Abstract
Type 1 diabetes mellitus subjects millions to a daily burden of disease management, life threatening hypoglycemia and long-term complications such as retinopathy, nephropathy, heart disease, and stroke. Cell transplantation therapies providing a glucose-regulated supply of insulin have been implemented clinically, but are limited by safety, efficacy and supply considerations. Stem cells promise a plentiful and flexible source of cells for transplantation therapies. Here we show that cells derived from human embryonic germ (EG) cells express markers of definitive endoderm, pancreatic and β-cell development, glucose sensing, and production of mature insulin. These cells integrate functions necessary for glucose-responsive regulation of preproinsulin mRNA and expression of insulin C-peptide in vitro. Following transplantation into mice, cells become insulin and C-peptide immunoreactive and produce plasma C-peptide in response to glucose. These findings suggest that EG cell derivatives may eventually serve as a source of insulin producing cells for the treatment of diabetes.
Keywords: Diabetes, stem, pancreas, endoderm, transplantation, development
The promise of a cellular therapy for type 1 diabetes has been sparked by the introduction of novel immunosuppressive regimens used in conjunction with cadaveric islet transplantation [1]. However, scarcity of cadaveric islets motivates the search for an alternate source of glucose-responsive insulin-producing (GRIP) cells. Pluripotent stem cells are a promising source for cellular based therapies. Spontaneous insulin production by mouse and human ES cell derivatives [2–5], as well as induced expression following manipulation of in vitro growth conditions [6–11] or genetic modification [12–14] have been reported.
Human embryonic germ (EG) cells are pluripotent stem cells derived from primordial germ cells [15]. Like ES cells, EG cells differentiate in vitro to form embryoid bodies (EBs) comprised of mature cell types and rapidly proliferating progenitor cells. Outgrowth of cells from disaggregated human EBs yields embryoid body-derived (EBD) cell cultures that proliferate robustly with a normal diploid karyotype and express progenitor and differentiated cell markers [16]. EBD culture LVEC has been differentiated into cells of the musculoskeletal lineages in vitro [17]. In this report we describe conditions under which LVEC cells express markers of definitive endoderm, pancreatic development and function. These cells achieve a GRIP phenotype in vitro and in vivo.
MATERIALS AND METHODS
Cell culture
LVEC cells were plated on type I collagen coated plates at 1.9 × 104 cells/cm2 and grown in 5 mM glucose, 5% fetal calf serum, basic fibroblast growth factor, insulin like growth factor I, vascular endothelial growth factor, epidermal growth factor, hydrocortisone, ascorbic acid, gentamycin, and amphotericin (EGM2MV, Clonetics) as described [16]. Day of plating is D0, media was replaced on D1, 3, 6 and 9. Mean cell number (n=3 counts) was determined by automated counting of cell nuclei (Nucleocounter, New Brunswick Scientific). Viability was determined by incubation with 0.4% solution of Trypan blue (Sigma).
Glucose Exposure in vitro
Cultures were supplemented daily to 5 mM glucose, 1 hr prior to RNA harvest, media was adjusted to 5mM or 20mM. To test for glucose responsive insulin release, on D6 and 8, cells plated in triplicate were incubated 2 hrs in media supplemented serially with 5 mM, 20 mM then 5 mM glucose or equivalent concentrations of mannitol. C-peptide levels were determined by using the 1-2-3 Ultrasensitive human C-peptide ELISA kit (Mercodia/Alpco), readings were done in duplicate. Significance was determined by 2-tailed paired Student’s T-test.
Reverse Transcriptase-Polymerase Chain Reactions
cDNA was synthesized by using oligo (dT) primers in a standard Moloney Murine Leukemia Virus reaction. Twenty to 35 cycles of PCR were carried out with 1 min. each for denaturation at 95°C, annealing and polymerase at 72°C. Oligonucleotide primer sequences, number of cycles and annealing temperatures are provided (Supplementary Table 1). Amplimers were resolved on 1–3% agarose gels, imaged by using a BioRad Chemidoc XRS and DNA sequence confirmed. All reactions were performed at a minimum in biological duplicate. The mRNA levels of preproinsulin and Cyclophilin A were determined by using Taqman® gene expression assays Hs00355733_m1 and Hs99999904_m1, respectively. Mean levels (3–5 readings/sample) of insulin were normalized to mean levels of Cyclophilin A. Significance was determined by 2-tailed heteroscedastic Student’s T-test. Threshold cycle (Ct) numbers are reported in Supplementary Table 2.
