Abstract
Background
Obesity may reduce fecundity. We examined the obesity-fecundity association in relation to menstrual cycle regularity, parity, smoking habits, and age to gain insight into mechanisms and susceptible subgroups.
Methods
Data were provided by 7,327 pregnant women enrolled in the Collaborative Perinatal Project at 12 study centers in the United States from 1959 to 1965. Prepregnancy body mass index was analyzed continuously and categorically (underweight (<18.5 kg/m2), optimal weight (18.5 kg/m2 – 24.9 kg/m2), overweight (25.0 kg/m2 – 29.9 kg/m2), and obese (≥30.0 kg/m2)). Adjusted fecundability odds ratios (ORs) were estimated using Cox proportional hazards modeling for discrete time data.
Results
Fecundity was reduced for overweight (OR=0.92, 95% confidence interval (CI): 0.84, 1.01) and obese (OR=0.82, 95% CI: 0.72, 0.95) women compared to optimal weight women, and was more evident for obese primiparous women (OR=0.66, 95% CI: 0.49, 0.89). Fecundity remained reduced for overweight and obese women with normal menstrual cycles. Neither smoking habits nor age modified the association.
Conclusions
Obesity was associated with reduced fecundity for all subgroups of women and persisted for women with regular cycles. Our results suggest that weight loss could increase fecundity for overweight and obese women, regardless of menstrual cycle regularity, parity, smoking habits, or age.
Keywords: Fecundity, fertility, obesity, reproduction
INTRODUCTION
Since 1971, the total fertility rate in the United States has been below the threshold required to maintain a steady population size (i.e. less than 2.1 births per woman over her reproductive lifetime) (Hamilton 2004). This deficit can be explained partly by social changes in desired family size, increased availability and effectiveness of contraception, and increased availability and use of induced abortion (Sallmen et al 2005). However, between 1996 and 2002, the number of assisted reproductive technology procedures increased 78% and the number of conceptions due to assisted reproductive technology increased 120% (Wright et al 2005), suggesting that among other factors, the decreased fertility rate could be explained in part by a decrease in human fecundity.
Obesity has been associated with reduced fecundity (Green et al 1988; Zaadstra et al 1993; Rich-Edwards et al 1994; Lake et al 1997; Jensen et al 1999; Bolumar et al 2000; Rich-Edwards et al 2002; Hassan and Killick 2004), as well as impaired pregnancy success for women using assisted reproductive technologies (Franks 2006; Bellver 2006; Pasquali and Gambineri 2006; Mitchell et al 2005; Bellver et al 2003; Pasquali et al 2003). One hypothesized mechanism is that obesity affects the hypothalamic-pituitary-ovary axis resulting in irregular cycles (Pralong et al 2002; Pasquali et al 2003; Haslam and James 2005). However, some evidence indicates that the effect of obesity on fecundity persists for women with regular menstrual cycles (Jensen et al 1999; Bolumar et al 2000). Additionally, other evidence suggests fecundity may only be reduced for obese women who smoke (Bolumar et al 2000). Given the increasing prevalence of obesity for women in their prime reproductive years (20 to 39 years old) (Flegal et al 2002; Ogden et al 2004), a closer look at risk factors that modify the obesity-fecundity association is in order because it may provide insights into mechanisms and highlight susceptible subgroups.
Our objective was to examine the obesity-fecundity association in relation to parity, menstrual cycle regularity, smoking habits, and age. We hypothesized that 1) increasing body mass index would be associated with reduced fecundity, 2) the association would be stronger for previously nulliparous women since, as a group, they have a broader range of fertility represented; 3) the association would persist for women with normal menstrual cycle characteristics (Jensen et al 1999; Bolumar et al 2000), suggesting a mechanism other than irregular menstrual cycling; 4) smoking status would modify the association such that the effect would be stronger for smokers (Bolumar et al 2000), especially given the independent association between smoking and reduced fecundity (The Practice Committee of the American Society for Reproductive Medicine 2004); and 5) age would modify the association such that the effect would be stronger with increasing age given the independent association of age and reduced fecundity (ESHRE Capri Workshop Group 2005).
MATERIALS AND METHODS
From 1959 to 1965, when smoking prevalence was high, over 55,000 pregnant women were enrolled in the Collaborative Perinatal Project at 12 study centers across the United States (Broman 1984). The Collaborative Perinatal Project was a large prospective study designed to investigate the developmental consequences of complications arising during pregnancy or the perinatal period. Information was collected on pre-pregnancy weight, height, and time to pregnancy; demographic and smoking related data; and reproductive, medical, and gynecological history.
Information on time to pregnancy was assessed at the initial study visit (median 16 weeks gestation). Among other questions, women were asked, “Have you been trying to become pregnant?” Those who responded, “Yes” were asked, “How long did it take you to become pregnant?” The response was recorded in months starting at one month. We used this self-reported estimate as the time to pregnancy measure for our primary analysis. However, we also compared the results substituting the original time to pregnancy estimates with a time to pregnancy estimate corrected for miscarriage (recalculated starting from the date of recent miscarriage, if miscarriage date was included in the time to pregnancy interval), postpartum subfertility (crediting a two-month period of subfertility), and lactational amennorhea (crediting a four-month period of subfertility) (Gesink Law et al 2005).
