Abstract
When healthy individuals eat solid food, chewed food is usually transported to the oropharynx where it accumulates before swallowing (stage II transport). We tested the hypothesis that this transport process can be altered by volition. Eight healthy young subjects ate 8 g pieces of cookie with barium while movements were recorded with videofluorography. There were two trials for each subject, each with different instructions: 1) Without command: to eat the cookie in his/her usual manner; 2) With command: to chew the cookie, give a signal when ready to swallow, and then swallow on command of the investigator. We measured the number of chewing cycles, the duration of each stage in the feeding sequence, and the position of the leading edge of the barium at time of command and at swallow onset. Sequence duration was longer with than without command (P = 0.02), primarily because of an increase in the number of chewing cycles (P = 0.02). The leading edge was typically higher in the foodway at the time of swallow onset with than without command (P = 0.06). Under the command condition, stage II transport was delayed, and transport to the valleculae was inhibited. Volition alters swallow initiation in both the timing and location of the food bolus relative to the airway.
Keywords: Deglutition/Physiology, Mastication/Physiology, Pharynx, Feeding behavior, Volition, Movement, Fluoroscopy, Neurophysiology, Dysphagia
INTRODUCTION
When a healthy young subject swallows a bolus of liquid, the bolus is usually held in the oral cavity until the time of swallow onset. The swallow is initiated while the liquid remains in the oral cavity or as it reaches the fauces. Once the oral stage is initiated, the pharyngeal stage follows in rapid sequence [1]. The sequence of events is quite different when a healthy young subject eats natural-size bite of solid food. The food must first be processed in the mouth in preparation for swallowing. When a portion of the food has reached a consistency appropriate for swallowing, that portion is propelled through the fauces to the oropharynx (stage II transport) while oral food processing continues. Additional aliquots of prepared food can be transported to the oropharynx, where a bolus gradually accumulates (for up to ten seconds). When bolus aggregation in the oropharynx is completed, a swallow is initiated, and the bolus is transported through the upper esophageal sphincter to the esophagus [2–5].
Stage II transport is defined as propulsion of triturated solid food through the fauces to the pharynx. This transport is accomplished by the tongue squeezing food back along the palate. The anterior tongue surface first contacts the palate, followed by the middle portion of the tongue, and finally the posterior potion. This gradually expands the area of tongue-palate contact from anterior to posterior, gradually squeezing the triturated food into the oropharynx. Food can be transported either to the immediate post-faucial region or to the valleculae [2, 5]. This transport is active, and does not depend on gravity [4].
The occurrence of stage II transport in man has implications for airway protection during feeding and swallowing given the unique position of the larynx in hominids. In other mammals, the larynx is located in the nasopharynx. This intranarial position provides an airway that is structurally isolated from the foodway, reducing the risk for aspiration of food [6]. The human neonate also has an intranarial larynx, but the larynx descends in the neck to its adult position during the first few years of life. In the adult, the valleculae are located in the pharyngeal airway, only millimeters away from the laryngeal additus. Food collected in the valleculae may enter the larynx on deep or forceful inhalation. Airway patency and protection are primary concerns in this process, requiring careful coordination of respiration and food transport [7].
The impact of commands or instructions on the processes of eating, drinking or swallowing is not well known. Despite the substantial literature on their neural control mechanisms [8–11], little data exist on the role of volition in the processing and swallowing of solid food. Both mastication and swallowing are mediated by central pattern generators in the brain. Each is influenced by peripheral factors, including food consistency and other oral stimuli but is also modified by the input from the cortex. A swallow may be initiated by conscious decision, but it is largely automatic once started. In contrast, mastication is readily interrupted. Certainly it is possible to perform mastication and food transport without consciously attending to these activities. But it is not known to what extent feeding, and stage II transport in particular, may be altered by volitional control or conscious decision, as in the command swallow paradigm (hold food in the mouth, swallow on command).
The purpose of this study was to study the effect of volition on food transport and bolus aggregation during feeding on solid food. We hypothesized that subjects instructed to chew solid food but hold it in the mouth until instructed to swallow would inhibit stage II transport until the time of the command. In the absence of stage II transport, these subjects would retain the food bolus in the oral cavity until swallow onset and would not demonstrate bolus aggregation in the oropharynx before swallowing. Although this implies that volition will alter swallow initiation, we predicted no effect on the swallow itself, once initiated.
