org).
The online version of this article contains supplemental material at MBC Online (http://www.molbiolcell.org).
REFERENCES
- Bomont P., Maddox P., Shah J. V., Desai A. B., Cleveland D. W. Unstable microtubule capture at kinetochores depleted of the centromere-associated protein CENP-F. EMBO J. 2005;24:3927–3939. doi: 10.1038/sj.emboj.7600848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burkhardt J. K., Echeverri C. J., Nilsson T., Vallee R. B. Overexpression of the dynamitin (p50) subunit of the dynactin complex disrupts dynein-dependent maintenance of membrane organelle distribution. J. Cell Biol. 1997;139:469–484. doi: 10.1083/jcb.139.2.469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan G. K., Jablonski S. A., Starr D. A., Goldberg M. L., Yen T. J. Human Zw10 and ROD are mitotic checkpoint proteins that bind to kinetochores. Nat. Cell Biol. 2000;2:944–947. doi: 10.1038/35046598. [DOI] [PubMed] [Google Scholar]
- Cleveland D. W., Mao Y., Sullivan K. F. Centromeres and kinetochores: from epigenetics to mitotic checkpoint signaling. Cell. 2003;112:407–421. doi: 10.1016/s0092-8674(03)00115-6. [DOI] [PubMed] [Google Scholar]
- Coquelle F. M., et al. LIS1, CLIP-170's key to the dynein/dynactin pathway. Mol. Cell. Biol. 2002;22:3089–3102. doi: 10.1128/MCB.22.9.3089-3102.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dujardin D. L., Vallee R. B. Dynein at the cortex. Curr. Opin. Cell Biol. 2002;14:44–49. doi: 10.1016/s0955-0674(01)00292-7. [DOI] [PubMed] [Google Scholar]
- Earnshaw W. C., Rattner J. B. The use of autoantibodies in the study of nuclear and chromosomal organization. Methods Cell Biol. 1991;35:135–175. [PubMed] [Google Scholar]
- Echeverri C. J., Paschal B. M., Vaughan K. T., Vallee R. B. Molecular characterization of the 50-kD subunit of dynactin reveals function for the complex in chromosome alignment and spindle organization during mitosis. J. Cell Biol. 1996;132:617–633. doi: 10.1083/jcb.132.4.617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elbashir S. M., Harborth J., Lendeckel W., Yalcin A., Weber K., Tuschl T. Duplexes of 21-nucleotide RNAs mediate RNA interference in cultured mammalian cells. Nature. 2001;411:494–498. doi: 10.1038/35078107. [DOI] [PubMed] [Google Scholar]
- Faulkner N. E., Dujardin D. L., Tai C. Y., Vaughan K. T., O'Connell C. B., Wang Y., Vallee R. B. A role for the lissencephaly gene LIS1 in mitosis and cytoplasmic dynein function. Nat. Cell Biol. 2000;2:784–791. doi: 10.1038/35041020. [DOI] [PubMed] [Google Scholar]
- Feng Y., Walsh C. A. Mitotic spindle regulation by Nde1 controls cerebral cortical size. Neuron. 2004;44:279–293. doi: 10.1016/j.neuron.2004.09.023. [DOI] [PubMed] [Google Scholar]
- Guo J., Yang Z., Song W., Chen Q., Wang F., Zhang Q., Zhu X. Nudel contributes to microtubule anchoring at the mother centriole and is involved in both dynein-dependent and -independent centrosomal protein assembly. Mol. Biol. Cell. 2006;17:680–689. doi: 10.1091/mbc.E05-04-0360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi M. A., et al. Inhibition of NUDEL (nuclear distribution element-like)-oligopeptidase activity by disrupted-in-schizophrenia 1. Proc. Natl. Acad. Sci. USA. 2005;102:3828–3833. doi: 10.1073/pnas.0500330102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirokawa N. Kinesin and dynein superfamily proteins and the mechanism of organelle transport. Science. 1998;279:519–526. doi: 10.1126/science.279.5350.519. [DOI] [PubMed] [Google Scholar]
- Hoffman D. B., Pearson C. G., Yen T. J., Howell B. J., Salmon E. D. Microtubule-dependent changes in assembly of microtubule motor proteins and mitotic spindle checkpoint proteins at PtK1 kinetochores. Mol. Biol. Cell. 2001;12:1995–2009. doi: 10.1091/mbc.12.7.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holt S. V., Vergnolle M. A., Hussein D., Wozniak M. J., Allan V. J., Taylor S. S. Silencing Cenp-F weakens centromeric cohesion, prevents chromosome alignment and activates the spindle checkpoint. J. Cell Sci. 2005;118:4889–4900. doi: 10.1242/jcs.02614. [DOI] [PubMed] [Google Scholar]
