Abstract
Background
The efficacy of iron polymaltose complex (IPC) in the treatment of iron deficiency anemia (IDA) during pregnancy has not been well established, and the evidence is inconclusive.
Aims
The aim of the study was to compare efficacy, safety, compliance, and cost-effectiveness of IPC with ferrous sulphate (FS) in pregnant patients.
Settings and Designs
The randomized, double-blind, parallel-group study was conducted in the Department of Pharmacology in collaboration with the Department of Obstetrics and Gynaecology Postgraduate Institute of Medical Education and Research, Chandigarh, India.
Methods
One hundred pregnant women aged 20–40 years at14 to 27 weeks' gestation, with hemoglobin (Hb) < 9 g/dL, and serum ferritin < 12 mcg/L, were classified into 2 groups. One group received IPC (100 mg elemental iron), and the other group received FS (120 mg elemental iron) daily for 8 weeks. At Week 0 and Week 8, Hb, packed cell volume (PCV), mean corpuscular volume (MCV), mean corpuscular hemoglobin (MCH), mean corpuscular hemoglobin concentration (MCHC), serum iron, and serum ferritin were measured. Compliance with study medication was determined by pill counting at each visit. Cost minimization analysis was done to compare the costs of the 2 treatments.
Statistical Analysis
Data are expressed as mean ± SD. Paired and unpaired ‘t’ test were used to analyze differences within groups and between groups. Chi-square (x2) test was used to analyze primary efficacy parameters and adverse drug reactions (ADR).
Results
Statistically significant increases in Hb, PCV, MCV, MCH, MCHC, serum iron, and serum ferritin levels were seen at the end of 8 weeks of treatment in both groups. The overall adverse effects were more common in the FS group compared with the IPC group [41 (78%) vs 15 (31%), P < .001]. The compliance rate was significantly (P < .05) higher for the IPC (91%) group than for the FS (87%) group. The average total cost (direct + indirect) of treatment of anemia was comparable between the 2 groups.
Conclusion
The results of the present study suggest that IPC can be considered as a useful alternative formulation for the treatment of IDA during pregnancy for those patients who cannot tolerate other iron preparations (ferrous form); this is an important finding, as compliance is a significant concern during pregnancy.
Introduction
The World Health Organization (WHO) defines anemia in pregnancy as a hemoglobin (Hb) concentration of < 11 g/dL.[1] Iron deficiency anemia (IDA) is the most common type of anemia in pregnancy. The iron content of the body is normally kept constant by regulating the amount absorbed to balance the amount lost.
An increase in loss along with inadequate intake can lead to depletion of body iron stores, iron deficiency, and eventually to anemia. Iron requirements are increased during infancy, puberty, pregnancy, and during menstruation.[2] The WHO estimates that 58% of pregnant women in developing countries are anemic mainly because of iron deficiency.[3]
Anemia has a significant impact on the health of the fetus as well as that of the mother. It impairs the oxygen delivery through the placenta to the fetus and interferes with the normal intrauterine growth, leading to fetal loss and perinatal deaths.[2] Anemia is associated with increased preterm labor (28.2%), preeclampsia (31.2%), and maternal sepsis.[2,4] Severe anemia can lead to palpitations, tachycardia, breathlessness, and increased cardiac output leading to cardiac stress, which can cause decompensation and cardiac failure. Anemia is responsible for 40%–60% of maternal deaths in nonindustrialized countries.[2]
Almost all cases of iron deficiency anemia respond readily to treatment with iron supplementation.[5] However, patients do not always respond adequately to oral iron therapy because of noncompliance due to side effects.[6] Gastrointestinal disturbances characterized by colicky pain, nausea, vomiting, diarrhea, and gastric distress occur in about 6%–12% of patients taking iron preparations.[6] The most widely recommended oral iron is ferrous salts; however, the use of these salts is limited by low and variable absorption, chelation by food products, and free radical-mediated mucosal luminal damage.[7–10] Ferric compounds were introduced to avoid these problems. However, these compounds are generally less soluble at physiologic pH and precipitate intraluminally as hydroxide or phosphate and therefore have poor bioavailability.[11] A need for a ferric complex that could overcome these problems was realized.
Iron-polymaltose complex (IPC), a combination of ferric iron with maltol (a food additive), was developed as a molecule that is soluble at neutral pH and is not chelated by other substances.[12]
Despite the advantage of the IPC over ferrous salts, the efficacy of IPC has not been well established in pregnancy. Studies have shown that IPC is as effective as FS, or even more so.[13–15] But some studies contradict these results.[16,17] However, most of them do not involve pregnant anemic women.
Therefore, the present study was conducted to evaluate the efficacy, safety, and cost of iron polymaltose formulations with ferrous sulphate in pregnant women, and to determine the compliance rates associated with IPC use.
