Abstract
Transfer RNAs specific for Gln, Lys, and Glu from all organisms (except Mycoplasma) and organelles have a 2-thiouridine derivative (xm5s2U) as wobble nucleoside. These tRNAs read the A- and G-ending codons in the split codon boxes His/Gln, Asn/Lys, and Asp/Glu. In eukaryotic cytoplasmic tRNAs the conserved constituent (xm5-) in position 5 of uridine is 5-methoxycarbonylmethyl (mcm5). A protein (Tuc1p) from yeast resembling the bacterial protein TtcA, which is required for the synthesis of 2-thiocytidine in position 32 of the tRNA, was shown instead to be required for the synthesis of 2-thiouridine in the wobble position (position 34). Apparently, an ancient member of the TtcA family has evolved to thiolate U34 in tRNAs of organisms from the domains Eukarya and Archaea. Deletion of the TUC1 gene together with a deletion of the ELP3 gene, which results in the lack of the mcm5 side chain, removes all modifications from the wobble uridine derivatives of the cytoplasmic tRNAs specific for Gln, Lys, and Glu, and is lethal to the cell. Since excess of the unmodified form of these three tRNAs rescued the double mutant elp3 tuc1, the primary function of mcm5s2U34 seems to be to improve the efficiency to read the cognate codons rather than to prevent mis-sense errors. Surprisingly, overexpression of the mcm5s2U-lacking tRNALys alone was sufficient to restore viability of the double mutant.
Keywords: modification, mcm5s2U, tRNA, wobble nucleoside, translation
INTRODUCTION
Transfer RNA from all organisms contains modified nucleosides, which are derivatives of the four major nucleosides adenosine (A), guanosine (G), cytosine (C), and uridine (U). So far, about 100 different modified nucleosides in RNA have been characterized (http://medstat.med.utah.edu/RNAmods/). Two positions in tRNA—the wobble position (position 34) and the nucleoside next to and 3′ of the anticodon (position 37)—are frequently modified (Björk 1998). Modifications in these two positions appear to be required for proper decoding of the message. When tRNAs have uridine (U) in the wobble position, this U residue is almost universally modified; i.e., an unmodified U is normally not present in the wobble position. When tRNAs containing modified uridines in the wobble position read codons of the split codon boxes, which code for more than one amino acid, the wobble position modifications not only contain an addition to the 5-carbon of U, but also have other features by which they may be grouped into three general categories: (1) those that are thiolated at the 2-carbon of U; (2) those that contain a ribose methylation; and (3) those that have no additional modification other than on the 5-carbon (Fig. 1). All tRNAs that fall into the first of these groups, i.e., those that are thiolated, are specific for the split codon boxes that contain the codons for Gln, Lys, and Glu (Fig. 2). These tRNAs contain a uridine (U35) 3′ and adjacent to the wobble position, since they decode codons containing an A as second nucleoside of the codon (denoted AII); (NI, NII, and NIII denote the first, second, and third nucleoside of the codon; and N34, N35, and N36 denote the first, second, and third nucleoside of the anticodon; thus, N34 [wobble nucleoside] pairs with NIII, N35 with NII, and N36 with NI). Note that in all organisms (except Mycoplasma) and in all organelles studied to date there are no tRNAs containing U35 reading codons ending in A that are not thiolated at the wobble uridine. Apparently, tRNAs with U34–U35 in their anticodons make poor interaction with the A-rich codons, and therefore require a modification of the wobble nucleoside. If this interpretation is correct, we would predict that an organism having an unmodified U as wobble nucleoside in these tRNAs would be detrimental to cell growth.
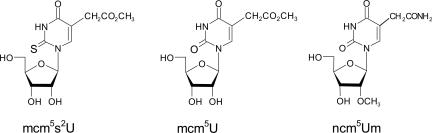
FIGURE 1.
Modified wobble uridines present in yeast tRNA reading twofold degenerate codon boxes (Fig. 2). 5-methylcarboxymethyl-2-thiouridine (mcm5s2U), 5-methylcarboxymethyl-uridine (mcm5U), and 5-carbamoylmethyl-2′-O-methyluridine (ncm5Um).
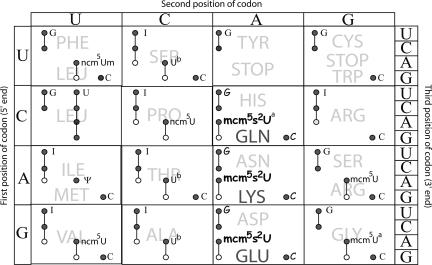
FIGURE 2.
Modified nucleosides in the wobble position and the coding capacities of the corresponding tRNAs. Letters outside the box, to the left, at top, and to the right indicate the first, second, and third position of the codon, respectively. Circles connected by a line, or a single circle, represent one tRNA species. A filled circle indicates the capability of that tRNA to base pair with a particular codon, either by Watson–Crick or by wobble according to the current wobble rules (Yokoyama and Nishimura 1995). An open circle suggests a restricted base pairing. To the right of each tRNA, the modification at positions 34 (the wobble position) is shown. Data compiled from Johansson and Byström (2005). Thus, tRNA specific for Lys, having mcm5s2U as wobble nucleoside, pairs well with AAA (•) but less well with AAG (○). (A) Presence of mcm5s2U in tRNAGln and mcm5U in tRNAGly as wobble nucleosides has been determined (Lu et al. 2005) (M.J.O. Johansson, pers. comm.) (B) tRNAs specific for Ser, Thr, and Ala have ncm5U, which synthesis is dependent on ELP3 (M.J.O. Johansson, pers. comm.).
Transfer RNAs having a U34–U35 in their anticodons are specific for Gln, Lys, and Glu codons, and have a modification of the type xm5s2U (x = any substitution) in the wobble position. In bacteria, these tRNAs have 5-methylaminomethyl-2-thiouridine (mnm5s2U34) as the wobble nucleoside. The mnmE and mnmA genes are required for the synthesis of the side chain at position 5 and sulfur at position 2, respectively (Björk and Hagervall 2005). The mnm5s2U nucleoside is also present in tRNA from Archaea, but so far, mnm5s2U34 has not been identified as a wobble nucleoside in any purified tRNA species from organisms of this domain of life, although there is strong circumstantial evidence that this is the case (Gupta 1984; McCloskey et al. 2001). In eukaryotes, the corresponding tRNAs have 5-methoxycarbonylmethyl-2-thiourdine (mcm5s2U34) as wobble nucleoside. In yeast, the synthesis of the side chain at position 5 requires several proteins including Elp3p (Huang et al. 2005). In order to test the above-mentioned prediction that an unmodified U as wobble nucleoside is detrimental to yeast, it was necessary to first identify a gene whose product is required for the insertion of sulfur at position 2 of U in the wobble position of these tRNAs. Although yeast has a homolog (MTU1) to the bacterial mnmA gene, this gene is not involved in the thiolation of cytoplasmic tRNA, but only in mitochondrial tRNA (Umeda et al. 2005). The product of the bacterial gene ttcA is required in the synthesis of 2-thiocytidine (s2C) at position 32 in a few tRNAs species (Jäger et al. 2004). We identified a gene (TUC1) in the yeast Saccharomyces cerevisiae that is similar to the bacterial ttcA gene, although no s2C32 is present in yeast tRNA. We could show that rather than synthesizing s2C32, the TUC1 gene is required for the thiolation of U in the wobble position of cytoplasmic tRNAs specific for Gln, Lys, and Glu. A double mutant (elp3∷KanMX4 tuc1∷TRP1), which lacks both the s2 and mcm5 groups, cannot survive at a normal level of these tRNA species. However, an excess level of hypomodified tRNALys UUU restored viability of the double mutant, although growth was very poor. Overexpression of hypomodified tRNAGln UUG, but not of tRNAGlu UUC, together with overexpressed hypomodified tRNALys UUU improved growth, but not to that of the wild-type strain. Our results suggest that the major role of the modification of wobble U is to enable tRNAs specific for Gln and Lys to read their cognate codons more efficiently rather than to increase their accuracy, since increased concentration of these tRNAs, still having an unmodified U in the wobble position, rescued growth of the double mutant (elp3∷KanMX4 tuc1∷TRP1).