- Howell B. J., McEwen B. F., Canman J. C., Hoffman D. B., Farrar E. M., Rieder C. L., Salmon E. D. Cytoplasmic dynein/dynactin drives kinetochore protein transport to the spindle poles and has a role in mitotic spindle checkpoint inactivation. J. Cell Biol. 2001;155:1159–1172. doi: 10.1083/jcb.200105093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang W., Hunter T. Analysis of cell-cycle profiles in transfected cells using a membrane-targeted GFP. Biotechniques. 1998;24:349–350. 352, 354. [PubMed] [Google Scholar]
- Kanda T., Sullivan K. F., Wahl G. M. Histone-GFP fusion protein enables sensitive analysis of chromosome dynamics in living mammalian cells. Curr. Biol. 1998;8:377–385. doi: 10.1016/s0960-9822(98)70156-3. [DOI] [PubMed] [Google Scholar]
- Karess R. Rod-Zw10-Zwilch: a key player in the spindle checkpoint. Trends Cell Biol. 2005;15:386–392. doi: 10.1016/j.tcb.2005.05.003. [DOI] [PubMed] [Google Scholar]
- King J. M., Hays T. S., Nicklas R. B. Dynein is a transient kinetochore component whose binding is regulated by microtubule attachment, not tension. J. Cell Biol. 2000;151:739–748. doi: 10.1083/jcb.151.4.739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King S. J., Schroer T. A. Dynactin increases the processivity of the cytoplasmic dynein motor. Nat. Cell Biol. 2000;2:20–24. doi: 10.1038/71338. [DOI] [PubMed] [Google Scholar]
- Kops G. J., Kim Y., Weaver B. A., Mao Y., McLeod I., Yates J. R., 3rd, Tagaya M., Cleveland D. W. ZW10 links mitotic checkpoint signaling to the structural kinetochore. J. Cell Biol. 2005;169:49–60. doi: 10.1083/jcb.200411118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lansbergen G., et al. Conformational changes in CLIP-170 regulate its binding to microtubules and dynactin localization. J. Cell Biol. 2004;166:1003–1014. doi: 10.1083/jcb.200402082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang Y., Yu W., Li Y., Yang Z., Yan X., Huang Q., Zhu X. Nudel functions in membrane traffic mainly through association with Lis1 and cytoplasmic dynein. J. Cell Biol. 2004;164:557–566. doi: 10.1083/jcb.200308058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao H., Winkfein R. J., Mack G., Rattner J. B., Yen T. J. CENP-F is a protein of the nuclear matrix that assembles onto kinetochores at late G2 and is rapidly degraded after mitosis. J. Cell Biol. 1995;130:507–518. doi: 10.1083/jcb.130.3.507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma L., Zhao X., Zhu X. Mitosin/CENP-F in mitosis, transcriptional control, and differentiation. J. Biomed. Sci. 2006;13:205–213. doi: 10.1007/s11373-005-9057-3. [DOI] [PubMed] [Google Scholar]
- Maiato H., DeLuca J., Salmon E. D., Earnshaw W. C. The dynamic kinetochore-microtubule interface. J. Cell Sci. 2004;117:5461–5477. doi: 10.1242/jcs.01536. [DOI] [PubMed] [Google Scholar]
- Merdes A., Heald R., Samejima K., Earnshaw W. C., Cleveland D. W. Formation of spindle poles by dynein/dynactin-dependent transport of NuMA. J. Cell Biol. 2000;149:851–862. doi: 10.1083/jcb.149.4.851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niethammer M., Smith D. S., Ayala R., Peng J., Ko J., Lee M. S., Morabito M., Tsai L. H. NUDEL is a novel Cdk5 substrate that associates with LIS1 and cytoplasmic dynein. Neuron. 2000;28:697–711. doi: 10.1016/s0896-6273(00)00147-1. [DOI] [PubMed] [Google Scholar]
- Sasaki S., et al. Complete loss of Ndel1 results in neuronal migration defects and early embryonic lethality. Mol. Cell. Biol. 2005;25:7812–7827. doi: 10.1128/MCB.25.17.7812-7827.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasaki S., Shionoya A., Ishida M., Gambello M. J., Yingling J., Wynshaw-Boris A., Hirotsune S. A LIS1/NUDEL/cytoplasmic dynein heavy chain complex in the developing and adult nervous system. Neuron. 2000;28:681–696. doi: 10.1016/s0896-6273(00)00146-x. [DOI] [PubMed] [Google Scholar]
