Abstract
Effects of postnatal hypothyroidism and recovery from this condition on regional growth of the rat hippocampus (HC) were studied using two-dimensional (2D) foldout, morphometric maps of HC and its constituent CA1–CA4 regions. The maps were derived from unfolding serial coronal sections of the rat forebrain, consisting of the entire rostrocaudal extent of HC pyramidal cell layer in the normal control and hypothyroid weanling (P25, postnatal day 25) and young adult (P90) male rats, as well as animals allowed to recover from hypothyroid-induced growth retardation at weaning. The maps revealed novel views of HC regions for assessment of topological relationships and measurement of surface areas of the HC cortical sheet (pyramidal cell layer). In normal control P90 rats, the unfolded HC on each side extended 4 times more laterally than rostrocaudally; total HC surface area was about 40 mm2, compared to 30 mm2 in the weanling, indicating 35% growth from P25 to P90; CA1 took up 52% of the total HC surface area, followed by CA3 (31%) and CA2 and CA4, 8% each. Hypothyroidism resulted in significant (p<0.01) 11% and 20% reductions in the HC surface area in P25 and P90 rats respectively; CA1 and CA4 regions suffered the most reductions while CA3 and CA2 regions the least. Recovering rats examined at P90, exhibited remarkable growth plasticity and recovery in HC regions, as evident by their near normal HC cortical surface area values, compared to age-matched controls. The 2-D maps also revealed growth deficits in all HC regions of the hypothyroid rats; recovery in these parameters occurred across all dimensions, although the anterior-posterior growth was more severely affected than the mediolateral one. These results are confirmed and extended by volumetric analysis of laminar volumes of HC regions presented in a companion paper (Farahvar et al., 2006). These results imply that HC regions, in contrast to whole brain, possess exceptional growth plasticity, as shown by ability to dramatically recover from early hypothyroid retardation; also 2D morphometric maps are useful tools to visualize complex and convoluted regional sheet of HC cortex and depict quantitative aspects of growth in normal and experimental conditions.
Keywords: Hippocampus maps, hippocampal growth and development, hippocampal morphometry, postnatal hypothyroidism, neurologic recovery, early brain retardation, brain rehabilitation, hippocampal plasticity, propylthiouracil (PTU), experimental cretinism
Introduction
Thyroid hormones (TH) are critical for normal growth and maturation of the developing brain; their absence during early period of mammalian brain development results in profound morphological, biochemical, and functional alterations in the central nervous system. In the rat THs deficiency during the suckling period results in marked retardation of brain growth and development (for reviews of earlier literature see Dussault & Ruel, 1987; Lauder, 1983; Paternostro & Meisami, 1993; Timiras, 1988; for reviews of more recent literature see Bernal, 2002; Morreale de Escobar et al., 2004; Santisteban & Bernal, 2005; Smith et al., 2002. Early studies on the effects of thyroid deficiency on brain development focused on structural abnormalities in the cerebral cortex (Eayrs, 1955, 1966; Rabie et al., 1979) and cerebellum (Lauder, 1983; Legrand et al., 1986, Patel & Rabie, 1980). A few previous studies investigated the deleterious effects of thyroid hormone deficiency on the developing hippocampus (HC) (Gould, et al. 1991, Lauder & Mugnaini, 1977, 1980; Rabie et al., 1979; Rami & Rabie, 1988; Rami et al., 1986a,b). Recently several molecular and functional studies have also appeared (Alvarez-Dolado et al., 2001; Alzoubi et al., 2005; Ambrogini et al., 2005; Desouza et al., 2005; Dong et al., 2005; Gerges & Alkadhi, 2004, Gilbert, 2004; Gilbert & Paczkowski, 2003; Martinez et al., 2001; Matos, et al. 2002; Meaney et al., 2000; Roskoden et al., 1999; Sui & Gilbert, 2003; Uchida et al., 2005; Vaidya et al., 2001; Vara et al., 2002, 2003).
Very few of these studies have focused on the possibility of recovery of the HC from hypothyroid-induced deficiencies. This is curious in light of the prominent role the HC plays in normal cognitive functions (Cohen & Eichenbaum, 1993; Olton et al. 1979; Zola-Morgan, et al., 1986) and the profound mental retardation and cognitive impairments associated with severe cases of neurological cretinism (Delange, 1994; Smith et al., 2002). In addition to its unique functions in congition, the HC has been shown to display marked neural plasticity, as well as pronounced vulnerability to ischemia and other hormonal and metabolic effects (Bothe et al., 1986; Gould et al. 1991; Masliah et al., 1992; Nawashiro et al., 1995; Sapolsky et al., 1985; Sloviter et al.1989; Smith et al., 1994; Zola-Morgan et al., 1986).
Since brain development is rapid during the early postnatal (P) period, and since TH replacement therapy after the suckling period is not able to reverse the retarding effects, the early postnatal period has often been referred as a “critical period” (Dobbing, 1974; Dobbing & Smart, 1974; Lauder & Krebs, 1986; Timiras, 1972). Therefore the prospect of recovery of the growth-retarded brain has been considered to be minimal. Paternostro & Meisami (1993, 1996) found essentially complete recovery of the olfactory epithelium and olfactory receptor neuron structure from early hypothyroid retardation; olfactory receptor tissue has unique regenerative ability, even in the adult (Farbman, 1992, Monti-Graziadei & Graziadei, 1979). A similarly marked ability for recovery appears to be true for the olfactory bulbs as well (Sendera, 1997). Furthermore, Meisami and collaborators (see Tamasy et al., 1986a,b) had noted considerable recovery in some learning tasks and behavioral functions after early hypothyroidism in the rat. It would therefore be interesting and important to investigate if the rat HC, with its own unique growth plasticity, also able to show recovery from early hypothyroid retardation.
In the present study we examine the retarding effects of THs deficiency during the postnatal period on rat HC growth. These effects were studied morphologically and morphometrically in growing rat pups that were made hypothyroid by administration of the reversible goitrogen PTU (n-propylthiouracil). Animals kept hypothyroid during the early postnatal life (suckling period, P1–P25) were compared with normal control rats and those in which hypothyroidism lasted for the entire duration of postnatal growth (pre- and postweaning periods) till early adulthood (P90). To determine the prospects of recovery from early hypothyroid retardation, HC growth in these hypothyroid rats were compared with pups that were allowed to recover normal thyroid function by withdrawal of PTU at weaning (P25). For morphological and morphometric parameters of the rat HC growth we developed novel two-dimensional (2D) fold-out maps of the pyramidal cell layer (PCL) of the HC; the latter was assumed to be the same as the cortical sheet of the HC in planar dimension. We then compared these parameters between the various control, hypothyroid and recovery groups. These maps exhibited details of the various HC regions (CA1–CA4) in the anterior-posterior and the mediolateral directions. The maps also allowed visual and graphic comparison of growth differences among the various experimental groups and permitted simultaneous morphometric determination of surface area of HC and its regions in the various ages and experimental groups.
In addition, in a companion paper submitted to this journal (Farahvar et al., 2006?), based on a study of cytochrome oxidase serial sections we provide detailed data on laminar volumes and densitometric analysis of individual HC layers in normal, hypothyroid and recovery group rats of the same ages as this study. Such data would be complementary to those presented in this paper.
Materials and methods
Animals
Pregnant albino rats of the Sprague-Dawley strain (Holtzman, Wisconsin) were kept in separate plastic cages until delivery. At birth, the litter size was reduced to eight pups per mother per cage. The conditions of the colony were kept constant (25 °C, 12 hr light-dark cycle). Food (Purina rat chow) and water were provided ad libitum. The litters were divided into two groups, one control and one experimental hypothyroid (see below). Pups of both groups were weighed regularly 2–3 times per week and allowed to suckle until day 25 postnatal (weaning). After weaning, the animals were separated by sex and the males were kept 2 per cage and weighed twice weekly until P90 days (young adult). Only male rats were utilized in these studies. Animal care and treatments were according to the approved protocols of the University of Illinois, Urbana-Champaign and standard NIH guidelines.
Induction of Hypothyroidism and Recovery
Experimental hypothyroidism was induced in the growing rats by administration of the reversible goitrogen, 6-n-propyl-2-thiouracil (PTU, Sigma, 0.1% w/vol. in the drinking water of the hypothyroid group from birth), following previously published procedures (Meisami, 1984; Paternostro & Meisami, 1993; Tamasy et al., 1986a,b). PTU inhibits the synthesis of thyroidal hormones and decreases their peripheral deiodination. PTU passes through the mother’s milk and depletes thyroid hormones in the mother and pups. Hypothyroid animals received PTU treatment until P25 (weaning) when, one group of hypothyroid animals were removed from PTU treatment and allowed to recover (recovery group). Removal from PTU allows re-establishment of normal serum levels of thyroid hormones. A second hypothyroid group was maintained on PTU until P90. Recovery animals were terminated on P90.
Measurement of Plasma T4 Levels
Blood was collected from animals of control and recovery group at P25 and P90, centrifuged to separate plasma (serum), which was collected and stored frozen. Total serum T3 (triiodothyronine) and T4 (thyroxine, tetraiodothyronine) concentrations were determined using a radioimmunoassay kits (Diagnostic Products, Los Angeles).
Histology
At P25 and P90, animals from control, hypothyroid and recovery groups were anesthetized with halothane and perfused intracardially withphosphate-buffered saline (PBS, pH 7.4) followed by 4% paraformaldehyde in PBS. The brains were carefully dissected out, weighed and placed in the same fixative overnight at 4 °C and transferred to a 30% sucrose solution to minimize tissue shrinkage and provide cryoprotection. Serial coronal (frontal) sections of the brains were cut frozen at 40 μm thickness on a sliding microtome equipped with a freezing stage. Every fifth section was placed on gelatin-subbed slides, air-dried overnight and Nissl stained with cresyl-violet acetate, according to standard procedures as modified in our laboratory (Meisami & Sendera, 1993).
Morphometry and Two-Dimensional (2D) Maps
Criteria for Identifying HC Regions
The pyramidal neurons comprising the four regions of the HC (CA1–CA4) were identified according to Lorente de No (1934), and Bayer (1985) (see also Witter & Amaral, 2004). CA1 cells were relatively small in diameter, rotund and densely packed occurring along the medial surface of the hemisphere directly adjacent to subiculum and cingulate cortex in coronal sections. CA2 cells were relatively large, irregularly shaped, located toward the lateral edge of the HC, in a small distinct band between CA1 and CA3. CA3 cells were large, loosely packed triangular cells, occurring laterally and extending inferio-medially towards the dentate hilar region. CA4 cells were similar to CA3 cells, with a less dense and scattered distribution and occurred throughout the hilus of the dentate gyrus.
