Abstract
Recent advances in the knowledge of the cellular effects of arsenic have provided insights into the molecular mechanisms of arsenic-associated carcinogenesis, immunotoxicity and cardiovascular disease. In the present experiments we tested the hypothesis that the arrival of arsenic to the gastrointestinal (GI) tract is detected by the gut-brain axis, which includes hindbrain and forebrain nuclei activated by GI stimulation. As a marker of neuronal activation we measured Fos expression using immunohistochemistry. Because Fos expression in these nuclei is closely linked to the development of conditioned flavor aversion (CFA) we also tested the effect of arsenic on CFA. Our experiments indicate that arsenic ingestion is readily detected by the brain, as shown by increased Fos expression after oral administration of arsenic. Furthermore, the vagus nerve, which supplies information from the GI tract to the brain, is not involved in this response because a complete subdiaphragmatic vagotomy did not reduce the effect of arsenic on brain Fos expression, but enhanced this response. In parallel, arsenic ingestion is associated with a robust, dose-dependent CFA, which started at doses as low as 0.1 mg/kg body weight. In summary, these data indicate that arsenic given by oral administration is detected by the brain in low concentrations, and activates specific nuclei, which might trigger behavioral responses, such as CFA.
Keywords: cFos, vagus, arsenate, brain, conditioned taste aversion
1. Introduction
Recent advances in the knowledge of the cellular effects of arsenic have raised considerable interest, and profuse research in this field is providing insights into the molecular mechanisms of arsenic-associated carcinogenesis, immunotoxicity and cardiovascular disease resulting from environmental exposure (Styblo et al. 2002; Xie et al. 2004). In addition, the therapeutic potential of arsenic due to its cytotoxic properties has greatly contributed to the interest in these investigations, and now arsenic compounds are widely used in the treatment of cancer (Miller, Jr. et al. 2002). Besides these arsenic effects, the neurotoxic actions of arsenic have been a primary focus of attention in the last few years since epidemiological studies indicate a significant presence of cognitive dysfunction in children exposed to low levels of arsenic (Calderon et al. 2001; Tsai et al. 2003; Wasserman et al. 2004) and these findings motivated experiments in animal models, where behavioral and neurochemical alterations have been reported.
Behavioral studies using rats and mice have shown that arsenic exposure affects locomotor activity (Itoh et al. 1990; Rodriguez et al. 2001) and learning tasks (Nagaraja and Desiraju 1994; Rodriguez et al. 2001; Rodriguez et al. 2003). Although neurochemical studies demonstrated changes of catecholamine content and release in several brain nuclei (Mejia et al. 1997; Rodriguez et al. 1998) and decreased nitric oxide production in the basal ganglia (Zarazua et al. 2006), there is not yet enough experimental evidence to attribute the observed behavioral changes to a specific action of arsenic on any particular brain region. Indeed, there is scarce information about how arsenic exposure leads to those alterations, from its entrance to the brain to its particular cellular and molecular targets. In addition to the demonstrated capacity of arsenic to produce oxidative stress in the central nervous system (Chaudhuri et al. 1999; Flora 1999; Garcia-Chavez et al. 2003; Goebel et al. 1990; Shila et al. 2005), other molecular events might trigger neurochemical changes because arsenic activates a great number of intracellular signaling pathways. Importantly, arsenic activates MAP kinase, ERK, JNK, p38, the transcription factor AP-1, and several heat shock proteins (Bernstam and Nriagu 2000; Del Razo et al. 2001; Yang and Frenkel 2002). The variety of mechanisms through which arsenic acts results in pleiotropic cellular effects, from differentiation and growth inhibition to cell proliferation, and from malignant transformation to apoptosis induction and cell death (Florea et al. 2005; Lau et al. 2004; Miller, Jr. et al. 2002).
In this context, the purpose of this work was to obtain information about the steps involved in arsenic contact with the CNS. Consequently, how early is arsenic detected after oral consumption and does the brain orchestrate a behavioral response to the contact with this toxin? Through the use of animal models designed to mimic human environmental exposure to arsenic we know oral administration of arsenic produces neurochemical and behavioral alterations, but we lack information about the initial events following arsenic ingestion.
The rationale for these experiments was to look for a direct association between arsenic ingestion, neuronal activation and a simpler behavioral response, since changes in locomotor activity reported previously represent a more complex event, which was both enhanced or decreased after arsenic exposure (Rodriguez et al. 2002; Rodriguez et al. 2001). For this purpose, we tested the hypothesis that the arrival of arsenic to the gastrointestinal (GI) tract is detected by brainstem nuclei related to the gut-brain axis such as the nucleus of the solitary tract (NTS) and area postrema (AP), as well as other forebrain nuclei commonly activated by GI stimulation (e.g., Tracy et al. 2004; Yamamoto et al. 1992). The response was measured using immunohistochemistry for the detection of the immediate early gene c-Fos, which is currently used as a marker of neuronal activation (e.g., Kovacs 1998; Olszewski et al. 2000; Sakai and Yamamoto 1997; Yamada et al. 2000). Before analyzing the early reaction to arsenic ingestion, namely hours after a single administration, we assayed Fos expression under conditions of repeated administration, at doses that do produce behavioral changes (Rodriguez et al. 2001). Next, we varied the arsenic dose, time of Fos measurement, and we assayed the effect of ablation of the vagus nerve, which innervates the GI tract. Afterwards, since C-Fos expression in those nuclei is closely linked to the development of certain aversive behaviors (e.g., Swank 2000) we approached the question of whether the early activation of some cerebral centers by arsenic can trigger a conditioned flavor aversion (CFA). We used two methods of CFA testing (one or two-stimulus choice tests), due to the sensitivity of these different testing methods for different kinds of aversive conditions (e.g., Garcia and Koelling 1967; Grote and Brown 1971; Siegel et al. 1995) and we varied also the conditions of strain and sex of the animals in order to confirm in different experimental settings the behavioral effects of arsenic ingestion.
2. Materials and Methods
2.1. Subjects
Female Wistar rats (200-250 g) were employed for Experiments 1, 2, and 4, and male Sprague-Dawley rats (250-300 g) were used in Experiments 2, 3 and 5. All animals were maintained using established standards of animal care (National Research Council, 2003a), on a 12:12 h light/dark cycle (lights on at 0700 h), with controlled temperature (23 °C) and humidity (60%). Animals were housed individually with food and water ad libitum, and before experimentation they underwent an adaptation period of 1 to 2 weeks, which consisted of daily handling and monitoring of body weight and water intake. For Fos expression experiments, food was removed 2 h prior to injection with saline or arsenic to eliminate the potential effect of feeding behavior on brain Fos expression. Prior to injecting saline or arsenic, animals were given at least two mock trials (once per day) by inserting the intragastric intubation needle but without an injection of liquid.
2.2. Fos immunohistochemistry and cell counting
At the time of sacrifice, rats were deeply anesthetized by injection of sodium pentobarbital (50 mg/kg, i.p.). Fos immunohistochemistry was performed as previously described (e.g., Horn et al. 1999; Horn et al. 2001). Briefly, rats were perfused transcardially with 2% acrolein (or 0.3% picric acid, used only in Experiment 1) plus 4% paraformaldehyde in 0.1 M phosphate buffer (pH 7.4). Brains were removed and cryoprotected in 30% sucrose/PBS (phosphate buffered saline) and then cut into 30-μm thick sections using a cryostat.