Continuous prepregnancy body mass index was calculated using self-reported height and prepregnancy weight, then categorized into underweight (<18.5 kg/m2), optimal weight (18.5 kg/m2 – 24.9 kg/m2), overweight (25.0 kg/m2 – 29.9 kg/m2), and obese (≥30.0 kg/m2). Subjects were asked about current smoking status, total years smoked, and number of cigarettes smoked per day. Women were classified as having irregular cycles if they reported irregular menstrual cycles, skipped one or more menstrual periods regularly, or if the usual length of their menstrual cycles varied by more than 7 days (Cooper et al 2005). Demographic data included age, race/ethnicity, education and occupation. Detailed information about each previous pregnancy and its outcome was also available. Information on alcohol and drug use ascertained severe abuse only, which was reported rarely, so we did not use these data. Data on waist size, waist-to-hip ratio, diet, caffeine consumption, knowledge of the fertile window, and frequency and timing of intercourse were not collected.
Of the 59,391 enrolled pregnancies, we excluded 47,399 unplanned pregnancies because they lacked time to pregnancy estimates, and 2,969 planned pregnancies that were missing time to pregnancy estimates. Of the remaining 9,023 planned pregnancies with time to pregnancy estimates, 671 were missing height, 151 were missing pre-pregnancy weight, and 225 were missing both height and pre-pregnancy weight estimates. Of the remaining 7,976 planned pregnancies, we retained: all entries for women with only one enrolled pregnancy (n=7,002); the first planned pregnancy for women with more than one enrolled pregnancy (n=403); and one pregnancy record for women with plural births since each twin or triplet had their own entry (n=71). Thus, there were 7,476 eligible pregnancies for analysis. However, our results reflect the fecundability of 7,327 women because 149 of the original 7,476 women were missing data on covariates in the final model (figure 1).
Figure I.
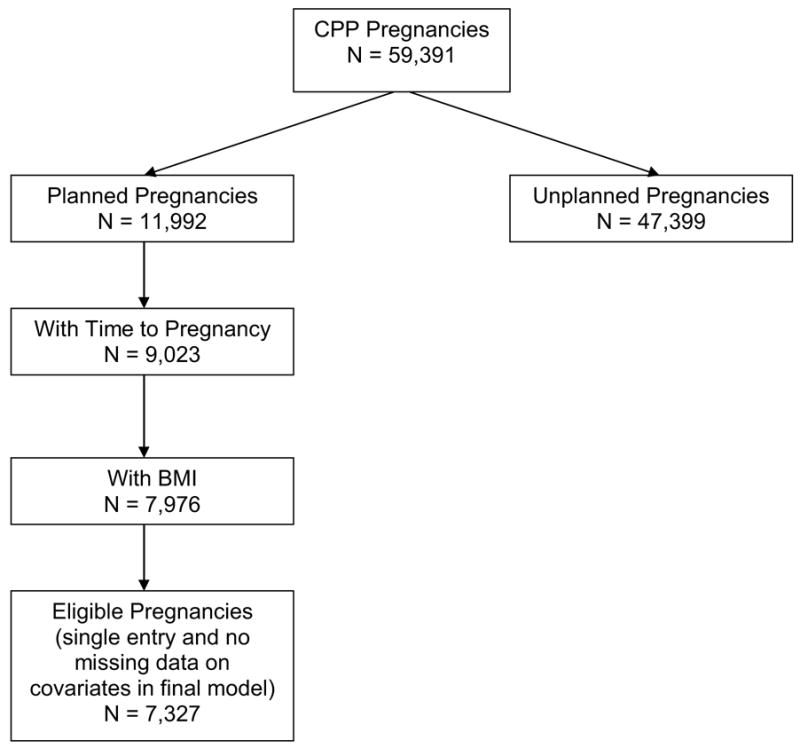
Derivation of the final subset of Collaborative Perinatal Project study participants used in the analysis of the association between body mass index and time to pregnancy. Pregnant women enrolled in the Collaborative Perinatal Project at 12 study centers in the United States from 1959 to 1965.
Data analysis
For descriptive purposes, we compared more fertile women (those who became pregnant in three months or less) with less fertile women (those who became pregnant in more than three months) according to risk factors known to increase time to pregnancy. We also compared women who planned their pregnancy with women who had not planned their pregnancy on body mass index, age, smoking habits, parity, and other demographic, gynecologic, and reproductive characteristics.
Fecundability odds ratios (Weinberg and Wilcox 1998) describing the association between time to pregnancy and body mass index were estimated using a Cox proportional hazards model (Cox 1972) modified for discrete time data (SAS 9.0; STATA/SE 9). Fecundability odds ratios less than one signified decreased fecundity, or increased time to pregnancy. Time to pregnancy was censored at 13 months in the event that women with longer times to pregnancy received treatment for infertility (Baird et al 1986). 1,193 (16%) pregnancies were censored at 13 months. Body mass index was examined categorically using under weight, optimal weight, overweight and obese categorizations, and continuously using a quadratic spline to allow more flexible estimation of the non-linear relationship between BMI and fecundability.
A priori, we decided our base model needed to adjust for age and investigated confounding in the age-adjusted model. Covariates that changed any of the beta coefficients describing the association between categorical body mass index and time-to-pregnancy by 10 percent or more were considered confounders (24). We evaluated the following covariates for confounding: current smoking status (yes; referent no), race (nonwhite; referent white), maternal education (number of years), maternal current or most recent occupation (referent never worked; blue collar; white collar), and study center (for methodologic reasons including interviewer and population differences among centers).
We evaluated effect modification by smoking habits and age, as well as by race and study center. We evaluated effect modification in age- and covariate-adjusted models using a likelihood ratio test. If an interaction term was significant at the α=0.10 level, we examined stratum-specific estimates to determine if effect modification was substantively important enough to report separate estimates. Smoking variables included smoking status (yes; referent no), number of cigarettes smoked per day, number of years smoked, and pack years (referent 0, >0 to 10, >10 to 20, >20).