RESULTS
The yeast protein Ygl211p, resembling the bacterial s2C32-forming protein TtcA, is required for s2U34 formation in cytoplasmic yeast tRNA
The bacterial TtcA protein, which is required for the synthesis of s2C at position 32 of a subset of tRNAs in Escherichia coli, belongs to a protein family with members from all three phylogenetic domains (Jäger et al. 2004). These proteins can be divided into two phylogenetic groups, where bacterial TtcA homologs are in Group 1 and eukaryotic and archaeal homologs are in Group 2 (Fig. 3). Blast analysis with E. coli TtcA toward the S. cerevisiae genome revealed that the protein coded by the YGL211 gene appears to be a homolog of the E. coli TtcA, suggesting that the yeast Ygl211p is involved in the synthesis of s2C32 in yeast tRNA (Ygl211p has 23% identical and 46% conserved amino acids compared with E. coli TtcA). However, no biochemical evidence exists for the presence of s2C in S. cerevisiae. If s2C was in fact present in yeast tRNA at a level found in tRNA from E. coli, it should be readily identifiable, yet is never observed in bulk tRNA samples (data not shown). Thus, it is likely that the Ygl211p is not involved in the synthesis of s2C32, but rather in some other sulfur transfer reaction.
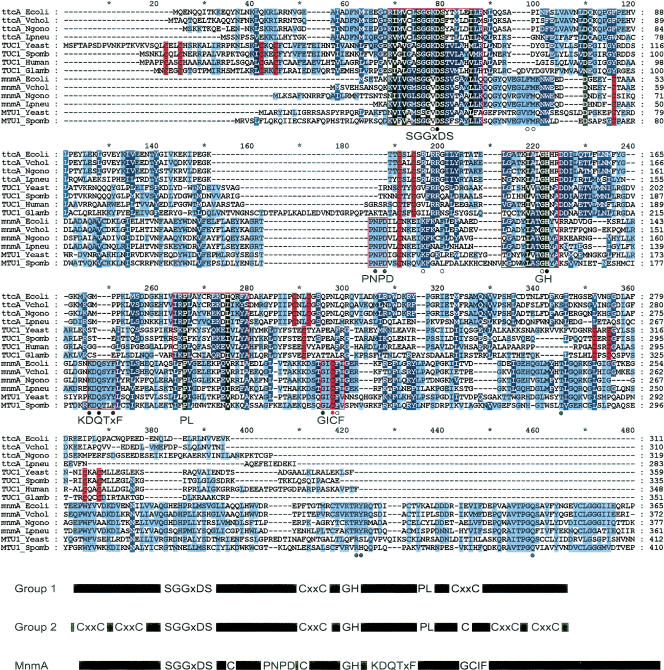
FIGURE 3.
Sequence alignment of four TtcA and four Tuc1p homologs and six MnmA/Mtu1p proteins. Letters shaded black, dark-blue, and light-blue indicate that the indicated amino acid is found in this position in at least 100%, 55%, and 25% of the sequences in the alignment, respectively. Letters shaded red indicate conserved cysteine residues. Cys-X1-X2 -Cys motifs in the central domain of Groups 1 and 2 and in the flanking regions in Group 2 are, consequently, seen as two red stripes close to each other. Conserved regions thought to be important for binding and catalytic function are boxed in red. Amino acids identified by Numata et al. (2006) important for binding of the tRNA substrate (black dots) and for catalytic function (open circles), as well as the two sulphur coordinating cysteines (C102 and C199 in E. coli MnmA, red dots) are indicated. At bottom is shown a schematic comparison of Group 1 (TtcA) and Group 2 (Ygl211p, renamed to Tuc1p, see text) as suggested by Jäger et al. (2004). The central part of MnmA, which is required for the synthesis of s2U34 in bacteria, is distinctly different from Groups 1 and 2 and is similar to the yeast protein Mtu1p, which is responsible for s2U34 formation in mitochondria (Umeda et al. 2005). The central domains of both Group 1 and Group 2 consist of the PP-loop (SGGxDS) motif, a Cys-X1-X2-Cys motif, a GH motif, and a PL motif. In addition to the conserved central domain, Group 2 proteins have two Cys-X1-X2-Cys motifs on each side of the central domain. Note that MnmA has the PP-loop motif and GH motif, but otherwise is not similar to any of the TtcA family proteins, but instead has conserved regions absent in Groups 1/2 thiolases.
The MTU1 gene has been demonstrated to be required for the synthesis of the s2 group of mnm5s2U34 in mitochondria (Umeda et al. 2005). Intriguingly, absence of Mtu1p does not affect the synthesis of mcm5s2U34 in cytoplasmic tRNA, despite the fact that this gene is extremely similar to MnmA from E. coli, which is required for the synthesis of s2U derivatives in this organism (Umeda et al. 2005; data not shown). Because the s2C32 and s2U34 modifications both involve 2-carbon thiolations of nucleosides in the anticodon loop of tRNAs, as well as the remarkably similar primary sequences of TtcA and Ygl211p (Fig. 3), YGL211 was considered as a candidate for the gene required for the synthesis of s2U in yeast cytoplasmic tRNAs. We have renamed YGL211 as TUC1 (thiolation of uridine in cytoplasmic tRNA).
