Abstract
Background
Pedicle screw fixation to stabilize lumbar spinal fusion has become the gold standard for posterior stabilization. A significant percentage of surgical candidates are classified as obese or morbidly obese. For these patients, the depth of the incisions and soft tissue makes it extremely difficult to insert pedicle screws along the pedicle axis. As such, the pedicle screws could only be inserted in a much more sagittal axis. However, biomechanical stability of the angled screw insertion has been controversial. We hypothesized that the straight or parallel screw was a more stable construct compared to the angled or axially inserted screw when subjected to caudal cyclic loading.
Methods
We obtained 12 fresh frozen lumbar vertebrae from L3 to L5 from five cadavers. Schantz screws (6.0mm) were inserted into each pedicle, one angled and along the axis of the pedicle and the other parallel to the spinous process. Fluoroscopic imaging was used to guide insertion. Each screw was then subjected to caudal cyclic loads of 50N for 2000 cycles at 2Hz. Analysis of initial damage, initial rate, and total damage during cyclic loading was undertaken.
Findings
Average total fatigue damage for straight screws measured 0.398±0.38 mm, and 0.689±0.96 mm for angled screws. Statistical analysis for total fatigue damage ratio of angled to straight screws revealed that a significant stability was achieved in straight- screw construct (p<0.03).
Interpretation
This study showed that straight screw insertion results in a more stable pedicle-screw construct. The angled screw insertion technique resulted in more scattered values of damage indicating that the outcome from the angled screw fixation is less predictable. This validates the use of this technique to implant pedicle screws across the axis of the pedicle rather than along the axis, (parallel to the midline sagittal line), and has broad implications in instrumented posterior lumbar spinal surgery.
Keywords: Pedicle screw, Cyclic loading, Biomechanical stability, Creep
1. Introduction
Pedicle screw fixation has become the mainstay of fixation for stabilization of the posterior lumbar spine. Originally described by Boucher in 1959, Roy-Camille popularized this technique in Europe in the 1960’s, and his spinal plating system has been called the “predecessor of most modern pedicular screw-plate fixation systems” (Boucher, 1959; Roy-Camille etal., 1976; Roy-Camille, 1992). Pedicle screw fixation is now readily accepted for treatment of fractures, tumors, and degenerative disease. Loosening due to fatigue loading and screw breakage are commonly cited reasons for failure, and numerous studies have been conducted to determine which factors are most important in determining biomechanical stability of the pedicle screw (Esses & Bednar, 1989; Willet, etal., 1993; Zdeblick etal., 1993). To date, biomechanical studies have for the most part examined pullout failure of the screw as the endpoint to determine stability ( Barber etal., 1998; Law etal., 1993; Yerby etal., 1997). Even those few reports that used a cyclic loading model utilized a displacement control rather than load control mechanism to determine relative stability ( Barber etal., 1998; Law etal., 1993; Soshi etal., 1991). While parameters studied included using bigger screws, drilling or probing the pilot hole, tapped and untapped screws, coupling, angular insertion, and augmentation with bushings and polymethylmethacrylate, fatigue failure based on the clinical scenario has rarely been reported.
Morphometric anatomic studies have determined that pedicles flare out laterally from the upper to lower lumbar spine (Ruland etal., 1991; Zindrick, 1991 ). Transverse pedicle angles of the lower lumbar spine range from 8.0-23.5° at L3 (mean 14.4°) to 19.0-44.0° at L5 (mean 29.8°) ( Zindrick etal., 1987). Some studies have suggested that convergent screws are a stronger construct, and have recommended that screws be inserted axially within the lumbar pedicle (Barber etal., 1998) On the basis of these studies and those testing pullout strength ( Law etal., 1993; Yerby etal., 1997), screw insertion technique along the axis of the pedicle has been described as superior, with increasing angular distance from the vertebral midline at lower levels of the lumbar spine (Cook etal., 2000). However, the patient population subjected to surgery includes a significant number of obese or morbidly obese individuals who present a challenge in exposure for pedicular insertion of screws. The incisions are deep and approaching the pedicle along its axis is difficult. As an alternative to pedicular insertion, in the technique described by Roy-Camille in 1976 and 1992, pedicle screws are inserted in a vertical fashion, crossing the axis of the pedicle rather than proceeding in line with it. While attempts have been made to determine the stability of these screws, most studies utilize a displacement control to determine pullout strength at the bone-screw interface, rather than examining them dynamically at sub-failure forces to determine relative stability based on amount of screw toggle acquired during fatigue testing (Barber etal., 1998; Brantley etal., 1994; Soshi etal., 1991).