Sections were collected from two locations based on previous work and knowledge of the viscerosensory pathways (e.g., Horn et al. 1999; Horn et al. 2001); from caudal hindbrain (approximately −14.5 to −12.5 mm bregma) and forebrain (approximately 0.2 to −3.6 mm bregma) (Paxinos and Watson 2004). Following peroxide and blocking treatments sections were then incubated at room temperature with gentle agitation in 1:40,000 polyclonal anti-Fos (Santa Cruz Biotechnology, Santa Cruz, CA; lot no. D172) for 20 h. Sections were placed in 1:400 biotinylated rabbit anti-rat (Elite kit, Vector Laboratories) for 3 h and then incubated in avidin–biotin (4.5 μl of avidin and biotin per ml PBS; Elite kit, Vector Laboratories, Burlingame, CA). Sections were placed in 3,3′-diaminobenzidine (DAB; 5 mg/ml) with nickel sulfate (25 mg/ml) for 1–3 min.
Fos cell staining was determined by the presence of a blue–black reaction product in the cell nuclei. Tissue sections were viewed with a Zeiss microscope (Axiostar Plus) equipped with a digital camera (Scion CFW-1312C). Brain regions and cells expressing Fos were imaged and the number of cells expressing Fos was manually counted by an experimenter who was blind to the experimental condition. Based on previous examinations of brain sections from rats treated with arsenic and on knowledge of the neural system of the gut-brain axis usually expressing Fos to a variety of treatments (e.g., Horn and Friedman 1998; Rowland 1998), cell counts were made in areas that consistently showed Fos expression. To standardize the analyses, cells from each area were counted in coronal sections at approximately the same level relative to bregma (Paxinos and Watson 2004). The brain areas analyzed and position relative to bregma (mm) were: 1) hindbrain: NTSc (nucleus of the solitary tract, caudal, −14.8), NTSm (middle, −13.9), NTSr (rostral, −13.3), AP (area postrema, −13.9), 2) forebrain: PVNp (paraventricular nucleus of the hypothalamus, parvocellular division, −1.9), PVNm (magnocellular division, −1.9), SON (supraoptic nucleus, −1.4), PVA (paraventricular nucleus of the thalamus, −1.9) CeA (central nucleus of the amygdala, −2.5), and BNST (bed nucleus of the stria terminalis, −0.1). Cell counts were obtained from 2 (consecutive and averaged) sections for each brain area and, because Fos expression was not lateralized in any of the bilateral structures examined, cell counts reflect the totals for both sides in these areas. Brains were processed in batches and each batch also included a positive control. Positive controls for Fos expression consisted of brain sections from rats treated with cholecystokinin (100 μMol/kg, i.p.) 1 h prior to sacrifice (e.g., Olson et al. 1992). These positive controls consistently showed a large number of Fos cell counts in the hindbrain and forebrain.
2.3. Brain Fos expression after daily or a single treatment with arsenic
2.3.1. Experiment 1, daily treatments with arsenic
Eighteen rats received gavage at 1100 – 1130 h of either saline (0.15 M NaCl), 5 mg/kg arsenic, or 20 mg/kg arsenic (n = 8 in each group) in daily administrations of approximately 6 ml/kg for 14 days. In all the experiments, arsenic dosage is expressed as mg of arsenic per kg of body weight, and all assayed doses were below those reported as lowest-observed-adverse-effect-levels (2003b). Two hours after the last gavage, on day 14, the animals were sacrificed and brains were extracted for Fos immunohistochemistry.
2.3.2. Experiment 2, a single treatment with arsenic
Twenty male Sprague-Dawley rats were used to test the effects of a single gastric intubation with saline or arsenic on brain Fos expression at 2 and 24 h after gavage. Rats were gavaged with saline (0.15 M NaCl, 10 ml/kg) or arsenic (5 mg/kg in 10 ml/kg) at 1000 – 1100 h. Animals were sacrificed 2 or 24 h after injection depending on treatment group: saline/2 h, saline/24 h, arsenic/2 h, and arsenic/24 h (n = 5 in each group).
Four additional animals, used in parallel with animals in Experiment 1, were used as a control for the difference in strain and sex between Experiments 1 and 2. Female Wistar rats received an acute gavage of saline (n=2) or 5 mg/kg arsenic (n=2) and were sacrificed at 2 h post injection.
2.4. Experiment 3: Effect of total subdiaphragmatic vagotomy on arsenic-induced Fos expression
Twenty rats were used for sham and vagotomy surgeries in order to determine brain Fos expression after arsenic treatment (the groups were sham/saline, sham/arsenic, vagotomy/saline, and vagotomy/arsenic; n = 5 in each group). After overnight fasting, the animals were anesthetized with a mixture of ketamine and xylazine (100 mg/kg ketamine plus 5 mg/kg, intramuscular injection). Through a midline abdominal incision, the ventral and dorsal vagal nerve trunks were localized below thediaphragm and transected with a thermal cautery. For sham surgeries the vagus was observed but left untouched. Rats received injections of an analgesic (buprenorphine 0.5 mg/kg, subcutaneous injection) and antibiotic (gentamicine, 5 mg/kg, intramuscular injection) for three days after surgery. To aid recover from the vagotomy surgery animals were given a liquid diet (50% sweetened condensed milk and 0.12% Poly Vi Sol, Enfamil Johnson & Johnson, diluted in water) for two weeks. At the time of testing with arsenic administration, both sham and vagotomized animals had recovered the weight lost after the surgery. During the test, animals were intubated with saline (0.15 M NaCl, 10 ml/kg) or arsenic (5 mg/kg in 10 ml/kg) and were sacrificed after 2 h.
2.5. Tests of CFA using high and low doses of arsenic
2.5.1. Experiment 4: High dose arsenic treatment with two-bottle testing for CFA
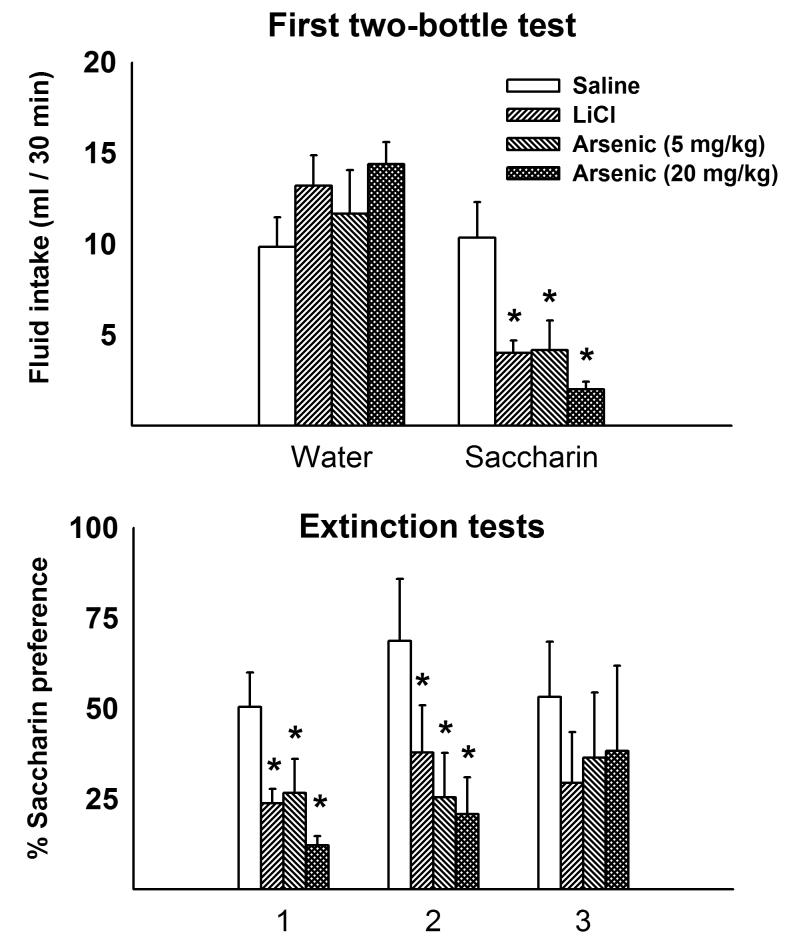
To test for CFA, twenty-four rats received gavage of either saline (0.15 M NaCl), 5 mg/kg arsenic, 20 mg/kg arsenic, or 21.2 mg/kg LiCl (a positive control) in volumes of 10 ml/kg (n = 6 for each group). To assure drinking of test liquids during training and testing, animals were deprived of water for 18 h (from 1600 to 1000 h). There was a 48 h delay between each mock, conditioning, and test day. Intubations occurred at 1000 h and the first two trials were mocks. Forty-eight hours after the second mock trail rats were injected with saline, arsenic, or LiCl and given a saccharin solution (0.15%) for 30 min. Forty-eight hours later, two bottles were presented for 30 min, water versus saccharin. To test for extinction of the CFA the two-bottle test was repeated two more times. One of the animals belonging to the group that received 20 mg/kg died before finishing the experiment, and none of the data collected from this animal are included.