Construction of 2D Fold-out Maps
To construct the 2D fold out maps of the HC, 10–15 complete series of brains were analyzed per experimental group. In each series, tracings were made in 20 equally spaced sections, approximately 0.2 mm apart along the anterior-posterior (A-P) extent of the HC. These sections, some of which are schematically and typically shown in Fig. 2, represented a fair distribution of the various pyramidal cell layers throughout the A-P extent of HC, including both the dorsal and ventral aspects. The 2D fold-out maps of HC and its four regions (CA1–CA4) were obtained by tracing microprojected images of the entire length of the HC pyramidal cell layer (PCL) in the serial sections.
Figure 2.
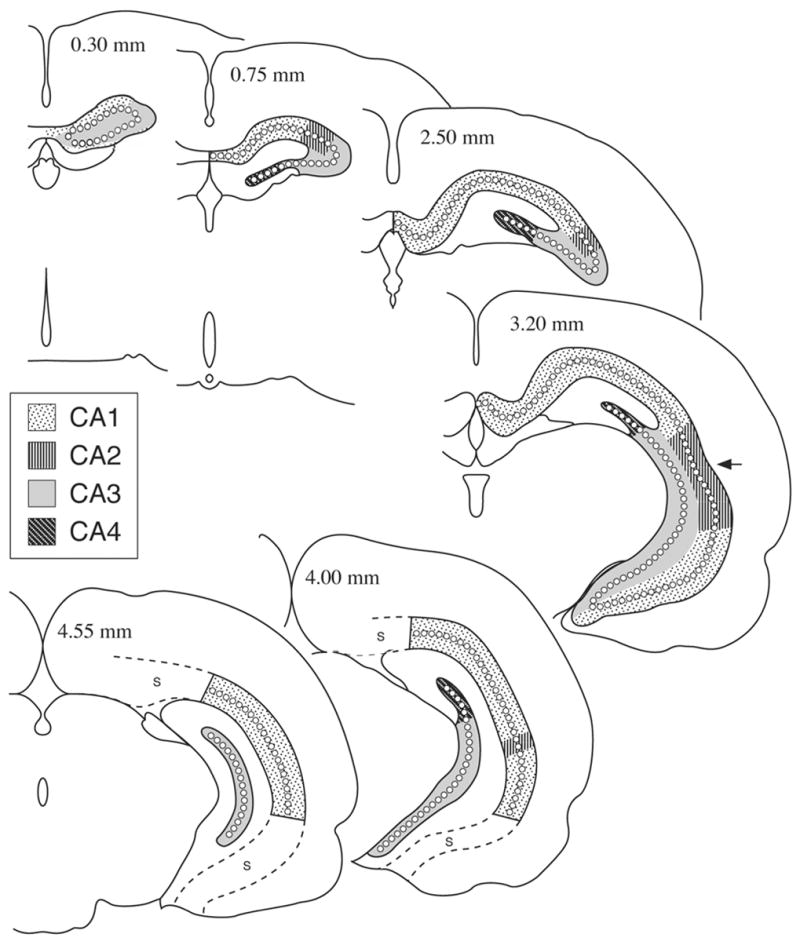
Representative schematic sections of the adult (P 90) rat brain hippocampus (HC) as appearing in frontally cut rostrocaudal series, indicating the exact borderline of the CA1–CA4 regions. Sections like these were used to develop the regional morphometric maps of the present report. Note that dorsal HC appears in the entire extent of HC, while the ventral HC appears mainly in the posterior half. Numbers in the upper portion of the sections indicate the distance in mm from the anterior pole of the HC; S, subiculum; arrow indicates the approximate dorso-ventral midline.
All tracings were done on the HC and its subregions of the left hemisphere of the animal. Diamond et al. (1983) had noted small and occasionally significant left-right differences in HC cortical thickness. We carried out several trial and similar measurements of the HC surface areas on right and left hemispheres but did not find definitive inter-hemispheric differences between the sizes and measures of the highly equivalent regions of the HC. Therefore all tracings and consequent measures and determinations of the surface area represent those of the HC on one hemisphere of the rat, namely the left. The different cytoarchitectonic regions of CA1–CA4 were differentiated and marked off on the tracings using the above morphologic criteria and the markers indicated for these regions in the rat brain stereotaxic atlas by Paxanos & Watson (1986). The tracings were made on the left hemisphere of HC only and included all portions of Ammon’s Horn (CA1–CA4) as well as the rostral subiculum. Three types of morphometric 2D maps were developed: Type I, Type II and Type III.
A. Types I and II maps
For Types I and II maps, total segmental length of HC regions were digitized using the SigmaScan Morphometric Program (Jandel Scientific) and the segmental lengths were summed for each section. These sectional sums were then plotted against the corresponding position of the sections along the A-P extent of the HC. This procedure was carried out for the individual regions as well. The resulting length curves for each HC region were then plotted as a single plot, in a superimposed fashion, utilizing the SigmaPlot Graphics Program (Jandel Scientific). The results provided a simple 2D- morphometric map of the surface area of HC and its regions in a single hemisphere. This map was called Type I. Adding the appropriate segmental length of subiculum to the type I map provided a more realistic 2D map as it displayed the distance of the folded out HC regions from the brain’s sagittal midline as well as exhibiting the topological relation of HC to subiculum. The resultant map was called Type II. The procedures used for unfolding and extending the HC regional segments from coronal sections to construct the Type I and II maps are demonstrated schematically in Fig. 3.
Figure 3.
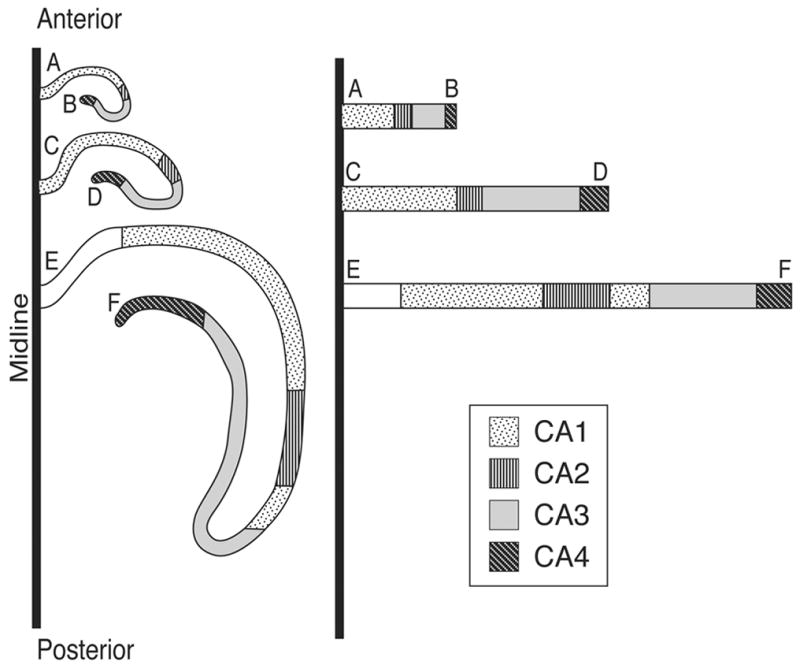
Schematic drawing demonstrating the unfolding procedures utilized to develop the two-dimensional (2D) fold-out maps of the hippocampus (HC) from microprojected tracings of the HC segments indicating the individual HC regions. On the left, 3 sections through the rostrocaudal extent of HC are shown in their folded position. On the right the same segments are unfolded and extended in a medio-lateral direction. The hatchings indicate the location of the various HC regions approximately and schematically. Note that the point of origin of each unfolded segment is at the midline of the brain. The letters indicate the point of origin and termination of the respective HC cell layer in the sections.
B. Type III maps
Although the Type I and II maps were sufficient for morphometric determination of the surface area of HC and its regions, they were not drawn to scale and therefore not appropriate from the anatomic and imaging points of view. Re-plotting the Type II maps using similar scales for the x- and y- axes yielded the Type III maps; this new type map of map provides a more realistic depiction of the surface of HC and its individual regions.
Surface Area of HC Regions
To calculate the surface area of individual HC regions, the Type I maps were utilized and the geometric area under the individual curves were obtained for all the brains of different age and experimental groups sing the SigmaScan Program. The sum of the surface areas for the individual HC subregions yielded the total surface area of the PCL, which is assumed to be equal to the total surface area of the HC cortical sheet. The surface area values thus obtained and shown in RESULTS section (see below) represent those of one hemisphere, the animal’s left hemisphere (see above for explanation). It is presumed that the values for the entire HC cortical sheet surface area in the whole brain are approximately twice these hemispheric values.
Statistical analysis
Statistical analyses were performed using the Student’s t-test to compare two sample means and the analysis of variance (ANOVA) to compare more than two sample means. A p-value of less than 0.05 was considered statistically significant.
Results
Somatic Growth
The hypothyroid rats showed reduced body weight gain, delayed ear and eye opening, infantile fur and face, and delayed motor development, as described previously (Meisami, 1984; Paternostro & Meisami, 1993). As shown in Fig. 1, mean body weights in the normal controls were 88, 312 and 502 g at P25, P50 and P90 respectively; hypothyroid rats weighed 52-, 78- and 73% less than age-matched controls respectively (p<0.01). These values and deficits were similar to those previously published from our laboratory (Paternostro & Meisami, 1993). One week after PTU withdrawal, the rats of the recovery group began compensatory body growth as reported before (Meisami, 1984; Paternostro & Meisami, 1993). As a result at P50 and P90, weight deficits were reduced to 47- and 30% respectively (p<0.01) (Fig. 1). Also, as previously described previously (Meisami, 1984; Paternostro & Meisami, 1993; Tamasy, et al., 1986a,b) the recovering rats showed markedly improved motor and behavioral recovery.
Figure 1.