This study was designed to determine the stability of pedicle screws that were inserted by both straight and angled techniques. Cyclic, sub-failure load control was used to simulate in vivo loading. The displacement of each screw was measured and compared with the contralateral screw that was inserted by the differing technique. Based on this work, the fatigue stability of the two different types of screw insertion technique was examined by answering two research questions: 1) is the rate of damage different between the two screw insertion methods? 2) is there a difference in stiffness and creep damage between the two methods?
2. Materials and Methods
Specimen Preparation and Screw Implantation
Use of human tissue was approved by our hospital Institutional Review Board. Five fresh frozen cadaveric spines were obtained from a tissue bank. These specimens were procured from T6 to the sacrum with minimal soft tissue attachment and were stored at -32°C. None had a history of metastatic disease. Fourteen total vertebral bodies were tested in this protocol. There were 4 males and 1 female with an average age of 67 years (range of 42 to 82 yrs.). The vertebral bodies of L3, L4, and L5 were dissected free of soft tissue and were disarticulated from their corresponding segments. All disc material was removed and the end plates were cleaned. One of the L5 vertebral bodies had been damaged during cadaveric extraction and was removed from our sample group. Fluoroscopic imaging confirmed absence of pathologic process other than osteoarthrosis, which was evident in three of the five specimens. A total of five L3 vertebral bodies, five L4 vertebral bodies, and four L5 vertebral bodies were instrumented. All screws were alternated with respect to left and right pedicles and angled versus straight screw insertion technique. Absent scoliosis or congenital malformations, previous literature has documented right and left symmetry within the same vertebral body specimen (Zindrick etal., 1987). Fourteen straight and fourteen angled screws were placed. Each specimen was labeled and stored at -32°C until implantation and testing.
Each vertebral body was visualized with fluoroscopic imaging in axial, sagittal, and coronal planes, and the appropriate insertion point for each pedicle was identified and marked. Using fluoroscopy allowed tight control of vertebral body orientation to help insure a standardized insertion technique. Utilizing a 2.7mm drill bit and a drill press, the starting hole was made in the pedicle. No specimens experienced cortical disruption through the pedicle wall or the anterior cortex of the vertebral body. For angled screws, a “Scotty Dog” was visualized and the screw inserted in the center of the pedicle. Under fluoroscopic guidance (Figure 1), 6.0mm Schantz screws (Synthes, Paoli, PA) were then inserted into the starting hole by hand. Anteroposterior, lateral and coronal images were obtained to confirm placement of the screws within the pedicle and the body during and after insertion. The screws were advanced to the anterior cortex of the body, but did not pierce the cortex. For the straight screws, the pedicle insertion point was identified utilizing fluoroscopy (Figure 1). The starting point was marked and the starting hole made utilizing the drill press. The 6.0mm Schantz screw was then inserted in similar fashion under fluoroscopic guidance. Once both screws were placed, the specimen was again stored at -32°C until testing could be performed.
Figure 1.
Two different screw insertion techniques (fluoroscopy).
Biomechanical Testing
Biomechanical testing was undertaken once the specimens had again thawed to room temperature. We fashioned a grip to hold the vertebral body and polymethylmethacrylate (Zimmer, Warsaw, IN) bone cement was utilized to pot the specimen. The cement was used to ensure uniform contact between the grip and the uneven vertebral body to prevent motion during testing and to distribute the forces being transmitted to the vertebral body by the loaded pedicle screw. The vertebra was fixed when the cement was in the doughy state and post testing analysis did not demonstrate cement intrusion into the vertebral body (Pfeiffer etal., 1996). The grip was firmly secured. Each specimen was placed in an upright anatomic orientation, and care was taken to prevent contact of the inferior or superior facets with the grip. To minimize the moment arm that existed outside of the vertebral body specimen, each pedicle screw was marked at two centimeters distance from their point of initial bony contact as a marker for the load contact point.