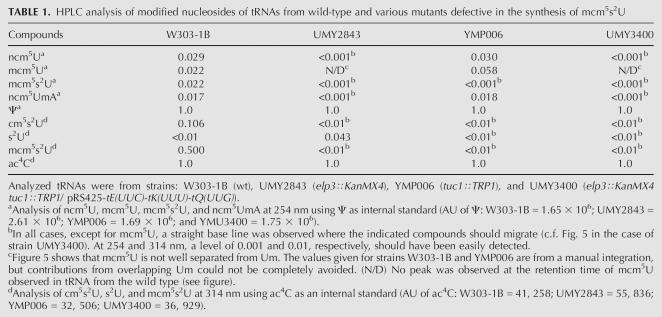
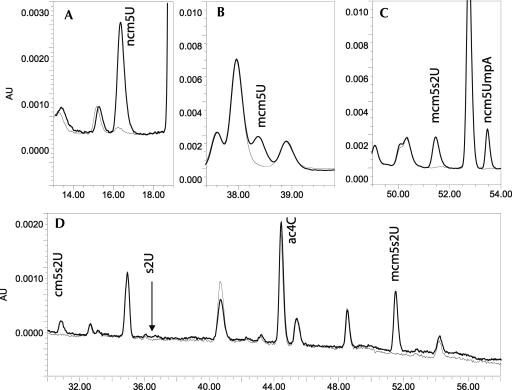
We analyzed both a strain from the yeast deletion collection (Research Genetics) and a deletion strain with a disruption covering the TUC1 ORF that we constructed in the W303-1B strain background. The results from these two strains were indistinguishable. Growth of the tuc1∷TRP1 strain was discernibly different from that of the wild-type strain cultivated on YEPD agar plates at 30°C (Fig. 4). HPLC analysis of nucleosides obtained from bulk tRNA from wild-type and tuc1∷TRP1 strains revealed two specific changes in the base composition of the mutant tRNA when monitored at 254 nm. First, the presence of mcm5s2U is abolished (Table 1) with a concomitant increase in the levels of methylcarboxymethyluridine (mcm5U). To improve the sensitivity of the analysis, tRNAGlu mcm5s2UUC was purified from the tuc1 mutant, but still no mcm5s2U was observed (data not shown). The only sulfur atoms previously known to exist in cytoplasmic yeast tRNA are in the context of mcm5s2U34 modifications present in tRNAGln mcm5s2UUG, tRNALys mcm5s2UUU, and tRNAGlu mcm5s2UUC. In the absence of an s2U sulfurtransferase, mcm5U should accumulate on Gln, Lys, and Glu tRNAs, explaining the increased level of this modified nucleoside in the tRNA from the tuc1∷TRP1 strain (Table 1). This mcm5U migrates close to and partially overlaps Um (Fig. 5). The disappearance of mcm5s2U34 in the tuc1∷TRP1 strain is more clearly visualized in the chromatogram by monitoring the absorbance at 314 nm (Table 1), as thiolated nucleosides generally absorb well at this wavelength, while nonthiolated nucleosides normally do not (Fig. 5). Additionally, at 314 nm it also becomes apparent that another compound with a shorter retention time than mcm5s2U is abolished in tRNA from the tuc1∷TRP1 strain (Fig. 5; Table 1). This compound is far less abundant than the standard modifications in yeast tRNA and its appearance is not observed at 254 nm as it is masked by more prominent compounds. It is clearly a thiolated derivative whose formation is TUC1 dependent, and it has been tentatively identified as an intermediate (cm5s2U) in the synthesis of mcm5s2U (Fig. 5). ELP3 is required for the formation of the modifications at the 5-carbon of U34 in tRNA (Huang et al. 2005). Accordingly, cm5s2U was also absent in the elp3∷KanMX4 mutant, further supporting that this compound is an intermediate in the synthesis of mcm5s2U (Table 1). Thus, this TUC1- and ELP3-dependent compound appears to be an intermediate in the biosynthesis of mcm5s2U34. As expected, we observed the appearance of s2U in the elp3∷KanMX4 mutant, as has also been shown before (Huang et al. 2005). We conclude that the Tuc1p protein is required for the thiolation step in the synthesis of mcm5s2U34 in yeast tRNA, although it resembles the bacterial s2C32-forming enzyme, TtcA.
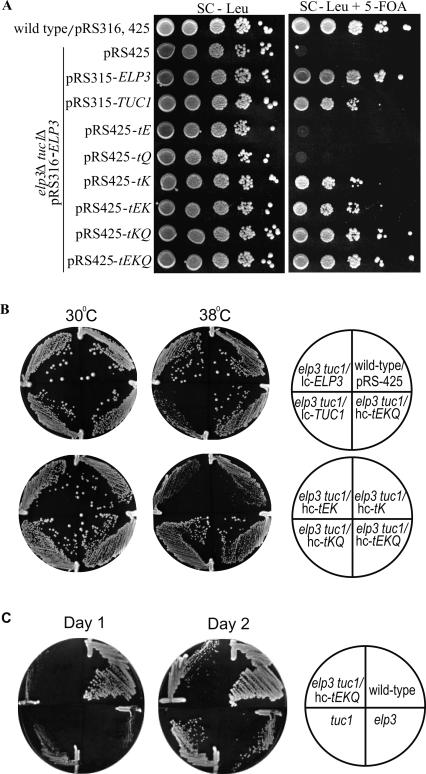
FIGURE 4.
Increased expression of tRNAs normally having mcm5s2U suppresses lethality induced by lack of this modification of wobble uridines. (A) Strains containing the elp3∷KanMX4 tuc1∷TRP1 mutations and carrying the URA3 plasmid pRS316-ELP3 and the LEU2 plasmids pRS425, pRS315-ELP3, pRS-425-tE(UUC), pRS425-tQ(UUG), pRS425-tK(UUU), pRS425-tK(UUU)-tQ(UUG), pRS425-tK(UUU)-tE(UUC), or pRS425-tE(UUC)-tK(UUU)-tQ(UUG) (tE, tK and tQ indicate the genes for tRNAGlu mcm5s2UUC, tRNALys mcm5s2UUU, and tRNAGln mcm5s2UUG, respectively), were grown in synthetic complete medium lacking leucine (SC-leu). Strains were diluted to 3 × 107 cells/mL, 10-fold serially diluted, and 5 μL was spotted onto either SC-leu plates or SC-leu plates containing 5-fluoroorotic acid (5-FOA). These plates were incubated at 30°C for 2 and 3 d, respectively. Cells containing a URA3 plasmid are unable to grow on 5-FOA-containing medium, with the result that these strains only contain the indicated LEU2 vectors when plated on 5-FOA plates (Boeke et al. 1984). (B) Growth of wild-type (W303-1B) and of the double mutant elp3∷KanMX4 tuc1∷TRP1 containing plasmids harboring genes encoding various tRNAs on SC-plates lacking leucine. Cells were picked from SC-Leu+5-FOA plates (Fig. 4A) and restreaked on SC plates lacking Leu. Plates were incubated at 30°C or at 38°C for 2.5 d. Plasmid pRS315 and pRS425 are denoted lc (low copy) and hc (high copy), respectively. (C) Growth of wild-type (W303–1B), tuc1∷TRP1 (YMP006), elp3∷KanMX4 (UMY2843), or the double mutant tuc1∷TRP1, elp3∷KanMX4/ pRS425-tEKQ (UMY3400) on rich medium (YEPD). Plasmid pRS425-tEKQ is denoted hc-tEKQ and its presence results in overexpression of tRNAGlu mcm5s2UUC, tRNALys mcm5s2UUU, and tRNAGln mcm5s2UUG. Growth at 30°C was scored after 1 or 2 d.
TABLE 1.
HPLC analysis of modified nucleosides of tRNAs from wild-type and various mutants defective in the synthesis of mcm5s2U
FIGURE 5.
HPLC analysis of modified nucleosides of tRNA from W303-1B (wild-type; thick line) and from strain UMY3400 (elp3∷KanMX4 tuc1∷TRP/pRS425-tE[UUC]-tK[UUU]-tQ[UUG]) (tE, tK and tQ indicate the genes for tRNAGlu mcm5s2UUC, tRNALys mcm5s2UUU, and tRNAGln mcm5s2UUG; thin line). Transfer RNA was degraded to nucleosides and the distribution of nucleosides was analyzed by HPLC. (A–C) The 13–19 min region, 37–41 min region, and 49.5–54 min region, respectively, of the HPLC chromatogram monitored at 254 nm. (D) The region 30–58 min was monitored at 314 nm. The expected position of s2U is indicated at bottom, and this compound was observed only in tRNA from UMY2843 (elp3∷KanMX4). The identities of cm5s2U and ncm5s2UmpA are tentative, since no markers for these compounds were available.