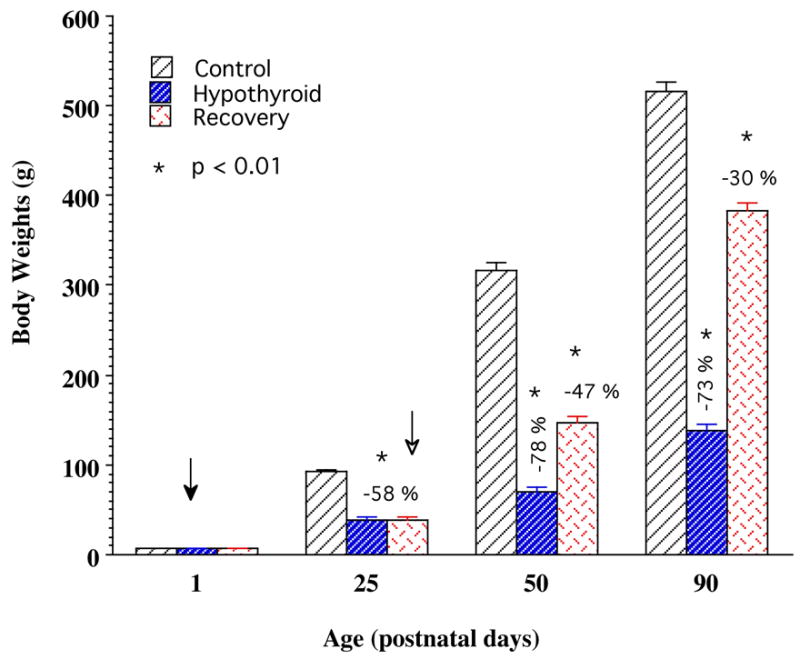
Body weights of the growing rats of the control, hypothyroid, and recovery groups at postnatal days 25, 50, and 90. Note that hypothyroid rats show significantly retarded body growth rates that persist over the entire duration of the study. Recovery rats exhibit accelerated body growth initiated after withdrawal of PTU, resulting in a marked gain in mean body weight. Bars represent mean body weights ± S.E.M. (n >10 animals at each data point). Filled arrow indicates the time of starting PTU treatment and open arrow the time of stopping PTU treatment (beginning rehabilitation).
Thyroid Hormonal Status
Results of the plasma TH levels (T4 and T3) for normal control, hypothyroid and recovery groups of growing and young adult rats are shown in Table 1 and were very similar to those reported previously (Paternostro & Meisami, 1993; Tamasy, et al., 1986a,b). Rats receiving PTU from birth showed completely suppressed plasma thyroxine (T4) levels at P25, P50 and P90 (below detection); in the recovery group (P90) these levels were restored to normal age-matched values (Table 1). As seen in the data of Table 1 and also based on previous measurements in our lab, the normalization of T4 levels occur by 2–3 weeks after withdrawal of PTU. Although T3 levels were markedly and highly significantly reduced compared to normal controls, detectable T3 levels were still present in the blood of hypothyroid rats, even though the rats showed all the somatic and behavioral signs and symptoms of experimental developmental cretinism. The T3 levels showed essentially complete recovery, similar to T4 levels, by 2–3 weeks after termination of PTU treatment and were the same as age-matched control levels by P50 and P90 (Table 1).
Table 1.
Plasma concentration of thyroid hormones (T4 and T3) in control, hypothyroid and recovery groups of growing rats at days 25, 50 and 90 postnatal.
| Age (days) | Control | Hypothyroid | Recovery |
|---|---|---|---|
| Thyroxine (T4) Levels (μg/ml) | |||
|
| |||
| 25 | 1.52 ± 0.12 | ND* | ND (est.) |
| 50 | 3.41 ± 0.45 | ND* | 2.65 ± 0.56 |
| 90 | 3.18 ± 0.27 | ND* | 3.57 ± 0.42 |
|
| |||
| Triiodothyronine (T3) Levels (ng/dl) | |||
|
| |||
| 25 | 103.2 ± 5.1 | 39.0 ± 5.9* | 39.0 (est.) |
| 50 | 73.7 ± 4.7 | 46.6 ± 5.6* | 76.0 ± 5.0 |
| 90 | 59.5 ± 3.9 | 27.1 ± 2.4* | 60.3 ± 0.4 |
Data are means ± s.e.m of hormone level in plasma; N >8 animals per mean; ND, not detectable; based on the radioimmunoassay method (see Methods); est., estimated to be same as or similar to 25-day hypothyroid value;
significant compared to control means (p<0.01); Age (days postnatal).
Two-Dimensional Regional Morphometric Maps of the Rat HC
A. Analysis of the maps based on 90-day control animals
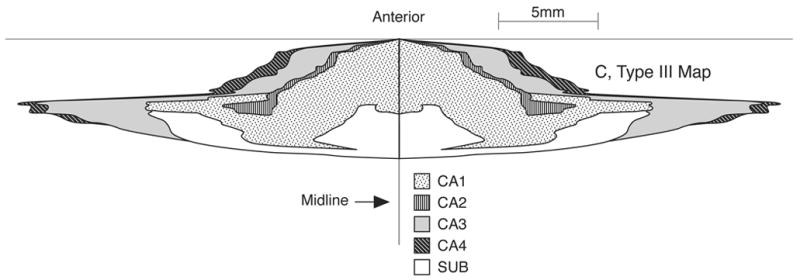
Examples of Type I, II & III, 2D fold-out morphometric maps of HC are shown in Figs. 4A–4C. Type III map (Fig. 4C), by depicting the anterior-posterior and medial-lateral dimensions of the HC on the same scale, represents a realistic 2D fold-out map of the entire cortical sheet of the HC (=PC layer of HC) and its four regions. In constructing Type III maps, data from the HC in a single hemisphere was duplicated graphically for the opposite hemisphere, in order to produce a 2D regional map of the entire rat brain HC (see Fig. 4C).
Figure 4.
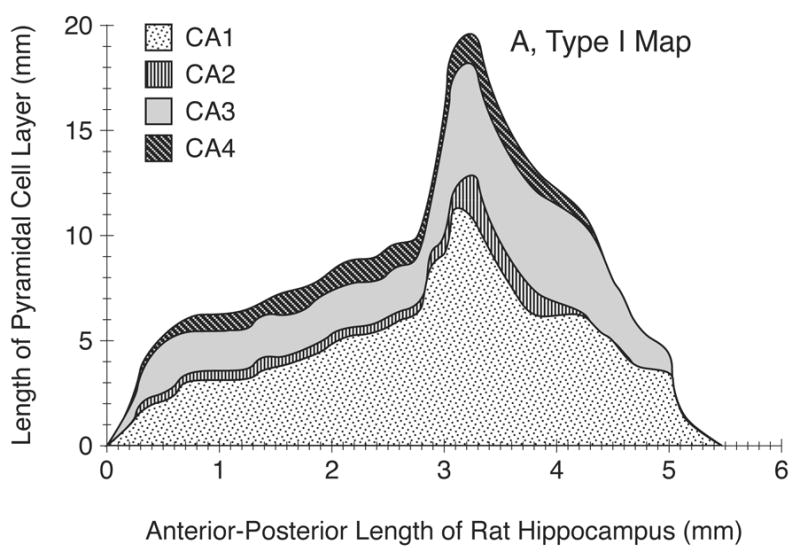
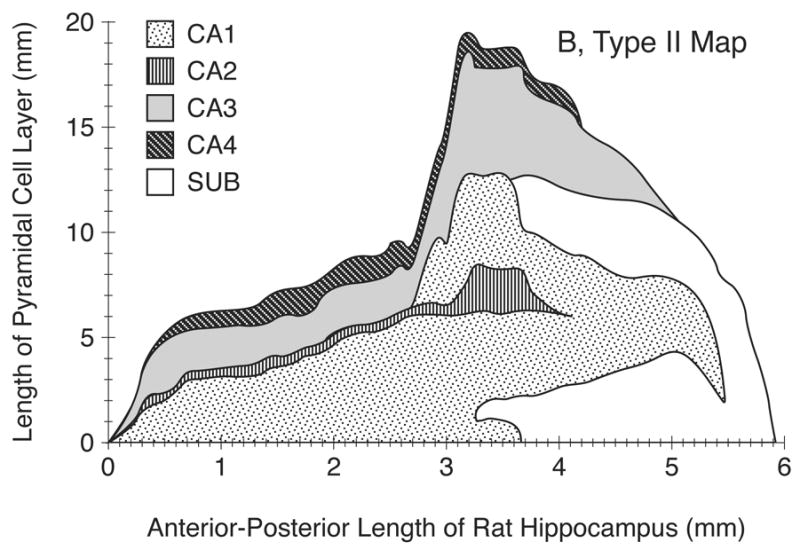
A) An example of Type I, two-dimensional (2D) unfolded map of the adult (P90) male rat hippocampus (HC) in a single hemisphere (left) including CA1–CA4 regions. In this basic and purely morphometric map, developed from a rostrocaudal coronal series, the y-axis indicates the linear extent of the unfolded HC cortex along the mediolateral plane and the HC regions are shown without regard to the position of subiculum.
B) An example of Type II 2D map of HC and its regions. This map is essentially the same as Type I (Fig. 4A) except that the interruption of the HC cortical regions by the subiculum (Sub), which begins about 2/3 of the HC rostrocaudally, are indicated, thus making it a more realistic morphometric map.
C) An example of the Type III 2D fold out map of the adult male rat HC. This is the same as type II map except that it is drawn to scale and it exhibits the true extent of surface area of HC and its regions in both left and right hemispheres. Note that HC extends several times more in the mediolateral plane than in the anterior-posterior plane; sub, subiculum.
In our samples, the HC of the P90 rat brain (representing young adult rats) typically began about 2.5 mm behind the anterior tip of the forebrain. As shown in Figs. 4A–C, the cytoarchitectonic regions of CA1–CA4 appear in sequence as one moves mediolaterally across a deconvoluted HC cortical sheet. The Type I map shows the first region encountered in coronal sections to be CA3, followed, after 0.2 mm, by CA1. Both CA1 and CA3 extend along essentially the entire A-P extent of HC. The CA3 region terminates at a point about 0.5 mm before CA1. The CA2 and CA4 regions begin at about 0.3–0.5 mm from the forebrain’s anterior tips and terminate 4 mm along the AP axis. All HC regions remain fairly constant in size through most of the dorsal HC. Type II map shows that subiculum begins at about 3.3–3.5 mm through the HC and terminates at about 8.5 mm from the forebrain’s anterior tip (not shown).
Figs. 4A–B also show a marked increase in the extent of CA1, CA2, and CA3 regions (but not CA4) at the midpoint of HC’s A-P extent (2.5 mm along the axis). This is mainly due to the emergence and inclusion of the ventral portion of the HC, which occurs mainly posteriorly.