Once the bone cement had hardened, the construct was placed in a servohydraulic materials test machine (8501M, Instron, Canton, MA) for fatigue testing. Specimens were maintained at room temperature and were kept moist throughout testing. A cylindrical loading rod attached to a load cell was then aligned with the screw such that the point of contact was at the 2 cm mark. The cylinder was brought down to the screw until initial contact was made. The load control testing protocol was programmed at 2000 cycles at 2Hz, with peak load of 50N with a load ratio R=0.1 (min. 5N / max. 50N), directed in a caudal fashion in sinusoidal pattern of compression; neither preconditioning nor tensile load was utilized. The anatomical right pedicle was tested first in all cases whether the screw was oriented straight or angled. Data acquisition was performed with acquisition rate set at 100 Hz. Following the conclusion of testing, the screws were carefully removed and a depth gauge was used to determine depth of screw insertion from the point where bony contact was initially made. Further, the coronal fluoroscopic images were used to calculate angles from the midline for each pedicle screw. The mid vertebral line was drawn at the measured center distance of the neural canal and tip of the spinous process.
Information from one specimen (L5, Specimen A) was lost due to an error in the data acquisition setup, and the specimen was removed from our sample set. The second specimen (L4) in our test group was used for preliminary tests. This left a total of 12 samples and 24 total pedicle screws within our test group for data analysis.
Data Analysis
Undamaged initial secant stiffness was measured by dividing the applied force (50N) by maximum displacement at the first cycle. The stiffness damage (ds_N= dN- d1) was determined by subtracting the amount of cyclic displacement at the first loading cycle (d1) from that for the current cycle (dN) (Figure 2). For example, the stiffness damage at 2000 cycles was calculated as ds_2000=d2000- d1. The creep damage (dc_N) was defined as the permanent displacement at the minimum (5N) applied load for each cycle. The total damage (dt_N= ds_N+ dc_N) was then calculated as the sum of the stiffness and creep damage. Differences between the straight and the angled screw types were examined using paired t-tests. To account for the high inter-specimen variability, the ratio of measured parameters from the angled-screw configuration to those from the straight-screw configuration within the same specimen was tested against a mean value of 1 using a one-sample t-test. All statistical analyses were performed using Statview software (SAS, NC) and statistical significance was set as p<0.05.
Figure 2.

A typical fatigue load-displacement curve. Stiffness damage (ds_N= dN- d1), creep damage (dc_N), and total damage (dt_N= ds_N+ dc_N).
3. Results
Preliminary testing revealed that a 200N force would cause traumatic fracture of the pedicle within the first and second cycles. Further testing at 50N demonstrated the first and second phases of the standard three-phase response of fatigue could be obtained. A specimen was tested at 25N but revealed no measurable damage, and the decision was made to proceed with testing the remainder of the specimens at 50N. At these small loads, no preliminary or experimental specimen was cycled to failure despite early specimens being run to 10,000 cycles.
Average angle and depth of insertion (relative to vertebral midline) were significantly different between the straight and the angled screw (p<0.001 for angle and p<0.02 for depth, respectively) (Table 1). Although none were cycled to the final third phase of failure, our fatigue test data followed a standard model for a two phase loading cycle fatigue test, as demonstrated previously: a rapid early phase and a constant second rate phase (Figure 3) ( Kim et al., 2004a, b). We observed stiffening of the construct, rather than loss of stiffness, for 15 out of 24 tests. We attribute this to the compaction of failed trabeculae at the screw-bone contact surface. Stiffness changes, however, were about 70 times less than creep damage. Thus, it is concluded that creep (dc) dominated the cyclic behavior for both screw types. The magnitudes of the initial stiffness, total damage at 2000 cycles (dt_2000), creep damage at 2000 cycles (dc_2000), the stiffness damage at 2000 cycles (ds_2000), initial damage rate (Δdt_10/Δt), and secondary damage rate (Δdt_2000/Δt) were measured higher for the angled screw than those for the straight screw. However, the difference of those magnitudes between the screw types turned out to be not significant (p>0.104 and p=0.063) for the stiffness damage at 2000 cycles (Table 1). This result was attributed to the significantly large variability (standard deviation) of the values of total damage, creep damage and secondary rate from the angled-screw construct than those from the straight-screw construct (F-test, p<0.01).