The double mutant elp3∷KanMX4 tuc1∷TRP1 is not viable, but is rescued by overexpression of only hypomodified tRNALys UUU
The three cytoplasmic tRNAs, which are affected by the loss of the TUC1 gene, are each members of split codon boxes and they decode codons of the general type NAA and can wobble onto NAG codons (Fig. 2). Because numerous genes have been demonstrated to be required for formation of the mcm5 group of mcm5s2U (Huang et al. 2005), it should be possible to effectively strip the uridines in the wobble position in tRNAGln mcm5s2UUG, tRNALys mcm5s2UUU, and tRNAGlu mcm5s2UUC of both the mcm5 and the s2 modification by combining a deletion of one of these genes (e.g., ELP3) with a TUC1 disruption. However, since such a elp3tuc1 double mutant might not be viable, the double mutant was generated in a cross where it was rescued by a wild-type ELP3 gene located on a URA3 plasmid. This strain, which also is leu2, was transformed with a LEU2 vector having ELP3, TUC1, or no chromosomal gene inserted (empty vector). To investigate whether or not these strains could survive without the URA3 plasmid carrying the ELP3 gene, they were grown on a medium containing 5-fluoro-orotic acid (5-FOA). These cells, being Ura+, i.e., having the URA3 plasmid, are unable to grow on a medium containing 5-FOA (Boeke et al. 1984). Growth of cells was obtained on the 5-FOA plates in the presence of the LEU2 plasmids harboring the wild-type allele of TUC1 or ELP3, but not together with the LEU2 plasmid without the chromosomal gene insertion (empty vector) (Fig. 4). Thus, the double mutant is not viable. Recently, it has been shown that overexpression of tRNALys mcm5s2UUU and tRNAGln mcm5s2UUGcounteracted the various phenotypes induced by the elp3∷KanMX4 mutation (Esberg et al. 2006). This result prompted us to test whether a double mutant was viable in the presence of plasmid harboring the genes for the three tRNAs normally having mcm5s2U. Indeed, stable constructs were also obtained if tRNAs specific for Gln, Lys, and Glu were overexpressed when the elp3∷KanMX4 and tuc1∷TRP1 mutations were combined (Fig. 4). Presence of the plasmid harboring the genes for tRNAGln mcm5s2UUG, tRNALys mcm5s2UUU, and tRNAGlu mcm5s2UUC resulted in overexpression of these tRNAs (Fig. 6). However, the strain (UMY3400) overexpressing these tRNAs grew less well than the wild-type strain W303-1B (Fig. 4). Thus, even an excess of the hypomodified forms (see below) of the tRNA normally having mcm5s2U did not fully counteract the growth defect mediated by the lack of mcm5s2U.
FIGURE 6.
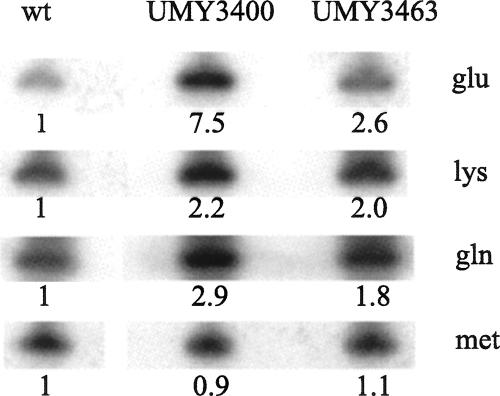
Levels of tRNAGlu mcm5s2UUC, tRNAGln mcm5s2UUG, and tRNALys mcm5s2UUU in strains W303-1B (wt), UMY3400 (elp3∷KanMX4 tuc1∷TRP1/pRS425-tE(UUC)-tK(UUU)-tQ(UUG)), and UMY3463 (ELP3 TUC1/pRS425-tE(UUC)-tK(UUU)-tQ(UUG)). The tRNA levels were monitored by Northern hybridization using radioactive oligonucleotides complementary to nucleotides 57–72 of tRNAGln, 57–73 of tRNALys, 52–72 of tRNAGlu, and 30–49 of tRNAMet. Use of the last oligonucleotide, which monitors the levels of a tRNA not having mcm5s2U, served as controls for how much material was loaded on the gel. The numbers below each lane represent the levels relative to the level of the corresponding tRNAs in strain W303-1B (wild type).
To further evaluate whether or not overexpression of all three tRNAs normally having mcm5s2U as wobble nucleoside was required for viability, plasmids with different combinations of genes encoding the three mcm5s2U-containing tRNAs were introduced into the double mutant containing the URA3 plasmid harboring the ELP3 gene (Fig. 4). When plasmids containing only one of the genes for these three tRNAs was present in conjunction with the complementing URA3 ELP3 plasmid, only cells containing the plasmid harboring the gene for tRNALys mcm5s2UUU resulted in colonies on the 5-FOA plates. Thus, such cells could grow in the absence of the complementing ELP3 gene (Fig. 4). Overexpressed hypomodified tRNAGln UUG, but not overexpressed hypomodified tRNAGlu UUC, together with overexpressed hypomodified tRNALys UUU stimulated growth (Fig. 4). However, even excess of all three tRNAs did not still restore growth to that of the wild-type level on rich medium (Fig. 4). We conclude that viability of the double mutant requires excess of only hypomodified tRNALys UUU. Moreover, excess of hypomodified tRNAGln UUG together with excess of hypomodified tRNALys UUU further stimulated growth of the double mutant elp3∷KanMX4 tuc1∷TRP1.
The double mutant elp3∷KanMX4 tuc1∷TRP1 in the presence of excess hypomodified tRNAGln UUG, tRNALys UUU, and tRNAGlu UUC lacks mcm5s2U
As stated above, it was not possible to obtain the double mutant elp3∷KanMX4 tuc1∷TRP1 except in the presence of excess hypomodified tRNAGln UUG, tRNALys UUU, and tRNAGlu UUC as in strain UMY3400. HPLC analysis of nucleosides obtained from bulk tRNA purified from this strain revealed five specific changes in nucleoside composition (Table 1). First, the disappearance of all mcm5s2U from tRNA, as expected; second, the absence of mcm5U; third, the absence of ncm5U; fourth, the disappearance of a compound with a retention time slightly longer than t6A (Fig. 5). We suggest that this compound is the dinucleotide ncm5UmpA originating from tRNALeu, which has the anticodon sequence ncm5UmpApA. The 2′-O-methylation to the sugar of this nucleoside renders it less susceptible to endonucleolytic cleavage by nuclease P1 and a significant fraction of dinucleotides is retained using our digestion procedure. The complete digestion product, ncm5Um, is masked by other more prominent peaks in the total tRNA digest. Fifth, the disappearance of the intermediate is tentatively identified as cm5s2U above (Table 1). These five nucleosides were all expected to disappear in the elp3∷KanMX4 tuc1∷TRP1 double mutant, as each one is abolished in one or both of the single mutants elp3∷KanMX4 or tuc1∷TRP. It should also be noted that the residual s2U present in the elp3∷KanMX4 mutant is absent in the elp3∷KanMX4 tuc1∷TRP1 double mutant (Table 1).