Immunocytochemistry
Staining LVEC at high cell density was carried out at D1, 6 and 8. LVEC cells and cultured human cadaveric islets were disaggregated by incubation in 0.25% trypsin/EDTA for 5–10 min. at 37°C. Cells were resuspended in calcium/magnesium free Dulbecco’s phosphate buffered saline (DPBS) and affixed to glass microscope slides by using a cytospin. LVEC cells were grown at low density on glass chamber slides coated with type I collagen. Cells were fixed in ice-cold 4% paraformaldehyde (PFA) in DPBS for 4 min., washed in 10 mM glycine in DPBS, incubated in DPBS, 0.3% Triton-X100, 1% BSA, 5% animal serum for 30 min. at room temperature, then incubated overnight at 4°C in primary antibody: guinea pig anti-porcine insulin (DAKO), rabbit anti-human C-peptide (Linco). Rabbit anti-glucagon (Zymed), rabbit anti-somatostatin (Zymed), goat anti-SOX17 (R&D Biosystems), goat anti-FOXA2 (R&D Biosystems), rabbit anti-SOX7 (Santa Cruz). Secondary antibodies were conjugated to Alexa-488 or -555 (Invitrogen). Cells were counterstained with 4′,6-diamidino-2-phenylindole (DAPI). Quantitative analyses were performed by manual counting stained cells in 4 or more random fields.
Immunohistochemistry
Five to 8μ sections of human pancreas and mouse tissues following LVEC cell injection were blocked in 10% animal serum, 1% BSA in DPBS for 1 hr at room temperature. Sections were stained with antibodies developed against human C-peptide (Linco), porcine insulin (DAKO) or pan cytokeratin (Chemicon) overnight at 4°C. Secondary antibodies were fluorescently labeled or conjugated to horseradish peroxidase (DAKO). Cells were counterstained with DAPI or hematoxylin.
Cell transplantation
NOD/LtSz-Prkdcscid/J mice fed ad libitum were given an intraperitoneal (IP) injection of 125 mg/kg body weight streptozotocin (STZ) in 0.1M sodium citrate pH 4.5 or citrate alone. Blood glucose levels were monitored to establish diabetes onset. To facilitate cell tracking but minimize cell toxicity, 20% of LVEC cells at 2.0×104 cells/cm2 were labeled by incubation with 500 ng/ml Hoechst 33342 (Sigma) and 5 μM Vybrant DiD (Invitrogen) for 1 hr. Cells were resuspended in calcium/magnesium-free DPBS at a concentration of 107 cells/ml. Animals were anesthetized by inhalation of 3–5% Enthrane. Approximately 0.1 ml cells were injected into the spleen or mixed with an equal volume of Matrigel™ and injected under the kidney capsule. An IP glucose tolerance test (2 g glucose/kg BW) was performed 22 days after splenic injection. Glucose > 600 mg/dL was plotted as 600 mg/dL. Human C-peptide was determined by using ELISA. Readings for each animal were made in duplicate (n= 4 animals). A 2-tailed heteroscedastic Student’s T-test was used to determine significant difference in blood glucose concentration. A 2-tailed paired Student’s T-test was used to determine significance of C-peptide values. Thirty days following cell injection kidneys were harvested.
RESULTS
LVEC cells express markers of glucose sensing, insulin production, and processing
Proliferating LVEC cells express low or undetectable levels of insulin mRNA. When LVEC cells were plated at 1.9×104 cells/cm2 and allowed to increase to 9×104 cells/cm2 (Supplementary Fig. 1) expression of preproinsulin (INS), glucose transporter 2 (GLUT2), glucokinase (GCK), ATP-sensitive inward rectifier potassium channel 11 (KIR6.2) and prohormone convertase 1/3 (PC1/3) mRNA was detected throughout the time course. INS and KIR6.2 mRNAs were not detected on D1 but were detected by D6 (Supplementary Fig. 2). >90% of adherent cells remained viable (data not shown).
On D8, approximately 5% of LVEC cells express cytoplasmic insulin protein (17/355) (Fig. 1A) or C-peptide (14/270) (Fig. 1C). With limited disaggregation prior to staining, most insulin+ or C-peptide+ cells appeared in multicellular clusters. Fewer insulin (3.8%, 8/208) and C-peptide (3.8%, 13/340) positive cells were present on D6 and no staining was observed on D1 (data not shown). No expression of glucagon or somatostatin (Fig. 1E,G) was detected.
Figure 1.