Table 1.
Comparison of parameters between straight and angled screw types. Average±Standard Deviation. n=12.
| Parameters | Straight | Angled | Paired t-test |
|---|---|---|---|
| Angle (°) | 7.0±3.2 | 26.3±6.9 | p<0.001 |
| Depth (mm) | 51.3±4.6 | 54.2±5.3 | p<0.020 |
| Initial stiffness (N/mm) | 1.533±0.075 | 1.690±0.272 | p=0.139 |
| Total damage at 2000 cycles (dt_2000) (mm) | 0.398±0.38 | 0.690±0.96 | p=0.286 |
| Creep damage at 2000 cycles) (dc_2000) (mm) | 0.430±0.37 | 0.673±0.92 | p=0.341 |
| Stiffness damage at 2000 cycles (ds_2000) (mm) | -0.032±0.051 | 0.0167±0.049 | p=0.063 |
| Initial damage rate (Δdt_10/Δt) (mm/sec) | 0.023±0.0004 | 0.048±0.0064 | p=0.275 |
| Secondary damage rate (Δdt_2000/Δt) (mm/sec) | 8.50*10-5 ±6.55*10-9 | 15.31*10-5 ±18.90*10-9 | p=0.104 |
Figure 3.
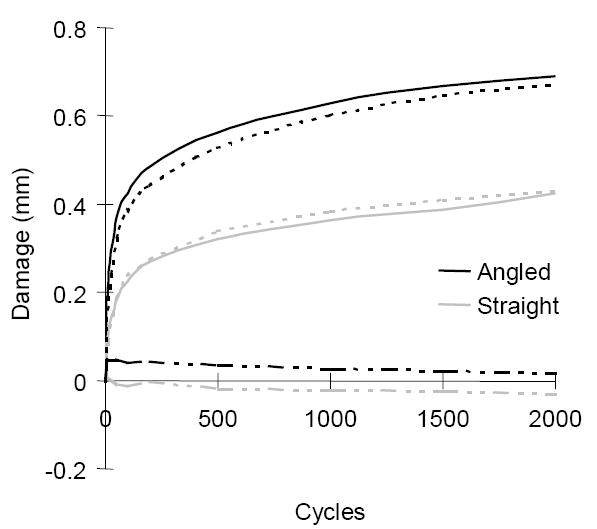
The response curve of the averaged damage vs. cycle. Black lines are for the angled and gray lines are for the straight. ─ : total damage, --- : creep damage, and –··– : stiffness damage. Creep dominated the fatigue damage caused by screw insertion.
Analysis of ratios demonstrated that the total damage in the angled-screw system was indeed significantly (1.6-fold) greater than in the straight-screw system at 2000 cycles (p<0.034) but other parameters remained to be non-significant (p>0.08).