B. 2D maps enable analysis of HC regional growth and show retarding effects of hypothyroidism
One of the advantages of Type III maps is that they allow comparison of the growth of HC regions in different directions (rostrocaudal vs. mediolateral). Comparison of the Type III maps for the control animals at P25 (Fig. 5, top) and P90 (Fig. 6 top) reveals that the HC cortical sheet grows considerably across its total surface area as well as its individual regions. Also the HC regions appear to grow more in the A-P direction than in the mediolateral directions. Comparison of Type III maps between the normal and hypothyroid animals (Figs. 5 and 6) reveal that early TH deficiency clearly diminishes the HC growth, since the overall dimensions and size of the maps are markedly reduced in the hypothyroid animals of both P25 and P90, compared to respective controls. The maps also allow comparison of the effects of early (Fig. 5) and long-term (Fig. 6) hypothyroidism (i.e., during P1–P25 and P1–P90 respectively) on growth of the individual regions of HC and show whether a particular region is selectively and adversely affected. Careful comparison of the maps of Figs. 5 and 6 shows that some areas such as CA1 and CA3 are affected more severely than others by hypothyroidism.
Figure 5.
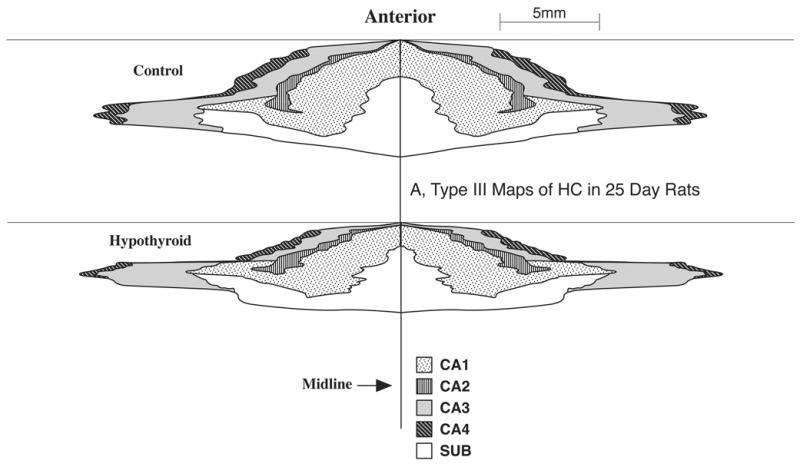
Comparison of Type III 2D fold-out maps of the regional hippocampus (HC) surface area in the P25 control (top) and hypothyroid animals (bottom). Note how the maps graphically depict the growth changes in the size of the HC cortical sheet and its constituent regions and how hypothyroidism reduces this growth markedly. The maps shown here represent the extent of the HC in both the left and right hemispheres.
Figure 6.
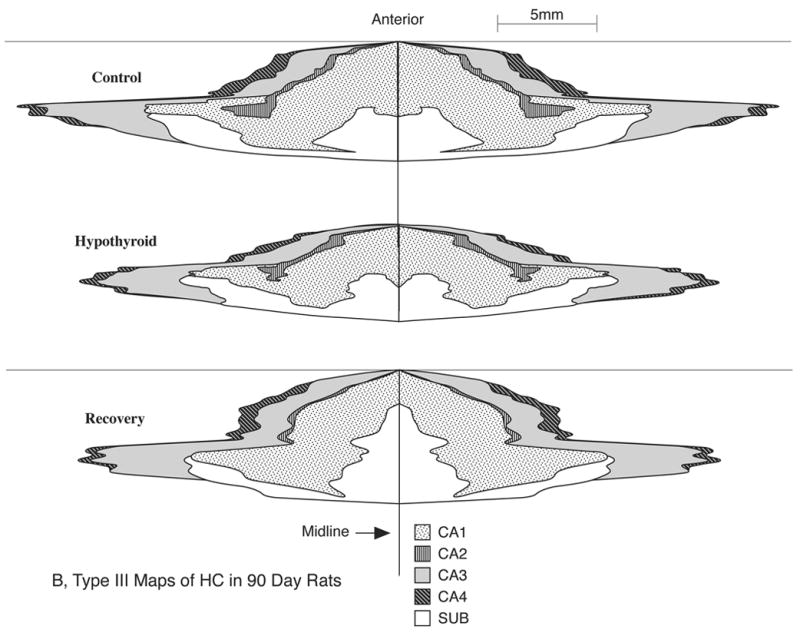
Comparison of Type III 2D fold-out maps of the regional hippocampus (HC) surface area in typical P90 control (top), hypothyroid (middle) and recovery (bottom) group male rats. Note graphic depiction of the growth changes in size of HC cortical sheet and its constituent regions and how prolonged hypothyroidism reduces this growth markedly, particularly for CA1 and CA3 regions; also note how the growth reduction is more affected in the anterior-posterior direction than in the lateral direction; note also the marked ability of the HC, particularly CA1 and CA3 regions to show recovery from the early effects of hypothyroidism. These maps represent the entire extent of the HC in both the left and right hemispheres.
C. 2D maps reveal HC growth recovery following reversal of hypothyroidism
Figure 6 compares a Type III regional map of the HC in a P90 animal of the recovery group with the age-matched control and hypothyroid animals. It is evident that both the entire pyramidal cell sheet of the HC and those of its subregions undergo a marked degree of growth recovery in animals in which euthyroid condition was restored by withdrawal of PTU. Animals recovering from the effects of early thyroid deficiency show compensatory growth in all HC regions but the CA1 and CA3 areas exhibit particularly active growth recovery. Interestingly these 2D maps reveal that the compensatory growth was more marked in the A-P direction than in the mediolateral direction (Fig. 6).
Morphometric and Quantitative Aspects
The mean total cortical surface area of the P90 normal control male rat HC (i.e. PCL surface area) was found to be just under 40 mm2 per hemisphere (Fig. 7). As shown in Table 2, CA1 and CA3 regions comprised 53% and 32% of this total respectively while CA2 and CA4 each occupied about 8%. Interestingly these proportions remained fairly similar in the various experimental and age groups (Table 2).
Figure 7.
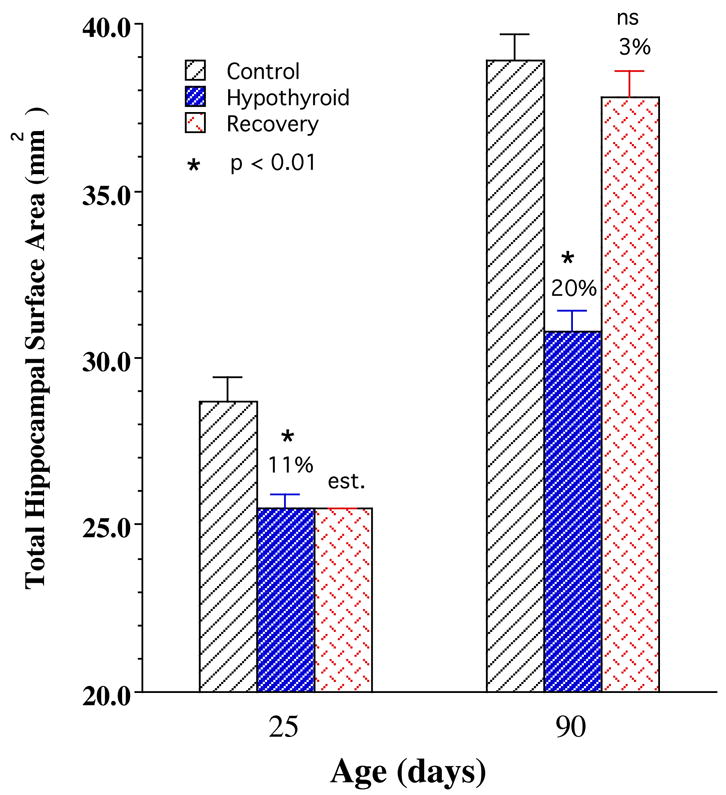
Mean total surface area values for the entire hippocampal (HC) cortical sheet (CA1+CA2+CA3+CA4)] for control, hypothyroid, and recovery groups of male rats at postnatal days 25 and 90. Note that the mean values represent those of a single HC hemisphere (left). Note also significant reductions in the 25- and 90-days hypothyroid means and how the mean values in the P90 recovery group is essentially the same as age-matched controls. Values are obtained from tracing of the length of the pyramidal cell layer (PCL) and their morphometric transformation into area values which is assumed to be equal to that of HC cortical sheet. Bars represent means ± SEM; N=15; *, p<0.01; est., “estimated”, i.e., same as hypothyroid P25 value; ns, not significant.
Table 2.
Percent amount of surface area of CA1–CA4 regions of hippocampus in the control and experimental groups relative to corresponding total HC value at the respective age.
| Relative Amounts of Surface Area of HC Regions (CA1–CA4) (% of total HC) | |||||
|---|---|---|---|---|---|
| HC Region | Control (25 day) | Control (90 day) | Hypothyroid (25 day) | Hypothyroid (90 day) | Recovery (90 day) |
| CA1 | 52 | 53 | 52 | 50 | 51 |
| CA3 | 30 | 32 | 31 | 32 | 32 |
| CA4 | 10 | 8 | 9 | 10 | 9 |
| CA2 | 8 | 7 | 8 | 8 | 7 |
CA1–CA4, specific hippocampal regions; HC, hippocampus. Values are % of total amount of surface area for each region and experimental group at the respective age (25 and 90 days). Rows show values for each HC region and are arranged in decreasing order of relative magnitude; columns show values for each experimental group.
A. Effects of hypothyroidism during the suckling period (days 1–25)
Hypothyroidism during the suckling period significantly reduced the postnatal growth of the HC cortical surface area (i.e., PCL surface area) in the developing rats (Fig. 7). Total HC surface area in the P25 hypothyroid rat was reduced by 11% (p<0.01) compared to a mean value of about 29 mm2 in the P25 controls. As shown in Fig. 8, this reduction was reflected in all four subfields of HC although to different extents: CA4, 21%, CA3, 7%, CA1, 12% (all significant, p<0.01) and CA2, 2% (not significant). Thus while CA1 and CA4 regions were most susceptible to the deleterious effects of early thyroid deficiency, CA3 was affected at a moderate level while CA2 was not affected significantly.
Figure 8.