METHODS
Subjects
Eight healthy young adults (four females and four males) with a median age of 23 yrs (range 21 to 25 years) participated in the study after giving fully informed consent. The protocol was approved by the applicable Institutional Review Boards. Subjects had brief medical and dental examinations that revealed them to be in excellent health with normal dentition and normal occlusion. A routine liquid barium swallow with videofluorography (VFG) in lateral and postero-anterior projections confirmed that swallowing was normal in each subject [12]. One subject was omitted from statistical data analysis (see below).
Data Collection
Small lead discs (4 mm × 0.4 m) were cemented to the buccal surfaces of the upper and lower canines and first molars of both sides. These are used as radiopaque markers in data analysis [13]. VFG recordings in lateral projection were made at 30 fps while each subject ate 8 g of hard cookie (shortbread fingers, Walkers, Aberlour on Spey, Scotland) dusted with barium sulfate powder [5]. There were two trials for each subject:
Trial 1 - No command (NC)
The subject was asked to chew and swallow the cookie in his/her usual manner;
Trial 2 - With command (WC)
The subject was instructed to a) Chew the cookie but don’t swallow; b) Hold it in the mouth and raise a hand to signal when ready to swallow; and c) Swallow on command of the investigator. The command was given immediately after the subject signaled readiness to swallow.
One subject had a saliva swallow early in food processing during the WC swallow; this subject was deleted from the statistical analysis.
Data Reduction
The videotapes were first analyzed using the slow motion and stop-frame features on the VCR [2, 13] and then digitized for further analysis.
Most recordings included more than one swallow. The median number of swallows was 2 for recordings both with and without command (with command [WC], range 1 – 3 swallows with command; no command [NC], 2 – 4 swallows; P = 0.16). Further analysis is limited to the first sequence in each recording (from the time food entered the mouth until the end of the first swallow). The time of the swallow command was defined as the end of the command “Swallow now” and was established with a graph of the acoustic signal over time.
The start of the swallow was defined as the onset of rapid hyoid elevation. We noted the position of the leading edge of the barium at swallowing onset and at the time of the command, and classified it in one of the following four regions (Fig. 1): 1) in the oral cavity; 2) in the upper oropharynx (past the posterior nasal spine, but above the level of the lower border of the mandible); 3) in the valleculae (past the lower border of the mandible but not passing the edge of the epiglottis or aryepiglottic folds); 4) in the hypopharynx. The leading edge of the barium never reached the hypopharynx prior to swallow onset in the present study. The lower border of the mandible is clearly external to the pharynx and does not delineate a distinct internal space. There is, however, no comparable internal pharyngeal reference. The radiographic shadow of the mandible is commonly used as a reference point in studies of human swallowing [1, 2, 4, 5, 12, 14].
Figure 1.
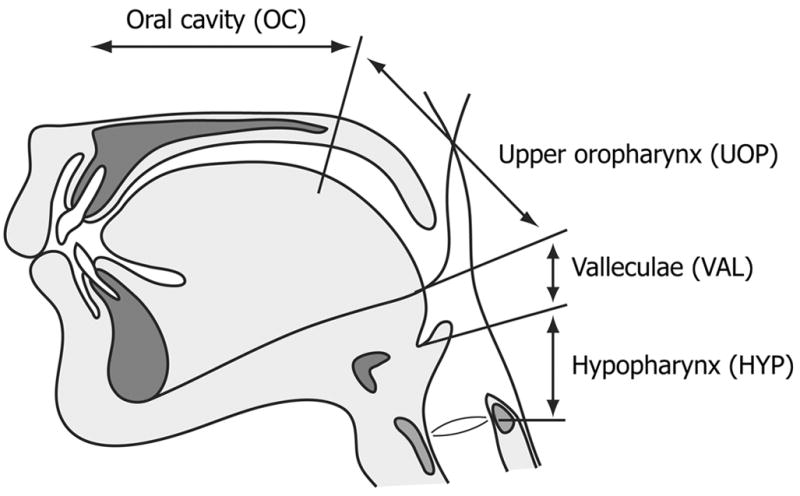
Four regions of the foodway used to define position of the leading edge of the barium at the time of the command and the time of swallow onset.