Aminoacylation and stability of tRNAGlu mcm5s2UUC, tRNAGln mcm5s2UUG, and tRNALys mcm5s2UUU are not affected by hypomodification
Modifications in the wobble position may in some cases improve the aminoacylation of tRNA (Giege et al. 1998). Therefore, the severe phenotype of the double mutant might be explained by low concentration of aminoacylated hypomodified tRNAs. However, this is not the case, since hypomodified tRNAGln UUC, tRNAGln UUG, and tRNALys UUU had the same charging levels as the ones observed in the wild type (Table 2). The mutation in elp3∷KanMX4 abolishes the synthesis of all xm5U derivatives (Fig. 2) (Huang et al. 2005), and such a modification deficiency does not influence the charging of these tRNAs (data not shown). Moreover, the wobble nucleosides in tRNAGlu mcm5s2UUC, tRNAGln mcm5s2UUG, and tRNALys mcm5s2UUU from yeast do not influence the aminoacylation in vitro, although the wobble nucleoside mnm5s2U34 in the corresponding tRNAs from E. coli is an identity element in aminoacylation (Giege et al. 1998).
TABLE 2.
Relative levels of aminoacylated tRNA
Defects in tRNA may also influence its stability; and the severe growth phenotype induced by the hypomodification might be caused by extensive degradation of these tRNAs, resulting in low steady-state levels of hypomodified tRNAGlu UUC, tRNAGln UUG, and tRNALys UUU. To test the stability of the hypomodified tRNAs in the double mutant, we compared the steady state levels of these tRNAs overproduced in the wild-type background (strain UMY3463) with the overproduced levels of the hypomodified tRNAs in the double mutant (strain UMY3400). The steady-state levels of the hypomodified tRNAs were not, in any case, lower than the corresponding fully modified tRNA, suggesting that the lack of this modification did not significantly reduce the stability of these tRNAs (Fig. 6).
We conclude that the induced severe growth phenotype due to lack of mcm5s2U is caused neither by poor aminoacylation nor by a reduced steady-state level of the hypomodified tRNAs. Therefore, the lethality caused by the two mutations should primarily be caused by a poor decoding of the message.
DISCUSSION
The identification of the TUC1 gene as responsible for the formation of the sulfur group of the conserved wobble nucleoside mcm5s2U34 allowed us to construct a yeast strain devoid of both the mcm5 and the s2 modifications in tRNAGln mcm5s2UUG, tRNALys mcm5s2UUU, and tRNAGlu mcm5s2UUC, which read codons in the His/Gln, Asn/Lys, and Asp/Glu split codon boxes, respectively (Figs. 2, 5; Table 1). This strain, which has an unmodified U as wobble nucleoside in these tRNAs, is not viable unless an excess of an unmodified form of one of these tRNAs, tRNALys mcm5s2UUU, is present (Fig. 4). The presence of excess hypomodified tRNAGln UUG together with hypomodified tRNALys UUU stimulated growth further. However, an excess of the unmodified form of all three of these tRNAs did not restore the growth to that of the wild-type level, demonstrating that mcm5s2U deficiency exerts a severe growth defect even in the presence of excess hypomodified tRNA (Fig. 4). Importantly, neither the aminoacylation nor the stability of these tRNAs were affected by the hypomodification. Taken together, these results suggest that the primary effect of mcm5s2U34 is not to reduce mis-sense errors caused by reading the noncognate codons ending with U or C in the split codon boxes, but to improve the efficiency of reading the cognate codon ending with A (the G-ending codons are read also by an alternative tRNA) (Fig. 2).
A possible explanation for the nonviability of the double mutant elp3∷KanMX4 tuc1∷TRP1 would be that Elp3p and Tuc1p have some uncharacterized function(s) beside being required for the synthesis of mcm5s2U. If so, deficiency in these unknown activities of these proteins would cause the nonviability of the double mutant and not the hypomodification of the tRNA. However, the fact that excess of the hypomodified form of the mcm5s2U-containing tRNAs counteracted the lethality induced by the mutations elp3∷KanMX4 and tuc1∷TRP1 argues against such an explanation and rather suggests that it is a translational defect due to mcm5s2U deficiency that causes nonviability of the double mutant elp3∷KanMX4 tuc1∷TRP1.
Formation of s2U34 in tRNA constitutes an interesting example of convergent evolution. This modification is present in hypermodified form in tRNAs specific for, tRNAGln, tRNALys, and tRNAGlu from all organisms except Mycoplasma and in all organelles tested to date. With the discovery that TUC1 is required for the synthesis of s2U34, it becomes evident that it may serve the same role as MnmA in bacteria. The yeast Tuc1p, which thiolates U34, is more similar to the bacterial TtcA, which thiolates C32, than to MnmA (Fig. 3). Thus, it appears unlikely that Tuc1p has been derived from MnmA, even though MnmA is universally present in Bacteria and Eukarya; but rather, it has evolved from an earlier form of the nonessential bacterial protein TtcA, which makes the different, although related, tRNA modification s2C32.
Whereas modification at position 5 of U is present in many tRNAs, the thiolation of U is only present in tRNAs reading the A- and G-ending codons in the His/Gln, Asn/Lys, and Asp/Glu split codon boxes, apparently making this modification of special importance to these tRNAs. Unlike tRNAs decoding in all other codon boxes, tRNAs decoding in the His/Gln, Asn/Lys, and Asp/Glu split codon boxes have U35. Since uracil is the base with the lowest stacking potential, the anticodon of the tRNAs reading the A- and G-ending codons in these split codon boxes are, when unmodified, inherently unstable, since the first two encoded nucleosides in the anticodon of these tRNAs are U34 and U35. Structural analyses using various model systems have revealed that the xm5s2 modification improves stacking and induces a proper conformation of the anticodon loop adapting it to efficient decoding (Yokoyama et al. 1979, 1985; Houssier et al. 1988; Kumar and Davis 1997; Grosjean et al. 1998; Ashraf et al. 1999; Sundaram et al. 2000; Durant et al. 2005; Agris et al. 2006). The lethality induced by lack of mcm5s2U, as observed, supports this suggestion and demonstrates the pivotal role that this conserved modification has on the viability of yeast. Interestingly, an excess of hypomodified tRNALys UUU by itself restored viability to the elp3∷KanMX4 tuc1∷TRP1 double mutant (Fig. 4), suggesting that the mcm5s2U modification is of special importance to this tRNA. The reason for the pivotal role of mcm5s2U in this tRNA is most likely because it is the only tRNA having the anticodon sequence U34–U35–U36, which, unmodified, has a poor stacking capacity and is highly flexible. In fact, the unmodified tRNALys UUU does not form a canonical anticodon loop unless it contains mcm5s2U (Ashraf et al. 1999; Durant et al. 2005).
Although the mcm5 group by itself has only limited impact on the remodeling of the anticodon, and thereby its stability, compared with the s2 group (Durant et al. 2005), lack of the mcm5 modification in the elp3∷KanMX4 mutant reduced growth more than that caused by the s2 deficiency in the tuc1∷TRP1 mutant (Fig. 4). An explanation of this apparent inconsistency may be that the various xm5 groups are present in many more tRNAs than the s2 group is (Fig. 1), and the final phenotypic outcome may be an additive effect by the xm5 deficiency of all of the xm5-containing tRNAs. This also explains why overexpression of unmodified tRNALys UUU, tRNAGln UUG, and tRNAGlu UUC did not fully suppress the growth phenotype to that exhibited by the wild type.
