Abstract
L1210 murine leukemia cells exposed to an LD90 concentration of the Bcl-2/Bcl-xL antagonist HA14-1 rapidly undergo apoptosis but also develop numerous intracellular vacuoles with double membranes, exhibit enhanced labeling by monodansylcadaverine, and convert the cytosolic protein LC3-I to LC3-II. These are hallmarks or autophagy. Autophagic vacuoles develop rapidly, preceding the appearance of an apoptotic nuclear morphology and can be observed in both non-apoptotic and apoptotic cells. Inhibition of autophagy by the PI 3-kinase inhibitor wortmannin promoted apoptosis; conversely inhibition of caspase-3/7 with zDEVD-fmk promoted autophagy. Neither process was dependent on calcium translocation. These results indicate that pharmacological suppression of Bcl-2 function can mimic the induction of autophagy that can occur following the down-regulation of Bcl-2 expression by molecular approaches.
Keywords: apoptosis, autophagy, Bcl-2, HA14-1, vacuoles, L1210
1. Introduction
The drug HA14-1 (ethyl 2-amino-6-bromo-4(1-cyano-2-ethoxy-2-oxoethyl)-4Hchromene-3 carboxylate) was initially identified as a non-peptidic ligand for the surface pocket of the anti-apoptotic protein Bcl-2 with pro-apoptotic activity [1]. Additional studies with HL-60 cells suggested that HA14-1 facilitated the release of Ca2+ from the endoplasmic reticulum (ER), contributing to mitochondrial dysfunction and the release of cytochrome c [2]. Initiation of apoptosis by HA14-1 could therefore be antagonized by either co-incubation with the calcium chelating agent EGTA or Ru360 [2], a ruthenium complex that inhibits Ca2+ uptake by the mitochondrial ‘uniporter’ [3].
Macroautophagy (hereafter termed ‘autophagy’) is a process whereby cytosol and organelles become enclosed in double membrane structures termed autophagosomes. The latter ultimately fuse with lysosomes and a subsequent proteolytic process permits the engulfed material to become available for recycling [4,5]. Although originally described as a survival response evoked by nutrient deprivation, it is now clear that autophagy can also serve as a cell death pathway [6–8].
Antisense-mediated down-regulation of Bcl-2 expression has been reported to induce autophagy in HL-60 cells [9]. Similarly, siRNA-mediated down regulation of Bcl-2 in MCF-7 cells dramatically enhanced the induction of autophagy by nutrient deprivation [10,11]. Conversely, Bcl-2 over expression has been reported to suppress the induction of autophagy by nutrient deprivation in several cell types [10]. In this study we determined if a functional inactivation of Bcl-2/Bcl-XL, mediated by HA14-1, also causes the induction of autophagy. As a test system we used murine leukemia L1210 cells. We also assessed the role of Ca2+ on HA14-1-initiated responses in this cell line. We found that HA14-1 promoted the development of both autophagy and apoptosis in L1210 cells, and that these processes were Ca2+ independent.
2. Materials and methods
2.1 Chemicals and biologicals
Amino acids, tissue culture media, wortmannin, monodansylcadaverine (MDC), ruthenium complex Ru360, and the caspase inhibitor zDEVDFMK were obtained from Sigma-Aldrich (St. Louis, MO). Sterile horse serum was purchased from Atlanta Biologicals (Lawrenceville, GA). Höchst dye HO33342 (HO342), tetramethylrhodamine methyl ester (TMRM), and the cell-permeable calcium-chelating agent BAPTA-AM were obtained from Invitrogen (Molecular Probes; Carlsbad, CA). The Bcl-2 antagonist HA14-1 was provided by Ryan Scientific Inc. (Isle of Palms, SC). Since this reagent slowly loses activity in the presence of water, solutions were made up in anhydrous dimethyl sulfoxide, and stored in small aliquots at −20°C under nitrogen.
