Abstract
This study investigated the effects of gonadal steroids on sexual motivation in male Syrian hamsters, using partner preference as a model. Male hamsters were assigned to 5 groups: control (n=4), Intact→Orchx (n=8), Orchx→Orchx+T (n=7), olfactory bulbectomy (BulbX, n=5), and vomeronasal organ lesion (VnoX, n=8). Each male was tested for partner preference before and after sexual experience. Unlike rats, sexually-inexperienced gonad-intact male hamsters preferred the receptive female to a stimulus male. However, sexual experience did not enhance preference for the stimulus female. Castration (orchx) reduced sexual motivation: OrchX males showed no significant preference for the stimulus female. Subsequently, intact males were castrated (Intact→Orchx) and OrchX males received a testosterone implant (Orchx→Orchx+T) to determine the time course of gonadal hormones on partner preference and mating behavior. Partner preference changed significantly in both groups within 6 weeks. In Intact→Orchx males, preference for the stimulus female decreased while Orchx→Orchx+T males increased their preference for the stimulus female. However, significant changes in mating behavior preceded the alterations in partner preference. Chemosensory cues are also important for partner preference. After BulbX, preference for the stimulus female significantly decreased. However, VnoX failed to block partner preference. These results show that partner preference may be even more dependent on testosterone than is sexual behavior. Furthermore, while chemosensory cues are essential for sexual motivation, the vomeronasal organ is not required for partner preference.
Keywords: testosterone, sex behavior, animal, vomeronasal organ, olfactory bulb
INTRODUCTION
The aim of the present study was to determine the importance of gonadal steroids, chemosensory cues, and sexual experience on sexual motivation in male hamsters, using partner preference as a model for appetitive sexual behavior. In the context of male sexual behavior, testicular steroid hormones have two important purposes: to promote sexual motivation and to induce copulation [1]. When endogenous steroid levels are low (i.e. before puberty and during seasonal reproductive suppression) or eliminated by castration (Orchx), mating is abolished [2, 3]. However, gonadal steroids also promote sexual motivation. Previous studies have shown that castrated male hamsters spend less time in contact with an estrous female and show less chemoinvestigatory behavior towards females or their odors [4–7]. In rats, castration eliminates partner preference [8] and reduces operant responding for an estrous female [9]. These effects are reversed by testosterone replacement. In the present study, we used partner preference in male hamsters to compare hormonal responsiveness of sexual motivation and copulation.
Although hormones are essential for mating, sexual behavior is only expressed in response to sensory stimuli from a potential sexual partner. In most male rodents, attraction to females is mediated by chemosensory cues which are detected in the vomeronasal organ and olfactory mucosa [see 10]. The olfactory mucosa responds to volatile odors, while the vomeronasal organ is especially sensitive to non-volatile stimuli from conspecifics. Sexual attraction is initiated through odors transduced in the olfactory mucosa, leading to investigation at close range and subsequent activation of the vomeronasal system. Even at low concentrations (1/100), gonad-intact male hamsters are highly attracted to female hamster vaginal secretion (FHVS; [11]). Removal or deafferentation of the olfactory bulbs abolishes consummatory sexual behavior, and eliminates preference for vaginal secretions from conspecific females [12]. To determine the importance of chemosensory cues from the olfactory mucosa and vomeronasal organ on sexual motivation, the present study compared partner preference after bilateral olfactory bulbectomy (BulbX) or removal of the vomeronasal organ (VnoX).
In rats, sexual experience modulates attraction to females [13]. Sexually-naïve male rats do not express a consistent preference for female odors [14]. By contrast, hamsters are attracted to FHVS even when sexually inexperienced [12]. The present study determined if prior sexual experience is required for partner preference in male hamsters.
MATERIALS AND METHODS
Animals
33 adult male Syrian hamsters (Mesocricetus auratus, 130–150 g, Charles River Laboratories) were housed under a long-day photoperiod (14:10 LD) and stable ambient temperature (24°C). Food and water were available at all times, except during testing. All experimental procedures were in accordance with the “Guide for the Care and Use of Laboratory Animals” [15].
Initially, all males were sexually-inexperienced. Males were pair-housed with another male from the same experimental group. 8 female hamsters used as stimulus animals were ovariectomized via bilateral dorsal flank incision, and received a 4-mm Silastic estradiol implant sc (id: 1.98 mm, od: 3.18 mm; Dow Corning, MI) to maintain chronic physiologic levels of estrogen. To induce estrus, females received 250 ug progesterone in cottonseed oil sc approximately 4h prior to testing (see [16]).
Based on the observation that a sexually-motivated male will prefer a receptive female to a stimulus male, partner preference was used to measure sexual motivation [10]. Mating tests were used to measure sexual behavior. To compare the time course of extinction and recovery of sexual motivation and mating behavior, males were tested at intervals before, during, and after castration and testosterone replacement.
Partner Preference Tests
Males were tested for partner preference in a clear plastic cage (see Fig 1: 40 × 23.5 × 21 cm, Penn Plax, Garden City, NY). A gonad-intact male and estrous female used as stimulus animals were confined in small clear plastic compartments (13 × 13 × 12.5 cm) attached at either end of the test cage via a perforated concave bubble cap (5 cm diam, 4.5 cm deep; Penn Plax) to provide visual, auditory and volatile chemosensory cues. The location of the male and female stimulus animal was randomized between tests to eliminate side preferences.
Figure 1.