Nulliparous women represent a broader spectrum of fertility than parous women (Axmon et al 2006). Consequently, the effect of obesity on fecundity may be more apparent for primiparous women than multiparous women. Therefore, we stratified our analysis by parity. We also restricted our analyses to women with regular menstrual cycle characteristics (Cooper 2004), and again to women with normal cycle lengths (27 to 29 days), to test the hypothesis that reduced fecundity is the result of irregular cycles in overweight and obese women.
We conducted several secondary analyses to verify the robustness of our results. We restricted our analysis to women with singleton births, without indication of metabolic or endocrine dysfunction (including diabetes), and without indication of prior gynecologic condition (vaginitis, infertility, incompetent cervix, surgery for incompetent cervix, gynecologic surgery, leiomyoma, other gynecologic tumor, other gynecologic problem).
RESULTS
Compared to women in the Collaborative Perinatal Project who had not planned their pregnancies, a greater proportion of women in our analysis were white, married, more highly educated, employed in a white-collar job, and had not been pregnant before. However, these two groups of women did not differ on body mass index, age, smoking habits, gynecologic characteristics, or frequency of spontaneous abortion (Table I). Furthermore, among primiparas, body mass index did not differ between planners and nonplanners (data not shown).
Table I.
Characteristics of women in the Collaborative Perinatal Project who planned their pregnancies (planners) compared to those who did not (non-planners). Women in the Collaborative Perinatal Project were enrolled at 12 study centers in the United States from 1959–1965.
| Characteristic | Planners (N = 8,514)
|
Non-Planners (N = 39,683)
|
|---|---|---|
| % or Median (Q1, Q3)* | % or Median (Q1, Q3) | |
| BMI† | 21.3 (19.3, 23.8) | 21.5 (19.4, 24.5) |
| Underweight (<18.5kg/m2) | 17 | 15 |
| Optimal weight (18.5–24.9 kg/m2) | 63 | 60 |
| Overweight (25.0–29.9 kg/m2) | 11 | 13 |
| Obese (≥30kg/m2) | 8 | 8 |
| Age (years) | 23 (20, 27) | 23 (19, 28) |
| <20 | 20 | 26 |
| 20–23 | 25 | 23 |
| 23–27 | 32 | 25 |
| ≥28 | 23 | 26 |
| Current smokers | 46 | 46 |
| Prior Pregnancies | ||
| 0 | 40 | 32 |
| 1 | 27 | 18 |
| 2+ | 33 | 47 |
| Prior Miscarriages among women with prior pregnancy | ||
| 0 | 67 | 74 |
| 1 | 24 | 19 |
| 2+ | 9 | 7 |
| Irregular cycles | 8 | 11 |
| Occupation | ||
| Never worked | 13 | 16 |
| White collar | 44 | 27 |
| Blue collar | 44 | 56 |
| Maternal Education | ||
| Elementary | 17 | 19 |
| < High school | 32 | 41 |
| High school | 33 | 29 |
| > High school | 18.5 | 11 |
| Race | ||
| White | 64 | 42 |
| Non-white | 36 | 58 |
Q = quartile
BMI = body mass index; categorized into underweight (<18.5 kg/m2), optimal weight (18.5 kg/m2 – 24.9 kg/m2), overweight (25.0 kg/m2– 29.9 kg/m2), and obese (≥30.0 kg/m2).
Most of the women in our analysis were young, white, and working (Table II). Ninety-four percent were married or common law. Sixty-four percent of the women had been pregnant before, 21 percent had a history of miscarriage, and 45 percent were current smokers. On average, smokers smoked 13 cigarettes per day for 7 years (mean pack-years = 5). The mean and median body mass indices were within the optimal range; 13 percent were overweight and 5 percent were obese. As expected, fecundity was reduced (longer time to pregnancy) for older women, smokers, nonwhites, and women with irregular cycles. Fecundity was also lower for women with less education. Women in white collar occupations had increased fecundity (shorter time to pregnancy), possibly because they were in better health (Artazcoz et al 2004), or because they were more highly educated and knowledgeable about their fertile window. The relation of education to fecundity, however, varies across studies (Axmon 2006).
Table II.
Characteristics of women in the Collaborative Perinatal Project and their association with time to pregnancy. Women in the Collaborative Perinatal Project were enrolled at 12 study centers in the United States from 1959–1965.