4. Discussion
Multiple studies have been conducted to examine stability characteristics of lumbar pedicle screw systems that have been developed for posterior fixation. Zindrick et al. (1986)performed a thorough biomechanical study performing axial pullout and cyclic loading modes (displacement control) with multiple screw designs at various depths. The construct was assumed failed when 50% of the initial force was required to displace a total of 6 mm (3mm caudad, 3mm cephalad). Further, screws were inserted along the axis of the pedicle for the lumbar spine, though the authors did examine sacral fixation at medial and lateral angles. Authors concluded that screws inserted to a greater depth achieved better stability. However, they did not specifically compare the fatigue behavior of constructs with axial versus angular insertion of screws. Ruland et al. (1991)put forth that axial pullout during forward bending is the main mode of failure, despite the paucity of clinical literature to support this. Authors since that time have demonstrated linear correlation between bone density and pull-out strength. Soshi et al. (1991)assessed inline pedicle pullout, which may not represent in vivo behavior accurately, and found a linear relationship with osteoporosis. Willet et al. (1993)also studied pullout strength in Schanz screws, but again did so using displacement control modalities to show that the 6.0 mm Schantz screw was a better biomechanical construct than the 5.0 mm construct. Zdeblick et al. (1993)correlated insertional torque with increased pullout strength. Part of this study also looked at bone mineral density and found this to be a less effective predictor of pedicle screw stability. Zdeblick’s study also examined probed versus drilled screws and found no significant difference in insertional torque, cycles to failure or ultimate failure load. The pullout test was also combined with caudad/cephalad toggling by offsetting the force vector from the screw axis. High forces leading to destructive failure were used, rather than non-catastrophic forces observed clinically (Yerby etal., 1997). Zdeblick’s data was confirmed by Meyers et al. (1996)who demonstrated that quantitative CT imaging combined with stiffness and insertion torque were the strongest predictors of pull-out strength. The authors used a ramped sinusoidal load, again utilizing destructive failure methods. Law et al. (1993)examined pedicle fill with augment bushings to increase cortical contact within the pedicle. This study used caudad/cephalad loading and noted toggle loosening with a fulcrum at the base of the pedicle. These screws were given high loads up to 200N, and the screw construct was only able to withstand three cycles. Large displacements up to 8 mm were observed, which certainly would be considered failure. Our preliminary testing experienced fracture of the pedicle at 200N loads within the first two to three cycles, confirming earlier testing by Law. Brantley et al. (1994)examined non-destructive mechanical testing to mimic in vivo forces to examine the effect of screw size on stability. Again, displacement control was used rather than load control. Most of these specimens were inserted in line with the pedicular axis and data analysis of angular orientation was not performed. Finally, Cook et al. (2000), in studying an expansible pedicle screw design in cases of compromised bone quality, utilized an axial pullout model of screws inserted inline with the axis of the pedicle.
We feel that the loading protocol employed in the current study is more relevant to the progressive failure of vertebra/screw constructs. It was indicated that, for cortical bone, fully reversed cyclic loading to one half of the yield strain caused fatigue fracture in 1000 cycles (Carter etal., 1981). However, to date, no fatigue failure characteristics of human cancellous bone have been reported. Recently, Lu et al. (2004)found that cyclic loading with 30% of the yield load of human vertebrae significantly increased microcrack density in the vertebral trabeculae at 20000 cycles of loading but the vertebrae did not fail. In the preliminary phases of the present study, we found that the pedicle screw system failed at 150N. Therefore, the load level of 50N we used for cyclic loading is about 30% of the system failure load. This level of cyclic load may be insufficient to cause failure of the entire pedicle screw system in a vertebra but sufficient to cause damage in bone adjacent to the screw.
To the author’s knowledge, fatigue testing utilizing load control methods at nondestructive levels has not been utilized to better understand the behavior of pedicle screw stability. Further, though pullout testing has become the main in vitro predictor for stability, this mode of failure in vivo is rare. Converging pedicle screws have been advocated in osteoporotic bone, but the axial pullout method does not truly test the stability of these screws, and significant “butterfly shaping” within the vertebral body have been demonstrated (Law etal., 1993). Our two-phase fatigue test of angled versus straight pedicle screws revealed that total damage of the angled screw was higher when compared to the straight screws. As a component of the total damage, stiffness loss at the initial phase of loading cycles is likely attributed to the local compressive damage in trabecular bone around the metal screw. The locally damaged trabeculae at the contact surface between the screw and the bone compacted in progression with increasing cycles of loading. The compaction of failed trabeculae seemed to maintain the stiffness at the second-rate phase of fatigue. Overall, creep that was measured about 70 times more than stiffness changes dominated the displacement developed in the fatigued screw system. This observation indicated that creep was a major type of fatigue failure of pedicle screw systems, consistent with the results of other bone-interface fatigue testing that showed creep damage as the primary mode of failure ( Kim et al., 2004a, b). This finding suggested that loosening between the pedicle and the vertebral bone observed in clinical situations (Pihlajamaki etal., 1997) could be a consequence of the increase in creep displacement with in vivo fatigue cycles. Other notable differences between the two sample groups occurred. For example, depth of screw insertion was significantly different between angled and straight screws. Though the straight screws were inserted shallower with less bone to distribute load and resist strain, the average deformation was less than the more deeply implanted angular screws. Angular screws demonstrated greater variability of results compared to straight screws. These results indicate that the angled screw system is less predictable than the straight-screw insertion.