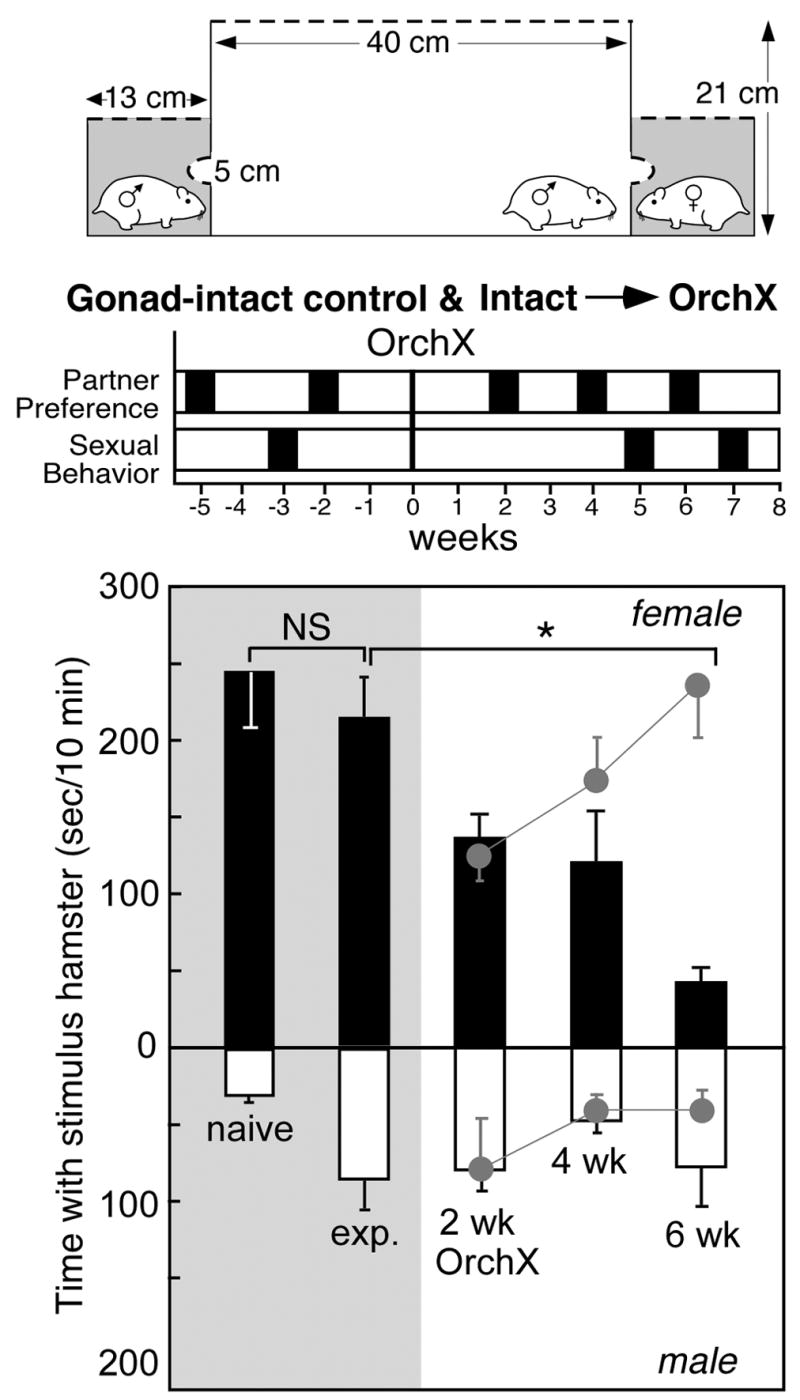
Top: Schematic of apparatus for testing partner preference in male hamsters. Middle: Timeline for partner preference and copulation tests in male hamsters before and after sexual experience and castration (orchX). Bottom: Time (mean±SEM) with an estrous female (black bars) or male stimulus animal (white bars) in 10-minute partner preference tests before (n=12, shaded area) and after castration (n=8) in male Syrian hamsters. Asterisk indicates significant effect of time post-castration. Gray symbols indicate behavior of gonad-intact control males tested at the same intervals.
Partner preference was recorded for 10 minutes on a PDA (Zire 21, Palm One Inc.) running Spectator Go! software (Biobserve GmbH, Bonn, Germany). Spectator Go! allows the user to enter time-stamped states or events on a PDA which are later analyzed with a standard spreadsheet application (Microsoft Excel). For partner preference testing, the parameters measured were time spent with the male and time spent with the female. Investigation of the stimulus animal was defined as the time the test male spent with his head in the bubble cap past the level of his eyes. Preference was calculated as the time spent investigating the stimulus female minus the time with the stimulus male.
Mating Tests
Mating behavior with an estrous female was tested for 10 min in the female’s home cage. The behavior of the test male was recorded each second on a PDA as described previously [17]. Behaviors measured included self-grooming, investigation of the female, anogenital investigation, mounting, intromission, and ejaculation.
Experimental Groups
There are 5 experimental groups: Control, Intact→Orchx, Orchx→Orchx+T, BulbX, and VnoX. The time-course of surgical procedures, hormonal manipulations and behavioral testing for each group is shown in Figures 1–3.
Figure 3.
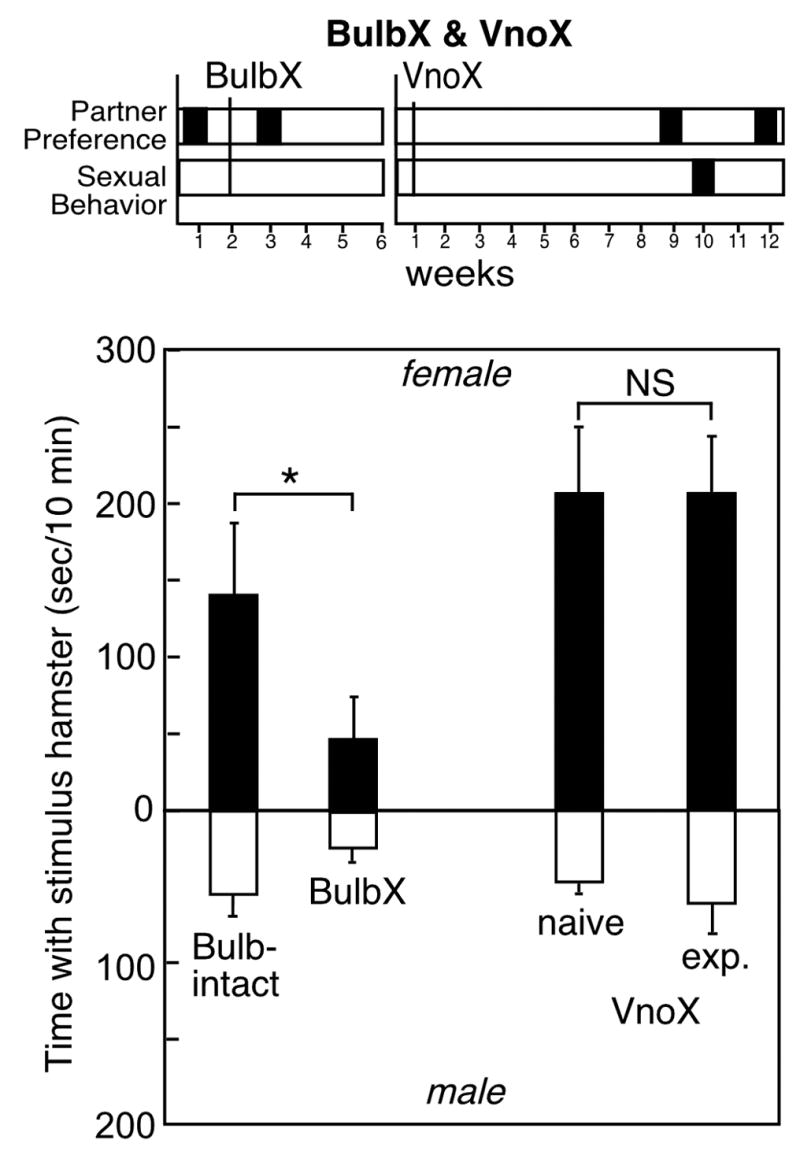
Top: Timeline for partner preference and copulation tests in male hamsters before and after olfactory bulbectomy (BulbX) or vomeronasal organ removal (VnoX). Bottom: Time (mean±SEM) with an estrous female (black bars) or male stimulus animal (white bars) in 10-minute partner preference tests before and after surgery or sexual experience in male Syrian hamsters (n=8/group). Asterisk indicates significant effect of BulbX on partner preference.