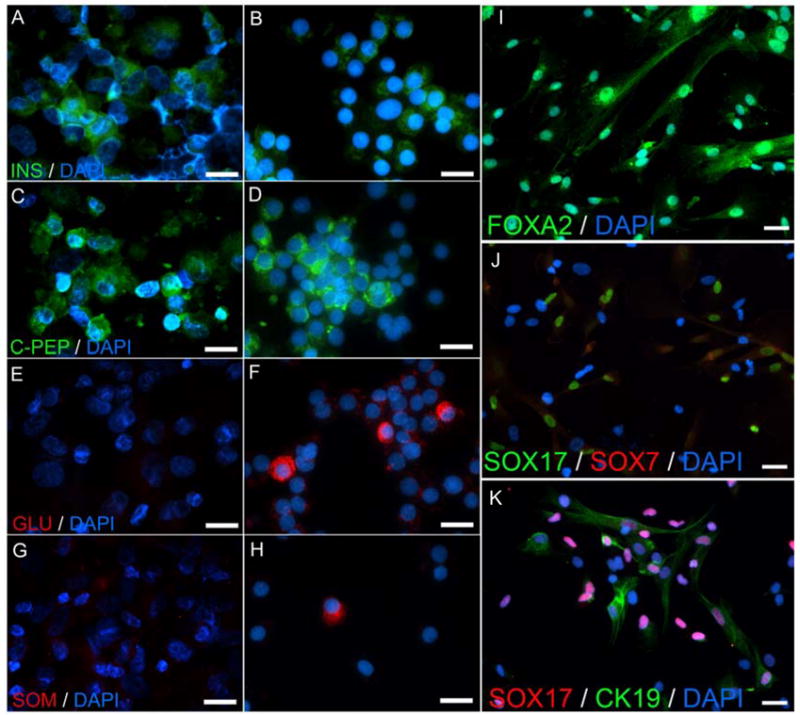
Immunocytochemical staining of LVEC cells and human islet cells. A–H, Disaggregated D8 LVEC cells (A,C,E,G) and cadaveric islets (B,D,F,H) stained for: A–B, insulin (INS); C–D, human C-peptide (C-PEP); E–F, glucagon (GLU); G–H, somatostatin (SOM). I–K, LVEC cells stained for: I, FOXA2, J, SOX17 and SOX7, K, SOX17 and Cytokeratin 19 (CK19). Nuclei counterstained with DAPI. Scale bar: 20 μm.
LVEC cells express transcription factors involved in the development of endoderm and endocrine pancreas
The mRNA expression of endoderm and endocrine pancreas transcription factors were detected during the 10 day time course (Supplementary Fig. 2). This includes sex determining region Y-box 17 (SOX 17), paired box gene 6 (PAX6), paired box gene 4 (PAX4), NK2 transcription factor related, locus 2 (NKX2.2), neurogenin 3 (NGN3), islet 1 (ISL1), hepatocyte nuclear factor 4α (HNF4α), hepatocyte nuclear factor 1α (HNF1α), homeobox HB9 (HB9), forkhead box A2 (FOXA2), neurogenic differentiation factor 1 (NEUROD1) and pancreatic and duodenal homeobox 1 (PDX1). Expression of NK6 transcription factor related, locus 1 (NKX6.1) was not detected. SOX17, ISL 1, HNF1α, HB9 and PAX6 expression was detected on D1, 6 and 8. PAX 4 expression was detected on D1 and 8 but not 6. NKX2.2, NGN3 and FOXA2 were expressed on D6 and 8. HNF4α was expressed on D6. NEUROD was expressed on D1 and PDX1 was expressed on D5.
LVEC cells express markers of definitive endoderm
Immunocytochemical staining was done to determine the frequency and co-expression of the early endodermal markers FOXA2, SOX17, sex determining region Y-box 7 (SOX7), and the ductal epithelial marker cytokeratin 19 (CK19). At low cell density (<1.9×104 cells/cm2) nearly all LVEC cells express FOXA2 (Fig. 1I). Approximately 68% (97/141) of LVEC are FOXA2+/SOX17+, 21% (33/152) are SOX17+/SOX7−, and 30% (45/152) are SOX17+/SOX 7+ (Fig. 1J). Coexpression of SOX17 and SOX7 suggests a population of visceral endoderm. Approximately 38% (37/97) of the FOXA2+/SOX17+ cells express CK19 (Fig 1K).
INS mRNA and C-peptide levels are glucose responsive in vitro
To assess glucose responsiveness we compared INS mRNA expression levels when cultures were exposed to 5 mM or 20 mM glucose (Fig. 2A). There was no significant difference in INS mRNA expression at D6 with respect to glucose concentration. However, when supplemented to 20 mM INS mRNA peaked at D8 and was elevated 2.9-fold (22.64±8.33 to 66.32±15.56, P<0.01) compared to cells supplemented to 5 mM. This expression level is 1000-fold lower than levels observed in the adult pancreas.
Figure 2.