| Characteristic | All Women (N=7327)
|
Time to pregnancy ≤ 3 months (n=3801)
|
Time to pregnancy> 3 months (n=3526)
|
Age-Adjusted Fecundability Odds Ratio† |
|
|---|---|---|---|---|---|
| % or Median(Q1, Q3) ‡ | % or Median (Q1, Q3) | % or Median (Q1, Q3) | FOR | 95% CI | |
| Prepregnancy BMI* | 21.4 (19.6, 23.7) | 21.2 (19.6, 23.3) | 21.8 (19.8, 24.2) | ||
| Under-weight | 12 | 12 | 12 | 0.94 | 0.86, 1.03 |
| Optimal weight | 71 | 73 | 69 | 1 | -- |
| Over-weight | 13 | 11 | 14 | 0.84 | 0.77, 0.92 |
| Obese | 5 | 4 | 5 | 0.72 | 0.63, 0.83 |
| Median Age (years) | 23 (20, 27) | 22 (20, 26) | 24 (21, 28) | 0.95 | 0.94, 0.96 |
| Smokers (%) | 45 | 44 | 47 | 0.91 | 0.86, 0.96 |
| Race (%) | |||||
| White | 63 | 68 | 57 | 1 | -- |
| Non-white | 37 | 32 | 43 | 0.65 | 0.62, 0.70 |
| Prior Pregnancies (%) | |||||
| 0 | 36 | 39 | 33 | 1 | -- |
| 1 | 30 | 31 | 30 | 0.98 | 0.91, 1.05 |
| 2+ | 34 | 30 | 37 | 1.04 | 0.97, 1.13 |
| Prior Miscarriage | |||||
| 0 | 79 | 82 | 77 | 1 | -- |
| 1 | 15 | 14 | 17 | 0.96 | 0.88, 1.04 |
| 2+ | 6 | 4 | 6 | 0.97 | 0.86, 1.11 |
| Irregular cycles (%) | 8 | 7 | 9 | 0.82 | 0.74, 0.91 |
| Occupation (%) | |||||
| Never worked | 12 | 12 | 13 | 1 | -- |
| White collar | 44 | 49 | 39 | 1.47 | 1.34, 1.61 |
| Blue collar | 44 | 39 | 48 | 1.02 | 0.93, 1.11 |
| Education | |||||
| Elementary | 16 | 12 | 19 | 0.44 | 0.40, 0.49 |
| Less than HS | 33 | 32 | 33 | 0.54 | 0.49, 0.59 |
| HS | 33 | 33 | 33 | 0.63 | 0.58, 0.68 |
| More than HS | 19 | 23 | 14 | 1 | -- |
BMI = body mass index; categorized into underweight (<18.5 kg/m2), normal weight (18.5 kg/m2–24.9 kg/m2), overweight (25.0 kg/m2–29.9 kg/m2), and obese (≥30.0 kg/m2).
Cox discrete time survival analysis used to model time-to-pregnancy, fecundability odds ratios and 95 percent (%) confidence interval (CI) reported
% = percent, Q1 = quartile 1, Q3 = quartile 3, FOR = fecundability odds ratio, CI = confidence interval
Planners had a median (first quartile, third quartile) time to pregnancy of 3 months (1 month, 9 months). By body mass index, median time to pregnancy was 3 months (1 month, 8 months) for underweight women, 3 months (1 month, 9 months) for optimal weight women, 4 months (2 months, 12 months) for overweight women and 5 months (2 months, 18 months) for obese women. Multiparous women had higher fecundity, or shorter time to pregnancy (fecundability odds ratio (OR): 1.17, 95 percent confidence interval (CI): 1.09, 1.24), compared to primiparous women, after adjusting for body mass index, age, smoking, race, occupation, education and study center.
After adjusting for age, fecundability was reduced for underweight (fecundability OR = 0.94; 95 percent CI: 0.86, 1.03), overweight (fecundability OR= 0.84; 95 percent CI: 0.77, 0.92), and obese (fecundability OR = 0.72; 95 percent CI: 0.63, 0.83) women, compared to women with an optimal body mass index.
Adjustment for smoking, race, occupation, education, and study center changed the beta coefficients for each category of body mass index by more than 10 percent when compared with the age-adjusted model. Time to pregnancy increased for overweight and obese women compared to women with an optimal body mass index, after adjusting for age, smoking, race, education, occupation, and study center (Table III). The probability of conception had an inverse-U shape with increasing body mass index, indicating that fecundability was highest for women in the lower end of the optimal range and decreased for underweight, overweight, and obese women (Figure II). The upward turn for women with very high BMIs is due to the small number of women in that range.
Table III.
Association between body mass index (BMI) and time to pregnancy based on 7,327 pregnancies for women in the Collaborative Perinatal Project who were trying to become pregnant.* Women in the Collaborative Perinatal Project were enrolled at 12 study centers in the United States from 1959–1965.
| BMI model† | Underweight
|
Optimal
|
Overweight
|
Obese
|
|---|---|---|---|---|
| FOR (95% CI) ‡ | FOR (95% CI) | FOR (95% CI) | FOR (95% CI) | |
| Age-adjusted model (N=7,327) | 0.94 (0.86, 1.03) | 1 | 0.84 (0.77, 0.92) | 0.72 (0.63, 0.83) |
| Final adjusted model§ (N=7,327) | 0.95 (0.87, 1.04) | 1 | 0.92 (0.84, 1.01) | 0.82 (0.72, 0.95) |
| Final model by smoking# | ||||
| Non-Smoker | 1.03 (0.90, 1.16) | 1 | 0.89 (0.79, 1.00) | 0.81 (0.67, 0.99) |
| Smoker | 0.89 (0.78, 1.01) | 0.97 (0.85, 1.11) | 0.83 (0.68, 1.02) | |
| Final model by pack years# | ||||
| 0 | 1.03 (0.91, 1.17) | 1 | 0.87 (0.78, 0.98) | 0.81 (0.66, 0.98) |
| > 0–10 | 0.85 (0.74, 0.97) | 0.97 (0.83, 1.13) | 0.84 (0.67, 1.05) | |
| >10–20 | 1.06 (0.73, 1.52) | 0.75 (0.42, 1.33) | ||
| > 20 | 1.05 (0.39, 2.79) | 0.55 (0.24, 1.26) | 0.77 (0.27, 2.26) | |
| Final model by parity | ||||
| Primiparous | 0.91 (0.79, 1.04) | 1 | 0.84 (0.71, 1.00) | 0.66 (0.49, 0.89) |
| Multiparous | 0.98 (0.88, 1.10) | 0.95 (0.86, 1.05) | 0.91 (0.78. 1.07) | |
| Final Model for Irregular Menstrual Cycles | 0.97 (0.88, 1.07) | 1 | 0.92 (0.83, 1.00) | 0.85 (0.74, 0.99) |
1193 pregnancies had time-to-pregnancy estimates longer than 13 months that were censored at 13 months. Number of subjects missing values for smoking (20), education (122), occupation (7).