Control
Control males remained gonad-intact and olfactory bulb-intact. They were first tested for partner preference when they were sexually-naïve, and again 2 weeks after receiving sexual experience. Thereafter, partner preference testing was repeated at 2, 4, and 6 weeks to determine the effects of repeated testing on preference for a receptive female.
Intact→Orchx
As with control males, we first determined the effects of sexual experience on preference for an estrous female. Sexually-naïve males were tested for partner preference. 2 weeks later, mating behavior with an estrous female was recorded. The males were again tested for partner preference 2 weeks following mating. Subsequently, these males were castrated under sodium pentobarbital anesthesia (15 mg/100 g) to determine the time-course of the decline in partner preference and sexual behavior. Partner preference was recorded 2, 4 and 6 weeks after castration; mating behavior was tested at 5 and 7 weeks post-orchidectomy (Fig 1).
Orchx→Orchx+T
We determined partner preference in long-term castrates before and after exposure to a receptive female. Sexually-naïve adult males were castrated at least 6 weeks before testing. This long duration is essential because mating behavior persists for several weeks after castration [3]. These long-term castrates were tested for partner preference. 2 weeks later, they were exposed to a receptive female, and their sexual behavior was scored. The males were again tested for partner preference 2 weeks following sexual experience. Afterwards, all castrates received a 10-mm Silastic implant filled with crystalline testosterone (OrchX+T) to produce physiologic levels of testosterone sufficient for restoration of copulation [18]. Partner preference was recorded 2, 4 and 6 weeks after testosterone replacement; mating behavior was tested at 5 and 7 weeks post-implantation (Fig 2).
Figure 2.
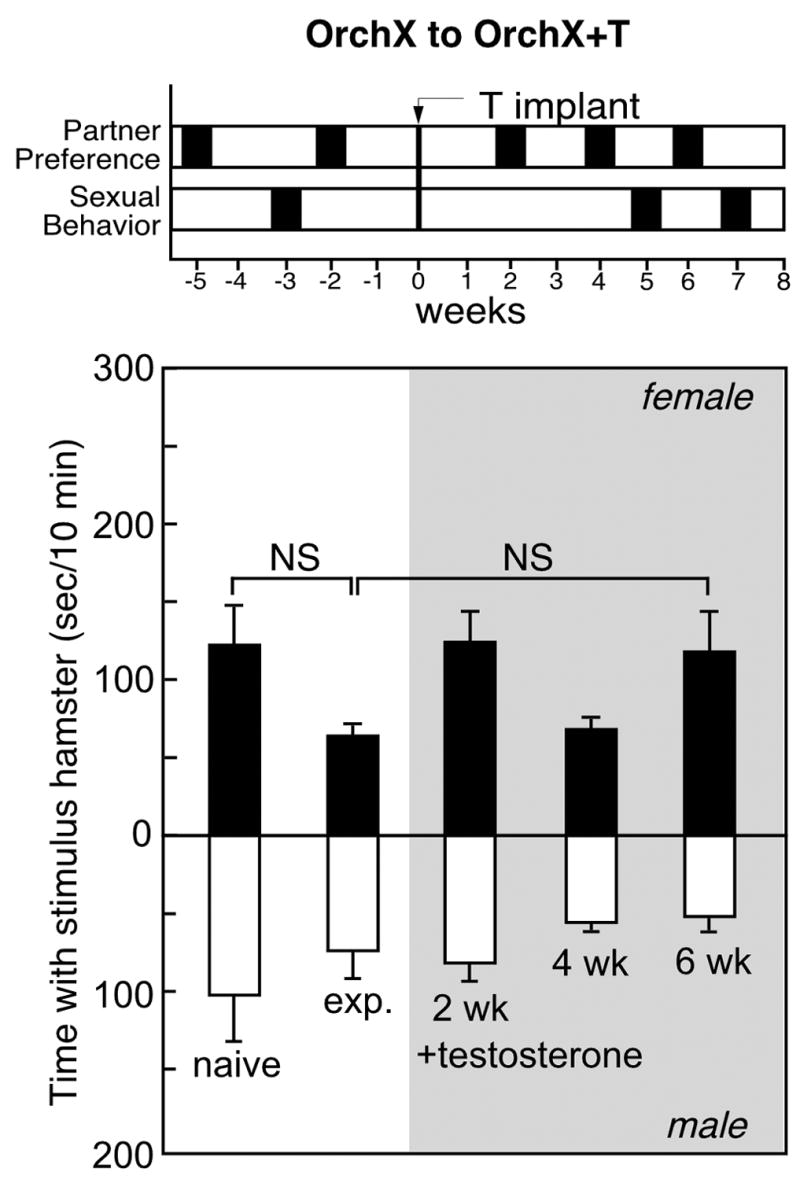
Top: Timeline for partner preference and copulation tests in castrated (OrchX) male hamsters before and after sexual experience and testosterone replacement (OrchX+T). Bottom: Time (mean±SEM) with an estrous female (black bars) or male stimulus animal (white bars) in 10-minute partner preference tests before and after (shaded area) testosterone replacement in male Syrian hamsters (n=8).