The Process model paradigm was used to divide each recording into four temporal stages, each beginning immediately upon termination of the last [2]. This model was selected because it was developed specifically to define the events occurring during feeding on sold food, and has been validated in several studies [5, 7, 14]. The four stages were:
Stage I transport
Food was transported from the anterior oral cavity to the molar region for chewing. Stage I transport began when food passed the incisors, and ended with the start of mastication.
Processing
The food was chewed and mixed with saliva, and particles were reduced in size. Processing ended when the leading edge of the barium reached the level of the lower border of the mandible. Processing included some stage II transport cycles in which triturated food was propelled through the fauces into the immediate post-faucial portion of the oropharynx. No pouching of food in the buccal recesses was observed. (Although the duration of the Processing stage was defined in this way, the actual processing of food could actually continue during the next stage if food remained in the mouth.)
Vallecular Aggregation Time (VAT)
Fully prepared food was propelled to the valleculae via stage II transport and collected there for bolus formation prior to swallowing. VAT ended when the leading edge of the barium passed the edge of the epiglottis.
Hypopharyngeal Transit Time (HTT)
The bolus passed through the hypopharynx and upper esophageal sphincter. HTT ended when the trailing edge of the barium passed the upper esophageal sphincter.
The motion of the mandible was plotted over time. Each chewing cycle (jaw motion cycle) was identified on this graph. We measured the number of chewing cycles (from the start of processing until swallow onset) in each sequence and the duration of each chewing cycle.
Each subject served as his/her own control for comparisons of the “no command” and “with command” sequences. Differences in chewing cycle duration were analyzed with a two-tailed paired T-test. Stage durations were not normally distributed, so they were analyzed as ordinal rather than continuous variables. Differences in number of swallows, stage duration, number of chewing cycles, and position of the barium leading edge were analyzed with the Wilcoxon Signed Ranks Test.
RESULTS
Stage Duration (Figs. 2 and 3)
Figure 2.
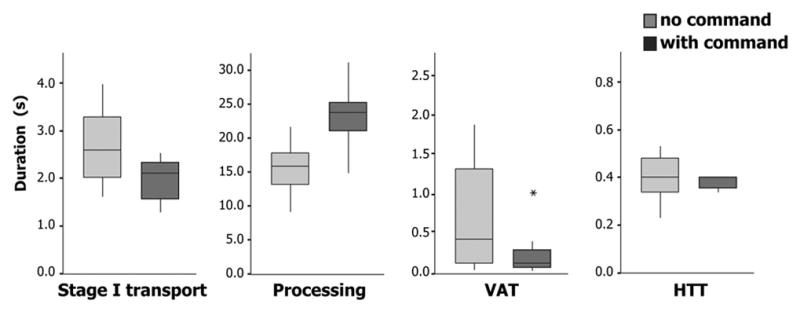
Timelines showing the duration of each recorded sequence in each subject, including sequences with command (WC) and with no command (NC). Times are as recorded, not averaged). The length of each horizontal bar reflects the total sequence length. The shading of the bar indicates the stage duration for Stage I transport, Processing, Vallecular aggregation time (VAT), and Hypopharyngeal transit time (HTT). WC, with command; NC, no command. The black dot shows the time of the command.
Figure 3.