Statistical analysis was difficult given the wide variability in results, but accounting for this by matching each screw with the opposite pedicle screw did demonstrate significant differences. Average total damage of the angled screw was higher than that of the straight screw, and the ratio analysis demonstrated that this difference was significant. All of the damage indices tested in this study, which are total damage at 2000 cycles, creep damage at 2000 cycles, the stiffness damage at 2000 cycles, initial damage rate, and secondary damage rate (Table 1), showed higher values for the angled screw construct than those for the straight screw construct indicating that the angled screw construct is inferior in fatigue performance compared to the straight screw technique. One would expect that the greater bone interface would yield a more stable construct, but this did not hold true. Previous studies have put forth that angular screws, contrary to our results, have more resistance to pullout strength and therefore should be used preferably, especially with osteoporotic bone (Cook etal., 2000). However, this was based on pullout of the pedicle screw in which the angular screw caused fracture of the pedicle as it was continuously loaded in tensile stress in the direction parallel to the midline of the vertebral body. Failure of the pedicle screw – vertebral body interface does not typically occur in vivo by this mechanism. From our experience with removal of failed screws we felt that the majority of them are loose within the vertebra but that the vertebra does not break. Therefore, dynamic fatigue testing over thousands of cycles would yield more clinically relevant data regarding the stability of these constructs. In addition, we think that the stability of the straight screw system can be justified by the three-point fixation that holds the straight screw closer to the cortical part of the vertebra in three regions; in the insertion point, across the pedicle and at the end point. The straight screw end point is closer to the edge where there is less cancellous and more cortical type hard bone. On the other hand, angled screws pass through the middle of the pedicle and rely on size for cortical contact; placing the larger screw in the safe zone is technically more difficult. The end point of the angled screw sits in cancellous bone, relying more on the weaker cancellous structure of the vertebra.
We identified several limitations within this study. In vivo testing is theoretically more likely to give accurate information regarding frequency of forces about the pedicle screw, displacement of the screw, and evidence for mode of failure. In vitro testing, however, is inevitably without the presence of the body’s immune response or ability to heal and adapt to load changes in the environment. In our specimens, the visual appearance of failure was not examined. Although the pedicle is generally cylindrical providing a straight path for screw insertion, it is possible that there is pivoting at some instances of loading and that toggling is involved in the damage mechanism. Nonetheless, if its presence is significant, this is an inherent part of the screw-technique and should be represented in engineering definitions of damage.
Another limitation was that the quality of specimens likely varied between ages of donor and between levels of vertebra. Although taking bone mineral density into consideration could account for this variability, we felt that a side-to-side comparison study would obviate the need for knowing what the density of these specimens truly was. However, in retrospect, bone mineral density may have shown a separate correlation with how each specimen behaved. It is possible that screws interacted in the paired configuration, however, by alternating the order of testing of straight and angled screws between vertebrae, the potential effect of this interaction was equally distributed between groups. This probably increased the variability in the data, however, this matching was deemed necessary given the more difficult task of matching properties between bones from different sources if separate groups were used.
5. Conclusions
This study showed that straight screw insertion results in a pedicle-screw construct that has a better fatigue performance. From a clinical perspective, insertion of the pedicle-screws in a straight fashion is certainly more practical as it does not require extensive dissection, retraction, or excision of paraspinal musculature to achieve screw insertion along transverse pedicle angles that can range up to 38º from the midline. Further, this technique, though with less support from the literature, is likely more widely practiced already than currently reported. In large patients or those in whom minimally invasive techniques are attempted, insertion along the pedicular axis is particularly difficult and may require percutaneous screw placement. Our results support this method of pedicle screw insertion.