BulbX
To determine the effects of chemosensory cues on partner preference, 5 sexually-naive males were tested for partner preference. The olfactory bulbs were removed by aspiration. Briefly, the animal’s head was secured in a stereotaxic apparatus (David Kopf Instruments, Tujunga, CA) under pentobarbital anesthesia. An incision was made medial to the orbits to expose the skull overlying the olfactory bulbs. The olfactory bulbs were accessed via holes drilled through the skull under direct visualization. Tissue within the bony capsule surrounding the olfactory bulbs was aspirated with a blunt 21-gauge needle connected to a vaccuum pump. The cavity was packed with gel-foam and the skin was sutured. 1 week after surgery, males were again tested for partner preference (Fig 3). Anosmia was confirmed via conditioned taste avoidance, according to the methods of Darling & Slotnick [19]. Briefly, hamsters were offered water containing a neutral odor (0.1% isoamyl acetate) paired with a bitter flavor (0.5% quinine). After 2 10-minute exposures to the odor/flavor combination, bulb-intact hamsters avoid water containing isoamyl acetate alone. Bulb-intact controls consumed 0.4±0.1 ml, while BulbX males drank 3.8±0.4 ml.
VnoX
We determined the role of the vomeronasal organ in partner preference in 8 sexually-naive males. 8 weeks before testing, vomeronasal organs were removed bilaterally through an incision in the hard palate. Briefly, with the anesthetized animal supine, an incision was made through the roof of the mouth to expose the incisive foramen. The palatine processes connecting to the vomer bone were drilled at rostral and caudal ends. The entire vomer bone encasing the vomeronasal organ was removed with fine forceps. The resulting cavity was packed with gel-foam and the skin was sutured. VnoX males were tested for partner preference before and 2 weeks after sexual experience with a receptive female (Fig 3).
Data Analysis
In each experimental group, time spent with each stimulus animal was averaged for all males. The effects of sexual experience, olfactory bulbectomy, and vomeronasal organ lesion were evaluated by 2-factor ANOVA, comparing time with the stimulus male and female before and after mating or surgery. The effects of hormone manipulations were evaluated by 2-factor repeated measures ANOVA, comparing time with the stimulus male and female in sexually-experienced males over time. Likewise, measures of copulatory behavior (mounts, intromissions and ejaculations) following hormone manipulations were compared by repeated measures ANOVA after square-root transformation to reduce variance. The number of males in each group preferring the stimulus female at each time point, as well as the number of males expressing copulatory behavior, was compared by a test of significance of the difference between two independent proportions [20]. Statistical tests were completed using Statview 5.0.1 (SAS Institute Inc., Cary, NC). For all tests, p<0.05 was considered significant.
RESULTS
Control
Effects of sexual experience
To determine the effects of sexual experience on partner preference, data from males in the control (n=5) and gonad-intact groups (n=7) were combined. Behavior in the partner preference test showed a significant effect for the sex of the stimulus animal [F (1, 44)=51.7, p<0.05], but no effect of sexual experience, and no interaction (Fig. 1). Even without prior sexual experience, all 12 males showed a preference for the stimulus female (+218.5±38.6 sec/10 min). During their first mating behavior test, all males mounted, and 9 of 12 males achieved ejaculation. This is typical of sexually-naïve male hamsters [21]. Intromissions averaged 14.6±2.8/10 min; ejaculations averaged 3.8±0.8/10 min. In a subsequent partner preference test, sexual experience did not affect preference for the stimulus female. 11 of 12 sexually-experienced males preferred the stimulus female, with an average preference score of +131.8±32.4 sec/10 min.
Effects of repeated testing
With repeated partner preference testing every 2 weeks for 6 weeks, there was a significant effect for the sex of the stimulus animal [F(3, 18)= 33.6, p<0.05], a significant effect of time [F(3, 18)= 5.7], as well as a significant time x sex interaction [F(3, 18)= 3.7, p<0.05]. However, even at the end of the study, all 4 males retained a substantial preference for the stimulus female (+200.3±22.5 sec/10 min).
Intact→Orchx
Effects of short-term orchidectomy
Castration caused a significant decrease in preference for the stimulus female within 6 weeks (Fig. 1). As determined by repeated measures ANOVA, there was a significant effect for the sex of the stimulus animal [F(3, 42)= 14.9, p<0.05], a significant effect of time [F(3, 42)= 3.7, p<0.05], as well as a significant time x sex interaction [F(3, 42)= 6.1, p<0.05]. At 4 weeks post-orchidectomy, 7 of 8 males preferred the stimulus female, and the mean preference score (+72.0±28.7 sec/10 min) was similar to their behavior pre-castration. However, by 6 weeks after castration, only 3 males retained a preference for estrous females (p<0.05 vs gonad-intact). Intact→Orchx males spent an average of 42.0±10.4 sec/10min (7.0±1.7% of the test) with the stimulus female and 77.6±27.3 sec/10 min (12.9±4.5%) with the stimulus male, for an average preference score of −35.6±30.8 sec/10 min.
Castration also reduced sexual behavior (Fig 4). There was a significant reduction in mounts [F(2, 14)=7.4], intromissions [F(2, 14)=10.3] and ejaculations [F(2, 14)=14.9] over time. At 5 weeks post-orchidectomy, there were no ejaculations, and intromissions averaged only 1.8±1.3/10 min (4 of 8 males). Sexual behavior was further reduced during mating tests 7 weeks after castration. Intromissions and ejaculations were eliminated. 2 of 8 Intact→Orchx males mounted the female for an average of 1.4±1.2 mounts/10 min (vs 19.1±4.6 mounts/10 min when gonad-intact). Furthermore, anogenital investigation decreased to 56.6±5.8 sec/10 min (p<0.05 vs 107.5±12.8 sec/10 min before orchx).
Figure 4.