In a separate control study, twelve rats were assigned to three groups: saline, 5 mg/kg arsenic, and 20 mg/kg arsenic. The procedure, in general, was the same as described above except that on the two mock trial days animals had 30 min access to saccharin and, on the treatment day, intubations with saline and arsenic were not followed by saccharin access. A two-bottle test with saccharin and water was given 48 h after gavage with saline and arsensic.
2.5.2. Experiment 5: Low dose arsenic treatment with one- and two-bottle testing for CFA
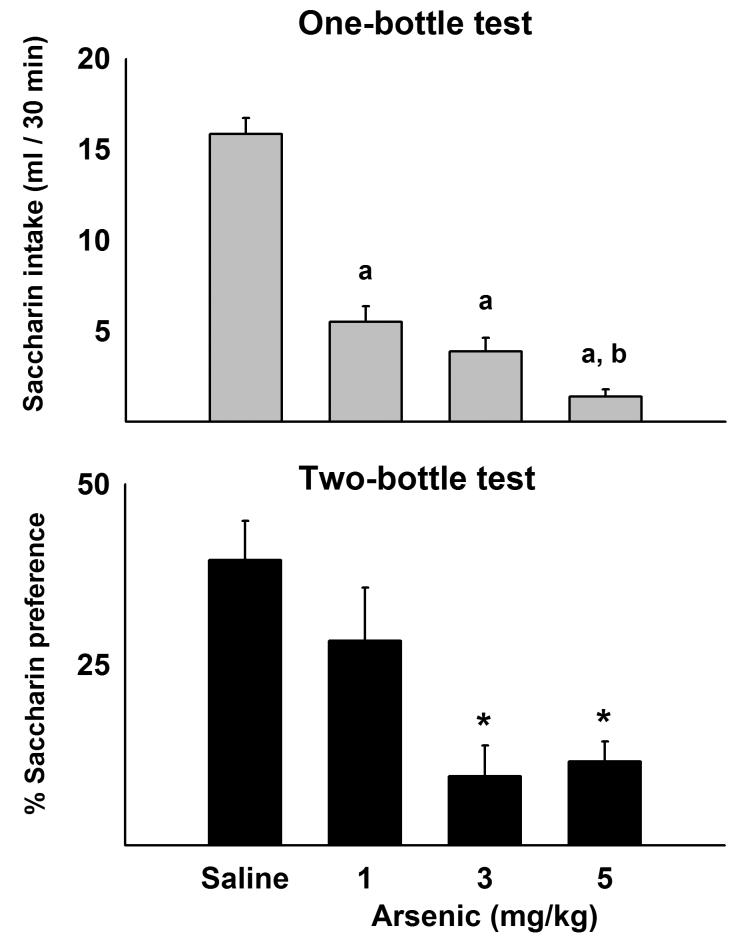
Thirty-two rats were administered saline (0.15 M, NaCl), 1, 3, or 5 mg/kg arsenic in 10 ml/kg (n = 8 in each group). Animals underwent the same adaptation period and conditioning procedures as described in Experiment 4. In the test session, however, only one bottle containing saccharin (0.15%) was presented during 30 min. A second test was conducted 48 h later using two bottles, saccharin versus water.
Eight additional animals were used to test the effects of a very low dose of arsenic on CFA. Rats were administered saline (0.15 M, NaCl) or 0.1 mg/kg arsenic in 10 ml/kg (n = 4 in each group). Animals underwent the same adaptation period and conditioning procedures as described in Experiment 4. In the test session, saccharin (0.15%) was presented for 30 min.
2.6. Statistical analysis
For Experiments 1 and 5, one-way ANOVAs were employed to compare control groups and different doses of arsenic, and, when statistically significant, Tukey's HSD tests were used to make post-hoc comparisons between means. A separate ANOVA was used to evaluate each brain area in Experiment 1. The effect of the lowest dose of arsenic, tested in only in 4 treated and 4 control animals, was compared using a Student's t-test.
For Experiments 2 and 3, two-way (2 × 2) ANOVAs (analysis of variance) were used to compare brain Fos expression after a single arsenic administration considering treatment (saline or arsenic) and time (2 or 24 h; Experiment 2) or vagotomy (sham or vagotomy; Experiment 3) as factors. A separate ANOVA was conducted for each brain region. When significant effects for treatment or treatment by time were found, post hoc comparisons were performed comparing saline and arsenic groups using Tukey's HSD tests.
For Experiment 4, fluid intakes were evaluated using a two-way (2 × 4) ANOVA using the factors of solution (saccharin or water) and treatment (saline, LiCl, 5, or 20 mg/kg arsenic). For the extinction test data, a two-way (3 × 4) ANOVA was applied using only the percentage preference data, including a within subjects factor for test days (1, 2, or 3) and a between subjects factor of treatment group (saline, LiCl, 5, or 20 mg/kg arsenic). A probability level of p < 0.05 was considered to indicate statistically significant differences for all experiments.
3. Results
3.1. Experiment 1. Fos expression after daily treatment with arsenic
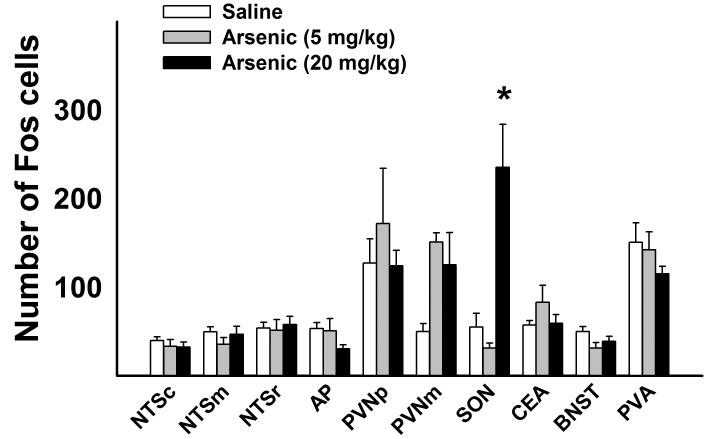
Fourteen days of daily treatment with arsenic produced a significant effect on Fos expression in the SON [F( 2,21) = 14.2, p< 0.05, one-way ANOVA for the SON]. Only the highest dose of arsenic, 20 mg/kg, generated a significant increase in Fos expression in the SON (Fig. 1). Although all groups gained weight during the 2 weeks of arsenic administration (6.1 % increase for saline, 6.7 % increase for 5 mg/kg arsenic, and 2.6 % increase for 20 mg/kg arsenic group), these increases were not significant when compared with the initial weight and also there were no significant differences of final weight among groups. The highest dose of arsenic elicited diarrhea. There were no other statistically significant effects of arsenic on Fos expression (one-way ANOVAs).