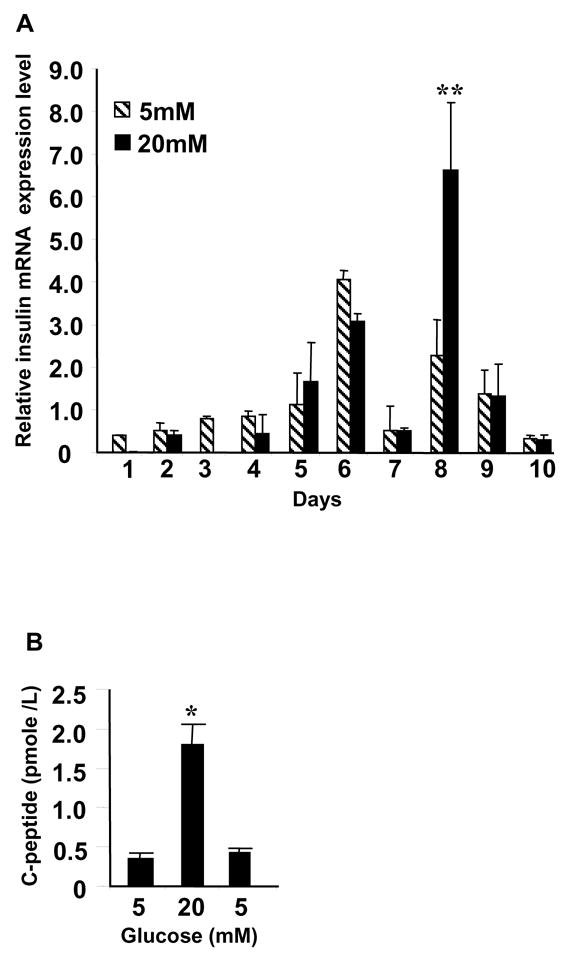
LVEC cell preproinsulin mRNA expression and C-peptide release. A, Normalized INS mRNA expression on Y-axis, day of RNA harvest on X-axis. Media supplemented to 5 mM (diagonal) or 20 mM (solid) glucose prior to RNA harvest. Significant difference between INS mRNA levels in 20mM and 5 mM glucose on D8 indicated by **, P<0.01. B, Human C-peptide released into media. Glucose concentrations indicated on X-axis, C-peptide values reported as mean±s.d on Y-axis. Significant difference between 5mM and 20 mM indicated by *, P<0.05.
We next investigated C-peptide release in response to glucose concentration. At D6 and 8 we exposed cells serially to 5mM, 20 mM and then 5 mM glucose. C-peptide increased 5-fold (0.35±0.08 pmole/L to 1.8±0.26 pmole/L, P<0.05) in 20 mM glucose then decreased (0.43±0.06 pmole/L) when re-exposed to 5 mM glucose (Fig. 2B). This pattern of release occurred only on D8 and was not due to an osmolar effect since it did not occur with 20 mM mannitol (data not shown).
LVEC cells engraft and produce C-peptide when transplanted under the kidney capsule
LVEC cells expressing low levels of INS mRNA and undetectable levels of INS protein were suspended in Matrigel™ and injected under the kidney capsule of normoglycemic mice. Cells immunoreactive to the C-peptide antibody were scattered within the matrix and in duct-like epithelial structures opening into a central lumen (Fig 3A). All of these structures stained positively for cytokeratins and often appear to branch (Fig 3B).
Figure 3.
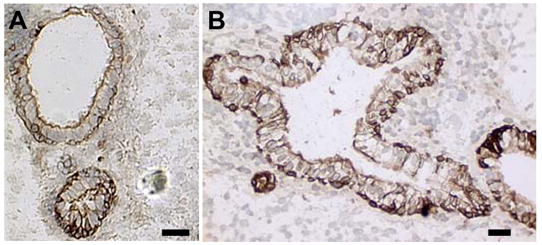
LVEC cells in Matrigel™ 30 days after transplantation under the kidney capsule. Immunoreactivity to: A, human C-peptide, B, Pan cytokeratin. Nuclei counterstained with hematoxylin. Scale bar: 50 μm.
Human C-peptide accumulation in blood following injection of LVEC cells
Mean glucose levels of untreated (no STZ, n=3 animals), mock treated (STZ, no cells, n=3 animals) and cell treated animals (STZ, cells, n=5 animals) were 107.8±10.3, 127.8±18.8 and 161.0±44.2 mg/dL, respectively, at time of transplantation. Glucose levels of STZ-treated animals were elevated compared to untreated animals (P<0.001, data not shown). Approximately 2 weeks after LVEC cell administration into the spleen, human C-peptide became detectable in the plasma of 4 of 5 animals injected with a mean (omitting the non-responder) of 3.8±1.4 pmole/L. This level increased in all 4 responding animals at 22 days to a mean of 6.6±1.5 pmole/L (Fig 4A). Failure to detect human C-peptide in the non-responder and in mock injected negative control animal plasma establishes the species-specificity of our ELISA (data not shown). Mean blood glucose concentration of the cell-treated animals was not different than mock-treated controls.