BMI = body mass index; categorized into underweight (<18.5 kg/m2), optimal weight (18.5 kg/m2–24.9 kg/m2), overweight (25.0 kg/m2–29.9 kg/m2), and obese (≥30.0 kg/m2).
Cox discrete time survival analysis used to model time-to-pregnancy; fecundability odds ratios (FOR) and 95 percent (%) confidence interval (CI) reported.
Adjusted for age, smoking, race, education, occupation, study center.
Adjusted for age, race, education, occupation, study center.
Figure II.
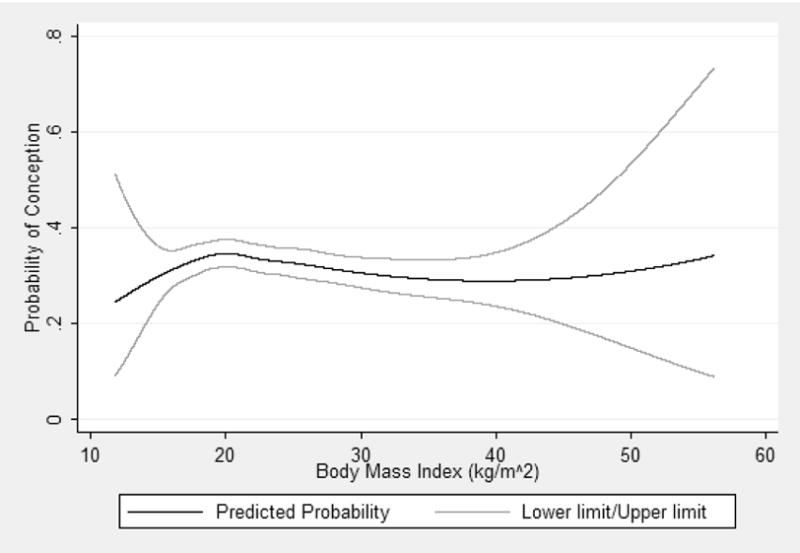
The predicted probability of conception with changing body mass index (kg/m2), after adjusting for age, smoking, race, education, occupation, and study center. The graph was constructed for 23 year old, non-smoking, white women with a high school diploma in white collar occupations enrolled at the Boston clinic. Pregnant women enrolled in the Collaborative Perinatal Project between 1959 and 1965.
Age, race, and study center did not modify the association between obesity and fecundity. However, evidence of effect modification by smoking habits was mixed. The association was not modified by number of cigarettes smoked per day (p=0.21) nor by number of years smoked (p=0.15). Effect modification by smoking status was of borderline statistical significance (p=0.12), but for the obese subjects the fecundability ORs in different smoking categories were similar (Table III). Statistical evidence for modification by pack years smoked was stronger (p=0.04), but again, obese subjects in different smoking categories had similar odds ratios and for the underweight and overweight, the odds ratios showed no suggestion of dose-response relations. Overall, evidence of effect modification by smoking was not compelling.
The association between body mass index and time-to-pregnancy was essentially unchanged when we restricted our analyses to women with regular menstrual cycles or menstrual cycles 27 to 29 days long. When we stratified our analysis on parity, the association was stronger for primiparous women, especially obese primiparous women (Table III).
The association between body mass index and time-to-pregnancy was unchanged when we restricted our analyses to singleton pregnancies, normal metabolic and endocrine function, or women without gynecologic conditions. Similarly, correcting 344 time-to-pregnancy estimates for unaccounted miscarriage, postpartum subfertility, and lactational amenorrhea (see methods; results not shown) did not alter the observed associations either.
DISCUSSION
Fecundability, or the probability of conceiving a pregnancy for a given cycle (Baird et al 1986), was reduced for overweight and obese women compared to women with an optimal body mass index enrolled in the Collaborative Perinatal Project. Overall, the probability of conceiving in a given cycle was reduced 8% for overweight women and 18% for obese women. This translated to a median one month longer for overweight women to become pregnant, and two months longer for obese women to become pregnant compared to optimal weight women. Cumulatively, this meant it took about three months longer before 75% of overweight women became pregnant and nine months longer before 75% of obese women became pregnant compared to optimal weight women. Focusing on the results for previously nulliparous women (primiparous in our analysis), the probability of conceiving per cycle was reduced even further: by about 16% for overweight and 34% for obese nulliparous women.
Our investigation confirms those of Jensen and colleagues (1999), Bolumar and colleagues (2000) and Hassan and England (2004). Similar to Jensen and colleagues (1999), and Bolumar and colleagues (2000), the associations we observed were maintained when we restricted our analyses to women with regular menstrual cycles. However, contrary to Bolumar and colleagues (2000), we only found subtle differences between smokers and nonsmokers. Bolumar and colleagues reported that reduced fecundability among underweight, overweight and obese women occurred only among smokers, but compared to our analysis, they had a much smaller sample, a lower percentage of smokers, and a lower proportion of overweight and obese women. We conclude that there was no effect modification by smoking, nor was there any by age. The greater reduction in fecundity for overweight and obese primiparous women further supports our central hypothesis.