Acknowledgments
This publication was, in part, made possible by Grant Number AR049343 from the National Institutes of Health. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the NIH. The authors gratefully acknowledge the late Mr. James Sapp for his contribution to design and building of the loading fixtures.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errorsmaybe discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Barber JW, Boden SD, Ganey T, Hutton WC. Biomechanical study of lumbar pedicle screw: does convergence affect axial pullout strength? Journal of Spinal Disorders. 1998;11(3):215–220. [PubMed] [Google Scholar]
- 2.Boucher HH. A method of spinal fusion. Journal of Bone and Joint Surgery. 1959;41A:248–259. doi: 10.1302/0301-620X.41B2.248. [DOI] [PubMed] [Google Scholar]
- 3.Brantley AG, Mayfield JK, Koeneman JB, Clark KR. The effects of pedicle screw fit: an in vitro study. Spine. 1994;19(15):1752–1758. doi: 10.1097/00007632-199408000-00016. [DOI] [PubMed] [Google Scholar]
- 4.Carter DR, Cale WE, Spengler DM, Frankel VH. Fatigue behavior of adult cortical bone: the influence of mean strain and strain range. Acta Orthopaedica Scandinavica. 1981;52(5):481–490. doi: 10.3109/17453678108992136. [DOI] [PubMed] [Google Scholar]
- 5.Cook SD, Salkeld SL, Whitecloud TS, III, Barbara J. Biomechanical evaluation and preliminary clinical experience with an expansive pedicle screw design. Journal of Spinal Disorders. 2000;13(3):230–236. doi: 10.1097/00002517-200006000-00006. [DOI] [PubMed] [Google Scholar]
- 6.Esses SI, Bednar DA. The spinal pedicle screw: techniques and system. Orthopaedic Review. 1989;18(6):676–682. [PubMed] [Google Scholar]
- 7.Kim DG, Miller MA, Mann KA. Creep dominates tensile fatigue damage of the cement-bone interface. Journal of Orthopaedic Research. 2004a;22:633–640. doi: 10.1016/j.orthres.2003.09.007. [DOI] [PubMed] [Google Scholar]
- 8.Kim DG, Miller MA, Mann KA. A fatigue damage model for the cement-bone interface. Journal of Biomechanics. 2004b;37:1505–1512. doi: 10.1016/j.jbiomech.2004.01.011. [DOI] [PubMed] [Google Scholar]
- 9.Law M, Tencer AF, Anderson PA. Caudo-Cephalad Loading of Pedicle Screws: Mechanisms of loosening and Methods of Augmentation. Spine. 1993;18:2438–2443. doi: 10.1097/00007632-199312000-00012. [DOI] [PubMed] [Google Scholar]
- 10.Lu WW, Luk KD, Cheung KC, Gui-Xing Q, Shen JX, Yuen L, Ouyang J, Leong JC. Microfracture and changes in energy absorption to fracture of young vertebral cancellous bone following physiological fatigue loading. Spine. 2004;29(11):1196–201. doi: 10.1097/00007632-200406010-00007. [DOI] [PubMed] [Google Scholar]
- 11.Magerl FP. Stabilization of the lower thoracic and lumbar spine with external skeletal fixation. Clinical Orthopaedics and Related Research. 1984;189:125–141. [PubMed] [Google Scholar]
- 12.McAfee PC, Weiland DJ, Carlow JJ. Survivorship analysis of pedicle spinal instrumentation. Spine. 1991;16(8 Suppl):S422–427. [PubMed] [Google Scholar]
- 13.Myers BS, Belmont PJ, Richardson WJ, Yu JR, Harper, Nightingale RW. The role of imaging and in situ biomechanical testing in assessing pedicle screw pull-out strength. Spine. 1996;21:1962–1968. doi: 10.1097/00007632-199609010-00004. [DOI] [PubMed] [Google Scholar]