2.2 Fluorescent probes
Nuclear morphology/condensed chromatin, mitochondrial membrane potential and enhanced lysosomal/endosomal activity were probed by staining cells with HO342, TMRM and MDC, respectively, as previously described [12]. After labeling, cells were washed once with fresh growth medium and examined by phase-contrast and fluorescence microscopy. Excitation wavelengths were 360–380 nm (HO33342, MDC) and 510–560 nm (TMRM). Interference filters were used to isolate the emission fluorescence of HO342 (400–450 nm), MDC (520–560 nm) and TMRM (550–650 nm).
2.3 Cells and maintenance
Murine leukemia L1210 cells were grown in sealed flasks, using an approximation of Fisher’s Medium consisting of α-MEM (GIBCO-BRL, Grand Island, NY) supplemented with MgCl2 (45 mg/l), methionine (75 mg/l), phenylalanine (30 mg/l), valine (30 mg/l), folic acid (9 mg/l), 1 mM glutamine, 1 mM mercaptoethanol, gentamicin, and 10% horse serum. Cells were maintained in suspension culture at 37°C in a 5% CO2 atmosphere.
Western blots for LC3
The conditions used for cell lysis and western blot analyses have been described [12]. Protease inhibitors (10 μg/ml each of E64d and pepstatin A) were added to inhibit degradation of LC3-II [13,14]. Samples containing 40 μg of protein were used for these assays. Immune complexes were detected with Vistra ECF western blot reagent (Amersham Biosciences Corp., Piscataway, NJ) using the ‘Storm’ imaging system (Molecular Dynamics, Sunnyvale, CA). A rabbit polyclonal antibody raised against the microtubule-associated protein LC3 was the gift of Dr. Masahiro Shibata, Osaka University Graduate School of Medicine, Japan.
2.4 Phase/fluorescence microscopy
Images were acquired using a Nikon Eclipse E600 microscope and a SenSys CCD camera (Photometrics, Tucson, AZ), and further processed using MetaMorph software (Universal Imaging, Downington, PA). A Uniblitz shutter was used to control exposure to the excitation source. This was configured to open and close with the camera shutter, thus minimizing photobleaching.
2.5 Electron Microscopy
For these studies, L1210 cells were fixed with glutaraldehyde and osmium tetroxide, treated with uranyl acetate + lead citrate for enhanced protein and lipid staining, and then dehydrated in ethanol. The cell pellets were embedded in epon resin and cut with an ultramicrotome to a 70 nm thickness.
2.6 HA14-1 treatment protocols
L1210 cells were treated with LD50 or LD90 doses of HA141, previously determined to be 20 and 40 μM, respectively [15], for specified times at 37°C. In some studies, cultures were co-treated with the caspase 3/7 inhibitor zDEVD-fmk (50 μM) or the phosphatidylinositol 3-kinase (PI 3-kinase) inhibitor wortmannin (100 nM). To assess the role for translocation of cytosolic calcium in HA14-1 initiated responses, some incubations were carried out in the presence of BAPTA-AM (10 μM) plus the ruthenium complex Ru360 (5 μM). After incubation, cells were labeled with HO342, TMRM or MDC, as described above. Because of the lack of spectral overlap, it was feasible to label cells simultaneously with HO342 and TMRM.
3. Results and discussion
Exposure of L1210 cultures to 40 μM HA14-1 for 60 min caused a partial loss of mitochondrial membrane potential (ΔΨm) in some cells, and the total loss in others (as indicated by TMRM staining, Fig. 1). HO342 staining indicated that many of the latter also exhibited condensed chromatin (Fig. 1). Many HA14-1 treated cells, including some with condensed chromatin, also increased in size and developed numerous intracellular vacuoles. Monodansylcadaverine (MDC) staining, commonly used as a marker of auophagic vacuoles and lysosomes [16], was primarily punctate in control cultures (Fig. 1, top row). HA14-1 treated cultures exhibited enhanced levels of punctate MDC staining, as well as diffuse vacuole staining (Fig. 1, second row). PI 3-kinase activity is required for formation of autophagic vacuoles [4,17]. Vacuole formation was strongly suppressed in cultures co-treated with HA14-1 and the PI 3-kinase inhibitor wortmannin, whereas the formation of condensed chromatin was not suppressed by inclusion of wortmannin (Fig. 1, third row). Conversely, inclusion of the caspase-3/7 inhibitor zDEVD strongly suppressed HA14-1-induced development of condensed chromatin, but potentiated the formation of vacuoles whose periphery stained with MDC (Fig. 1, fourth row).