We restricted our analysis to women who planned their pregnancy. This could be a problem if women who did not plan their pregnancy had different body mass indices or differed from women who planned their pregnancies in other important characteristics. When we compared planners with non-planners, we did not find a difference in body mass index, age, smoking habits, cycle length, cycle regularity, or previous gynecologic condition. However, more planners were primiparous. This difference could explain some of the association we observed if planners had lower fecundity than nonplanners.
The association between obesity and fecundity could be weaker than we observed. Obesity and infertility are common characteristics of polycystic ovary syndrome, which occurs in approximately four percent of women (Guzick 2004). Given that the prevalence of obesity was lower in the 1960’s (five percent) than it is today (over 25 percent), it is possible that a higher proportion of obese women in our study had polycystic ovary syndrome. The fertility problems women with polycystic ovary syndrome experience, such as anovulation, may not be a consequence of their obesity (Pralong et al 2002; Pasquali et al 2003). Polycystic ovary syndrome was not recognized at the time of this study, but during the physical exam, physicians noted whether hirsutism and obesity were “normal” or “abnormal” for a subset of women in our analysis (n=4,115). Less than 10 women were identified as potentially having polycystic ovary syndrome regardless of whether we defined polycystic ovary syndrome as women with hirsutism and menstrual cycle length greater than 36 days (n=7); hirsutism and obesity (n=8); or hirsutism, obesity, and menstrual cycle length greater than 36 days (n=3) (Hartz et al 1979). However, polycystic ovary syndrome can exist without hirsutism, and while polycystic ovary syndrome is likely to explain some of the associations we observed, we believe the prevalence is low in our participants and unlikely to explain all of our results.
The association between obesity and reduced fecundity could be stronger than we observed since our sample did not include women who did not become pregnant. Consequently, we may be missing a group of women whose ability to conceive a detectable pregnancy is particularly sensitive to the mechanism(s) by which obesity reduces fecundity. This is further supported by the stronger association between overweight and obesity and fecundity we observed when we stratified our analysis on parity.
The biologic mechanism responsible for the association between body mass index and fecundity is unclear. One hypothesis is that obesity affects the hypothalamic-pituitary-ovary axis (Pralong et al 2002; Pasquali et al 2003; Haslam and James 2005). Excess free estrogen, resulting in part from increased peripheral conversion of androgens to estrogens in adipose tissue, combined with decreased availability of gonadotropin releasing hormone, could interfere with hypothalamic-pituitary regulation of ovarian function, causing irregular or anovulatory cycles (Pralong et al 2002; Hartz et al 1979; Haslam and James 2005). However, like Jensen (Jensen et al 1999), we found that fecundity remained reduced for overweight and obese women with regular menstrual cycles, which suggests that anovulation despite regular menses, or the release of ova with reduced fertilization potential, or even endometrial abnormalities, may be the more likely mechanism.
Another possibility is that obese women have reduced fecundity because of a complex interplay of psychosocial, sociobiological, and physiological factors. Obese people do not have sexual intercourse as frequently as slimmer people, even if they have a cohabiting sexual partner (Brody 2004). This could be explained, in part, by decreased sex drive resulting from decreased dopamine activity and increased serotonin levels in the brain due to overeating (Brody 2004), and increased sexual dysfunction (Trischitta 2003). Additionally, chronic fat or sugar consumption could have psychopharmacological effects, relabeling sexual desire as a cue to eat (Brody 2004). For obese women then, especially primiparous obese women, knowledge of the fertile window and timing of intercourse is even more important, since capturing the fertile window by chance is lowered with decreased frequency of sexual intercourse.
Our conclusions are based on an analysis of 7,327 women who were trying to get pregnant and conceived in the late 1950’s/early 1960’s, when the prevalence of maternal smoking was high (46%), but the prevalence of obesity (5%) and oral contraceptive use were low (less than 2%), and maternal age was young (median 23 years). We found that fecundity was reduced for all subgroups of obese women, and was particularly evident for nulliparous women. We hypothesize that obesity-induced excess estrogen interferes with the hypothalamic-pituitary-ovary axis such that women experience anovulatory cycles with regular menstruation, or release of ova with reduced fertilization potential, or endometrial abnormalities. Today, maternal smoking is much lower (20%; Yeh and Shelton 2005), but the prevalence of obesity (30%; Flegal et al 2002), and maternal age at first birth (27 years; Hamilton et al 2003) have increased dramatically, which could still have an overall negative impact on fertility trends.
Weight loss among overweight and obese women has been shown to improve ovarian function and fecundity, suggesting that the adverse effects of obesity could be reversible (Norman et al 2004; Moran et al 2003; Moran and Norman 2002; Rich-Edwards et al 2002; Clark et al 1998; Clark et al 1995; Kiddy et al 1992). If costly assisted reproductive technology use has risen in recent years because of a true decline in fecundity, and obesity is a contributing factor, then weight loss and improved knowledge of the fertile window should be encouraged as a non-invasive first attempts at treating infertility for overweight and obese women.
Acknowledgments
The authors wish to thank Drs. Olga Basso and Donna Baird for their helpful comments and insights during development of this manuscript. This research was supported in part by the Intramural Research Program of the National Institutes of Health, National Institute of Environmental Health Sciences.