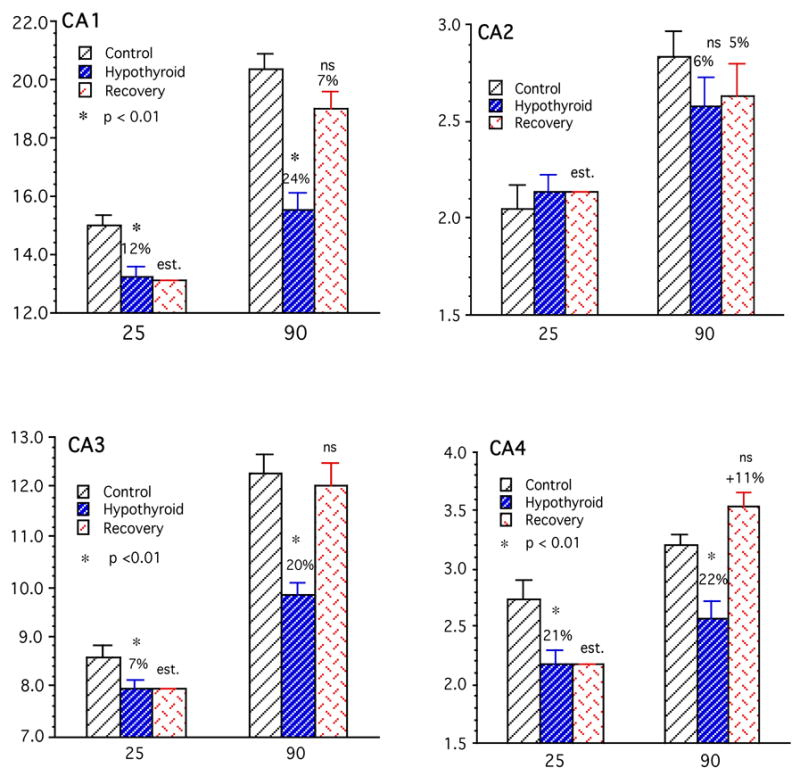
The mean surface areas (y-axis, mm2) of the cortical sheet of the individual hippocampal (HC) regions (CA1–CA4) in the control, hypothyroid and recovery groups of rats at postnatal days 25 and 90 (x-axis). The hypothyroid animals show reductions of surface area in all regions (except CA2) at both ages. Recovery group animals show markedly and significantly increased growth and expansion of their respective HC region cortical surface areas so that their mean values are not statistically different from controls by P90. Bars represent means ± SEM; N=15; *, p<0.01; est., “estimated”, i.e., same as hypothyroid P25 value; ns, not significant; y-axis, surface area (mm2); x-axis, age (days postnatal); other details same as legend to Fig. 7.
B. Normal HC growth and effects of hypothyroidism during the combined postnatal and postweaning growth (P1–P90)
Between days P25 to P90, total HC surface area in control rats increased by 33% (Table 3) to attain a mean value of about 39 mm2 at P90; this increase was only 21% in the hypothyroid animals, resulting in a significant deficit of 20% in whole HC surface area (p<0.01) (Fig. 7). Between P25 to P90 all regions of HC in control rats showed significant growth: highest growth occurred in CA3 region (41%) followed by CA1 (33%), CA2 (27%), and CA4 18% (Fig. 8 and Table 3). The HC regions of the hypothyroid animals also showed growth but at much lower rates: CA1, CA3 and CA4 showed nearly half as much growth compared to controls while the CA2 region was very little affected. Figure 8 and Table 3 indicate growth deficits in surface area of HC regions. As a result, compared to P90 controls, HC regions of P90 hypothyroid animals showed marked and significant growth deficits. The CA3, CA4 and CA1 were most severely affected (20–24%, p<0.01) while CA2 was least affected (6%, not significant).
Table 3.
Changes in amount of growth of surface area in the whole HC and its specific regions (CA1–CA4) between 25 day to 90 days of age shown as growth ratios
| Growth Ratios of Surface Areas of HC Regions (CA1–CA4) (90 day mean/25 day mean) | |||
|---|---|---|---|
| HC Region | Control | Hypothyroid | Recovery |
| CA1 | 1.36 | 1.17 | 1.44 |
| CA2 | 1.46 | 1.21 | 1.23 |
| CA3 | 1.47 | 1.24 | 1.51 |
| CA4 | 1.18 | 1.19 | 1.63 |
| Total HC | 1.35 | 1.21 | 1.45 |
Values are ratios of 90 day means over 25 means for each group. Rows show values for each HC region, columns for each experimental group; CA–CA, specific hippocampal regions; HC, hippocampus.
C. Recovery of regional HC surface areas following restoration TH
As shown Fig. 7, the mean total surface area of the HC in the P90 rehabilitated animals was about 38 mm2, only 3% less than the control P90 value (not significant), indicating that this parameter shows essentially complete recovery from hypothyroid retardation in the animals of the recovery group. The increase in growth of total HC surface area between P25 and P90 for the recovery group was 48%, that is 33% more than controls and 2.3 x more than hypothyroid rats (Table 3). This rapid growth rate may explain how the recovering animals were able to show essentially catch-up growth.
This remarkable ability to recover growth deficit in surface area expansion was manifested at the individual regions of HC as well. Thus the CA1, CA3 and CA4 showed high compensatory growth rates while that of CA2 was less marked (see Table 3). Growth ratios, defined as ratios of 90 day/25 day values, were 1.63 and 1.51 times for CA4 and CA3 and 1.44 and 1.23 times for CA1 and CA2 respectively (Table 3). As a result of these intense growth rates, by age 90-days the mean surface areas of the CA1–CA4 regions of the rehabilitated rats were not significantly different from those in age-matched (P90) control rats. In fact mean CA4 surface area in the rehabilitated rats was actually higher by 11% than the age-matched controls, although not significantly (Fig. 8).
Discussion
Useful Features of Regional 2D Maps
Despite its convoluted appearance and complex 3D structure (Amaral & Witter, 1989), the HC may be considered as a relatively simple infolded cortical sheet of cytoarchitecturally distinct neurons, well suited for folded out 2D representations. Our 2D maps can be made using relatively simple plotting software like SigmaPlot and can provide useful visual and graphic means for regional analysis of surface area and topology along the A-P axis and in the medial-lateral direction. Thus the properly scaled Type III map (Fig. 4C) shows that the HC extends nearly four times more in the mediolateral plane than in the AP plane. The maps can also be extended to describe topological relation between HC and its inter-communicating structures like the dentate gyrus and subiculum.
To test the accuracy of our delineation of HC regions (CA1–CA3) and estimates of surface areas, we traced the HC regions from the coronal sections of the stereotaxic atlas of the rat brain by Paxanos and Watson (1986) and calculated the surface areas for these according to our methods and found a value of 37.8 mm2 for the entire HC and 19.3-, 3.0 - and 15.5 mm2 for CA1, CA2 and CA3 respectively. These results vary from our estimates by only about 2–3%. Our morphometric maps clearly demonstrate the marked variation in surface area in the different regions of the adult rat HC. The largest region is CA1, with over one-half of the total surface area; CA1 and CA3 together account for about 85% of the total surface area (see Table 2). Although CA2 and CA4 regions are small compared to CA1 and CA3, their total surface area (15%) warrant a closer attention to the contribution of these regions to HC function. Swanson et al. (1978) and Gaarskjaer (1978) developed the first unfolded and extended maps of the rat HC (see also Swanson, 1998).
Swanson et al. (1978) constructed a 3D, large-scale model and realigned tracings of the cell layer in frontal sections in such a way as to provide a representation of the HC as longitudinal cortical fields in a septo-temporal direction. Our 2D maps attempt to unfold, flatten and extend the HC using the midline of the brain as a reference point. Unfolding begins from the ventral surface towards the dorsal surface, resulting in a map where HC of each hemisphere is extended and flattened across the medio-lateral plane. Our maps, being morphometric in essence, do not attempt to reconstruct the HC but unfold it in order to show the details of the cortical sheet (surface area) and the relative proportions and the topographic relations of the various HC regions.
Due to the relatively simple methods used in their construction, our morphometric maps may be applicable in regional studies of HC in experimental animals. These studies have indicated regional differences in metabolism, function and vulnerability among the HC regions. In short, our 2D, fold out, morphometric maps of the HC are useful means of visualizing and measuring the relative size and complexity of the various HC regions. These maps provide further analytic tools to demonstrate, demarcate and measure regional changes during normal and abnormal development and aging, and in experimental regional studies on cell death, lesioning, electrophysiology and functional mapping of the rat HC.
Regional Changes in Morphology and Surface Area of the HC in Experimental Conditions
Early hypothyroidism causes gross morphological alterations in the brain. Growth in the anterior-posterior (or rostral-caudal) axis of the forebrain appears to be severely restricted in hypothyroid rats (Eayrs & Taylor, 1951). Rabie et al. (1979) also found that both forebrain and hippocampal length was decreased in neonatal hypothyroidism. Our morphometric study confirms and extends these observations regarding HC length. However, Rabie et al. (1979) found that neonatal hypothyroidism had no significant effect on height or width of the HC and HC length remained markedly reduced in rehabilitated animals.
Our data however shows considerable longitudinal growth of the HC during the rehabilitation phase. The observed changes in the gross HC morphology of the hypothyroid and recovering animals may be a consequence of differential thyroid hormonal effects on the different hippocampal areas (i.e. dorsal versus ventral portions of the HC). On the other hand, these unique morphological changes in the brain may be an indirect consequence of thyroid hormone effects on skull growth. Characteristic craniofacial malformations occur in neurological cretinism including, midface hypoplasia, vertical displacement of the cranial base, flattened frontal bones, and diminutive nasal bones, leading to infantile appearance of the head (Israel et al., 1983). Also craniosynostosis, or early closure of the cranial sutures has been implicated in maternal thyroid status (Cohen, 1988). Akita et al. (1994) noted that congenital or juvenile hyperthyroidism accelerates skeletal bone formation leading to premature closure of the calvarial sagittal suture and excess thyroxine lead to prominently decreased size in the left-right direction of the skull. Whether or not the increased thyroxine levels during the rehabilitation stages of our study lead to premature closure of the cranial sutures, and thus restrict brain growth, is not known.
Selective Vulnerability of the CA1–CA4 Regions to Neurological Insult
Experimental and clinical findings have noted selective vulnerability of different hippocampal neuronal populations to various neurological pathologies. These include transient cerebral ischemia (Bothe et al., 1986; Zola-Morgan et al., 1986), neurodegenerative disease states, human immunodeficiency virus encephalitis (Masliah et al., 1992) and mild concussive brain injury (Nawashiro et al., 1995; Smith et al., 1994). Others have implicated altered hormonal status as a causal factor in the differential degeneration of HC neurons (Gould et al., 1991; Lauder, 1983). Sloviter et al. (1989) found that adrenalectomy and consequent reductions of corticosterone in adult rats lead to selective loss of dentate granule cells. Alternatively, prolonged exposure to excess corticosteroids can cause degeneration of CA3 pyramidal neurons (Sapolsky et al., 1985).