Fig. 1.
Experiment 1: Fos expression in brainstem and forebrain nuclei after daily intragastric saline or arsenic (5 or 20 mg/kg) treatment for 14 days. Rats were sacrificed at 2 h after the last injection. Values represent means ± SEM. * = p < 0.05, saline versus arsenic, Tukey HSD test. Hindbrain = NTSc (nucleus of the solitary tract, caudal), NTSm (middle), NTSr (rostral), AP (area postrema), and forebrain = PVNp (paraventricular nucleus of the hypothalamus, parvocellular division), PVNm (magnocellular division), SON (supraoptic nucleus), CeA (central nucleus of the amygdala), BNST (bed nucleus of the stria terminalis), and PVA (paraventricular nucleus of the thalamus).
3.2. Experiment 2. Fos expression after a single treatment with arsenic
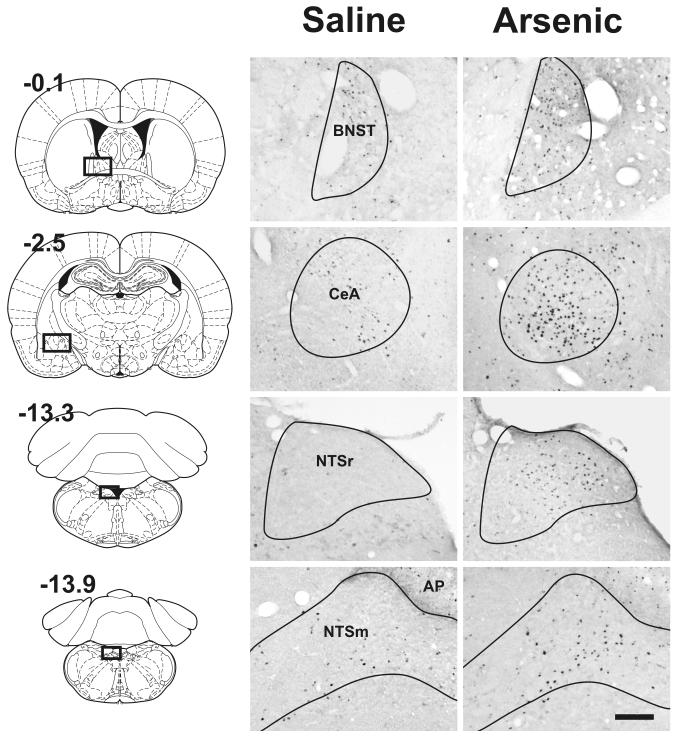
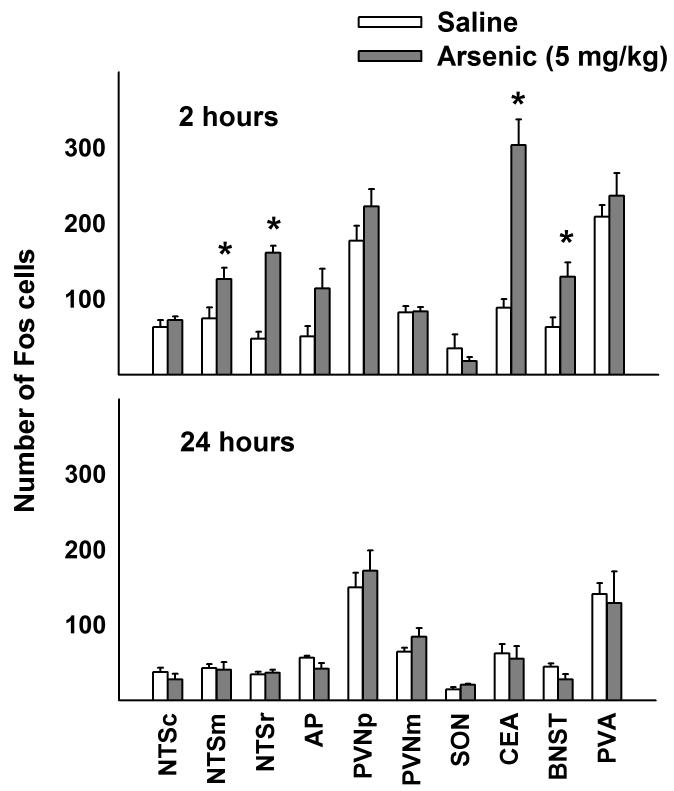
Representative Fos cell staining is shown in Fig. 2. Arsenic treatment significantly increased brain Fos expression in the NTSm, NTSr, CeA, and BNST compared to saline control [Fs(1,15) > 4.0, ps < 0.05; treatment by interaction effect]. Only at 2 h after arsenic administration were these effects statistically significant (Fig. 3). There were no other statistically significant effects on Fos expression (two-way ANOVAs for main effect of treatment or interaction effects).
Fig. 2.
Experiment 2: Representative brain images showing the effect of acute intragastric intubation with saline or arsenic (5 mg/kg) on Fos expression in the NTSm, NTSr, CeA, and BNST at 2 h after injection. These brain areas showed statistically significant Fos expression after arsenic treatment (see Fig. 3). Diagrams on the left show the location of the respective regions in whole brain slices (values represent position relative to bregma, mm; reprinted from “The rat brain in stereotaxic coordinates,” G. Paxinos and C. Watson, Copyright, 2005, with permission from Elsevier). The Calibration bar equals 200 μm. See legend in Figure 1 for key to abbreviations.
Fig 3.
Experiment 2: Fos expression in brainstem and forebrain nuclei after intragastric saline or arsenic (5 mg/kg). Values represent means ± SEM. * = p < 0.05, saline versus arsenic, Tukey HSD test. See legend in Figure 1 for key to abbreviations.
In female Wistar rats acute arsenic treatment produced an effect on brain Fos expression greater than saline treatment in brainstem and amygdala. Cell counts (sum total of NTSm, NTSr, and CeA) were 143 and 150 for saline treatment (n = 2) and 184 and 465 for arsenic treatment (n = 2).