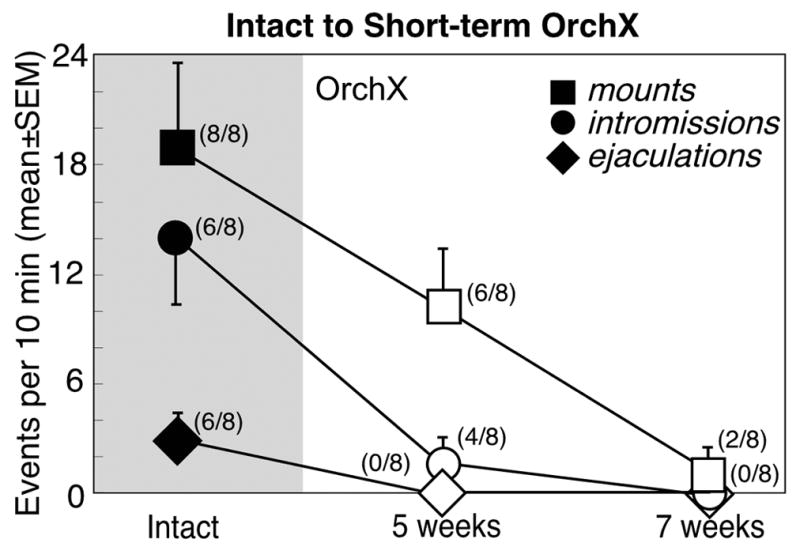
Copulatory behaviors (mean±SEM) in male Syrian hamsters (n=8) before (black symbols, shaded) and at 5 and 7 weeks after castration (white symbols). Numbers in parentheses reflect the number of animals expressing behavior at each time point.
Orchx→Orchx+T
Effects of sexual experience
Before hormone replacement, orchx hamsters did not show a significant preference for the stimulus female, and there was no significant effect of sexual experience (Fig. 2). During initial testing, 5 of 7 sexually-naïve castrates preferred the stimulus female, and the average preference score was only +23.6±45.7 sec/10 min. Not surprisingly, when paired with a receptive female, none of the OrchX males mated, and preference for the stimulus female (−5.3±18.4 sec/10 min) was unchanged in subsequent partner preference testing. OrchX males spent an average of 64.7±7.8 sec/10min with the stimulus female (10.8±1.4%) and 70.0±18.5 sec/10 min (11.7±3.3%) with the stimulus male.
Effects of testosterone replacement
During 6 weeks of testosterone replacement in Orchx→Orchx+T males, there was a significant effect on partner preference for the sex of the stimulus animal [F(2,36)=7.3], but no effect of time and no interaction. After 6 weeks of testosterone exposure, Orchx→Orchx+T males averaged 117.3±27.0 sec/10min (19.5±4.8%) with the stimulus female vs 48.4±9.8 sec/10 min (8.1±1.7%) with the stimulus male, for a mean preference score of +68.9±29.8 sec/10 min. However, testosterone replacement significantly stimulated the number of mounts [F(2,12)=4.7], intromissions [F(2,12)=5.5], and ejaculations [F(2,12)=6.1] (Fig. 5). Even 5 weeks of testosterone exposure increased the proportion of males expressing copulatory behaviors (p<0.05). 6 of 7 OrchX+T males mounted, and 4 ejaculated. By 7 weeks, 6 males achieved intromission (10.6±3.1 intromissions/10 min), and 5 ejaculated (4.6±2.3 ejaculations/10min).
Figure 5.
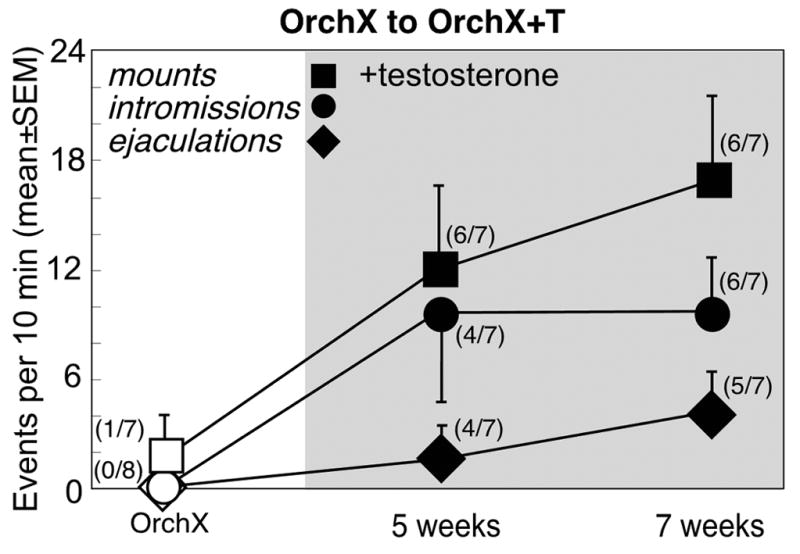
Copulatory behaviors (mean±SEM) in castrated male Syrian hamsters (n=8) before (white symbols) and at 5 and 7 weeks after testosterone replacement (black symbols, shaded). Numbers in parentheses reflect the number of animals expressing behavior at each time point.
BulbX
Bilateral removal of the olfactory bulbs eliminated sexual behavior and significantly attenuated partner preference in male hamsters (Fig. 3). By ANOVA, there was a significant effect for the sex of the stimulus animal [F(1,16)=5.1], and an effect of bulbectomy [F(1,16)=4.5], but no interaction. Before olfactory bulbectomy, all 5 males preferred the stimulus female. Hamsters spent an average of 142.0±46.2 sec/10 min (23.7±7.7%) with the stimulus female vs 52.4±12.9 sec/10 min (8.7±2.1%) with the stimulus male, for an average preference score of +89.6±53.7 sec/10 min. After surgery, only 3 of 5 males maintained a preference for the stimulus female, and the mean preference score decreased to +27.8±31.6.
VnoX
Despite bilateral removal of the vomeronasal organs, all 7 VnoX males expressed a preference for the stimulus female in partner preference tests (Fig. 3). VnoX males averaged 206.6±41.8 sec/10min (34.4±7.0%) with the stimulus female and 45.1±5.7 sec/10 min (7.5±1.0%) with the stimulus male (preference score +161.5±45.7 sec/10 min). Moreover, when paired with a receptive female, removal of the vomeronasal organ did not suppress precopulatory or copulatory behaviors in male hamsters (see Figure 4). VnoX males spent an average of 115.4±9.4 sec/10 min in anogenital investigation. Every VnoX male mated to ejaculation. Mounts averaged 24.5±1.6/10 min, with 19.4±1.8 intromissions, and 4.8±0.6 ejaculations per 10 min. When partner preference was retested following mating experience, sexually-experienced VnoX males spent an average of 210.0±36.0 sec/10min (35.0±6.0%) with the female hamster and 59.3±18.5 seec/10 min (9.9±3.1%) with the male hamster. Accordingly, as determined by ANOVA, VnoX males demonstrated a significant effect for sex of the stimulus animal [F(1,28)=28.6], but there was no effect of sexual experience and no interaction.