Our morphometric measurements also indicate selective and differential vulnerability of the various HC regions to early and prolonged hypothyroidism. Thus the CA4 surface area was most adversely affected by early hypothyroidism (postnatal days 1–25), while long-term hypothyroidism (days 1–90 postnatal) seems to greatly affect the CA1 region. The CA1 and CA4 regions are particularly vulnerable to neuronal injury caused by ischemia, epileptiform activity, and hypoglycemia (Griffiths et al., 1984; Schmidt-Kaster & Freund, 1991; Schwartzkroin & Wyler, 1980; Vornov et al., 1991; Wieloch et al., 1985). The CA3 region was also decreased in surface area, albeit to lesser degree than in the CA1 and CA4 regions. The CA2 region does not show a significant change in surface area in early or in long-term hypothyroidism. It is noteworthy that the CA4 region of the HC (dentate hilar region) is most adversely affected by early hypothyroidism (postnatal days 1–25). This may, in part, be due to the fact that this region is the latest to develop during the postnatal period (Bayer, 1980).
The cellular bases of the observed morphometric changes are not clear and will require studies on changes in the neuronal cell size, density and number as well as dendritic parameters in the CA1–CA4 regions to determine the underlying changes in surface area. Rami et al. (1986) showed morphological alterations in the pyramidal cells of the HC as a consequence of early hypothyroidism but the reversibility of these effects upon rehabilitation has not been studied.
Prospects for Rehabilitation from Hypothyroid Brain Retardation
Recovery of surface area was documented in all regions that were affected by early hypothyroidism, indicating a strong potential for structural recovery of HC and its regions from developmental growth retardation induced by early postnatal hypothyroidism. Previous studies by Meisami and collaborators have shown that rats have a remarkable ability to spontaneously recover somatic growth from PTU-induced growth retardation (Meisami, 1984; Paternostro & Meisami, 1993; Tamasy et al., 1986a,b). In fact, female rats recovering from hypothyroidism can completely catch up in body growth to the level of age-matched controls, by age 90 days postnatal. Recovering male rats, however, were not able to fully recover to the level of the body weights of normal control rats, despite the fact that they showed higher growth rates than their female counterparts (Meisami, 1984).
It is a widely held belief that permanent deficits in growth are an inevitable outcome of early brain retardation (Dobbing, 1974; Dobbing & Smart, 1974; Brazier, 1975; Meisami & Brazier, 1979; Meisami & Timiras, 1982; Meisami & Timiras, 1988b; Timiras, 1972). Compensatory or “catch-up” growth following transient growth retardation has long been recognized both in clinical medicine and experimental animal studies (for review see Moiser, 1986; Smart et al. 1977). Attempts to “catch-up” growth of body and brain weight during the postnatal phase have been observed in response to a divergent group of adverse insults during the perinatal period. These include under- and malnutrition, cerebral ischemia, cranial x-irradiation, and exposure to teratogens and drugs (Beach et al., 1982; Clayton et al., 1988; Groziak et al., 1984; Meyer et al., 1990; Slotkin et al., 1986; Weichsel & Clark, 1977; Williams & Hughes, 1978). Once the growth-retarding agent is removed there is resumption of growth rather than a rebound of growth activity. Complete catch up growth may or may not occur depending on the kind and intensity of the growth retarding effect.
Examination of hippocampal growth ratios in our study however, reveals that the brain, in fact, shows accelerated growth velocity between postnatal day 25 and 90 rather than continued growth (see Table 3). Thus the rate of neural growth following removal of PTU far surpasses that of normal development. The high growth ratio, or rate of growth between 25 days and 90 days in the recovery rat HC indicates that the central nervous system has a great deal of plasticity in the developing hypothyroid animals. Analysis of HC cortical surface area indicates that the hippocampal formation, in particular, has a substantial capacity for renewed growth after the so-called “critical period” of development in the rat (postnatal days 1–25). This plasticity may be unique to only a few regions of the rat brain. For example, unpublished data from our laboratory concerning the weights of the cerebellum in similarly treated rehabilitating animals indicate that cerebellar recovery is far from complete by postnatal day 90.
The HC is a brain structure with marked plasticity possibly due to its unique role in learning and memory functions (Cohen & Eichenbaum, 1993; Holscher, 2003; Olton et al., 1979; Rawlins, 1985; Zola-Morgan, et al., 1986). Our results show that HC is an excellent model to study developmental plasticity and recovery from neurological insult; they also question the widely accepted notion that thyroid hormone deficiency imparts permanent damage to the developing brain unless replacement therapy is initiated early on. Our results point to considerable structural and functional plasticity of the brain after the critical period of brain development and suggest that ideas concerning this critical period should be re-evaluated. These studies also bring to light the extraordinary ability of the central nervous system to grow during the postweaning period at a pace that far exceeds normal developmental parameters.
Further studies are needed to analyze the cellular basis and possible mechanisms of this accelerated neural growth and the prospects of HC plasticity and potential for recovery. In a companion quantitative study (Farahvar et al., 2006) on serial sections of the rat hippocampal formation stained with cytochrome oxidase we found significant reductions in laminar volumes and staining density in the postnatal hypothyroid rats. Similar to the results of this study, the effects on the laminar volumes and cytochrome oxidase activity were also largely reversed at later ages upon withdrawal of PTU and recovery of the rats. Furthermore preliminary results from our lab on volumes of whole HC and its individual regions (CA1–CA4) reveal results which are similar to those found for the surface area, indicating that the observed changes in the hypothyroid and recovery group rats are not limited to the surface area of the cortical sheet of the HC and occur in other dimensions of the HC cortex as well.
One mechanism which may explain the accelerated growth of hippocampal formation in the recovery animals may be increased neurogenesis which is known to occur in the postnatal and adult HC; indeed there is evidence that this proliferation is stimulated by thyroid hormones and retarded in their absence (Ambrogini et al., 2005; Desouza et al., 2005; Uchida et al., 2005). However, active neuronal proliferation is mainly restricted to the dentate gyrus of the hippocamus and is known to occur in the HC proper where our results were obtained.
A second and more plausible explanation of the marked recovery in HC growth may involve the neurotrophins. Given the important role of neurotrophins (e.g. nerve growth factory, NGF) in early brain development and their suggested interaction with thyroid hormones (Clos & Legrand, 1990; Roskoden et al., 1999), we suggest that NGF and its receptors may play a key role in this accelerated neural growth process. Studies from our laboratory have indicated marked changes in NGF and its receptor (p75) during developmental hypothyroidism as well as in the recovery process (Farahvar & Meisami, 1994, 1995; Sendera & Meisami, 1995; Sendera, 1997; Smith et al., 2002). Further work on the possible involvement of neurotrophins and other growth factors in the recovery process can yield greater insight into developmental plasticity following brain retardation as well as plasticity following many other types of neurological insult.
Acknowledgments
This work was supported by funds from the NIH grant (GM07143) and the Research Funds of the University of Illinois. Dr. Arash Farahvar was a recipient of NIH, SITG (Systems & Integrative Biology Training Grant) predoctoral fellowship. Preliminary reports of this study were presented at the previous meetings of the Society for Neuroscience. The authors are grateful to Prof. Michael Gabriel of the University of Illinois for constructive suggestions and to Mona Meisami for the graphic designs of the morphometric maps presented in this paper.
Abbreviations
- 2D
two-dimensional
- HC
hippocampus
- P
postnatal
- PCL
pyramidal cell layer
- PTU
n-propylthiouracil
- T3
triiodothyronine
- T4
thyroxine, tetraiodothyronine
- TH
thyroid hormones
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Akita S, Nakamura T, Hirano A, Fujii T, Yamashita S. Thyroid hormone action on rat calvarial sutures. Thyroid. 1994;4:99–106. doi: 10.1089/thy.1994.4.99. [DOI] [PubMed] [Google Scholar]
- Alzoubi KH, Gerges NZ, Alkadhi KA. Levothyroxin restores hypothyroidism-induced impairment of LTP of hippocampal CA1: electrophysiological and molecular studies. Exp Neurol. 2005;195:330–341. doi: 10.1016/j.expneurol.2005.05.007. [DOI] [PubMed] [Google Scholar]
- Amaral DG, Witter MP. The three-dimensional organization of the hippocampal formation: A review of anatomical data. Neuroscience. 1989;31:571–591. doi: 10.1016/0306-4522(89)90424-7. [DOI] [PubMed] [Google Scholar]
- Ambrogini P, Cuppini R, Ferri P, Mancini C, Ciaroni S, Voci A, Gerdoni E, Gallo G. Thyroid hormones affect neurogenesis in the dentate gyrus of adult rat. Neuroendocrinology. 2005;81:244–253. doi: 10.1159/000087648. [DOI] [PubMed] [Google Scholar]