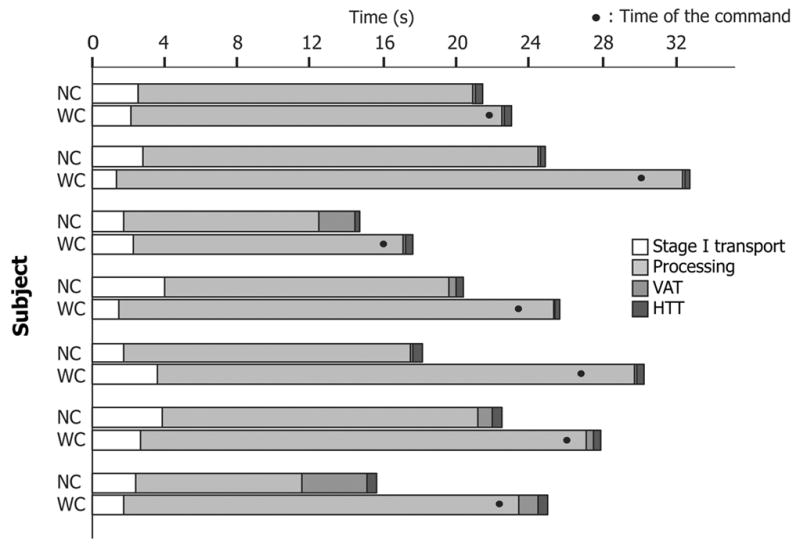
Duration of each stage in the sequence (box and whisker plot), comparing sequences with command and with no command. Note that the amplitude of the Y axis varies. The box represents median, and 1st and 3rd quartile values. The ends of the whiskers show the largest and smallest observed values that are less than 1.5 box lengths from either end of the box. The asterisk represents an outlier.
Total sequence duration was significantly longer for sequences with than without command (with command [WC], 26.0 ± 5.0 s, mean ± S.D.; no command [NC], 19.6 ± 3.7 s sec; P = 0.02). This was due to the substantially longer duration of Processing in sequences with command (WC, 23.2 ± 5.0 s; NC, 15.5 ± 4.3 s; P = 0.02). The prolongation of Processing (7.7 s) was much greater than the time from the command until the onset of swallowing. Indeed, the duration of Processing in sequences with command was longer than the total sequence duration in sequences with no command. Inversely, VAT and its variation were reduced in sequences with command; the mean difference (0.72 s) approached statistical significance (WC, 0.28 ± 0.35 s; NC, 1.0 ± 1.3 s; P = 0.08). There was little difference between WC and NC duration for stage I transport (WC, 2.1 ± 0.79 s; NC, 2.7 ± 0.92 s; P = 0.24) or HTT (WC, 0.37 ± 0.08 s; NC, 0.40 ± 0.11 s; P = 0.55).
Chewing Cycles
The longer duration of Processing in sequences with command reflected a greater number of chewing cycles, not an increase in cycle duration. The mean duration of chewing cycles did not differ significantly between sequences with and without command (WC, 0.74 ± 0.2 s; NC, 0.76 ± 0.13 s; P = 0.42). The number of chewing cycles, however, was greater in sequences with command for every subject (NC, median 23, interquartile range 20–25; WC, median 31, interquartile range 24.5–34; P = 0.02).
Swallow initiation
The location of the leading edge at the time of swallow onset was, on the average, higher in the foodway in sequences with than without command (P = 0.06, Table). Without command, the leading edge of the barium was usually in the valleculae at swallow onset (five out of seven cases). With command, the subject invariably signaled “ready to swallow” before the leading edge of the barium reached the valleculae (Fig. 2, Table). In three cases, the leading edge was in the oral cavity at the time of the command to swallow, and in the remaining four cases, it was in the upper oropharynx (above the level of the lower border of the mandible).
Table. Number of swallows in which the leading edge of the barium was located in each anatomical region at the time of command and at swallow onset
| With command
|
No command
|
||
|---|---|---|---|
| at command | swallow onset | swallow onset | |
| Oral cavity | 3 | 3 | 0 |
| Upper oropharynx | 4 | 1 | 2 |
| Valleculae | 0 | 3 | 5 |
|
| |||
| Hypopharynx | 0 | 0 | 0 |
DISCUSSION
This study shows that food processing, transport, and bolus formation are subject to modification by conscious processes. In sequences with command, the number of chewing cycles was greater, the duration of Processing was longer, the VAT was shorter, and the leading edge of the barium was higher in the foodway at swallow onset. The duration of Processing in sequences with command was longer than the entire sequence duration without command. These findings imply that the mastication, food transport, and bolus formation are affected by volition.