3.3. Experiment 3: Effect of total subdiaphragmatic vagotomy on arsenic induced Fos expression
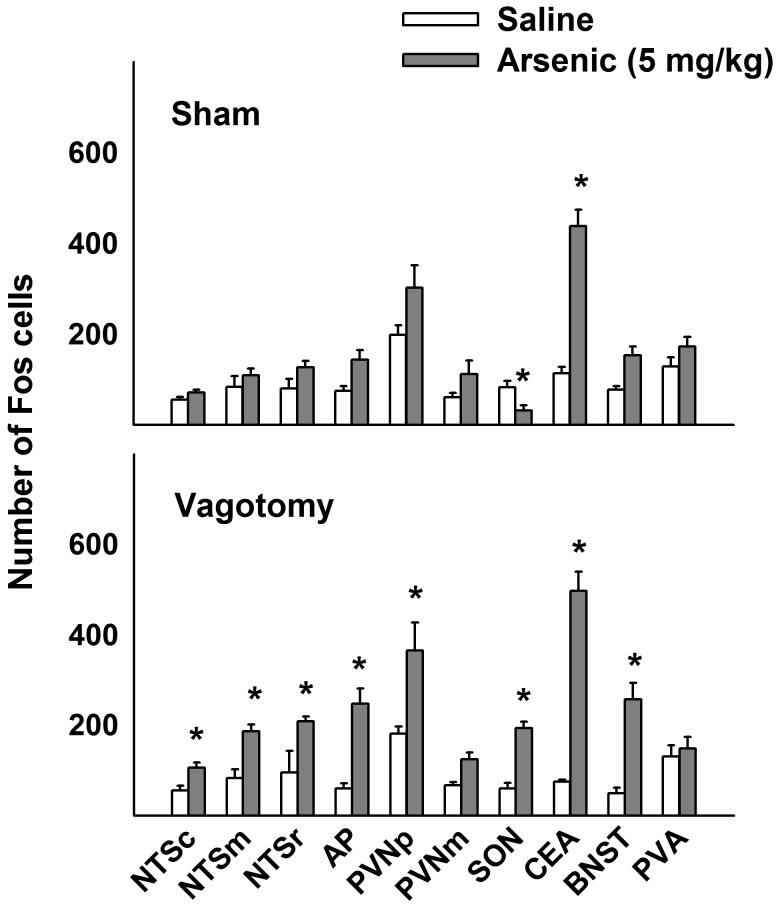
Arsenic treatment significantly increased brain Fos expression in the NTSc, NTSm, NTSr, AP, PVNp, SON, CeA, and BNST compared to saline control [Fs(1,15) > 4, ps < 0.05; treatment by surgery interaction effect for AP, SON, and BNST, and main effect of treatment for NTSc, NTSm, NTSr, PVNp, and CeA]. Mean comparisons revealed that arsenic treatment increased Fos expression in vagotomized animals in the NTSc, NTSm, NTSr, AP, PVNp, SON, CeA, and BNST compared to saline treatment (ps < 0.05, Tukey's HSD test; see Fig. 4). In contrast, arsenic treatment only increased Fos expression in the CeA of sham operated animals (p < 0.05; see Fig. 4). There were no other statistically significant effects of arsenic on Fos expression (two-way ANOVAs for main effect of treatment or interaction effects).
Fig 4.
Experiment 3: Effect of total subdiaphragmatic vagotomy on Fos expression in brainstem and forebrain nuclei 2 h after intragastric saline or arsenic (5 mg/kg) treatment. Values represent means ± SEM. * = p < 0.05, saline versus arsenic, Tukey's HSD test. See legend in Figure 1 for key to abbreviations.
3.4. Experiment 4: High dose arsenic treatment with two-bottle testing for CFA
Rats that received LiCl (as a positive control) or arsenic treatment (5 or 20 mg/kg) developed CFAs [F(3,19) = 6.7, p = 0.003; treatment by solution interaction effect]. Although water consumption was unchanged across treatment conditions, LiCl and arsenic treatments significantly reduced saccharin intake relative to the saline control group (Fig. 5). Furthermore, saccharin preference was also reduced for the LiCl and arsenic treatment groups on the second extinction test, but was not significantly different from saline control by the third test [F(3,19) = 3.8, p = 0.028; F(3,19) = 0.9, p = 0.46, for the second and third tests, respectively; treatment by day interaction effect; Fig. 5]. In the control study when arsenic treatment was not paired with saccharin there were no significant differences between saline (68 ± 7 % preference for saccharin), 5 mg/kg arsenic (49 ± 11 %), or 20 mg/kg arsenic (66 ±12 %) groups [F(2,9) = 1.0, p = 0.4, one-way ANOVA, these data are not plotted].
Fig 5.
Experiment 4: Conditioned flavor aversion produced by arsenic using a 2-bottle preference test. Top panel shows the first two-bottle preference test, water versus saccharin intake, 48 h after pairing of saccharin with intragastric injection of saline, LiCl, or arsenic (5 or 20 mg/kg) treatment. Bottom panel shows the effect of conditioning on all two-bottle extinction tests (2, 4, and 6 days after pairing of saccharin with intragastric injection) as measured by percentage of preference for saccharin solution. Values represent means ± SEM. * = p < 0.05, saline versus arsenic, Tukey HSD test. LiCl injection was used as a positive control.
3.5. Experiment 5: Low dose arsenic treatment with one- and two-bottle testing for CFA
All arsenic treatments (1, 3, or 5 mg/kg) produced CFA [F(3,27) = 34.3, p < 0.05; one-way ANOVA]. One-bottle tests showed significant reductions in saccharin intake produced by previous pairing of arsenic with saccharin (Fig. 6). Two days later, the 3 and 5 mg/kg arsenic groups showed significant reductions in two-bottle percent preference for saccharin compared to the saline control group [F(3,27) = 6.9, p < 0.05, one-way ANOVA; ps < 0.05, Tukey's HSD test, saline versus arsenic groups; see Fig. 6]. However, 1 mg/kg arsenic was not significantly different from saline control in the two-botttle test. Lastly, in the separate test using 2 groups of 4 naïve animals each, 0.1 mg/kg arsenic (mg per kg body weight) produced a significant decrease in saccharin consumption in a one-bottle test of CFA [ t (6 ) = 3.6, p < 0.05; 5.8 ± 2.5 versus 14.8 ± 2.3 for arsenic and saline groups, respectively; these data are not plotted).
Fig 6.
Experiment 5: Conditioned flavor aversion produced by low doses of arsenic. Saccharin solution was paired with intragastric injection of saline or arsenic (1, 3, or 5 mg/kg) on the conditioning day. Top panel shows a one bottle-test using saccharin solution, 48 h after pairing of saccharin with intragastric injection of saline or arsenic. a = p < 0.001 compared to saline control, b = p < 0.01 compared to the 1 mg/kg arsenic group. Bottom panel shows a two-bottle preference test for saccharin versus water four days after pairing saccharin with intragastric delivery of saline or arsenic. Values are percent preference for saccharin. Values represent means ± SEM. * = p < 0.05, saline versus arsenic, Tukey HSD test.
4. Discussion
These results indicate that arsenic ingestion is readily detected by the brain, as shown by significant increases in Fos expression in NTSr and CEA at 2 h after oral administration. Moreover, arsenic triggers a robust dose-dependent behavioral response measured by CFA, and a significant CFA was apparent at even the lowest dose of arsenic (0.1 mg/kg). Arsenic-induced Fos expression did not appear to depend on the integrity of the vagus because vagotomy did not affect this response. In the vagotomy experiment, the surgical stress also did not induce changes in basal Fos expression; however, a response to arsenic administration was observed, and in many regions enhanced, in vagotomized animals compared to sham operated rats. The exception was the CeA, where arsenic induced Fos expression to the same extent in both sham operated and vagotomized animals.
The use of Fos immunostaining as a strategy to map which CNS areas are activated following arsenic ingestion was based on reports showing that a wide range of stimuli induce Fos expression with a specific anatomical location and temporal pattern (Kovacs 1998). Those stimuli range from emotional situations, such as restraint, immobilization or fear, to physical challenges like exposure to drugs, painful stimuli, or osmotic challenges through salt-loading (Navarro et al. 2000; Penny et al. 2005; Pirnik et al. 2004). It is clear from Experiment 2 (see Figs. 2 and 3) that acute treatment with arsenic produced activation of many of the same brain areas, such as the NTS and CeA that are activated by other stress associated stimuli. In general, although there are exceptions (e.g., Horn et al. 2006), brain Fos expression is an acute phenomenon that reaches maximal levels within 1-3 h after a stimulus treatment (e.g., Kovacs 1998), therefore the lack of significant increases in 24 h Fos expression in the current study can be explained on the basis of the temporal course of Fos induction.