Figure 4.
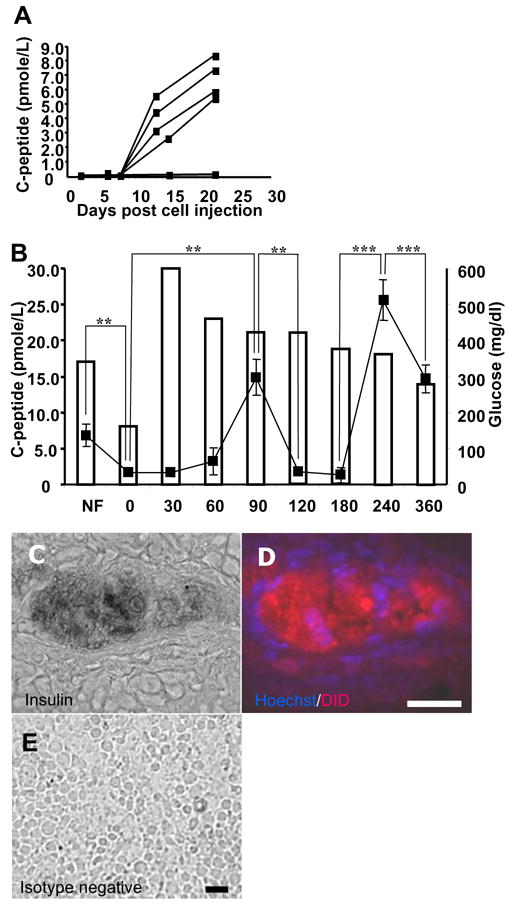
Human C-peptide in transplanted mice. A, Mean individual plasma C-peptide on Y-axis by black box, days following transplantation on X-axis. B, mean C-peptide of responders by black boxes on left Y-axis. Blood glucose of one animal by unshaded bars and right Y-axis. On X-axis, NF, not fasting, 0, overnight fast, 30–360, minutes following glucose challenge. Significant differences in C-peptide by ** and ***, for P<0.01 and P<0.001, respectively. C, insulin immunoreactivity of splenic LVEC cells overlayed on a phase contrast image. D, fluorescent imaging of section in C, human cells stained with Hoechst 33342 nuclear (blue) and DiD lipid (red) dyes. D, Parallel section stained with negative control antibody. Scale bar: 50 μm.
At 22 days, an IP glucose tolerance test (IPGTT) was performed on the 4 human C-peptide immunoreactive animals. Mean C-peptide level decreased from 6.95±1.65 pmole/L to 1.7±0.5 pmole/L following overnight fast concomitant with a fall in blood glucose (P<0.01). Following glucose administration, mean C-peptide level increased at 90 min. to 14.9±2.5 pmole/L (P<0.01) then decreased between 90 and 180 min (P<0.01). A second peak of 25.6±2.9 pmole/L was observed at 240 min. (P<0.001) followed by a decrease to 14.7±2.0 pmole/L at 360 min. (P<0.001) (Fig 4B). There was no difference in the blood glucose levels between the cell- and mock-injected animals (Data not shown). Islands of insulin-immunoreactive cells in responding animals were of human origin based on co-localization of Hoechst 33342-labeled nuclei and DiD-labeled cell membranes and cytoplasm (Fig 4C–D). Labeled insulin negative cells were also detected in the spleen of responding animals. No labeled cells were detected in the non-responding animal (data not shown). This finding is consistent with a mixed cell population observed in vitro.
DISCUSSION
A cure for type 1 diabetes requires a closed loop system of continuous glucose monitoring coupled to insulin delivery. For a cellular treatment, this implies functional integration of gene products involved in glucose sensing and mature insulin production. We have demonstrated that LVEC cells express markers of definitive endoderm, pancreatic development and β-cell function and provide evidence of integrated glucose sensing and insulin production in vitro and in vivo.
Definitive endoderm identified by coexpression of FOXA2 and SOX17 in 60–80% of differentiated human ES cells [18] is comparable to our finding of 68% in LVEC cultures. SOX17 expression in the absence of SOX7 has also been used to mark definitive endoderm following human ES cell derivation [18]. The 21% SOX17+/SOX7- population present in LVEC cultures may be a subpopulation of cells coexpressing FOXA2 and SOX17.