DISCUSSION
This study compared partner preference and mating behavior in male hamsters to determine the effects of sexual experience, the time-course of castration and testosterone replacement, and the effects of chemosensory stimuli on sexual motivation. The results demonstrate that male hamsters do not require sexual experience to express a partner preference for females. In addition, partner preference develops even when contact with the stimulus animals is prevented, suggesting that the combination of visual, auditory and volatile chemosensory cues from an estrous female are attractive to males. Indeed, partner preference was reduced after bilateral removal of the olfactory bulbs, but not following vomeronasal organ removal. Furthermore, the present study supports previous findings [8, 9] that testosterone stimulates both appetitive and consummatory aspects of sexual behavior. In particular, sexual motivation may be even more dependent on testosterone than sexual behavior. In this regard, partner preference was attenuated within 6 weeks after castration, yet 6 weeks of testosterone replacement did not restore partner preference. On the other hand, copulatory behavior was significantly reduced 5 weeks after castration and returned after 7 weeks of testosterone replacement. These results indicate that hamsters possess some flexibility with regard to sensory cues for sexual motivation, but have a strong reliance on gonadal steroids.
Male sexual behavior includes both appetitive and consummatory behaviors (reviewed in [10]). Appetitive behaviors encompass pursuit and investigation of the female; consummatory sexual behavior consists of mounting, intromission, and ejaculation. Both sexual motivation and copulation are modified by gonadal steroids, sensory stimuli from females, and learned associations (sexual experience). In contrast to male rats, mating in male hamsters is considered to be relatively less flexible: more dependent on chemosensory cues, less sensitive to prior sexual experience.
Partner preference is well-established method to study sexual motivation independent of copulatory behavior [23]. A sexually-motivated male will prefer to spend time with a receptive female over a stimulus male [10]. Partner preference requires that the male identify, interpret, and respond to cues from the stimulus animals, and has been shown to depend on hormonal cues and prior experience, at least in rats [1, 8, 13]. Importantly, partner preference has not previously been demonstrated in male hamsters.
Other approaches have been developed to measure sexual motivation. With second-order reinforcement, male rats to repeatedly press a lever previously paired with a stimulus light to gain access to a female [24]. This paradigm has been used to demonstrate the importance of gonadal steroid hormones [9]. However, extensive training of the test subject is necessary, which makes this method unsuitable for sexually-naïve animals. Level-changing in a bilevel chamber is another popular model for sexual motivation. Mendelson and Pfaus [25] showed that sexually-experienced male rats increase their level changing behavior in the presence of a receptive female, but not when presented with a non-receptive female. This model takes advantage of the high motor activity of both male and female rats during mating. By contrast, female hamsters are largely stationary during copulation. Hence, the bilevel chamber is not well-suited to the typical pattern of mating behavior in hamsters.
In rats, prior sexual experience is necessary for the male to show a preference for a receptive female or her odors [13, 14, 26]. While sexually-experienced male rats prefer urine from estrous females over diestrous urine, sexually-inexperienced rats do not [14]. Furthermore, sexually-naïve male rats do not prefer estrous urine over water. Matuszczyk & Larsson [13] extended these observations to show that sexually-naïve male rats alternate preference for the stimulus male and female. Sexual experience made the preference for the female rat consistent. Thus, partner preference in male rats is influenced by learning and experience. Conversely, male hamsters are more sensitive to specific environmental and hormonal signals. Previous studies have shown that sexually-naïve male hamsters prefer FHVS to other odors [4, 12, 27, 28]. Our study supports these observations by showing that gonad-intact male Syrian hamsters prefer estrous females over gonad-intact males even without sexual experience. Partner preference was not enhanced after the males gained sexual experience, but was maintained with repeated testing. These results reinforce the concept that learning and experience play a relatively minor role in hamster sexual motivation.
The recognition of sexually-relevant chemosensory cues appears to be major component of learned responses for mating, at least in male rats. Volatile and non-volatile chemosensory cues are transduced in the olfactory mucosa and vomeronasal organ, which project to the main and accessory olfactory bulbs, respectively (reviewed in [22]). While sexually-naïve male rats do not show a preference for female urine [14], removal of the olfactory bulbs significantly impairs copulation (reviewed in [10]). However, sexually-experienced BulbX male rats continue to copulate. By contrast, hamsters depend on chemosensory cues for mating. Anosmia eliminates copulation, even in sexually-experienced males [29]. BulbX also substantially reduces preference for odors from conspecific females [12, 28].
It is thought that male hamsters are particularly responsive to the non-volatile components of FHVS detected in the vomeronasal organ. Although VnoX was not verified histologically here, our behavioral results are consistent with previous work. Earlier studies have found it difficult to eliminate the entire vomeronasal organ, whether by electrocautery [30] or surgical ablation [28]. Importantly, Pfeiffer and Johnston [28] found no correlation between the extent of lesion damage and behavior or hormone secretion. Most sexually-experienced male hamsters still mate after removal of the VNO [12, 28, 30–32], and investigation of vaginal secretions and partner preference is unimpaired [12, 30]. Similarly, our results suggest that the vomeronasal organ may not be necessary for attraction to females. Instead, male hamsters may rely on a combination of visual, auditory, and volatile odor cues. In support of this conclusion, work by Pfeiffer & Johnston [28] demonstrated that most VnoX male hamsters did not have an androgen surge when exposed to FHVS, but still exhibited an androgen surge when exposed to an estrous female. In the present study, the testing apparatus used prevented physical contact, and hence transfer of non-volatile stimuli, between the test male and the two stimulus animals. Furthermore, VnoX males continued to show a preference for a receptive female. This result may reflect the relative importance of volatile and non-volatile chemosensory cues in attraction to females vs copulation. Initially, the male may seek out a receptive female in response to volatile odor cues. This is the behavior approximated by our partner preference test. Non-volatile stimuli detected by the vomeronasal organ may play a more important role in the transition from investigation to copulation.