A unmodified U34 may base pair with UIII and CIII under certain conditions (Lim 1994; Yokoyama and Nishimura 1995), and structural data of the decoding center on the 30S ribosomal subunit show that there are no constraints precluding a U34–UIII or U34–CIII pairing (Murphy et al. 2004). Such decoding can be allowed in the family codon boxes because it would not induce any mis-sense errors. Similar decoding by U34 is not allowed in the split codon family boxes His/Gln, Asn/Lys, and Asp/Glu, since such base pairing would result in mis-sense errors (see Fig. 2). It is, therefore, reasonable to suppose that the ubiquitous presence of the xm5s2U modification in tRNA reading codons in these split codon boxes are there to prevent mis-sense errors. It follows that the major cause of the nonviability of the elp3∷KanMX4 tuc1∷TRP1 double mutant could be the misreading of the U- and C-ending codons in the split codon boxes His/Gln, Asn/Lys, and Asp/Glu by U34 (Fig. 2) (as discussed above). Alternatively, the mcm5 and s2 modifications of the wobble uridine in these tRNAs may be present primarily to improve the decoding efficiency of the cognate codons. It is possible that a combination of both of these alternatives is in operation. One way to increase the reading efficiency of the cognate codons would be to increase the concentration of the corresponding hypomodified tRNAs, provided that such an increase of the aminoacylated tRNA would also result in a corresponding increase of the ternary complex. Indeed, excess of unmodified variants of the tRNALys mcm5s2UUU encoded from a plasmid restored viability of the elp3∷KanMX4 tuc1∷TRP1 double mutant (Fig. 4), and the presence of hypomodified tRNAGln mcm5s2UUG partially suppressed the poor growth obtained by the presence of tRNALys mcm5s2UUU (Figs. 4). As stated above, complete suppression by overexpression of tRNALys UUU, tRNAGln UUG, and tRNAGlu UUC cannot be expected, since the elp3 mutation also removes the xm5 modification of eight other tRNA species (Fig. 1). We therefore suggest that the primary function of mcm5s2U34 is to improve the reading of the cognate codons rather than to prevent mis-sense errors, since an excess of unmodified tRNA specific for Gln, Lys, and Glu should increase rather than decrease misreading. A direct test for the hypothesis that these modifications should improve reading fidelity did not support such a role of the modifications (Hagervall et al. 1998). Moreover, deficiency of the wobble nucleoside 5-taurinomethyl-2-thiouridine (τm5s2U34) of mitochondrial tRNALys, which induces the mitochondrial disease myoclonus epilepsy associated with ragged-red fibers (MERRF), reduces the efficiency of reading the cognate codons AAA/G (Yasukawa et al. 2001). These and other experiments (for review, see Björk and Hagervall 2005) support our suggestion that the primary function of mcm5s2U34 is to improve the reading of the cognate codons. This is also consistent with the postulated function of the xm5s2U modifications as deduced from structural data (see above) (Murphy et al. 2004; Durant et al. 2005). However, in certain organisms, some of these split codon boxes are read only with an xm5s2U34-containing tRNA (e.g., in the Asn/Lys and Asp/Glu split codon boxes in E. coli and Salmonella enterica Serovar Typhimurium). Thus, in these cases the efficiency of this modified nucleoside to wobble toward GIII must be sufficient, and no other tRNA is required. Yeast (Fig. 2), as well as many other organisms, has other tRNAs with C34 as wobble nucleoside, which efficiently read the G-ending codons in the His/Gln, Asn/Lys, and Asp/Glu split codon boxes, suggesting that in these organisms the ability of the xm5s2U34 to base pair with G is limited.
MATERIALS AND METHODS
Media and yeast strains
Media and genetic procedures used have been described (Burke et al. 2000). Yeast strains are listed in Table 3. A one-step gene replacement was performed to disrupt the TUC1 gene (Brachmann et al. 1998). Oligonucleotides5′-TTTGGCGATGAGACGATATGGTAAGAGTAAAGCAAAGGA ACCGTCAGATTGTACTGAGAGTGCAC and 5′-ATTATGTTACGCTGCATTCTT CTACTGCGAGCTATATATATGTCACTGTGCGGTATTTCACACCG were used to PCR amplify a DNA fragment containing the TRP1 marker and 45 nt of TUC1 flanking sequences on both sides. Strain UMY3373, in which both the TUC1 and ELP3 genes are disrupted and where the viability of the strain is dependent on an ELP3 gene located on the URA3-based plasmid pRS316, was constructed in a cross between strains YMP006 and UMY2843. The diploid was transformed with plasmid pRS316-ELP3 and in the concomitant tetrad analysis, offspring were identified that were G418R, Trp+, and Ura+, which is indicative that the spore had the elp3∷KanMX4 tuc1∷TRP1 alleles and harbored the pRS316-ELP3 plasmid. The construction of the double mutant elp3∷KanMX4 tuc1∷TRP1 harboring plasmids encoding various tRNA genes is described below.
TABLE 3.
Saccharomyces cerevisiae strains
Plasmid shuffling assay
In strain UMY3373, the endogenous TUC1 and ELP3 genes are disrupted and the viability of the strain is dependent on an URA3-based plasmid carrying the ELP3 gene (pRS316-ELP3). These strains were transformed with an LEU2 plasmid carrying no insert (pRS425), ELP3 (pRS315-ELP3), TUC1 (pRS315-TUC1), tE(UUC) (pRS425-tE), tQ(UUG) (pRS425-tQ), tK(UUU) (pRS425-tK), tE(UUC) tK(UUU) (pRS425-tEK), tK(UUU) tQ(UUG) (pRS425-tKQ), or tE(UUC) tK(UUU) tQ(UUG) genes (pRS425-tEKQ) (Lu et al. 2005). To investigate whether or not the strains were able to grow in the absence of the URA3-ELP3 plasmid, strains were first grown in synthetic complete medium lacking leucine (SC-Leu), and thereafter serially diluted on SC-Leu plates and SC-Leu plates containing 5-fluoroorotic acid (5-FOA). Plates were incubated at 30°C. Cells maintaining the URA3 plasmid are unable to grow on plates containing 5-FOA (Boeke et al. 1984). Strains capable of growing in the absence of the URA3-ELP3-rescuing plasmid were analyzed for growth at 30°C and 38°C.
Analysis of modified nucleosides in tRNA
Cells were grown in YEPD medium at 25°C to about OD600 of 1.0. In all cases, the cultures used for tRNA preparations were homogenous and no revertants or contaminants were observed, as judged from single-cell streak on agar plates at the time of harvest. Cells were washed and resuspended in 0.9% NaCl and 2 vol of phenol were added. The mixture was shaken for 30 min at room temperature and then 0.1 vol of chloroform was added and the mixture was shaken for an additional 15 min. The water and phenol phases were separated by centrifugation for 20 min at 10–15.000 rpm in a Sorvall GS3 rotor. Adding 2.5 vol of ethanol and 0.1 vol of 20% potassium acetate precipitated the tRNA in the water phase. Transfer RNA was extracted by 2 M of LiCl as described earlier (Avital and Elson 1969) and precipitated with ethanol. To remove excess salt, another ethanol precipitation was performed, and the resulting tRNA pellet was washed with 70% ethanol. Such tRNA preparation was dissolved in R200 buffer (10 mM Tris-H3PO4 at pH 6.3; 15% ethanol, 200 mM KCl) and applied to a Nucleobond column (AX500) equilibrated with the same buffer. The column was washed with 6 mL of R200 and 2 mL of R600 buffer (similar to R200 buffer, but containing 600 mM KCl) and this combined eluate was discarded. Transfer tRNA was then eluted with 7 mL of buffer R600 and precipitated with 0.7 vol of cold isopropanol. The precipitate was dissolved in water and precipitated with ethanol, the precipitate was washed twice with 80% ethanol, and dried. The dried tRNA was dissolved in water, heated to 95°C for 2.5 min, and quickly put on ice. A portion of it was degraded to nucleosides by nuclease P1, followed by treatment with bacterial alkaline phosphatase (Gehrke et al. 1982). The hydrolysate was analyzed by HPLC (Gehrke and Kuo 1990) using a Develosil C30 reverse-phase column (250 × 4.6 mm; Phenomex Ltd.).