Prolonged exposure to arsenic in Experiment 1 produced a profile of Fos expression unlike that of acute exposure to arsenic. Only the SON, at the highest dose of arsenic (20 mg/kg), showed significant levels of Fos expression (see Fig. 3). The SON contains cells that secrete vasopressin (e.g., Hamamura et al. 1992) and is stimulated when animals are osmotically challenged (e.g., Kovacs and Sawchenko 1993). Prolonged exposure to high doses of arsenic provoke diarrhea due to irritation of the gastric mucosa (Rodriguez et al. 2001). Therefore, the Fos expression in the SON in the current experiment could have been the result of an osmotic challenge produced by a high dose of arsenic, a condition that was not present after a single administration. It should be noted, however, that c-Fos is induced in this region also in response to stimuli that do not bear any relationship with osmotic changes, such as spinal cord injury (Xu et al. 2006) and stress-related stimuli like foot-shock and immobilization (Kovacs 1998). The fact that we did not observe the same activated areas in experiments 1 and 2 probably reflects the use of chronic versus acute arsenic exposure. It is likely that the difference in Fos expression pattern between Experiments 1 and 2 is not due to differences in sex or strain because two female Wistar rats receiving acute arsenic treatment in Experiment 2 showed increased Fos expression in the brainstem and amygdala compared to two saline control animals, a pattern also observed in male Sprague-Dawley rats.
We found that arsenic administration (5 mg/kg) significantly increased Fos expression in NTSc, NTSm, NTSr, AP, PVNp, SON, CeA and BNST of vagotomized animals, but only the CeA showed significant increases in Fos expression in sham operated controls. It is worth noting that the number of stained cells in saline exposed rats, both sham and vagotomized, did not differ from those of intact animals (compare Figs. 3 and 4), therefore this observation is not due to variations in absolute Fos cell numbers between operated and unoperated animals. It is possible that vagotomized animals were more sensitive to the arsenic treatment in this experiment. Vagotomized rats have reduced gastric emptying (Kraly et al. 1985) and this might have enhanced the effect of arsenic in these animals because by slowing down gastric emptying there is a potential for more arsenic absorption. Furthermore, the use of a liquid diet, not used in Experiment 2, might have produced increased GI transit, which could have lessened the effect of arsenic on brain Fos expression in sham operated animals. Unfortunately, without the liquid diet administration complete subdiaphragmatic vagotomized animals usually do not recover from surgery, and many die several days later (Kraly et al. 1986; Phillips et al. 1997). There is a hint that arsenic might have produced a more global effect in sham operated controlled because all nine brain areas that do not show a statistically significant effect, nevertheless, have higher mean values compared to saline controls (see top panel, Fig. 4). Ultimately the results of this experiment are probably due to the influence of two stressors, vagotomy surgery and arsenic, that when combined produced a synergistic effect on brain Fos expression. In summary, this experiment suggests that the vagus is not necessary for arsenic-induced brain Fos expression, and this is clearly the case for activation of the CeA (see Fig. 4).
Although we did not measure simultaneously Fos expression and CFA, the employment of two different rat strains, different sexes, and CFA protocols (see Figs. 5 and 6) in this study provides solid evidence for the early detection of arsenic by the brain. Several CFA studies document that a decreased intake of saccharin on the test day, when this liquid is the only choice presented to the animal (a one-bottle test), may reflect the effect of the drug on overall fluid intake rather than on intake of a specific fluid (Na and Fitts 2001; Rowland et al. 2004). For this reason, 2-bottle tests are considered more sensitive than one-bottle tests, since the reduced intake of the non-preferred substance can be compensated by the supplementary intake of the other fluid (Grote and Brown 1971; Phillips et al. 1997). We found significant effects in both test paradigms. These results lead us to accept the hypothesis that a single oral exposure of small amounts of arsenic, as low as 0.1 mg/kg, can trigger a behavioral response directed to avoid further contact with the toxic substance.
An important issue is where arsenic is acting to produce a signal that leads to suppression of saccharin intake. A control study using unpaired arsenic treatment and saccharin exposure (see Experiment 4) indicated that arsenic (5 or 20 mg/kg) has no significant effect on flavor responses to saccharin. Further down the alimentary tract, it is possible that arsenic produces a CFA by action on GI neural pathways, including the vagus or splanchnic nerves innervating the gut, although the role of peripheral nerves in CFA is controversial (Kiefer et al. 1981; e.g., Martin et al. 1978). Alternatively, arsenic might be detected by a brain area, such as the area postrema, often referred to as the “chemoreceptive trigger zone” that is important in the production of CFA and other aversive responses, such as emesis (e.g., Borison 1959; Wang et al. 1997).
In summary, although arsenic is known for its capacity to trigger activation of a number of transcription factors in different kinds of cells and to affect a myriad of cellular functions, the c-Fos activation induced by arsenic in these experiments was specific to some CNS regions and temporally restrained. In addition, doses of arsenic that produce c-Fos activation are also able to trigger a behavioral response, CFA. These effects suggest the activation of specific signals in the brain by arsenic, and these observations open the possibility for further studies of the events triggered in the CNS by arsenic exposure.
Acknowledgements
This work was supported by funding in the USA (NIH--DK065971) and Mexico (CONACYT--P40627M and fellowship--194111).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- ToxProfiles 2003 . Arsenic. Agency for Toxic Substances and Disease Registry (ATSDR) Department of Health & Human Services; USA: 2003b. [Google Scholar]
- Guidelines for the care and use of mammals in neuroscience and behavioral research. The National Academies Press; Washington, DC: 2003a. [PubMed] [Google Scholar]
- Bernstam L, Nriagu J. Molecular aspects of arsenic stress. J Toxicol. Environ. Health B Crit Rev. 2000;3(4):293–322. doi: 10.1080/109374000436355. [DOI] [PubMed] [Google Scholar]