Compared with rats, it appears that hamsters show substantially less behavioral flexibility, at least with regard to mating. However, this is not to say that learning is impaired in male hamsters. Our laboratory has demonstrated robust operant responding for food reward, even in castrated male hamsters [33]. Similarly, both gonad-intact and castrated male hamsters will form a conditioned taste aversion to vaginal secretions paired with LiCl-induced gastrointestinal illness [34]. Furthermore, Kollack-Walker and Newman [35] demonstrated anticipatory Fos activation in neural circuits for sexual behavior of sexually-experienced male hamsters. Together, these observations suggest that hamsters are capable of modifying their behavior in response to reinforcing or aversive stimuli. It may be that the relative importance of prior sexual experience for sexual attraction in rats and hamsters reflects the biology of the two species. While rats are social animals, hamsters are solitary [36]. As such, hamsters have limited opportunities for learned social interactions. Thus, an attraction to females that does not depend on prior sex experience should enhance reproductive fitness.
It is not surprising that gonadal steroid hormones facilitate both partner preference and sexual behavior in hamsters. Loss of sexual motivation after castration and restoration by exogenous hormone replacement has been demonstrated in male rats by second-order responding [9], conditioned place preference [8] and partner preference [8, 13]. Furthermore, it has previously been observed that copulatory behaviors which persist the longest after castration are the first to return with testosterone replacement [37]. Compared with copulation, sexual motivation is relatively more responsive to hormone deprivation, and relatively more resistant to hormone reinstatement. In particular, partner preference was not restored after 6 weeks of testosterone treatment in Orchx→Orchx+T males, although copulation was significantly increased by 5 weeks. This supports the finding by Powers et al [6] that low levels of testosterone in male hamsters will maintain ejaculation, but will not sustain chemosensory investigation. However, castrated males can nonetheless detect FHVS even at low concentrations, suggesting that castration selectively reduces attraction to chemosensory cues, but not their detection [34]. Again, the differential hormonal control of appetitive and consummatory sexual behavior can be understood in a natural setting when endogenous testosterone levels are low, as at the beginning or end of the breeding season. Under these conditions, a male may be capable of copulation if he encounters a female in estrus. However, he is less likely to undertake the risk of searching for females that may or may not be sexually-receptive.
Acknowledgments
We thank Ms. Soobin Kim for assistance with behavioral testing. This work supported by a grant from the NIH (MH05534).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Everitt BJ. Sexual motivation: A neural and behavioural analysis of the mechanisms underlying appetitive and copulatory responses of male rats. Neurosci Biobehav Rev. 1990;14:217–232. doi: 10.1016/s0149-7634(05)80222-2. [DOI] [PubMed] [Google Scholar]
- 2.Sisk CL, Zehr JL. Pubertal hormones organize the adolescent brain and behavior. Front Neuroendocrinol. 2005;26(3–4):163–174. doi: 10.1016/j.yfrne.2005.10.003. [DOI] [PubMed] [Google Scholar]
- 3.Pospichal MW, Karp JD, Powers JB. Influence of daylength on male hamster sexual behavior: masking effects of testosterone. Physiol Behav. 1991;49(3):417–422. doi: 10.1016/0031-9384(91)90258-p. [DOI] [PubMed] [Google Scholar]
- 4.Gregory E, Engel K, Pfaff DW. Male hamster preference for odors of female hamster vaginal discharges: studies of experiential and hormonal determinants. J Comp Physiol Psychol. 1975;89:442–446. doi: 10.1037/h0077043. [DOI] [PubMed] [Google Scholar]
- 5.Steel E, Keverne EB. Effect of female odour on male hamsters mediated by the vomeronasal organ. Physiol Behav. 1985;35:195–200. doi: 10.1016/0031-9384(85)90335-x. [DOI] [PubMed] [Google Scholar]
- 6.Powers JB, Bergondy ML, Matochik JA. Male hamster sociosexual behaviors: effects of testosterone and its metabolites. Physiol Behav. 1985;35:607–616. doi: 10.1016/0031-9384(85)90149-0. [DOI] [PubMed] [Google Scholar]
- 7.Powers JB, Bergondy ML. Androgenic regulation of chemoinvestigatory behaviors in male and female hamsters. Horm Behav. 1983;17:28–44. doi: 10.1016/0018-506x(83)90013-2. [DOI] [PubMed] [Google Scholar]
- 8.Hughes AM, Everitt BJ, Herbert J. Comparative effects of preoptic area infusions of opioid peptides, lesions, and castration on sexual behaviour in male rats: studies of instrumental behaviour, conditioned place preference and partner preference. Psychopharmacology. 1990;102:243–256. doi: 10.1007/BF02245929. [DOI] [PubMed] [Google Scholar]
- 9.Everitt BJ, Stacey P. Studies of instrumental behavior with sexual reinforcement in male rats (Rattus norvegicus): II. Effects of preoptic area lesions, castration, and testosterone. J Comp Psychol. 1987;101(4):407–419. [PubMed] [Google Scholar]
- 10.Hull EM, Wood RI, McKenna KE. Male sexual behavior. In: Neill JD, editor. The Physiology of Reproduction. 3. New York: Raven Press; 2006. pp. 1729–1824. [Google Scholar]
- 11.Macrides F, Singer AG, Clancy AN, Goldman BD, Agosta WC. Male hamster investigatory and copulatory responses to vaginal discharge: relationship to the endocrine status of females. Physiol Behav. 1984;33:633–637. doi: 10.1016/0031-9384(84)90383-4. [DOI] [PubMed] [Google Scholar]