Fig. 1.

Loss of mitochondrial membrane potential and formation of condensed chromatin in L1210 cultures treated with HA14-1. Phase-contrast (first and fourth columns) and fluorescence images of L1210 cells stained with TMRM, HO342 and MDC. Row headings indicate additions of HA14-1 (40 μM), zDEVD-fmk (50 μM) and/or wortmannin (WM, 100 nM). All incubations were for 60 min. The first three columns are from the same field. The MDC labeling studies (columns 4 and 5) represent a separate experiment. White bar in top right panel = 20 μ.
A structural feature of autophagosomes that distinguishes them from other vacuoles is a double walled membrane [14]. An examination of L1210 cells 60 min after exposure to 40 μM HA14-1 by electron microscopy confirmed the appearance of cells with multiple vacuoles (Fig. 2 top) that were enclosed in a double membrane (Fig 2, bottom).
Fig. 2.
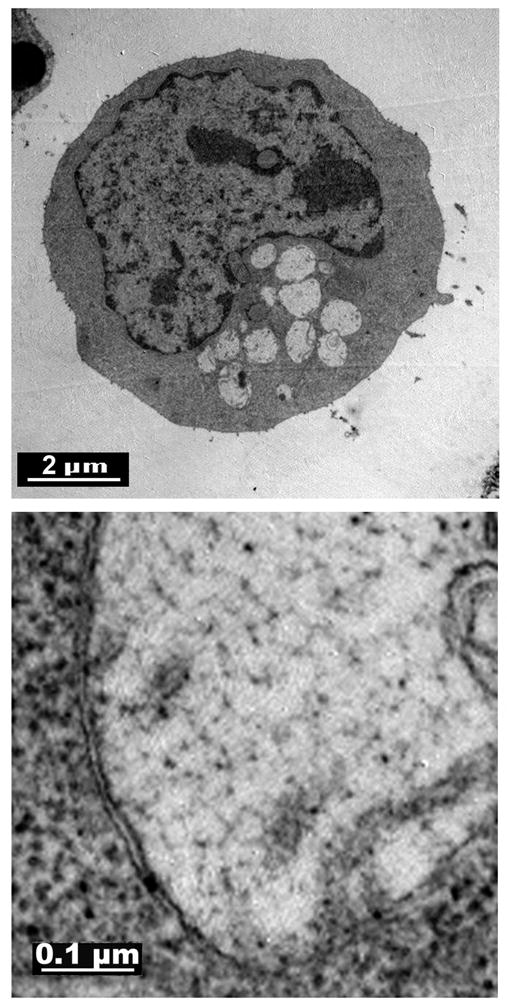
Electron micrographs of an L1210 cell after a 60 min exposure to an LD90 concentration of HA14-1. The bottom panel depicts a typical vacuole. Top = 5,000 x, bottom = 30,000 x.
Upon the induction of autophagy, phosphatidylethanolamine is covalently linked to the cytosolic protein LC3-I to yield LC3-II, which then associates with the autophagosome [14,18]. This conversion is commonly used as a marker for autophagy [14]. Exposure of L1210 cells to HA14-1 caused the conversion of LC3-I to LC3-II (Fig. 3). This effect was enhanced by the presence of the caspase inhibitor zDEVD-fmk, and antagonized by the PI 3-kinase inhibitor wortmannin (Fig. 3). The effects of these inhibitors on LC3-1 processing in HA14-1 treated cultures are consistent with the morphological effects reported in Fig. 1: suppression or enhancement of vacuolization by wortmannin or zDEVD-fmk, respectively. Collectively, the data presented in Figs. 1–3 suggest that HA14-1 treatment of L1210 cultures triggers both autophagy and apoptosis. Furthermore, the two processes are not mutually exclusive. Cells that eventually undergo apoptosis, may or may not exhibit features of autophagy. Similar observations have been reported for L1210 cells following PDT with the photosensitizer CPO [12].