- Borison HL. Effect of ablation of medullary emetic chemoreceptor trigger zone on vomiting responses to cerebral intraventricular injection of adrenaline, apomorphine and pilocarpine in the cat. J Physiol. 1959;147(1):172–177. doi: 10.1113/jphysiol.1959.sp006232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calderon J, Navarro ME, Jimenez-Capdeville ME, Santos-Diaz MA, Golden A, Rodriguez-Leyva I, Borja-Aburto V, az-Barriga F. Exposure to arsenic and lead and neuropsychological development in Mexican children. Environ. Res. 2001;85(2):69–76. doi: 10.1006/enrs.2000.4106. [DOI] [PubMed] [Google Scholar]
- Chaudhuri AN, Basu S, Chattopadhyay S, Das GS. Effect of high arsenic content in drinking water on rat brain. Indian J Biochem. Biophys. 1999;36(1):51–54. [PubMed] [Google Scholar]
- Del Razo LM, Quintanilla-Vega B, Brambila-Colombres E, Calderon-Aranda ES, Manno M, Albores A. Stress proteins induced by arsenic. Toxicol. Appl. Pharmacol. 2001;177(2):132–148. doi: 10.1006/taap.2001.9291. [DOI] [PubMed] [Google Scholar]
- Flora SJ. Arsenic-induced oxidative stress and its reversibility following combined administration of N-acetylcysteine and meso 2,3-dimercaptosuccinic acid in rats. Clin Exp. Pharmacol Physiol. 1999;26(11):865–869. doi: 10.1046/j.1440-1681.1999.03157.x. [DOI] [PubMed] [Google Scholar]
- Florea AM, Yamoah EN, Dopp E. Intracellular calcium disturbances induced by arsenic and its methylated derivatives in relation to genomic damage and apoptosis induction. Environ. Health Perspect. 2005;113(6):659–664. doi: 10.1289/ehp.7634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia J, Koelling RA. A comparison of aversions induced by x-rays, toxins, and drugs in the rat. Radiat. Res Suppl. 1967;7:439–450. [PubMed] [Google Scholar]
- Garcia-Chavez E, Santamaria A, Díaz-Barriga F, Mandeville P, Juarez BI, Jimenez-Capdeville ME. Arsenite-induced formation of hydroxyl radical in the striatum of awake rats. Brain Res. 2003;976(1):82–89. doi: 10.1016/s0006-8993(03)02697-0. [DOI] [PubMed] [Google Scholar]
- Goebel HH, Schmidt PF, Bohl J, Tettenborn B, Kramer G, Gutmann L. Polyneuropathy due to acute arsenic intoxication: biopsy studies. J Neuropathol. Exp. Neurol. 1990;49(2):137–149. doi: 10.1097/00005072-199003000-00006. [DOI] [PubMed] [Google Scholar]
- Grote F, Brown R. Conditioned taste aversion: Two-stimulus tests are more sensitive than one-stiumulus tests. Behavior Research Methods and Instrumentation. 1971;3(6):311–312. [Google Scholar]
- Hamamura M, Nunez DJ, Leng G, Emson PC, Kiyama H. c-fos may code for a common transcription factor within the hypothalamic neural circuits involved in osmoregulation. Brain Res. 1992;572(12):42–51. doi: 10.1016/0006-8993(92)90448-i. [DOI] [PubMed] [Google Scholar]
- Horn CC, Addis A, Friedman MI. Neural substrate for an integrated metabolic control of feeding behavior. Am. J. Physiol. 1999;276(1 Pt 2):R113–R119. doi: 10.1152/ajpregu.1999.276.1.R113. [DOI] [PubMed] [Google Scholar]
- Horn CC, Ciucci M, Chaudhury A. Brain Fos expression during 48 h after cisplatin treatment: Neural pathways for acute and delayed visceral sickness. Auton Neurosci Basic Clin. 2006 doi: 10.1016/j.autneu.2006.09.005. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horn CC, Friedman MI. Metabolic inhibition increases feeding and brain Fos-like immunoreactivity as a function of diet. Am. J. Physiol. 1998;275(2 Pt 2):R448–R459. doi: 10.1152/ajpregu.1998.275.2.R448. [DOI] [PubMed] [Google Scholar]
- Horn CC, Tordoff MG, Friedman MI. Role of vagal afferent innervation in feeding and brain Fos expression produced by metabolic inhibitors. Brain Res. 2001;919(2):198–206. doi: 10.1016/s0006-8993(01)02963-8. [DOI] [PubMed] [Google Scholar]
- Itoh T, Zhang YF, Murai S, Saito H, Nagahama H, Miyate H, Saito Y, Abe E. The effect of arsenic trioxide on brain monoamine metabolism and locomotor activity of mice. Toxicol. Lett. 1990;54(23):345–353. doi: 10.1016/0378-4274(90)90202-w. [DOI] [PubMed] [Google Scholar]
- Kiefer SW, Rusiniak KW, Garcia J, Coil JD. Vagotomy facilitates extinction of conditioned taste aversions in rats. J. Comp Physiol Psychol. 1981;95(1):114–122. [PubMed] [Google Scholar]
- Kovacs KJ. c-Fos as a transcription factor: a stressful (re)view from a functional map. Neurochem. Int. 1998;33(4):287–297. doi: 10.1016/s0197-0186(98)00023-0. [DOI] [PubMed] [Google Scholar]
- Kovacs KJ, Sawchenko PE. Mediation of osmoregulatory influences on neuroendocrine corticotropin-releasing factor expression by the ventral lamina terminalis. Proc. Natl. Acad. Sci. U. S. A. 1993;90(16):7681–7685. doi: 10.1073/pnas.90.16.7681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraly FS, Jerome C, Smith GP. Specific postoperative syndromes after total and selective vagotomies in the rat. Appetite. 1986;7(1):1–17. doi: 10.1016/s0195-6663(86)80038-1. [DOI] [PubMed] [Google Scholar]
- Kraly FS, Jerome C, Smith GP. Postvagotomy syndrome in rats is abolished by palatable liquid diet. Appetite. 1985;6:205–206. [Google Scholar]
- Lau AT, Li M, Xie R, He QY, Chiu JF. Opposed arsenite-induced signaling pathways promote cell proliferation or apoptosis in cultured lung cells. Carcinogenesis. 2004;25(1):21–28. doi: 10.1093/carcin/bgg179. [DOI] [PubMed] [Google Scholar]
- Martin JR, Cheng FY, Novin D. Acquisition of learned taste aversion following bilateral subdiaphragmatic vagotomy in rats. Physiol Behav. 1978;21(1):13–17. doi: 10.1016/0031-9384(78)90269-x. [DOI] [PubMed] [Google Scholar]
- Mejia JJ, Díaz-Barriga F, Calderon J, Rios C, Jimenez-Capdeville ME. Effects of lead-arsenic combined exposure on central monoaminergic systems. Neurotoxicol. Teratol. 1997;19(6):489–497. doi: 10.1016/s0892-0362(97)00066-4. [DOI] [PubMed] [Google Scholar]
- Miller WH, Jr., Schipper HM, Lee JS, Singer J, Waxman S. Mechanisms of action of arsenic trioxide. Cancer Res. 2002;62(14):3893–3903. [PubMed] [Google Scholar]
- Na ES, Fitts DA. Conditioned taste aversion and c-Fos expression in cholestatic rats. Brain Res. 2001;918(12):187–190. doi: 10.1016/s0006-8993(01)02982-1. [DOI] [PubMed] [Google Scholar]
- Nagaraja TN, Desiraju T. Effects on operant learning and brain acetylcholine esterase activity in rats following chronic inorganic arsenic intake. Hum. Exp. Toxicol. 1994;13(5):353–356. doi: 10.1177/096032719401300511. [DOI] [PubMed] [Google Scholar]
- Navarro M, Spray KJ, Cubero I, Thiele TE, Bernstein IL. cFos induction during conditioned taste aversion expression varies with aversion strength. Brain Res. 2000;887(2):450–453. doi: 10.1016/s0006-8993(00)03032-8. [DOI] [PubMed] [Google Scholar]
- Olson BR, Hoffman GE, Sved AF, Stricker EM, Verbalis JG. Cholecystokinin induces c-fos expression in hypothalamic oxytocinergic neurons projecting to the dorsal vagal complex. Brain Res. 1992;569:238–248. doi: 10.1016/0006-8993(92)90635-m. [DOI] [PubMed] [Google Scholar]