The mean duration of Processing was almost 50% longer in sequences with command (figure 3) although all subjects ingested identical volumes of cookie. This was primarily related to the greater number of chewing cycles in sequences with command, since their duration was not significantly longer. The reason for this prolongation of Processing is not clear. When eating solid food, it is common to have several swallows for a single bite. Each swallow accounts for a portion of the food, and more food may remain in the mouth after each swallow (for subsequent processing and swallow). Our subjects, when instructed to chew the food but hold it in the mouth until the command, may have tried to chew all the food before the first swallow. Chewing a larger amount of food would reasonably be expected to require a larger number of chewing strokes. The instruction to eat in a somewhat different manner (swallowing on command), or the expectation of an interruption of feeding, may have caused some reduction in the efficiency of mastication in reducing particle size. The efficiency of mastication could also have been reduced simply by drawing the subject’s attention to it.
The present study suggests that the vallecular region is perceived differently than the upper oropharynx in feeding. The leading edge of barium reached the valleculae before swallow onset in most subjects during sequences with no command, consistent with previously published studies of normal individuals eating solid food [2, 3, 5]. However, in sequences with command, subjects invariably held the food above the valleculae until after the command to swallow, and VAT was an average of 34% shorter (although four of these subjects transported food to the upper oropharynx, above the valleculae, before swallow onset). Given the semi-solid nature of the chewed food, it is unlikely that this movement to the pharynx was a result of gravity or leakage from the oral cavity [4, 15]. We infer that subjects inhibited stage II transport to the valleculae in the sequences with command. In a recent study on the coordination of breathing and eating, Palmer and Hiiemae (2003) showed an alteration in respiration during bolus aggregation in the valleculae [7]. In that study, airflow typically ceased or was in the expiratory direction when food was accumulating in the valleculae. This suggests that brain centers controlling respiration and feeding are sensitive to input from receptors in the valleculae.
Our findings indicate that the processes of bolus transport, bolus aggregation, and swallow initiation may be altered by volition. In particular, this study shows that subjects can consciously inhibit stage II transport to the valleculae. This could, in part, reflect a difference in the neural mechanisms controlling volitional and reflex swallowing. Several investigators have shown with brain imaging techniques that different but overlapping areas of the cerebrum are activated in volitional vs. automatic swallowing of saliva or water [16–18]. Recent work by Toogood et al (2005) also suggests that the processing of the cue to swallow engages different brain areas than the swallow itself [19].
These results also speak to the nature of airway protection given the unique laryngeal position in hominids relative to swallowing, as discussed above. The default behavior for feeding and swallowing, which moves food quickly past the laryngeal opening, minimizes the potential for aspiration. Human volition perturbs that system, reducing the time, and the variation in time, when the airway is at risk.
Acknowledgments
Chune Yang provided superb technical assistance. We thank Rebecca German for providing helpful comments on the manuscript. Supported in part by USPHS Award R01 DC 02123 from the NIH/National Institute on Deafness and other Communication Disorders, two Health Sciences Research Grants (H 12-choujyu-21 and H15-21 EBM-018) from the Ministry of Health, Labor and Welfare of Japan, and a grant from the Medstar Research Institute.
Footnotes