NKX6.1 was the only transcription factor not detected. NKX6.1 deficient mice develop a reduced number of mature β cells, but those that form are capable of replication at the same rate as wild type [19]. NKX6.2 has been shown to partially compensate for NKX6.1 activity in NKX6.1 deficient mice. However, β cells still form in NKX6.1/NKX6.2 double mutants, albeit in reduced numbers [20].
In the pancreas CK19 is expressed by ductal epithelia and marks stem/progenitor cell potential [21, 22]. CK19 expression has been used to track epithelial-to-mesenchymal transition of human islet-derived precursor cells from a ductal CK19+ INS- phenotype to a INS+ phenotype [23] as well as differentiation of non-endocrine epithelial cells of adult human pancreas into β-cells [22]. The CK19+ definitive endoderm population (38% FOXA2+/SOX17+/CK19+) may be precursors to INS+ cells but this requires confirmation by lineage tracing. CK19 immunoreactivity accounts for virtually 100% of pan cytokeratin immunoreactivity in vitro (data not shown). Transplanted LVEC cells acquire an INS+ cytokeratin+ epithelial phenotype, similar to that observed in human fetal pancreas [24].
When LVEC cells were exposed to high glucose INS mRNA increased 2.9-fold on D8, suggesting integration of glucose sensing and insulin production at the single cell level. This is supported by glucose responsive C-peptide release on D8. These results are consistent with 2- to 5-fold increases in INS mRNA levels observed in HIT-T15 insulinoma cells and isolated rat islets [25].
LVEC cells were transplanted into mildly hyperglycemic mice to expose them to potential regenerative signals while minimizing glucotoxicity. Following splenic transplantation, human C-peptide was detectable by D12 and reached a mean of 6.6 pmole/L in approximately 3 weeks. This is below the human fasting range of 170–660 pmole/L [26] but established that processed insulin produced by LVEC cells can enter the circulation. Following glucose administration, blood glucose levels increase followed by biphasic release of C-peptide. The peak C-peptide level of 25.6 pmole/L at 240 min is below the human stimulated C-peptide range of 500–3000 pmole/L [26] and stimulated levels of 532–1618 pmole/L [27] observed from 2000 human islet equivalents transplanted under the kidney capsule of NOD-SCID mice. However, these levels exceeded the 6.1±1.2 pmol/L C-peptide observed after glucose stimulation of mice transplanted with human neurosphere-derived insulin-producing cells [28]. Although C-peptide levels are at the low end of our assays reported range, statistical analyses of our technical and biological replicates confirms the significance of our findings.
Although there are two peaks of C-peptide following IPGTT, the kinetics of insulin release are delayed and of lower magnitude compared to that observed in rodents or humans. This may be a result of low percentage of LVEC cells with GRIP function, inefficient engraftment, incomplete terminal differentiation comparable to fetal rat [29] and fetal human islets [30] or hyperglycemia causing a loss of insulin gene expression, content, and/or secretion [31].
We have demonstrated glucose responsive insulin production in vitro, both at the level of mRNA and mature protein release. Concordantly, in animal models, we demonstrate that LVEC cells can form insulin and cytokeratin expressing duct-like structures, as well as release processed insulin in response to blood glucose. These findings represent a crucial first step towards the development of an expandable source of cells that may be used for cellular therapies for type 1 diabetes.
Supplementary Material
Acknowledgments