Fig. 3.

Conversion of LC3-I to LC3-II in L1210 cultures treated with HA14-1. A = Controls, B = cells treated with an LD90 concentration of HA14-1 for 60 min, C = same as B but with 100 nM wortmannin present, D = same as B with 50 μM zDEVD-fmk present.
Data shown in Fig. 4 demonstrate the formation of autophagic vacuoles prior to the appearance of an apoptotic nuclear morphology. Specifically, exposure to an LD50 concentration of HA14-1 (20 μM) for 60 minutes caused significant vacuolization, without loss of )Qm, or the development of condensed chromatin (Fig. 4, top row). At an LD90 concentration (40 μM) vacuoles were apparent within 15 min of treatment (Fig. 4, bottom row). Although the latter conditions promoted a coordinate loss of ΔΨm, no chromatin condensation had occurred. Hence, the processes whereby autophagosomes are generated following HA14-1 exposure are faster than those involved in activation of the endonuclease responsible for chromatin fragmentation.
Fig. 4.

Rate of appearances of autophagic vacuoles and apoptotic nuclear morphology, and loss of ΔΨm, after treatment with HA14-1. Cultures were treated with 20 μM HA14-1 for 60 min (top row) or 40 μM HA14-1 for 15 minutes before being stained with TMRM or HO342 and photographed. White bar in bottom right panel = 20 μ.
An et al. [2] reported that the induction of apoptosis in HL-60 cells by HA14-1 could be inhibited by co-incubation with either the acetoxymethyl ester of EGTA, a Ca2+ chelator, or by inhibition of mitochondrial Ca2+ uptake by Ru360. Co-treatment with the calcium chelator BAPTA-AM and the mitochondrial uniporter antagonist Ru360 did not, however, impair HA14-1 induced chromatin condensation or vacuole formation in L1210 cells (Fig. 5). In other studies, we found that co-treatment with these agents did not affect the lethality of HA14-1 (not shown). Thus, unlike the situation in HL-60 cells, Ca2+ does not appear to play a role in the mechanism of HA14-1-induced apoptosis or autophagy in L1210 cultures.
Fig. 5.
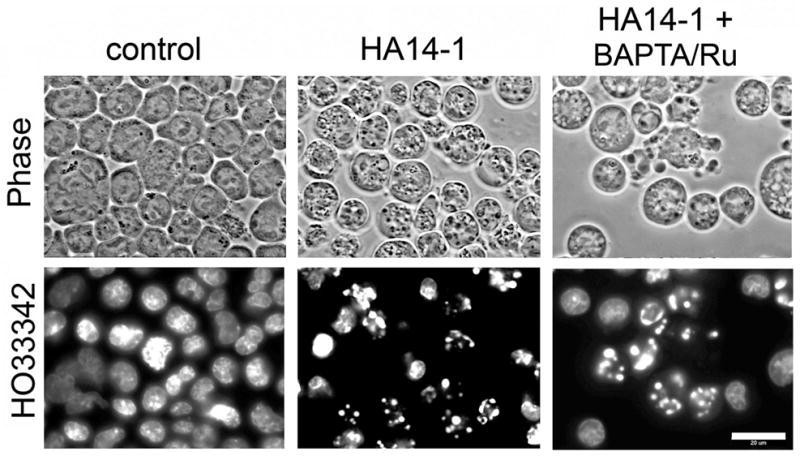
Role of calcium in HA14-1 induced apoptosis and autophagy. L1210 cultures were treated with 40 μM HA14-1 or HA14-1 + BAPTA-AM (10 μM) + Ru360 (5 μM) for 60 min prior to being stained with HO342 and photographed. White bar in bottom right panel = 20 μ.