Analysis of tRNA levels
Strains (W303-1B, wild type; UMY3400 (elp3∷KanMX4 tuc1∷TRP1/pRS425-tE(UUC)-tK(UUU)-tQ(UUG)), and UMY3463 (ELP3 TUC1/pRS425-tE(UUC)-tK(UUU)-tQ(UUG)) were grown in 10 mL of YEPD medium at 25°C to about OD600 of 1.0. Following centrifugation the pellet was suspended in 1 mL of ice-cold water. Cells were collected by centrifugation and resuspended in 400 μL of 10 mM Tris-EDTA, (pH 7.5). The same amount of acid phenol was added and the mixture was vigorously shaken for 10 sec and incubated for 45 min at 65°C with occasional shaking before the phases were separated by centrifugation. To the water phase, 400 μL of chloroform was added and the mixture was shaken, the water phase was transferred to a clean test tube, and 40 μL of 3 M sodium acetate (pH 5.3) and 1 mL of 100% ethanol was added. The precipitated RNA was washed once with 70% ethanol, centrifuged, and dissolved in 50 μL of water. About 5 μg of RNA was applied to an 8% polyacrylamide gel containing 8 M Urea in 89 mM Tris-borate buffer (pH 8.2) containing 2 mM EDTA. Following electrophoresis, the gel was transferred to a Zeta probe membrane and the RNA were UV cross-linked to the membrane. The tRNAs was detected by Northern hybridization using radioactive oligonucleotides complementary to nucleotides 57–72 of tRNAGln mcm5s2UUG, 57–73 of tRNALys mcm5s2UUU, 52–72 of tRNAGlu mcm5s2UUC, and 30–49 of tRNAMet CAU. The last oligonucleotide, which monitors the levels of two tRNAs not having mcm5s2U, served as controls for how much materials was loaded on the gel.
Determination of aminoacylation of tRNAGln mcm5s2UUG, tRNALys mcm5s2UUU, and tRNAGlu mcm5s2UUC in vivo
Cells were grown in 30 mL of YEPD medium at 25°C (except for strain UMY2843, which was grown at 30°C) to about OD600 of 1.0, and cells were collected by centrifugation. Cells were resuspended in 1 mL of water, washed once with 1 mL of water, and finally resuspended in 500 μL of cold 0.1 M NaAc (pH 4–5) containing 10 mM EDTA. To the suspension of cells, 200 uL of glass beads and 500 μL of 25:24:1 phenol-chloroform-isoamylalcohol mixture were added and the mixture was vortexed four times for 1 min with 1 min on ice between the shaking. Following centrifugation, the supernatant was transferred to a new tube and RNA was precipitated by adding 3 vol of ethanol. The RNA was dissolved in 50 μL of 10 mM NaAc (pH 4.5) containing 1 mM EDTA. Half of the sample was diluted with an equal volume of 0.5 M Tris HCl (pH 9.0) and incubated for 20 min at 37°C. The deacylated and nontreated samples were run on an acid gel containing 8% polyacrylamide, 8 M urea, and 0.1 M NaAc (pH 5.0). RNA was transferred to Zeta probe membrane and the tRNA was detected as above.
ACKNOWLEDGMENTS
We thank Drs. Tord G. Hagervall, Umeå, Marcus Johansson, Ivor Tittawella, Jaunius Urbonavicius, Umeå, and Hans Wolf-Watz, Umeå, for critical reading of the manuscript. We are also grateful to Mike G. Pollard, who, while working in the laboratory of G.R.B., pointed out that YLG211 might encode the s2U thiolase in yeast. We thank K. Jacobsson for skillful analysis of modified nucleosides by HPLC and Kristina Nilsson for technical assistance. This work was supported by grants from the Swedish Cancer Foundation (Projects 680 to G.R.B. and 3516-B05-12XAB to A.S.B.), Swedish Science Research council (Projects BU-2930 to G.R.B. and 621-2006-4269 to A.S.B.), and Margareta Dannbergs Foundation (Project 223-302-06 to A.S.B.)
Footnotes
Article published online ahead of print. Article and publication date are at http://www.rnajournal.org/cgi/doi/10.1261/rna.558707.
REFERENCES
- Agris, P.F., Vendeix, F.A., Graham, W.D. tRNA's wobble decoding of the genome: 40 years of modification. J. Mol. Biol. 2006;366:1–13. doi: 10.1016/j.jmb.2006.11.046. [DOI] [PubMed] [Google Scholar]
- Ashraf, S.S., Sochacka, E., Cain, R., Guenther, R., Malkiewicz, A., Agris, P.F. Single atom modification (O→S) of tRNA confers ribosome binding. RNA. 1999;5:188–194. doi: 10.1017/s1355838299981529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avital, S., Elson, D. A convenient procedure for preparing transfer ribonucleic acid from Escherichia coli . Biochim. Biophys. Acta. 1969;179:297–307. doi: 10.1016/0005-2787(69)90038-0. [DOI] [PubMed] [Google Scholar]
- Björk, G.R. Modified nucleosides in positions 34 and 37 of tRNAs and their predicted coding capacities. In: Grosjean H., Benne B., editors. Modification and editing of RNA. American Society for Microbiology; Washington, DC: 1998. pp. 577–581. [Google Scholar]
- Björk, G.R., Hagervall, T.G. 25 July 2005, posting date. Chapter 4.6.2. Transfer RNA modification. In Escherichia coli and Salmonella. In: Böck A., et al., editors. Cellular and molecular biology. ASM Press; Washington, DC: 2005. [Google Scholar]
- Boeke, J.D., LaCroute, F., Fink, G.R. A positive selection for mutants lacking orotidine-5′-phosphate decarboxylase activity in yeast: 5-Fluoro-orotic acid resistance. Mol. Gen. Genet. 1984;197:345–346. doi: 10.1007/BF00330984. [DOI] [PubMed] [Google Scholar]
- Brachmann, C.B., Davies, A., Cost, G.J., Caputo, E., Li, J., Hieter, P., Boeke, J.D. Designer deletion strains derived from Saccharomyces cerevisiae S288C: A useful set of strains and plasmids for PCR-mediated gene disruption and other applications. Yeast. 1998;14:115–132. doi: 10.1002/(SICI)1097-0061(19980130)14:2<115::AID-YEA204>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- Burke, D., Dawson, D., Stearns, T. Methods in yeast genetics. Cold Spring Harbor Laboratory Press; Cold Spring Harbor, NY: 2000. [Google Scholar]
- Durant, P.C., Bajji, A.C., Sundaram, M., Kumar, R.K., Davis, D.R. Structural effects of hypermodified nucleosides in the Escherichia coli and human tRNA(Lys) anticodon loop: The effect of nucleosides s2U, mcm5U, mcm5s2U, mnm5s2U, t6A, and ms2t6A. Biochemistry. 2005;44:8078–8089. doi: 10.1021/bi050343f. [DOI] [PubMed] [Google Scholar]
- Esberg, A., Huang, B., Johansson, M.J.O., Byström, A.S. Elevated levels of two tRNA species bypass the requirement for elongator complex in transcription and exocytosis. Mol. Cell. 2006;24:139–148. doi: 10.1016/j.molcel.2006.07.031. [DOI] [PubMed] [Google Scholar]