Research funded by NIDDK Beta Cell Biology Consortium Pilot and Feasibility Study VU CA9182 (M.J.S), JDRF Award 2-2002-306 (M.J.S), NIH T32 DK007751 (G.O.C), Diabetes Trust Foundation Fellowship (G.O.C) and the C. Michael Armstrong Stem Cell Fellowship (G.O.C). Islets were provided by the Islet Cell Resource Center. Under a licensing agreement between Geron Corporation and Johns Hopkins University, M.J.S is entitled to a share of royalty received by the University on sales of products described in this presentation. M.J.S and the University own Geron stock, which is subject to certain restrictions under University policy. The terms of this arrangement are being managed by the Johns Hopkins University in accordance with its conflict of interest policies.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Shapiro AM, Lakey JR, Ryan EA, Korbutt GS, Toth E, Warnock GL, Kneteman NM, Rajotte RV. Islet Transplantation in Seven Patients with Type 1 Diabetes Mellitus Using a Glucocorticoid-free Immunosuppressive Regimen. N Engl J Med. 2000;343:230–238. doi: 10.1056/NEJM200007273430401. [DOI] [PubMed] [Google Scholar]
- 2.Assady S, Maor G, Amit M, Itskovitz-Eldor J, Skorecki KL, Tzukerman M. Insulin production by human embryonic stem cells. Diabetes. 2001;50:1691–1697. doi: 10.2337/diabetes.50.8.1691. [DOI] [PubMed] [Google Scholar]
- 3.Houard N, Rousseau GG, Lemaigre FP. HNF-6-independent differentiation of mouse embryonic stem cells into insulin-producing cells. Diabetologia. 2003;46:378–385. doi: 10.1007/s00125-003-1041-8. [DOI] [PubMed] [Google Scholar]
- 4.Kahan BW, Jacobson LM, Hullett DA, Ochoada JM, Oberley TD, Lang KM, Odorico JS. Pancreatic precursors and differentiated islet cell types from murine embryonic stem cells: an in vitro model to study islet differentiation. Diabetes. 2003;52:2016–2024. doi: 10.2337/diabetes.52.8.2016. [DOI] [PubMed] [Google Scholar]
- 5.Shiroi A, Yoshikawa M, Yokota H, Fukui H, Ishizaka S, Tatsumi K, Takahashi Y. Identification of insulin-producing cells derived from embryonic stem cells by zinc-chelating dithizone. Stem Cells. 2002;20:284–292. doi: 10.1634/stemcells.20-4-284. [DOI] [PubMed] [Google Scholar]
- 6.Hori Y, Rulifson IC, Tsai BC, Heit JJ, Cahoy JD, Kim SK. Growth inhibitors promote differentiation of insulin-producing tissue from embryonic stem cells. Proc Natl Acad Sci U S A. 2002;99:16105–16110. doi: 10.1073/pnas.252618999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lumelsky N, Blondel O, Laeng P, Velasco I, Ravin R, McKay R. Differentiation of embryonic stem cells to insulin-secreting structures similar to pancreatic islets. Science. 2001;292:1389–1394. doi: 10.1126/science.1058866. [DOI] [PubMed] [Google Scholar]
- 8.Kim D, Gu Y, Ishii M, Fujimiya M, Qi M, Nakamura N, Yoshikawa T, Sumi S, Inoue K. In vivo functioning and transplantable mature pancreatic islet-like cell clusters differentiated from embryonic stem cell. Pancreas. 2003;27:e34–41. doi: 10.1097/00006676-200308000-00021. [DOI] [PubMed] [Google Scholar]
- 9.Vaca P, Berna G, Martin F, Soria B. Nicotinamide induces both proliferation and differentiation of embryonic stem cells into insulin-producing cells. Transplant Proc. 2003;35:2021–2023. doi: 10.1016/s0041-1345(03)00735-8. [DOI] [PubMed] [Google Scholar]
- 10.Segev H, Fishman B, Ziskind A, Shulman M, Itskovitz-Eldor J. Differentiation of human embryonic stem cells into insulin-producing clusters. Stem Cells. 2004;22:265–274. doi: 10.1634/stemcells.22-3-265. [DOI] [PubMed] [Google Scholar]
- 11.D’Amour KA, Bang AG, Eliazer S, Kelly OG, Agulnick AD, Smart NG, Moorman MA, Kroon E, Carpenter MK, Baetge EE. Production of pancreatic hormone-expressing endocrine cells from human embryonic stem cells. Nat Biotechnol. 2006 doi: 10.1038/nbt1259. [DOI] [PubMed] [Google Scholar]
- 12.Leon-Quinto T, Jones J, Skoudy A, Burcin M, Soria B. In vitro directed differentiation of mouse embryonic stem cells into insulin-producing cells. Diabetologia. 2004;47:1442–1451. doi: 10.1007/s00125-004-1458-8. [DOI] [PubMed] [Google Scholar]
- 13.Blyszczuk P, Czyz J, Kania G, Wagner M, Roll U, St-Onge L, Wobus AM. Expression of Pax4 in embryonic stem cells promotes differentiation of nestin-positive progenitor and insulin-producing cells. Proc Natl Acad Sci U S A. 2003;100:998–1003. doi: 10.1073/pnas.0237371100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Miyazaki S, Yamato E, Miyazaki J. Regulated expression of pdx-1 promotes in vitro differentiation of insulin-producing cells from embryonic stem cells. Diabetes. 2004;53:1030–1037. doi: 10.2337/diabetes.53.4.1030. [DOI] [PubMed] [Google Scholar]