References
- Artazcoz L, Borrell C, Benach J, Cortes I, Rohlfs I. Women, family demands and health: the importance of employment status and socio-economic position. Soc Sci Med. 2004 Jul;59(2):263–74. doi: 10.1016/j.socscimed.2003.10.029. [DOI] [PubMed] [Google Scholar]
- Axmon A, Rylander L, Albin M, Hagmar L. Factors affecting time to pregnancy. Human Reproduction. 2006 May;21(5):1279–84. doi: 10.1093/humrep/dei469. [DOI] [PubMed] [Google Scholar]
- Baird DD, Wilcox AJ, Weinberg CR. Use of Time to Pregnancy to Study Environmental Exposures. American Journal of Epidemiology. 1986;124:470–80. doi: 10.1093/oxfordjournals.aje.a114417. [DOI] [PubMed] [Google Scholar]
- Bellver J, Busso C, Pellicer A, Remohi J, Simon C. Obesity and assisted reproductive technology outcomes. Reprod Biomed Online. 2006 May;12(5):562–8. doi: 10.1016/s1472-6483(10)61181-9. [DOI] [PubMed] [Google Scholar]
- Bellver J, Rossal LP, Bosch E, Zuniga A, Corona JT, Melendez F, Gomez E, Simon C, Remohi J, Pellicer A. Obesity and the risk of spontaneous abortion after oocyte donation. Fertil Steril. 2003 May;79(5):1136–40. doi: 10.1016/s0015-0282(03)00176-6. [DOI] [PubMed] [Google Scholar]
- Bolumar F, Olsen J, Rebagliato M, Saez-Lloret I, Bisanti L the European Study Group on Infertility and Subfecundity. Body mass index and delayed conception: A European multicenter study on infertility and subfecundity. American Journal of Epidemiology. 2000;151:1072–1079. doi: 10.1093/oxfordjournals.aje.a010150. [DOI] [PubMed] [Google Scholar]
- Broman S. The Collaborative Perinatal Project: An Overview. In: Mednick SA, Harway M, Finello KM, editors. Handbook of Longitudinal Research. New York: Praeger Pub; 1984. pp. 185–215. [Google Scholar]
- Brody S. Slimness is associated with greater intercourse and lesser masturbation frequency. Journal of Sex and Marital Therapy. 2004;30:251–261. doi: 10.1080/00926230490422368. [DOI] [PubMed] [Google Scholar]
- Clark AM, Thornley B, Tomlinson L, Galletley C, Norman RJ. Weight loss in obese infertile women results in improvement in reproductive outcome for all forms of fertility treatment. Hum Reprod. 1998 Jun;13(6):1502–5. doi: 10.1093/humrep/13.6.1502. [DOI] [PubMed] [Google Scholar]
- Clark AM, Ledger W, Galletly C, Tomlinson L, Blaney F, Wang X, Norman RJ. Weight loss results in significant improvement in pregnancy and ovulation rates in anovulatory obese women. Hum Reprod. 1995 Oct;10(10):2705–12. doi: 10.1093/oxfordjournals.humrep.a135772. [DOI] [PubMed] [Google Scholar]
- Cooper GS, Klebanoff MA, Promislow J, Brock JW, Longnecker MP. Polychlorinated biphenyls and menstrual cycle characteristics. Epidemiology. 2005;16:191–200. doi: 10.1097/01.ede.0000152913.12393.86. [DOI] [PubMed] [Google Scholar]
- Cox DR. Regression models and life-tables. Journal of the Royal Statistical Society Series B-Statistical Methodology. 1972;34:187–220. [Google Scholar]
- ESHRE Capri Workshop Group. Fertility and ageing. Human Reproduction Update. 2005;11:261–276. doi: 10.1093/humupd/dmi006. [DOI] [PubMed] [Google Scholar]
- Flegal KM, Carroll MD, Ogden CL, Johnson CL. Prevalence and trends in obesity among US Adults, 1999–2000. Journal of the American Medical Association. 2002;288:1723–1727. doi: 10.1001/jama.288.14.1723. [DOI] [PubMed] [Google Scholar]
- Franks S. Genetic and environmental origins of obesity relevant to reproduction. Reprod Biomed Online. 2006 May;12(5):526–31. doi: 10.1016/s1472-6483(10)61177-7. [DOI] [PubMed] [Google Scholar]
- Gesink Law DC, Klebanoff MA, Brock JW, Dunson DB, Longnecker MP. Maternal Serum Levels of Polychlorinated Biphenyls and 1,1-Dichloro-2,2-bis(p-chlorophenyl)ethylene (DDE) and Time to Pregnancy. American Journal of Epidemiology. 2005;162:1–10. doi: 10.1093/aje/kwi240. [DOI] [PubMed] [Google Scholar]
- Green BB, Weiss NS, Daling JR. Risk of ovulatory infertility in relation to body weight. Fertility and Sterility. 1988;50:721–726. [PubMed] [Google Scholar]
- Hamilton BE, Sutton PD, Ventura SJ. Revised birth and fertility rates for the 1990s and new rates for hispanic populations 2000 and 2001: United States. Vol. 51. 2003. National Vital Statistics Reports. [PubMed] [Google Scholar]
- Hamilton BE. National Vital Statistics Reports. Vol. 52. 2004. Reproduction rates for 1990–2002 and intrinsic rates for 2000–2001: United States. [PubMed] [Google Scholar]
- Haslam DW, James WPT. Obesity. Lancet. 2005;366:1197–209. doi: 10.1016/S0140-6736(05)67483-1. [DOI] [PubMed] [Google Scholar]