Autophagy was originally described as an adoptive survival response to nutrient deprivation [4,5]. Lemasters has proposed that cells utilize autophagy to rid themselves of damaged organelles [19]. Whereas the above situations envision autophagy as a survival mechanism, autophagy can also lead to cell death under some circumstances [6–8]. In the current study it is unclear as to whether the observed autophagic response results in death or survival. However, autophagic morphological features were expressed in over 90% of L1210 cells following exposure to an LD50 concentration of HA14-1 Hence, a large percentage of cells undergoing autophagy did not die.
In summary, our studies indicate that the pharmacological inhibition of Bcl-2 function, like molecular approaches that suppress Bcl-2 expression [9–11], is associated with the both apoptosis and autophagy in L1210 cells. Our findings are also pertinent in terms of recent developments in the field of photodynamic therapy (PDT). We, as well as others, have reported that Bcl-2 becomes rapidly inactivated/lost following irradiation of photosensitized cells where the targets for photodamage include the endoplasmic reticulum [20] or the ER + mitochondria [21]. Both apoptosis and autophagy are induced in L1210 cultures following ER photodamage [12]. Presumably, these responses are triggered by the rapid loss of Bcl-2 following irradiation [20].
Acknowledgments
Excellent technical assistance was provided by Ann Marie Santiago and Nakaiya Okan-Mensa. These studies were support in part by grant CA 23378 from the National Cancer Institute, NIH.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Wang JL, Liu D, Zhang ZJ, Shan S, Han X, Srinivasula SM, Croce CM, Alnemri ES, Huang Z. Structure based discovery of an organic compound that binds Bcl-2 protein and induces apoptosis of tumor cells. Proc Natl Acad Sci U S A. 2000;97:7124–7129. doi: 10.1073/pnas.97.13.7124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.An J, Chen Y, Huang Z. Critical upstream signals of cytochrome c release induced by a novel Bcl-2 inhibitor. J Biol Chem. 2004;279:19133–19140. doi: 10.1074/jbc.M400295200. [DOI] [PubMed] [Google Scholar]
- 3.Matlib MA, Zhou Z, Knight S, Ahmed S, Choi KM, Krause-Bauer J, Phillips R, Altschuld R, Katsube Y, Sperelakis N, Bers DM. Oxygen-bridged dinuclear ruthenium amine complex specifically inhibits Ca2+ uptake into mitochondria in vitro and in situ in single cardiac myocytes. J Biol Chem. 1998;273:10223–10231. doi: 10.1074/jbc.273.17.10223. [DOI] [PubMed] [Google Scholar]
- 4.Yorimitsu T, Klionsky DJ. Autophagy: molecular machinery for self-eating. Cell Death Differ. 2005;12(Suppl 2):1542–1552. doi: 10.1038/sj.cdd.4401765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Levine B, Klionsky DJ. Development by self-digestion: pathway of cellular degradation. Science. 2004;6:463–477. [Google Scholar]
- 6.Yu L, Lenardo MJ, Baehrecke EH. Autophagy and caspases: a new cell death program. Cell Cycle. 2004;3:1124–1126. [PubMed] [Google Scholar]
- 7.Tsujimoto Y, Shimizu S. Another way to die: autophagic programmed cell death. Cell Death Differ. 2005;12(Suppl 2):1528–1534. doi: 10.1038/sj.cdd.4401777. [DOI] [PubMed] [Google Scholar]