- Alvarez-Dolado M, Figueroa A, Kozlov S, Sonderegger P, Furley AJ, Munoz A. Thyroid hormone regulates TAG-1 expression in the developing rat brain. Eur J Neurosci. 2001;14:1209–1218. doi: 10.1046/j.0953-816x.2001.01745.x. [DOI] [PubMed] [Google Scholar]
- Bayer SA. Development of the hippocampal region in the rat. II. Morphogenesis during embryonic and early postnatal life. J Comp Neurol. 1980;190:115–134. doi: 10.1002/cne.901900108. [DOI] [PubMed] [Google Scholar]
- Bayer SA. Hippocampal regions. In: Paxinos G, editor. The Rat Nervous System. Academic Press; Sydney: 1985. pp. 335–352. [Google Scholar]
- Beach RS, Gershwin ME, Hurley LS. Reversibility of development retardation following murine fetal zinc deprivation. J Nutr. 1982;112:1169–1181. doi: 10.1093/jn/112.6.1169. [DOI] [PubMed] [Google Scholar]
- Bernal J. Action of thyroid hormone in brain. J Endocrinol Invest. 2002;25:268–288. doi: 10.1007/BF03344003. [DOI] [PubMed] [Google Scholar]
- Bothe HW, Bosma HJ, Hofer H, Hossmann KA, Angermeier WF. Selective vulnerability of hippocampus and disturbances of memory storage after mild unilateral ischemia of gerbil brain. Stroke. 1986;17:1160–1163. doi: 10.1161/01.str.17.6.1160. [DOI] [PubMed] [Google Scholar]
- Brazier MAB. Growth and development of the brain: nutritional, genetic, and environmental factors, International Brain Research Organization Monograph series. Vol. 1. Raven Press; New York: 1975. [Google Scholar]
- Clayton PE, Shalet SM, Price DA. Growth response to growth hormone therapy following cranial irradiation. Europ J Pediat. 1988;147:593–596. doi: 10.1007/BF00442470. [DOI] [PubMed] [Google Scholar]
- Clos J, Legrand C. An interaction between thyroid hormone and nerve growth factor promotes the development of hippocampus, olfactory bulbs and cerebellum: a comparative biochemical study of normal and hypothyroid rats. Growth Factors. 1990;3:205–220. doi: 10.3109/08977199009043905. [DOI] [PubMed] [Google Scholar]
- Cohen MM., Jr Craniosynostosis update. Am J Med Genet. 1988;4(Suppl):99–148. doi: 10.1002/ajmg.1320310514. [DOI] [PubMed] [Google Scholar]
- Cohen NJ, Eichenbaum H. Memory, Amnesia, and the Hippocampal System. MIT Press; Cambridge: 1993. [Google Scholar]
- Delange F. The disorders induced by iodine deficiency. Thyroid. 1994;4:107–128. doi: 10.1089/thy.1994.4.107. [DOI] [PubMed] [Google Scholar]
- Desouza LA, Ladiwala U, Daniel SM, Agashe S, Vaidya RA, Vaidya VA. Thyroid hormone regulates hippocampal neurogenesis in the adult rat brain. Mol Cell Neurosci. 2005;29:414–426. doi: 10.1016/j.mcn.2005.03.010. [DOI] [PubMed] [Google Scholar]
- Diamond MC, Johnson RE, Young D, Singh SS. Age-related morphologic differences in the rat cerebral cortex and hippocampus: male-female; right-left. Exp Neurol. 1983;81:1–13. doi: 10.1016/0014-4886(83)90153-x. [DOI] [PubMed] [Google Scholar]
- Dobbing J. The later growth of the brain and its vulnerability. Pediatrics. 1974;53:2–6. [PubMed] [Google Scholar]
- Dobbing J, Smart JL. Vulnerability of developing brain and behaviour. Br Med Bull. 1974;30:164–168. doi: 10.1093/oxfordjournals.bmb.a071188. [DOI] [PubMed] [Google Scholar]
- Dong J, Yin H, Liu W, Wang P, Jiang Y, Chen J. Congenital iodine deficiency and hypothyroidism impair LTP and decrease C-fos and C-jun expression in rat hippocampus. Neurotoxicology. 2005;26:417–426. doi: 10.1016/j.neuro.2005.03.003. [DOI] [PubMed] [Google Scholar]
- Dussault JH, Ruel J. Thyroid hormones and brain development. Annu Rev Physiol. 1987;49:321–334. doi: 10.1146/annurev.ph.49.030187.001541. [DOI] [PubMed] [Google Scholar]
- Eayrs JT. The cerebral cortex of normal and hypothyroid rats. Acta Anat. 1955;25:160–183. doi: 10.1159/000141068. [DOI] [PubMed] [Google Scholar]
- Eayrs JT. Scientific Basis of Medicine, Annual Review. Athlone Press; London: 1966. Thyroid and central nervous development; pp. 317–339. [PubMed] [Google Scholar]
- Eayrs JT, Taylor SH. The effect of thyroid deficiency induced by methyl thiouracil on the maturation of the central nervous system. J Anat. 1951;35:350–358. [PMC free article] [PubMed] [Google Scholar]
- Farahvar A, Meisami E. Changes in levels of low affinity NGF receptor in hippocampal and forebrain cholinergic systems of developing hypothyroid and rehabilitated rats. Soc Neurosci Abst. 1994;20:34. [Google Scholar]
- Farahvar A, Meisami E. NGF-Receptor immunocytochemistry provides evidence for increased neuronal processes in basal forebrain cholinergic neurons of rats recovering from early hypothyroid brain retardation. Soc Neurosci Abst. 1995;21:2014. [Google Scholar]
- Farahvar A, Darwish N, Sladek S, Meisami E. Quantitative cytochrome oxidase histochemistry and morphometry reveal recovery of functional metabolic activity and laminar volumes in the rat hippocampus and dentate gyrus following postnatal hypothyroid retardation. 2006 doi: 10.1016/j.expneurol.2006.12.019. Submitted Concurrently to Experimental Neurology. [DOI] [PubMed] [Google Scholar]
- Farbman AI. Cell Biology of Olfaction. Cambridge University Press; Cambridge, UK: 1992. [Google Scholar]
- Gaarskjaer FB. Organization of the mossy fiber system of the rat studied in extended hippocampi. J Comp Neurol. 1978;178:49–71. doi: 10.1002/cne.901780104. [DOI] [PubMed] [Google Scholar]
- Gerges NZ, Alkadhi KA. Hypothyroidism impairs late LTP in CA1 region but not in dentate gyrus of the intact rat hippocampus: MAPK involvement. Hippocampus. 2004;14:40–45. doi: 10.1002/hipo.10165. [DOI] [PubMed] [Google Scholar]
- Gilbert ME. Alterations in synaptic transmission and plasticity in area CA1 of adult hippocampus following developmental hypothyroidism. Dev Brain Res. 2004;148:11–18. doi: 10.1016/j.devbrainres.2003.09.018. [DOI] [PubMed] [Google Scholar]
- Gilbert ME, Paczkowski C. Propylthiouracil (PTU)-induced hypothyroidism in the developing rat impairs synaptic transmission and plasticity in the dentate gyrus of the adult hippocampus. Dev Brain Res. 2003;145:19–29. doi: 10.1016/s0165-3806(03)00191-3. [DOI] [PubMed] [Google Scholar]
- Griffiths T, Evans MC, Meldrum BS. Status epilepticus: the reversibility of calcium loading and acute neuronal pathological changes in the rat hippocampus. Neuroscience. 1984;12:557–567. doi: 10.1016/0306-4522(84)90073-3. [DOI] [PubMed] [Google Scholar]
- Groziak S, Kirksey A, Hamaker B. Effect of maternal vitamin B-6 restriction on pyridoxal phosphate concentrations in developing regions of the central nervous system in rats. J Nutr. 1984;114:727–732. doi: 10.1093/jn/114.4.727. [DOI] [PubMed] [Google Scholar]
- Gould E, Woolley CS, McEwen BS. The hippocampal formation: morphological changes induced by thyroid, gonadal and adrenal hormones. Psychoneuroendocrinology. 1991;16:67–84. doi: 10.1016/0306-4530(91)90071-z. [DOI] [PubMed] [Google Scholar]
- Holscher C. Time, space and hippocampal functions. Rev Neurosci. 2003;14:253–284. doi: 10.1515/revneuro.2003.14.3.253. [DOI] [PubMed] [Google Scholar]
- Israel H, Johnson GF, Fierro-Benitez R. Craniofacial malformations among endemic cretins in Ecuador. J Craniofac Genet Dev Biol. 1983;3:3–10. [PubMed] [Google Scholar]
- Lauder JM. Hormonal and humoral influences on brain development. Psychoneuro-endocrinology. 1983;8:121–155. doi: 10.1016/0306-4530(83)90053-7. [DOI] [PubMed] [Google Scholar]
- Lauder JM, Krebs H. Do neurotransmitters, neurohormones and hormones specify critical periods? In: Greenough WT, Juraska JM, editors. Developmental Neuropsychobiology. Academic Press; New York: 1986. pp. 119–175. [Google Scholar]
- Lauder JM, Mugnaini E. Early hyperthyroidism alters the distribution of mossy fibers in the rat hippocampus. Nature. 1977;268:335–337. doi: 10.1038/268335a0. [DOI] [PubMed] [Google Scholar]
- Lauder JM, Mugnaini E. Infrapyramidal mossy fibers in the hippocampus of the hyperthyroid rat. A light and electron microscopic study. Dev Neurosci. 1980;3:248–265. doi: 10.1159/000112397. [DOI] [PubMed] [Google Scholar]
- Legrand J. Thyroid hormone effects on growth and development. In: Hennemann G, editor. Thyroid Hormone Metabolism. Marcel Dekker, Inc; New York: 1986. pp. 503–534. [Google Scholar]
- Lorente de Nó R. Studies on the structure of the cerebral cortex: continuation of the study of the Ammonic system. J Psychol Neurol. 1934;46:113–177. [Google Scholar]
- Martinez B, del Hoyo P, Martin MA, Arenas J, Perez-Castillo A, Santos A. Thyroid hormone regulates oxidative phosphorylation in the cerebral cortex and striatum of neonatal rats. J Neurochem. 2001;78:1054–1063. doi: 10.1046/j.1471-4159.2001.00487.x. [DOI] [PubMed] [Google Scholar]
- Matos JA, Bruno AN, Oses JP, Bonan CD, Battastini AM, Barreto-Chaves ML, Sarkis JJ. In vitro effects of thyroid hormones on ectonucleotidase activities in synaptosomes from hippocampus of rats. Cell Mol Neurobiol. 2002;22:345–32. doi: 10.1023/A:1020776119612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meaney MJ, Diorio J, Francis D, Weaver S, Yau J, Chapman K, Seckl JR. Postnatal handling increases the expression of cAMP-inducible transcription factors in the rat hippocampus: the effects of thyroid hormones and serotonin. J Neurosci. 2000;20:3926–3935. doi: 10.1523/JNEUROSCI.20-10-03926.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meisami E. Complete recovery of growth deficits after reversal of PTU induced postnatal hypothyroidism in the female rat: A model for catch up growth. Life Sci. 1984;34:1487–1496. doi: 10.1016/0024-3205(84)90064-x. [DOI] [PubMed] [Google Scholar]