- Olszewski PK, Shi Q, Billington CJ, Levine AS. Opioids affect acquisition of LiCl-induced conditioned taste aversion: involvement of OT and VP systems. Am. J Physiol Regul. Integr. Comp Physiol. 2000;279(4):R1504–R1511. doi: 10.1152/ajpregu.2000.279.4.R1504. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The rat brain in stereotaxic coordinates. Academic Press; New York: 2004. [DOI] [PubMed] [Google Scholar]
- Penny ML, Bruno SB, Cornelius J, Higgs KA, Cunningham JT. The effects of osmotic stimulation and water availability on c-Fos and FosB staining in the supraoptic and paraventricular nuclei of the hypothalamus. Exp. Neurol. 2005;194(1):191–202. doi: 10.1016/j.expneurol.2005.02.004. [DOI] [PubMed] [Google Scholar]
- Phillips RJ, Baronowsky EA, Powley TL. Afferent innervation of gastrointestinal tract smooth muscle by the hepatic branch of the vagus. J Comp Neurol. 1997;384:248–270. [PubMed] [Google Scholar]
- Pirnik Z, Mravec B, Kiss A. Fos protein expression in mouse hypothalamic paraventricular (PVN) and supraoptic (SON) nuclei upon osmotic stimulus: colocalization with vasopressin, oxytocin, and tyrosine hydroxylase. Neurochem. Int. 2004;45(5):597–607. doi: 10.1016/j.neuint.2004.04.003. [DOI] [PubMed] [Google Scholar]
- Rodriguez VM, Carrizales L, Jimenez-Capdeville ME, Dufour L, Giordano M. The effects of sodium arsenite exposure on behavioral parameters in the rat. Brain Res Bull. 2001;55(2):301–308. doi: 10.1016/s0361-9230(01)00477-4. [DOI] [PubMed] [Google Scholar]
- Rodriguez VM, Carrizales L, Mendoza MS, Fajardo OR, Giordano M. Effects of sodium arsenite exposure on development and behavior in the rat. Neurotoxicol. Teratol. 2002;24(6):743–750. doi: 10.1016/s0892-0362(02)00313-6. [DOI] [PubMed] [Google Scholar]
- Rodriguez VM, Dufour L, Carrizales L, az-Barriga F, Jimenez-Capdeville ME. Effects of oral exposure to mining waste on in vivo dopamine release from rat striatum. Environ. Health Perspect. 1998;106(8):487–491. doi: 10.1289/ehp.106-1533203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez VM, Jimenez-Capdeville ME, Giordano M. The effects of arsenic exposure on the nervous system. Toxicol. Lett. 2003;145(1):1–18. doi: 10.1016/s0378-4274(03)00262-5. [DOI] [PubMed] [Google Scholar]
- Rowland NE. Brain mechanisms of mammalian fluid homeostasis: Insights from use of immediate early gene mapping. Neurosci. Biobehav. Rev. 1998;23:49–63. doi: 10.1016/s0149-7634(97)00068-7. [DOI] [PubMed] [Google Scholar]
- Rowland NE, Nasrallah NA, Robertson KL. LiCl-induced flavor avoidance compared between rats and mice using a nondeprivation protocol. Am. J Physiol Regul. Integr. Comp Physiol. 2004;286(2):R260–R268. doi: 10.1152/ajpregu.00312.2003. [DOI] [PubMed] [Google Scholar]
- Sakai N, Yamamoto T. Conditioned taste aversion and c-fos expression in the rat brainstem after administration of various USs. NeuroReport. 1997;8(910):2215–2220. doi: 10.1097/00001756-199707070-00025. [DOI] [PubMed] [Google Scholar]
- Shila S, Kokilavani V, Subathra M, Panneerselvam C. Brain regional responses in antioxidant system to alpha-lipoic acid in arsenic intoxicated rat. Toxicology. 2005;210(1):25–36. doi: 10.1016/j.tox.2005.01.003. [DOI] [PubMed] [Google Scholar]
- Siegel S, Parker LA, Moroz I. Morphine-induced taste avoidance is attenuated with multiple conditioning trials. Pharmacol Biochem. Behav. 1995;50(2):299–303. doi: 10.1016/0091-3057(94)00318-d. [DOI] [PubMed] [Google Scholar]
- Styblo M, Drobna Z, Jaspers I, Lin S, Thomas DJ. The role of biomethylation in toxicity and carcinogenicity of arsenic: a research update. Environ. Health Perspect. 2002;110(Suppl 5):767–771. doi: 10.1289/ehp.110-1241242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swank MW. Conditioned c-Fos in mouse NTS during expression of a learned taste aversion depends on contextual cues. Brain Res. 2000;862(12):138–144. doi: 10.1016/s0006-8993(00)02101-6. [DOI] [PubMed] [Google Scholar]
- Tracy AL, Phillips RJ, Chi MM, Powley TL, Davidson TL. The gastrointestinal tract “tastes” nutrients: evidence from the intestinal taste aversion paradigm. Am. J Physiol Regul. Integr. Comp Physiol. 2004;287(5):R1086–R1100. doi: 10.1152/ajpregu.00047.2004. [DOI] [PubMed] [Google Scholar]
- Tsai SY, Chou HY, The HW, Chen CM, Chen CJ. The effects of chronic arsenic exposure from drinking water on the neurobehavioral development in adolescence. Neurotoxicology. 2003;24(45):747–753. doi: 10.1016/S0161-813X(03)00029-9. [DOI] [PubMed] [Google Scholar]
- Wang Y, Lavond DG, Chambers KC. Cooling the area postrema induces conditioned taste aversions in male rats and blocks acquisition of LiCl-induced aversions. Behav. Neurosci. 1997;111(4):768–776. doi: 10.1037//0735-7044.111.4.768. [DOI] [PubMed] [Google Scholar]
- Wasserman GA, Liu X, Parvez F, Ahsan H, Factor-Litvak P, van GA, Slavkovich V, LoIacono NJ, Cheng Z, Hussain I, Momotaj H, Graziano JH. Water arsenic exposure and children's intellectual function in Araihazar, Bangladesh. Environ. Health Perspect. 2004;112(13):1329–1333. doi: 10.1289/ehp.6964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie Y, Trouba KJ, Liu J, Waalkes MP, Germolec DR. Biokinetics and subchronic toxic effects of oral arsenite, arsenate, monomethylarsonic acid, and dimethylarsinic acid in v-Ha-ras transgenic (Tg.AC) mice. Environ. Health Perspect. 2004;112(12):1255–1263. doi: 10.1289/txg.7152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Y, Zheng Z, Ho KP, Qian Z. Effects of spinal cord injury on c-fos expression in hypothalamic paraventricular nucleus and supraoptic nucleus in rats. Brain Res. 2006;1087(1):175–179. doi: 10.1016/j.brainres.2006.03.003. [DOI] [PubMed] [Google Scholar]
- Yamada Y, Tsukamoto G, Kobashi M, Sasaki A, Matsumura T. Abdominal vagi mediate c-Fos expression induced by X-ray irradiation in the nucleus tractus solitarii of the rat. Auton. Neurosci. 2000;83(12):29–36. doi: 10.1016/S0165-1838(00)00105-3. [DOI] [PubMed] [Google Scholar]
- Yamamoto T, Shimura T, Sako N, Azuma S, Bai WZ, Wakisaka S. C-fos expression in the rat brain after intraperitoneal injection of lithium chloride. NeuroReport. 1992;3(12):1049–1052. doi: 10.1097/00001756-199212000-00004. [DOI] [PubMed] [Google Scholar]
- Yang C, Frenkel K. Arsenic-mediated cellular signal transduction, transcription factor activation, and aberrant gene expression: implications in carcinogenesis. J Environ. Pathol. Toxicol. Oncol. 2002;21(4):331–342. [PubMed] [Google Scholar]
- Zarazua S, Perez-Severiano F, Delgado JM, Martinez LM, Ortiz-Perez D, Jimenez-Capdeville ME. Decreased nitric oxide production in the rat brain after chronic arsenic exposure. Neurochem. Res. 2006;31(8):1069–1077. doi: 10.1007/s11064-006-9118-7. [DOI] [PubMed] [Google Scholar]