- Starr D. A., Williams B. C., Hays T. S., Goldberg M. L. ZW10 helps recruit dynactin and dynein to the kinetochore. J. Cell Biol. 1998;142:763–774. doi: 10.1083/jcb.142.3.763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tai C. Y., Dujardin D. L., Faulkner N. E., Vallee R. B. Role of dynein, dynactin, and CLIP-170 interactions in LIS1 kinetochore function. J. Cell Biol. 2002;156:959–968. doi: 10.1083/jcb.200109046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vallee R. B., Williams J. C., Varma D., Barnhart L. E. Dynein: an ancient motor protein involved in multiple modes of transport. J. Neurobiol. 2004;58:189–200. doi: 10.1002/neu.10314. [DOI] [PubMed] [Google Scholar]
- van de Wetering M., Oving I., Muncan V., Pon Fong M. T., Brantjes H., van Leenen D., Holstege F. C., Brummelkamp T. R., Agami R., Clevers H. Specific inhibition of gene expression using a stably integrated, inducible small-interfering-RNA vector. EMBO Rep. 2003;4:609–615. doi: 10.1038/sj.embor.embor865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varis A., Salmela A. L., Kallio M. J. Cenp-F (mitosin) is more than a mitotic marker. Chromosoma. 2006;115:288–295. doi: 10.1007/s00412-005-0046-0. [DOI] [PubMed] [Google Scholar]
- Wang H., et al. Human Zwint-1 specifies localization of Zeste White 10 to kinetochores and is essential for mitotic checkpoint signaling. J. Biol. Chem. 2004;279:54590–54598. doi: 10.1074/jbc.M407588200. [DOI] [PubMed] [Google Scholar]
- Williams B. C., Gatti M., Goldberg M. L. Bipolar spindle attachments affect redistributions of ZW10, a Drosophila centromere/kinetochore component required for accurate chromosome segregation. J. Cell Biol. 1996;134:1127–1140. doi: 10.1083/jcb.134.5.1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wojcik E., Basto R., Serr M., Scaerou F., Karess R., Hays T. Kinetochore dynein: its dynamics and role in the transport of the Rough deal checkpoint protein. Nat. Cell Biol. 2001;3:1001–1007. doi: 10.1038/ncb1101-1001. [DOI] [PubMed] [Google Scholar]
- Wynshaw-Boris A., Gambello M. J. LIS1 and dynein motor function in neuronal migration and development. Genes Dev. 2001;15:639–651. doi: 10.1101/gad.886801. [DOI] [PubMed] [Google Scholar]
- Yan X., Li F., Liang Y., Shen Y., Zhao X., Huang Q., Zhu X. Human Nudel and NudE as regulators of cytoplasmic dynein in poleward protein transport along the mitotic spindle. Mol. Cell. Biol. 2003;23:1239–1250. doi: 10.1128/MCB.23.4.1239-1250.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Z., Guo J., Chen Q., Ding C., Du J., Zhu X. Silencing mitosin induces misaligned chromosomes, premature chromosome decondensation before anaphase onset, and mitotic cell death. Mol. Cell. Biol. 2005;25:4062–4074. doi: 10.1128/MCB.25.10.4062-4074.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Z. Y., Guo J., Li N., Qian M., Wang S. N., Zhu X. L. Mitosin/CENP-F is a conserved kinetochore protein subjected to cytoplasmic dynein-mediated poleward transport. Cell Res. 2003;13:275–283. doi: 10.1038/sj.cr.7290172. [DOI] [PubMed] [Google Scholar]
- Zhou X., Wang R., Fan L., Li Y., Ma L., Yang Z., Yu W., Jing N., Zhu X. Mitosin/CENP-F as a negative regulator of activating transcription factor-4. J. Biol. Chem. 2005;280:13973–13977. doi: 10.1074/jbc.M414310200. [DOI] [PubMed] [Google Scholar]
- Zhu X. Structural requirements and dynamics of mitosin-kinetochore interaction in M phase. Mol. Cell. Biol. 1999;19:1016–1024. doi: 10.1128/mcb.19.2.1016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu X., Mancini M. A., Chang K. H., Liu C. Y., Chen C. F., Shan B., Jones D., Yang-Feng T. L., Lee W. H. Characterization of a novel 350-kilodalton nuclear phosphoprotein that is specifically involved in mitotic-phase progression. Mol. Cell. Biol. 1995;15:5017–5029. doi: 10.1128/mcb.15.9.5017. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.