- Meisami E, Brazier MAB. Neural Growth and Differentiation, International Brain Research Organization Monographs. Vol. 5. Raven Press; New York: 1979. [Google Scholar]
- Meisami E, Sendera TJ. Morphometry of rat olfactory bulbs stained for cytochrome oxidase reveals that the entire population of glomeruli forms early in the neonatal period. Develop Brain Res. 1993;71:253–257. doi: 10.1016/0165-3806(93)90177-c. [DOI] [PubMed] [Google Scholar]
- Meisami E, Timiras PS. Normal and abnormal brain biochemical development after birth. In: Jones C, editor. Biochemical Development of the Fetus and Neonate. Elsevier; Amsterdam: 1988a. pp. 757–819. [Google Scholar]
- Meisami E, Timiras PS, editors. Handbook of Human Growth and Developmental Biology. 1, Part C Factors Influencing Brain Development. CRC Press; Boca Raton, Florida: 1988b. [Google Scholar]
- Meyer LS, Kotch LE, Riley EP. Neonatal ethanol exposure: functional alterations associated with cerebellar growth retardation. Neurotoxicol Teratol. 1990;12:15–22. doi: 10.1016/0892-0362(90)90107-n. [DOI] [PubMed] [Google Scholar]
- Moiser HD. The control of catch-up growth. Acta Endocrin. 1986;279(Suppl):1–7. doi: 10.1530/acta.0.112s001. [DOI] [PubMed] [Google Scholar]
- Monti-Graziadei GA, Graziadei PPC. Studies on neuronal plasticity and regeneration in the olfactory system: morphological and functional characteristic of the olfactory sensory neurons. In: Meisami E, Brazier MAB, editors. Neural Growth and Differentiation. Raven Press; New York: 1979. pp. 373–396. [Google Scholar]
- Morreale de Escobar G, Obregon MJ, Escobar del Rey F. Role of thyroid hormone during early brain development. Eur J Endocrinol. 2004;151(Suppl 3):U25–37. doi: 10.1530/eje.0.151u025. [DOI] [PubMed] [Google Scholar]
- Nawashiro H, Shima K, Chigasaki H. Selective vulnerability of hippocampal CA3 neurons to hypoxia after mild concussion in the rat. Neurol Res. 1995;17:455–460. [PubMed] [Google Scholar]
- Olton DS, Becker JT, Handelmann GE. Hippocampus, space, and memory. Behav Brain Sci. 1979;2:313–365. [Google Scholar]
- Paternostro M, Meisami E. Developmental plasticity of the rat olfactory receptor sheet as shown by complete recovery of surface area and cell number from early hypothyroid growth retardation. Develop Brain Res. 1993;76:151–161. doi: 10.1016/0165-3806(93)90203-m. [DOI] [PubMed] [Google Scholar]
- Paternostro MA, Meisami E. Marked restoration of density and number of mature (knob-bearing) olfactory receptor neurons in rats recovering from hypothyroid-induced growth retardation. Develop Brain Res. 1996;96:173–183. doi: 10.1016/0165-3806(96)00110-1. [DOI] [PubMed] [Google Scholar]
- Paxanos G, Watson C. The Rat Brain in Stereotaxic Coordinates. 2 and 3. Academic Press; San Diego: 1986 1997. [Google Scholar]
- Rabie A, Patel AJ, Clavel MC, Legrand J. Effect of thyroid deficiency on the growth of the hippocampus in the rat. A combined biochemical and morphological study. Develop Neurosci. 1979;2:183–194. doi: 10.1159/000112453. [DOI] [PubMed] [Google Scholar]
- Rami A, Rabie A. Effect of thyroid deficiency on the development of glia in the hippocampal formation of the rat: an immunocytochemical study. Glia. 1988;1:337–345. doi: 10.1002/glia.440010506. [DOI] [PubMed] [Google Scholar]
- Rami A, Patel AJ, Rabie A. Thyroid hormone and development of the rat hippocampus: morphological alterations in granule and pyramidal cells. Neuroscience. 1986;19:1217–1226. doi: 10.1016/0306-4522(86)90135-1. [DOI] [PubMed] [Google Scholar]
- Rawlins JPN. Associations across time: The hippocampus as a temporal memory store. Behav Brain Sci. 1985;8:479–496. [Google Scholar]
- Santisteban P, Bernal J. Thyroid development and effect on the nervous system. Rev Endocr Metab Disord. 2005;6:217–228. doi: 10.1007/s11154-005-3053-9. [DOI] [PubMed] [Google Scholar]
- Sapolsky RM, Krey LC, McEwen BS. Prolonged glucocorticoid exposure reduces hippocampal neuron number: implications for aging. J Neurosci. 1985;5:1222–1227. doi: 10.1523/JNEUROSCI.05-05-01222.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt-Kastner R, Freund TF. Selective vulnerability of the hippocampus in brain ischemia. Neuroscience. 1991;40:599–636. doi: 10.1016/0306-4522(91)90001-5. [DOI] [PubMed] [Google Scholar]
- Sendera TJ, Meisami E. Altered levels of NGF & NGF-receptor in olfactory bulbs of developing hypothyroid and recovering rats. Chemical Senses. 1995;20:776–777. [Google Scholar]
- Sendera TJ. PhD thesis. University of Illinois; Urbana-Champaign: 1997. Interactions of thyroid hormones, nerve growth factor and its receptors in development and plasticity of the rat olfactory bulb; p. 125. [Google Scholar]
- Slotkin TA, Cowdery TS, Orband L, Pachman S, Whitmore WL. Effects of neonatal hypoxia on brain development in the rat: immediate and long-term biochemical alterations in discrete regions. Brain Res. 1986;374:63–74. doi: 10.1016/0006-8993(86)90395-1. [DOI] [PubMed] [Google Scholar]
- Sloviter RS, Valiquette G, Abrams GM, Ronk EC, Sollas AL, Paul LA, Neubort S. Selective loss of granule cells in the mature rat brain after adrenalectomy. Science. 1989;243:535–538. doi: 10.1126/science.2911756. [DOI] [PubMed] [Google Scholar]
- Smart JL, Byrne EA, Dobbing J. New thoughts on catch-up growth. Proc Nutr Soc. 1977;36:99a. [PubMed] [Google Scholar]
- Smith DH, Lowenstein DH, Gennarelli TA, McIntosh TK. Persistent memory dysfunction is associated with bilateral hippocampal damage following experimental brain injury. Neurosci Lett. 1994;168:151–154. doi: 10.1016/0304-3940(94)90438-3. [DOI] [PubMed] [Google Scholar]
- Smith JW, Evans AT, Costall B, Smythe JW. Thyroid hormones, brain function and cognition: a brief review. Neurosci Biobehav Rev. 2002;26:45–60. doi: 10.1016/s0149-7634(01)00037-9. [DOI] [PubMed] [Google Scholar]
- Sui L, Gilbert ME. Pre- and postnatal propylthiouracil-induced hypothyroidism impairs synaptic transmission and plasticity in area CA1 of the neonatal rat hippocampus. Endocrinology. 2003;144:4195–4203. doi: 10.1210/en.2003-0395. [DOI] [PubMed] [Google Scholar]
- Swanson LW, Wyss JM, Cowan WM. An autoradiographic study of the organization of intrahippocampal association pathways in the rat. J Comp Neurol. 1978;181:681–716. doi: 10.1002/cne.901810402. [DOI] [PubMed] [Google Scholar]
- Swanson LW. Brain Maps: Structure of the Rat Brain. Elsevier; Amsterdam: 1998. [Google Scholar]
- Tamasy V, Meisami E, Vallerga A, Timiras PS. Rehabilitation from neonatal hypothyroidism: spontaneous motor activity, exploratory behavior, avoidance learning and responses of pituitary-thyroid axis to stress in male rats. Psychoneuroendocrinology. 1986a;11:91–103. doi: 10.1016/0306-4530(86)90035-1. [DOI] [PubMed] [Google Scholar]
- Tamasy V, Meisami E, Du JZ, Timiras PS. Exploratory behavior, learning ability and thyroid hormonal responses to stress in female rats rehabilitating from postnatal hypothyroidism. Develop Psychobiol. 1986b;19:537–553. doi: 10.1002/dev.420190606. [DOI] [PubMed] [Google Scholar]
- Timiras PS. Developmental Physiology and Aging. Macmillan; New York: 1972. [Google Scholar]
- Timiras PS. Thyroid hormones and the developing brain. In: Meisami E, Timiras PS, editors. Handbook of Human Growth and Developmental Biology. 1, Part C, Factors Influencing Brain Development. CRC Press; Boca Raton: 1988. pp. 59–82. [Google Scholar]
- Uchida K, Yonezawa M, Nakamura S, Kobayashi T, Machida T. Impaired neurogenesis in the growth-retarded mouse is reversed by T3 treatment. Neuroreport. 2005;16:103–106. doi: 10.1097/00001756-200502080-00005. [DOI] [PubMed] [Google Scholar]
- Vaidya VA, Castro ME, Pei Q, Sprakes ME, Grahame-Smith DG. Influence of thyroid hormone on 5-HT(1A) and 5-HT(2A) receptor-mediated regulation of hippocampal BDNF mRNA expression. Neuropharmacology. 2001;40:48–56. doi: 10.1016/s0028-3908(00)00094-0. [DOI] [PubMed] [Google Scholar]
- Vara H, Martinez B, Santos A, Colino A. Thyroid hormone regulates neurotransmitter release in neonatal rat hippocampus. Neuroscience. 2002;110:19–28. doi: 10.1016/s0306-4522(01)00541-3. [DOI] [PubMed] [Google Scholar]
- Vara H, Munoz-Cuevas J, Colino A. Age-dependent alterations of long-term synaptic plasticity in thyroid-deficient rats. Hippocampus. 2003;13:816–825. doi: 10.1002/hipo.10132. [DOI] [PubMed] [Google Scholar]
- Vornov JJ, Tasker RC, Coyle JT. Direct observation of the agonist-specific regional vulnerability to glutamate, NMDA, and kainate neurotoxicity in organotypic hippocampal cultures. Exp Neurol. 1991;114:11–22. doi: 10.1016/0014-4886(91)90079-r. [DOI] [PubMed] [Google Scholar]
- Weichsel ME, Clark BR. Pyrimidine metabolism during restorative brain growth after neonatal undernutrition in the rat. Pediat Res. 1977;11:293–297. doi: 10.1203/00006450-197704000-00007. [DOI] [PubMed] [Google Scholar]
- Wieloch T, Lindvall O, Blomqvist P, Gage FH. Evidence for amelioration of ischemic neuronal damage in the rat hippocampal formation by lesions of the perforant path. Neurol Res. 1985;7:24–26. doi: 10.1080/01616412.1985.11739695. [DOI] [PubMed] [Google Scholar]
- Williams JP, Hughes PC. Catch-up growth in the rat skull after retardation during the suckling period. J Embryol Exp Morphol. 1978;45:229–235. [PubMed] [Google Scholar]
- Witter MP, Amaral DG. Hippocampal formation. In: Paxinos G, editor. The Rat Nervous System. 3. Academic Press; Sydney: 2004. pp. 637–703. [Google Scholar]
- Zola-Morgan S, Squire LR, Amaral DG. Human amnesia and the medial temporal region: enduring memory impairment following a bilateral lesion limited to field CA1 of the hippocampus. J Neurosci. 1986;6:2950–2967. doi: 10.1523/JNEUROSCI.06-10-02950.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]