- Fiorentini, P., Huang, K.N., Tishkoff, D.X., Kolodner, R.D., Symington, L.S. Exonuclease I of Saccharomyces cerevisiae functions in mitotic recombination in vivo and in vitro. Mol. Cell. Biol. 1997;17:2764–2773. doi: 10.1128/mcb.17.5.2764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gehrke, C.W., Kuo, K.C., McCune, R.A., Gerhardt, K.O., Agris, P.F. Quantitative enzymatic hydrolysis of tRNAs: Reversed-phase high-performance liquid chromatography of tRNA nucleosides. J. Chromatogr. 1982;230:297–308. [PubMed] [Google Scholar]
- Gehrke, C.W., Kuo, K.C. Ribonucleoside analysis by reversed-phase high performance liquid chromatography. In: Gehrke C.W., Kuo K.C.T., editors. Chromatography and modification of nucleosides. Part A. Analytical methods for major and modified nucleosides. Vol. 45A. Elsevier; Amsterdam, The Netherlands: 1990. pp. A3–A71. J. Chromatography Library. [Google Scholar]
- Giege, R., Sissler, M., Florentz, C. Universal rules and idiosyncratic features in tRNA identity. Nucleic Acids Res. 1998;26:5017–5035. doi: 10.1093/nar/26.22.5017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grosjean, H., Houssier, C., Romby, P., Marquet, R. Modulatory role of modified nucleotides in RNA loop–loop interaction. In: Grosjean H., Benne R., editors. Modification and editing of RNA. ASM Press; Washington, DC: 1998. pp. 113–133. [Google Scholar]
- Gupta, R. Halobacterium volcanii tRNAs. Identification of 41 tRNAs covering all amino acids, and the sequences of 33 class I tRNAs. J. Biol. Chem. 1984;259:9461–9471. [PubMed] [Google Scholar]
- Hagervall, T.G., Pomerantz, S.C., McCloskey, J.A. Reduced misreading of asparagine codons by Escherichia coli tRNALys with hypomodified derivatives of 5-methylaminomethyl-2-thiouridine in the wobble position. J. Mol. Biol. 1998;284:33–42. doi: 10.1006/jmbi.1998.2162. [DOI] [PubMed] [Google Scholar]
- Houssier, C., Deg:ee, P., Nicoghosian, K., Grosjean, H. Effect of uridine dethiolation in the anticodon triplet of tRNAGlu on its association with tRNA(Phe) J. Biomol. Struct. Dyn. 1988;5:1259–1266. doi: 10.1080/07391102.1988.10506468. [DOI] [PubMed] [Google Scholar]
- Huang, B., Johansson, M.J., Bystrom, A.S. An early step in wobble uridine tRNA modification requires the Elongator complex. RNA. 2005;11:424–436. doi: 10.1261/rna.7247705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jäger, G., Leipuviene, R., Pollard, M.G., Qian, Q., Björk, G.R. The conserved Cys-X1-X2-Cys motif present in the TtcA protein is required for the thiolation of cytidine in position 32 of tRNA from Salmonella enterica serovar typhimurium. J. Bacteriol. 2004;186:750–757. doi: 10.1128/JB.186.3.750-757.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansson, M.J.O., Byström, A.S. Transfer RNA modifications and modifying enzymes. In: Grosjean H., editor. Fine-tuning of RNA functions by modification and editing. Springer-Verlag; New York: 2005. pp. 87–120. [Google Scholar]
- Kumar, R.K., Davis, D.R. Synthesis and studies on the effect of 2-thiouridine and 4-thiouridine on sugar conformation and RNA duplex stability. Nucleic Acids Res. 1997;25:1272–1280. doi: 10.1093/nar/25.6.1272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim, V.I. Analysis of action of wobble nucleoside modifications on codon–anticodon pairing within the ribosome. J. Mol. Biol. 1994;240:8–19. doi: 10.1006/jmbi.1994.1413. [DOI] [PubMed] [Google Scholar]
- Lu, J., Huang, B., Esberg, A., Johansson, M.J., Bystrom, A.S. The Kluyveromyces lactis {γ}-toxin targets tRNA anticodons. RNA. 2005;11:1648–1654. doi: 10.1261/rna.2172105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCloskey, J.A., Graham, D.E., Zhou, S., Crain, P.F., Ibba, M., Konisky, J., Soll, D., Olsen, G.J. Post-transcriptional modification in archaeal tRNAs: Identities and phylogenetic relations of nucleotides from mesophilic and hyperthermophilic Methanococcales . Nucleic Acids Res. 2001;29:4699–4706. doi: 10.1093/nar/29.22.4699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy, F.V., Ramakrishnan, V., Malkiewicz, A., Agris, P.F. The role of modifications in codon discrimination by tRNALys UUU . Nat. Struct. Mol. Biol. 2004;11:1186–1192. doi: 10.1038/nsmb861. [DOI] [PubMed] [Google Scholar]
- Numata, T., Fukai, S., Ikeuchi, Y., Suzuki, T., Nureki, O. Structural basis for sulfur relay to RNA mediated by heterohexameric TusBCD complex. Structure. 2006;14:357–366. doi: 10.1016/j.str.2005.11.009. [DOI] [PubMed] [Google Scholar]
- Sundaram, M., Durant, P.C., Davis, D.R. Hypermodified nucleosides in the anticodon of tRNALys stabilize a canonical U-turn structure. Biochemistry. 2000;39:12575–12584. doi: 10.1021/bi0014655. [DOI] [PubMed] [Google Scholar]
- Umeda, N., Suzuki, T., Yukawa, M., Ohya, Y., Shindo, H., Watanabe, K., Suzuki, T. Mitochondria-specific RNA-modifying enzymes responsible for the biosynthesis of the wobble base in mitochondrial tRNAs. Implications for the molecular pathogenesis of human mitochondrial diseases. J. Biol. Chem. 2005;280:1613–1624. doi: 10.1074/jbc.M409306200. [DOI] [PubMed] [Google Scholar]
- Yasukawa, T., Suzuki, T., Ishii, N., Ohta, S., Watanabe, K. Wobble modification defect in tRNA disturbs codon-anticodon interaction in a mitochondrial disease. EMBO J. 2001;20:4794–4802. doi: 10.1093/emboj/20.17.4794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yokoyama, S., Nishimura, S. Modified nucleosides and codon recognition. In: Söll D., Rajbhandary U.L., editors. tRNA: Structure, biosynthesis, and function. ASM Press; Washington, D.C: 1995. pp. 207–223. [Google Scholar]
- Yokoyama, S., Yamaizumi, Z., Nishimura, S., Miyazawa, T. 1H NMR studies on the conformational characteristics of 2-thiopyrimidine nucleotides found in transfer RNAs. Nucleic Acids Res. 1979;6:2611–2626. doi: 10.1093/nar/6.7.2611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yokoyama, S., Watanabe, T., Murao, K., Ishikura, H., Yamaizumi, Z., Nishimura, S., Miyazawa, T. Molecular mechanism of codon recognition by tRNA species with modified uridine in the first position of the anticodon. Proc. Natl. Acad. Sci. 1985;82:4905–4909. doi: 10.1073/pnas.82.15.4905. [DOI] [PMC free article] [PubMed] [Google Scholar]