- 15.Shamblott MJ, Axelman J, Wang S, Bugg EM, Littlefield JW, Donovan PJ, Blumenthal PD, Huggins GR, Gearhart JD. Derivation of pluripotent stem cells from cultured human primordial germ cells. Proc Natl Acad Sci U S A. 1998;95:13726–13731. doi: 10.1073/pnas.95.23.13726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shamblott M, Axelman J, Littlefield J, Blumenthal P, Huggins G, Cui Y, Cheng L, Gearhart J. Human embryonic germ cell derivatives express a broad range of developmentally distinct markers and proliferate extensively in vitro. Proc Natl Acad Sci U S A. 2001;98:113–118. doi: 10.1073/pnas.021537998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kim MS, Hwang NS, Lee J, Kim TK, Leong K, Shamblott MJ, Gearhart J, Elisseeff J. Musculoskeletal differentiation of cells derived from human embryonic germ cells. Stem Cells. 2005;23:113–123. doi: 10.1634/stemcells.2004-0110. [DOI] [PubMed] [Google Scholar]
- 18.D’Amour KA, Agulnick AD, Eliazer S, Kelly OG, Kroon E, Baetge EE. Efficient differentiation of human embryonic stem cells to definitive endoderm. Nat Biotechnol. 2005;23:1534–1541. doi: 10.1038/nbt1163. [DOI] [PubMed] [Google Scholar]
- 19.Sander M, Sussel L, Conners J, Scheel D, Kalamaras J, Dela Cruz F, Schwitzgebel V, Hayes-Jordan A, German M. Homeobox gene Nkx6.1 lies downstream of Nkx2.2 in the major pathway of beta-cell formation in the pancreas. Development. 2000;127:5533–5540. doi: 10.1242/dev.127.24.5533. [DOI] [PubMed] [Google Scholar]
- 20.Henseleit KD, Nelson SB, Kuhlbrodt K, Hennings JC, Ericson J, Sander M. NKX6 transcription factor activity is required for alpha- and beta-cell development in the pancreas. Development. 2005;132:3139–3149. doi: 10.1242/dev.01875. [DOI] [PubMed] [Google Scholar]
- 21.Bouwens L. Cytokeratins and cell differentiation in the pancreas. J Pathol. 1998;184:234–239. doi: 10.1002/(SICI)1096-9896(199803)184:3<234::AID-PATH28>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- 22.Hao E, Tyrberg B, Itkin-Ansari P, Lakey JR, Geron I, Monosov EZ, Barcova M, Mercola M, Levine F. Beta-cell differentiation from nonendocrine epithelial cells of the adult human pancreas. Nat Med. 2006;12:310–316. doi: 10.1038/nm1367. [DOI] [PubMed] [Google Scholar]
- 23.Gershengorn MC, Hardikar AA, Wei C, Geras-Raaka E, Marcus-Samuels B, Raaka BM. Epithelial-to-mesenchymal transition generates proliferative human islet precursor cells. Science. 2004;306:2261–2264. doi: 10.1126/science.1101968. [DOI] [PubMed] [Google Scholar]
- 24.Bouwens L. Islet morphogenesis and stem cell markers. Cell Biochem Biophys. 2004;40:81–88. doi: 10.1385/cbb:40:3:81. [DOI] [PubMed] [Google Scholar]
- 25.Leibiger B, Moede T, Schwarz T, Brown GR, Kohler M, Leibiger IB, Berggren PO. Short-term regulation of insulin gene transcription by glucose. Proc Natl Acad Sci U S A. 1998;95:9307–9312. doi: 10.1073/pnas.95.16.9307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Greenspan FS, Gardner DG, editors. Basic & Clinical Endocrinology. 7. Lange Medical Books/McGraw-Hill; New York: 2004. [Google Scholar]
- 27.Gaber AO, Fraga DW, Callicutt CS, Gerling IC, Sabek OM, Kotb MY. Improved in vivo pancreatic islet function after prolonged in vitro islet culture. Transplantation. 2001;72:1730–1736. doi: 10.1097/00007890-200112150-00005. [DOI] [PubMed] [Google Scholar]
- 28.Hori Y, Gu X, Xie X, Kim SK. DIfferentiation of insulin-producing cells from human neural progenitor cells. PLOS Medicine. 2005;2:347–356. doi: 10.1371/journal.pmed.0020103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Asplund K, Westman S, Hellerstrom C. Glucose stimulation of insulin secretion from the isolated pancreas of foetal and newborn rats. Diabetologia. 1969;5:260–262. doi: 10.1007/BF01212095. [DOI] [PubMed] [Google Scholar]
- 30.Obenshain SS, Adam PA, King KC, Teramo K, Raivio KO, Raiha N, Schwartz R. Human fetal insulin response to sustained maternal hyperglycemia. N Engl J Med. 1970;283:566–570. doi: 10.1056/NEJM197009102831104. [DOI] [PubMed] [Google Scholar]
- 31.Robertson RP, Zhang HJ, Pyzdrowski KL, Walseth TF. Preservation of insulin mRNA levels and insulin secretion in HIT cells by avoidance of chronic exposure to high glucose concentrations. J Clin Invest. 1992;90:320–325. doi: 10.1172/JCI115865. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