Presented in part at the annual meeting of the Dysphagia Research Society, March, 2006.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Dodds WJ, Stewart ET, Logemann JA. Physiology and radiology of the normal oral and pharyngeal phases of swallowing [see comments] AJR Am J Roentgenol. 1990;154(5):953–63. doi: 10.2214/ajr.154.5.2108569. [DOI] [PubMed] [Google Scholar]
- 2.Palmer JB, Rudin NJ, Lara G, Crompton AW. Coordination of mastication and swallowing. Dysphagia. 1992;7(4):187–200. doi: 10.1007/BF02493469. [DOI] [PubMed] [Google Scholar]
- 3.Dua KS, Ren J, Bardan E, Xie P, Shaker R. Coordination of deglutitive glottal function and pharyngeal bolus transit during normal eating. Gastroenterology. 1997;112(1):73–83. doi: 10.1016/s0016-5085(97)70221-x. [DOI] [PubMed] [Google Scholar]
- 4.Palmer JB. Bolus aggregation in the oropharynx does not depend on gravity. Arch Phys Med Rehabil. 1998;79(6):691–6. doi: 10.1016/s0003-9993(98)90046-6. [DOI] [PubMed] [Google Scholar]
- 5.Hiiemae KM, Palmer JB. Food transport and bolus formation during complete feeding sequences on foods of different initial consistency [see comments] Dysphagia. 1999;14(1):31–42. doi: 10.1007/PL00009582. [DOI] [PubMed] [Google Scholar]
- 6.German RZ, Palmer JB. Anatomy and Development of Oral Cavity and Pharynx. GI Motility Online. 2006 doi: 10.1038/gimo5. http://www.nature.com/gimo/contents/pt1/full/gimo5.html. [DOI]
- 7.Palmer JB, Hiiemae KM. Eating and breathing: interactions between respiration and feeding on solid food. Dysphagia. 2003;18(3):169–78. doi: 10.1007/s00455-002-0097-9. [DOI] [PubMed] [Google Scholar]
- 8.Lund JP. Mastication and its control by the brain stem. Crit Rev Oral Biol Med. 1991;2(1):33–64. doi: 10.1177/10454411910020010401. [DOI] [PubMed] [Google Scholar]
- 9.Nakamura Y, Katakura N. Generation of masticatory rhythm in the brainstem. Neurosci Res. 1995;23(1):1–19. [PubMed] [Google Scholar]
- 10.Jean A. Brain stem control of swallowing: neuronal network and cellular mechanisms. Physiol Rev. 2001;81(2):929–69. doi: 10.1152/physrev.2001.81.2.929. [DOI] [PubMed] [Google Scholar]
- 11.Thexton AJ, Crompton AW. The control of swallowing. In: Linden RWA, editor. The Scientific Basis of Eating Frontiers of Oral Biology. Vol. 9 Karger Basel; 1998. pp. 168–222. [Google Scholar]
- 12.Palmer JB, Kuhlemeier KV, Tippett DC, Lynch C. A protocol for the videofluorographic swallowing study. Dysphagia. 1993;8(3):209–14. doi: 10.1007/BF01354540. [DOI] [PubMed] [Google Scholar]
- 13.Palmer JB, Hiiemae KM, Liu J. Tongue–jaw linkages in human feeding: a preliminary videofluorographic study. Arch Oral Biol. 1997;42(6):429–41. doi: 10.1016/s0003-9969(97)00020-4. [DOI] [PubMed] [Google Scholar]
- 14.Matsuo K, Hiiemae KM, Palmer JB. Cyclic motion of the soft palate in feeding. J Dent Res. 2005;84(1):39–42. doi: 10.1177/154405910508400106. [DOI] [PubMed] [Google Scholar]
- 15.Saitoh E, Shibata S, Matsuo K, Baba M, Fujii W, Palmer JB. Chewing and Food Consistency: Effects on Bolus Transport and Swallow Initiation. Dysphagia. doi: 10.1007/s00455-006-9060-5. in press. [DOI] [PubMed] [Google Scholar]
- 16.Dziewas R, Soros P, Ishii R, Chau W, Henningsen H, Ringelstein EB, Knecht S, Pantev C. Neuroimaging evidence for cortical involvement in the preparation and in the act of swallowing. Neuroimage. 2003;20(1):135–44. doi: 10.1016/s1053-8119(03)00285-4. [DOI] [PubMed] [Google Scholar]
- 17.Kern MK, Jaradeh S, Arndorfer RC, Shaker R. Cerebral cortical representation of reflexive and volitional swallowing in humans. Am J Physiol Gastrointest Liver Physiol. 2001;280(3):G354–60. doi: 10.1152/ajpgi.2001.280.3.G354. [DOI] [PubMed] [Google Scholar]
- 18.Martin RE, Goodyear BG, Gati JS, Menon RS. Cerebral cortical representation of automatic and volitional swallowing in humans. J Neurophysiol. 2001;85(2):938–50. doi: 10.1152/jn.2001.85.2.938. [DOI] [PubMed] [Google Scholar]
- 19.Toogood JA, Barr AM, Stevens TK, Gati JS, Menon RS, Martin RE. Discrete functional contributions of cerebral cortical foci in voluntary swallowing: a functional magnetic resonance imaging (fMRI) “Go, No-Go” study. Exp Brain Res. 2005;161(1):81–90. doi: 10.1007/s00221-004-2048-1. [DOI] [PubMed] [Google Scholar]