- Hassan MAM, Killick SR. Negative lifestyle is associated with a significant reduction in fecundity. Fertility and Sterility. 2004;81:384–392. doi: 10.1016/j.fertnstert.2003.06.027. [DOI] [PubMed] [Google Scholar]
- Jensen TK, Scheike T, Keiding N, Schaumburg I, Grandjean P. Fecundability in relation to body mass and menstrual cycle patterns. Epidemiology. 1999;10:422–428. doi: 10.1097/00001648-199907000-00011. [DOI] [PubMed] [Google Scholar]
- Kiddy DS, Hamilton-Fairley D, Bush A, Short F, Anyaoku V, Reed MJ, Franks S. Improvement in endocrine and ovarian function during dietary treatment of obese women with polycystic ovary syndrome. Clin Endocrinol (Oxf) 1992 Jan;36(1):105–11. doi: 10.1111/j.1365-2265.1992.tb02909.x. [DOI] [PubMed] [Google Scholar]
- Lake JK, Power C, Cole TJ. Women’s reproductive health: the role of body mass index in early and adult life. Int J Obes Relat Metab Disord. 1997 Jun;21(6):432–8. doi: 10.1038/sj.ijo.0800424. [DOI] [PubMed] [Google Scholar]
- Moran LJ, Noakes M, Clifton PM, Tomlinson L, Galletly C, Norman RJ. Dietary composition in restoring reproductive and metabolic physiology in overweight women with polycystic ovary syndrome. J Clin Endocrinol Metab. 2003 Feb;88(2):812–9. doi: 10.1210/jc.2002-020815. [DOI] [PubMed] [Google Scholar]
- Moran LJ, Norman RJ. The obese patient with infertility: a practical approach to diagnosis and treatment. Nutr Clin Care. 2002 Nov–Dec;5(6):290–7. doi: 10.1046/j.1523-5408.2002.05604.x. [DOI] [PubMed] [Google Scholar]
- Mitchell M, Armstrong DT, Robker RL, Norman RJ. Adipokines: implications for female fertility and obesity. Reproduction. 2005 Nov;130(5):583–97. doi: 10.1530/rep.1.00521. [DOI] [PubMed] [Google Scholar]
- Norman RJ, Noakes M, Wu R, Davies MJ, Moran L, Wang JX. Improving reproductive performance in overweight/obese women with effective weight management. Hum Reprod Update. 2004 May–Jun;10(3):267–80. doi: 10.1093/humupd/dmh018. [DOI] [PubMed] [Google Scholar]
- Ogden CL, Fryar CD, Carroll MD, Flegal KM. Advance data from vital and health statistics. Hyattsville, Maryland: National Center for Health Statistics; 2004. Mean body weight, height, and body mass index, United States 1960–2002. [PubMed] [Google Scholar]
- Pasquali R, Gambineri A. Metabolic effects of obesity on reproduction. Reprod Biomed Online. 2006 May;12(5):542–51. doi: 10.1016/s1472-6483(10)61179-0. [DOI] [PubMed] [Google Scholar]
- Pasquali R, Pelusi C, Genghini S, Cacciari M, Gambineri A. Obesity and reproductive disorders in women. Human Reproduction Update. 2003;9:359–372. doi: 10.1093/humupd/dmg024. [DOI] [PubMed] [Google Scholar]
- Pralong FP, Castillo E, Raposinho PD, Aubert ML, Gaillard RC. Obesity and the reproductive axis. Ann Endocrinol. 2002;63:129–134. [PubMed] [Google Scholar]
- Rich-Edwards JW, Spiegelman D, Garland M, et al. Physical activity, body mass index, and ovulatory disorder infertility. Epidemiology. 2002;13:184–190. doi: 10.1097/00001648-200203000-00013. [DOI] [PubMed] [Google Scholar]
- Sallmen M, Weinberg CR, Baird DD, Lindbohm M, Wilcox AJ. Has human fertility declined over time? Why we may never know. Epidemiology. 2005;16:494–499. doi: 10.1097/01.ede.0000165391.65690.e1. [DOI] [PubMed] [Google Scholar]
- SAS 9.0. The SAS Institute. Cary: North Carolina, USA; [Google Scholar]
- STATA/SE 9. Statacorp; College Station, Texas, USA: [Google Scholar]
- The Practice Committee of the American Society for Reproductive Medicine. Smoking and infertility. Fertility and Sterility. 2004;81:1181–6. doi: 10.1016/j.fertnstert.2003.11.024. [DOI] [PubMed] [Google Scholar]
- Trischitta V. Relationship between obesity-related metabolic abnormalities and sexual function. Journal of Endocrinol Invest. 2003;26:62–64. [PubMed] [Google Scholar]
- Weinberg CR, Wilcox AJ. Reproductive Epidemiology. In: Rothman KJ, Greenland S, editors. Modern Epidemiology. Chapter 29. Philadelphia: Lippincott-Raven Publishers; 1998. [Google Scholar]
- Wright VC, Schieve LA, Reynolds MA, Jeng G. Assisted reproductive technology surveillance - United States, 2002. MMWR. 2005;54:1–24. [PubMed] [Google Scholar]
- Yeh J, Shelton JA. Increasing prepregnancy body mass index: analysis of trends and contributing variables. Am J Obstet Gynecol. 2005 Dec;193(6):1994–8. doi: 10.1016/j.ajog.2005.05.001. [DOI] [PubMed] [Google Scholar]
- Zaadstra BM, Seidell JC, Van Noord PAH, et al. Fat and female fecundity: prospective study of the effect of body fat distribution on conception rates. British Medical Journal. 1993;306:484–7. doi: 10.1136/bmj.306.6876.484. [DOI] [PMC free article] [PubMed] [Google Scholar]