- 8.Levine B, Yuan J. Autophagy in cell death: an innocent convict? J Clin Invest. 2005;115:2679–2688. doi: 10.1172/JCI26390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Saeki K, You A, Okuma E, Susin SA, Kroemer G, Takaku GF. Bcl-2 down-regulation causes autophagy in a caspase-independent manner in human leukemic HL60 cells. Cell Death Differ. 2000;7:1263–1269. doi: 10.1038/sj.cdd.4400759. [DOI] [PubMed] [Google Scholar]
- 10.Pattingre S, Tassa A, Qu X, Garuti R, Liang XH, Mizushima N, Packer M, Schneider MD, Levine B. Bcl-2 antiapoptotic proteins inhibit Beclin 1-dependent autophagy. Cell. 2005;122:927–939. doi: 10.1016/j.cell.2005.07.002. [DOI] [PubMed] [Google Scholar]
- 11.Pattingre S, Levine B. Bcl-2 inhibition of autophagy: a new route to cancer? Cancer Res. 2006;66:2885–2888. doi: 10.1158/0008-5472.CAN-05-4412. [DOI] [PubMed] [Google Scholar]
- 12.Kessel D, Vicente MGH, Reiners JJ., Jr Initiation of apoptosis and autophagy by photodynamic therapy. Lasers Surg Med. 2006;38:482–488. doi: 10.1002/lsm.20334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tanida I, Minematsu-Ileguchi N, Ueno T, Kominami E. Lysosomal turnover, but not a cellular level of endogenous LC3 is a marker for autophagy. Autophagy. 2005;1:84–91. doi: 10.4161/auto.1.2.1697. [DOI] [PubMed] [Google Scholar]
- 14.Mizushima N. Methods for monitoring autophagy. Int J Biochem Cell Biol. 2004;36:2491–2502. doi: 10.1016/j.biocel.2004.02.005. [DOI] [PubMed] [Google Scholar]
- 15.Kessel D, Castelli M, Reiners JJ., Jr Apoptotic response to photodynamic therapy versus the Bcl-2 antagonist HA14-1. Photochem Photobiol. 2002;76:314–319. doi: 10.1562/0031-8655(2002)076<0314:artptv>2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 16.Biederbick A, Kern HF, Elsasser HP. Monodansylcadaverine (MDC) is a specific in vivo marker for autophagic vacuoles. Eur J Cell Biol. 1995;66:3–14. [PubMed] [Google Scholar]
- 17.Blommaart EF, Krause U, Schellens JP, Vreeling-Sindelarova H, Meijer AJ. The phosphatidylinositol 3-kinase inhibitors wortmannin and LY294002 inhibit autophagy in isolated rat hepatocytes. Eur J Biochem. 1997;243:240–246. doi: 10.1111/j.1432-1033.1997.0240a.x. [DOI] [PubMed] [Google Scholar]
- 18.Kabeya Y, Mizushima N, Yamamoto A, Oshitani-Okamoto S, Ohsumi Y, Oshimori T. LC3, GABARAP and GATE1 localize to autophagosomal membrane depending on form-II formation. J Cell Sci. 2004;117:2805–2812. doi: 10.1242/jcs.01131. [DOI] [PubMed] [Google Scholar]
- 19.Lemasters JJ. Selective mitochondrial autophagy, or mitophagy, as a targeted defense against oxidative stress, mitochondrial dysfunction, and aging. Rejuvenation Res. 2005;8:3–5. doi: 10.1089/rej.2005.8.3. [DOI] [PubMed] [Google Scholar]
- 20.Kessel D, Castelli M, Reiners JJ., Jr Ruthenium red-mediated suppression of Bcl-2 loss and Ca2+ release initiated by photodamage to the endoplasmic reticulum: scavenging of reactive oxygen species. Cell Death Differ. 2005;12:502–511. doi: 10.1038/sj.cdd.4401579. [DOI] [PubMed] [Google Scholar]
- 21.Xue LY, Chiu SM, Oleinick NL. Photochemical destruction of the Bcl-2 oncoprotein during photodynamic therapy with the phthalocyanine photosensitizer Pc 4. Oncogene. 2001;20:3420–3427. doi: 10.1038/sj.onc.1204441. [DOI] [PubMed] [Google Scholar]