- 14.Pihlajamaki H, Myllynen P, Bostman O. Complications of transpedicular lumbosacral fixation for non-traumatic disorders. Journal of Bone and Joint Surgery. 1997;79B:183–189. doi: 10.1302/0301-620x.79b2.7224. [DOI] [PubMed] [Google Scholar]
- 15.Pfeiffer M, Gilbertson LG, Goel VK, Griss P, Keller JC, Ryken TC, Hoffman HE. Effect of specimen fixation method on pullout tests of pedicle screws. Spine. 1996;21(9):1037–44. doi: 10.1097/00007632-199605010-00009. [DOI] [PubMed] [Google Scholar]
- 16.Roy-Camille R. Posterior Screw Plate Fixation in Thoracolumbar Injuries. American Academy of Orthopaedic Surgeons Instructional Course Lectures. 1992;41:157–163. [PubMed] [Google Scholar]
- 17.Roy-Camille R, Saillant G, Berteaux D, Salgado V. Osteosynthesis of thoraco-lumbar spine fractures with metal plates screwed through the vertebral pedicles. Recontruction Surgery Traumatology. 1976;15:2–16. [PubMed] [Google Scholar]
- 18.Roy-Camille RR, Saillant G, Mazel C. Internal fixation of the lumbar spine with pedicle screw plating. Clinical Orthopaedics. 1986;203:7–17. [PubMed] [Google Scholar]
- 19.Ruland CM, McAfee PC, Warden KE, Cunningham BW. Triangulation of pedicular instrumentation: a biomechanical analysis. Spine. 1991;16( supple ):S270–276. doi: 10.1097/00007632-199106001-00019. [DOI] [PubMed] [Google Scholar]
- 20.Saillant G. Anatomical study of vertebral pedicles, surgical application.(French) Revue de chirurgie orthopédique et réparatrice de l’appareil moteur. 1976;62(2):157. [PubMed] [Google Scholar]
- 21.Soshi S, Shiba R, Kondo H, Murota K. An experimental study on transpedicular screw fixation in relation to osteoporosis of the lumbar spine. Spine. 1991;16:1335–40. doi: 10.1097/00007632-199111000-00015. [DOI] [PubMed] [Google Scholar]
- 22.Willet K, Hearn TC, Cuncins AV. Biomechanical testing of a new design for Schanz pedicle screws. Journal of Orthopaedic Trauma. 1993;7(4):375–80. doi: 10.1097/00005131-199308000-00015. [DOI] [PubMed] [Google Scholar]
- 23.Yamagata M, Kitahara H, Minami S, Takahashi K, Iso Moriya H, Tamaki T. Mechanical stability of the pedicle fixation systems for the lumbar spine. Spine. 1992;17(Suppl 3):S51–S54. doi: 10.1097/00007632-199203001-00011. [DOI] [PubMed] [Google Scholar]
- 24.Yerby SA, Ehteshami JR, McLain RF. Loading of pedicle screws within the vertebra. Journal of Biomechanics. 1997;30(9):951–4. doi: 10.1016/s0021-9290(97)00037-7. [DOI] [PubMed] [Google Scholar]
- 25.Zdeblick TA, Kunz DN, Cooke ME, McCabe R. Pedicle Screw Pullout Strength: correlation with insertional torque. Spine. 1993;18(12):1673–6. doi: 10.1097/00007632-199309000-00016. [DOI] [PubMed] [Google Scholar]
- 26.Zindrick MR. The role of transpedicular fixation systems for stabilization of the lumbar spine. Orthopaedic Clinics of NA. 1991;22(2):333–44. [PubMed] [Google Scholar]
- 27.Zindrick MR, Witse LL, Doornik A, Widell EH, Knight GW, Patwardhan AG, Thomas JC, Rothman SL, Fields BT. Analysis of the morphometric characteristics of the thoracic and lumbar pedicles. Spine. 1987;12(2):160–166. doi: 10.1097/00007632-198703000-00012. [DOI] [PubMed] [Google Scholar]
- 28.Zindrick MR, Wiltse LL, Widell EH, Thomas JC, Holland WR, Field BT, Spencer CW. A biomechanical study of interpeduncular screw fixation in the lumbosacral spine. Clinical Orthopaedics and Related Research. 1986;203:99–112. [PubMed] [Google Scholar]