- 12.Murphy MR. Sexual preferences of male hamsters: importance of preweaning and adult experience, vaginal secretion, and olfactory or vomeronasal sensation. Behav Neural Biol. 1980;30:323–340. doi: 10.1016/s0163-1047(80)91210-8. [DOI] [PubMed] [Google Scholar]
- 13.Matuszczyk JV, Larsson K. Experience modulates the influence of gonadal hormones on sexual orientation of male rats. Physiol Behav. 1994;55(3):527–531. doi: 10.1016/0031-9384(94)90112-0. [DOI] [PubMed] [Google Scholar]
- 14.Lydell K, Doty RL. Male rat odor preferences for female urine as a function of sexual experience, urine age, and urine source. Horm Behav. 1972;3:205–212. doi: 10.1016/0018-506x(72)90033-5. [DOI] [PubMed] [Google Scholar]
- 15.National Research Council. Guide for the Care and Use of Laboratory Animals. Washington DC: National Academy Press; 1996. [Google Scholar]
- 16.Carter CS. Female Sexual Behavior. In: Siegel HI, editor. The Hamster: Reproduction and Behavior. New York: Plenum Press; 1985. pp. 173–189. [Google Scholar]
- 17.Wood RI, Newman SW. The medial amygdaloid nucleus and medial preoptic area mediate steroidal control of sexual behavior in the male Syrian hamster. Horm Behav. 1995;29:338–353. doi: 10.1006/hbeh.1995.1024. [DOI] [PubMed] [Google Scholar]
- 18.Noble RG, Alsum PB. Hormone dependent sex dimorphisms in the golden hamster (Mesocricetus auratus) Physiol Behav. 1975;14(5):567–574. doi: 10.1016/0031-9384(75)90183-3. [DOI] [PubMed] [Google Scholar]
- 19.Darling FM, Slotnick BM. Odor-cued taste avoidance: a simple and efficient method for assessing olfactory detection, discrimination and memory in the rat. Physiol Behav. 1994;55(5):817–822. doi: 10.1016/0031-9384(94)90065-5. [DOI] [PubMed] [Google Scholar]
- 20.Ferguson GA. Statistical Analysis in Psychology and Education. New York: McGraw-Hill; 1966. [Google Scholar]
- 21.Wood RI. Oral testosterone self-administration in male hamsters: dose-response, voluntary exercise, and individual differences. Horm Behav. 2002;41(3):247–258. doi: 10.1006/hbeh.2002.1769. [DOI] [PubMed] [Google Scholar]
- 22.Wood RI, Newman SW. Hormonal influence on neurons of the mating behavior pathway in male hamsters. In: Micevych P, Hammer RP, editors. Neurobiological Effects of Sex Steroid Hormones. Cambridge: Cambridge University Press; 1995. pp. 3–39. [Google Scholar]
- 23.Adkins-Regan E, Mansukhani V, Thompson R, Yang S. Organizational actions of sex hormones on sexual partner preference. Brain Res Bull. 1997;44(4):497–502. doi: 10.1016/s0361-9230(97)00231-1. [DOI] [PubMed] [Google Scholar]
- 24.Everitt BJ, Fray P, Kostarczyk E, Taylor S, Stacey P. Studies of instrumental behavior with sexual reinforcement in male rats (Rattus norvegicus): I. Control by brief visual stimuli paired with a receptive female. J Comp Psychol. 1987;101(4):395–406. [PubMed] [Google Scholar]
- 25.Mendelson SD, Pfaus JG. Level searching: a new assay of sexual motivation in the male rat. Physiol Behav. 1989;45:337–341. doi: 10.1016/0031-9384(89)90136-4. [DOI] [PubMed] [Google Scholar]
- 26.Carr WJ, Loeb LS, Dissinger ML. Responses of rats to sex odors. J Comp Physiol Psychol. 1965;59(3):370–377. doi: 10.1037/h0022036. [DOI] [PubMed] [Google Scholar]
- 27.Huck UW, Lisk RD, Kim S, Evans AB. Olfactory discrimination of estrous condition by the male golden hamster (Mesocricetus auratus) Behav Neural Biol. 1989;51:1–10. doi: 10.1016/s0163-1047(89)90608-0. [DOI] [PubMed] [Google Scholar]
- 28.Pfeiffer CA, Johnston RE. Hormonal and behavioral responses of male hamsters to females and female odors: roles of olfaction, the vomeronasal system, and sexual experience. Physiol Behav. 1994;55:129–138. doi: 10.1016/0031-9384(94)90020-5. [DOI] [PubMed] [Google Scholar]
- 29.Murphy MR, Schneider GE. Olfactory bulb removal eliminates mating behavior in the male golden hamster. Science. 1970;167:302–304. doi: 10.1126/science.167.3916.302. [DOI] [PubMed] [Google Scholar]
- 30.Powers JB, Fields RB, Winans SS. Olfactory and vomeronasal system participation in male hamsters’ attraction to female vaginal secretions. Physiol Behav. 1979;22(1):77–84. doi: 10.1016/0031-9384(79)90407-4. [DOI] [PubMed] [Google Scholar]
- 31.Johnston RE, Rasmussen K. Individual recognition of female hamsters by males: role of chemical cues and of the olfactory and vomeronasal systems. Physiol Behav. 1984;33:95–104. doi: 10.1016/0031-9384(84)90019-2. [DOI] [PubMed] [Google Scholar]
- 32.Meredith M. Vomeronasal organ removal before sexual experience impairs male hamster mating behavior. Physiol Behav. 1986;36:737–743. doi: 10.1016/0031-9384(86)90362-8. [DOI] [PubMed] [Google Scholar]
- 33.Chu L, Wood RI. Testosterone, dopamine, and food choice in a cost-benefit test with male hamsters. Behav Brain Res. 2002;136:137–142. doi: 10.1016/s0166-4328(02)00101-8. [DOI] [PubMed] [Google Scholar]
- 34.Peters K, Hom SM, Wood RI. Testosterone and chemosensory detection in the male Syrian hamster. Horm Behav. 2004;46(3):341–348. doi: 10.1016/j.yhbeh.2004.04.004. [DOI] [PubMed] [Google Scholar]
- 35.Kollack-Walker S, Newman SW. Mating-induced expression of c-fos in the male Syrian hamster brain: role of experience, pheromones, and ejaculations. J Neurobiol. 1997;32(5):481–501. doi: 10.1002/(sici)1097-4695(199705)32:5<481::aid-neu4>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- 36.Ferris CF, Messenger T, Sullivan R. Behavioral and neuroendocrine consequences of social subjugation in adolescence and adulthood. Front Zool. 2005;2:7. doi: 10.1186/1742-9994-2-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Merkx J. Effect of castration and subsequent substitution with testosterone, dihydrotestosterone and oestradiol on sexual preference behavior in the male rat. Behav Brain Res. 1984;11:59–65. doi: 10.1016/0166-4328(84)90008-1. [DOI] [PubMed] [Google Scholar]