Materials and Methods
The study was conducted in the Department of Pharmacology in collaboration with the Department of Obstetrics and Gynaecology at the Postgraduate Institute of Medical Education and Research, Chandigarh, India. A total of 120 patients were screened, and 100 were enrolled (20 patients did not fulfill the inclusion criteria). Fifty-two patients were assigned to the FS group, and 48 patients were assigned to the IPC group.
In one group, iron polymaltose (Ind-swift limited, India), 100 mg elemental iron + folic acid 500 mcg, was given for 8 weeks (1 tablet orally once daily). The other group received FS (GlaxoSmithKline, India), 60 mg elemental iron + folic acid 500 mcg, for 8 weeks (1 tablet orally twice daily). Folic acid was also given to both groups. To ensure blinding in the IPC group, a placebo of FS was given. Thus, in both groups, patients received 2 tablets per day. Since FS tablets containing 50 mg or 100 mg of elemental iron are not available, the tablet containing 60 mg of elemental iron was chosen and given twice daily.
Patients were selected in accordance with the inclusion criteria:
Pregnant;
Age 20–40 years;
Gestation 14–27 weeks;
Hb < 9 g/dL (moderate anemia); and
Serum ferritin < 12 mcg/L.
Patients with a history of anemia due to any other causes such as chronic blood loss, hemolytic anemia, and thalassemia (including thalassemic trait) were excluded from the study. Pregnant women with Hb < 7 g/dL (severe anemia) were also excluded. Other exclusion criteria were as follows: clinical and/or laboratory evidence of hepatic, renal, hematologic, cardiovascular abnormalities; history of acid-peptic disorders, esophagitis, or hiatal hernia; family history of thalassemia, sickle cell anemia, or malabsorption syndrome; history of any other medical disorder; hypersensitivity to iron preparations; history of ingestion of hematinic within 24 hours prior to inclusion, and treatment with any other investigational drug in the last 1 month before study entry.
Written informed consent was obtained from all patients prior to screening and enrollment. The study protocol was approved by the Institute ethics committee.
This was an 8-week, prospective, double-blind, randomized, parallel-group, single-center study. Both of the drugs were dispensed in identical capsules to ensure blinding.
The primary efficacy parameter was the proportion of women achieving normal Hb level (> 11 g/dL) in the treatment groups within 8 weeks of treatment. Other efficacy parameters were packed cell volume (PCV), mean corpuscular volume (MCV), mean corpuscular hemoglobin (MCH), mean corpuscular hemoglobin concentration (MCHC), serum iron, and serum ferritin. At Week 0 and Week 8, Hb, PCV, MCV, MCH, MCHC, serum iron, serum ferritin were measured. Hb and ADR were also measured at 2, 4, and 6 weeks.
Hemoglobin concentration was measured by spectrophotometry.[18] PCV was determined by micro-hematocrit method.[19]
MCV was calculated using the following formula:[20]
MCV = PCV/RBC count (femtoliter)
MCH was calculated by the formula:[20]
MCH = Hb/liter of blood/RBC count (picogram)
MCHC was calculated by the formula:[20]
MCHC = Hb/liter of blood/PCV (g/L)
Serum iron was estimated by the colorimetric method without deproteinization by using the Ferrozene kit (ORgen Tec Kupferbergterrasse, Germany).[20]
Serum ferritin was measured using the human ferritin enzyme immunoassay test kit (Bio Plus, Inc; South San Francisco, California).[21]
The patients were asked to report any unusual or unpleasant symptoms during the study period. This report included a detailed description of the signs and symptoms of the event, time of onset and duration, whether treatment was discontinued, corrective measures, outcome, and other possible causes. Compliance with study medication was determined by pill counting at each visit.
Cost minimization analysis was done to compare the direct and indirect costs of both treatments. Direct costs include total cost of drug treatment, cost of all investigations (eg, estimation of Hb, PCV, MCV, MCH, MCHC, serum iron, and serum ferritin, etc). Indirect costs included the cost of treatment of adverse drug reactions (ADRs) related to iron therapy (eg, cost of antacids) and the cost of all visits (traveling cost) and wages lost due to visits to hospital.
Statistical Analysis
Forty (40) patients per group were required to detect a 5% difference in the percentage of patients achieving normal Hb level (≥ 11 g/dL) within 8 weeks of treatment at an alpha of 0.05 and power of 80%. Estimating a dropout rate of about 5%–10%, it was decided to recruit at least 45 patients per group.
For statistical analysis, data for all the parameters were first subjected to a test of normality. Data showing normal distribution were analyzed by using parametric tests. Accordingly, differences between the groups for change (baseline vs 8 weeks) in hemoglobin, PCV, MCV, MCH, MCHC, serum iron, serum ferritin, compliance (pill counting), and cost were compared by using student's 2-tailed unpaired 't' test. The difference between the 2 groups with regard to primary efficacy parameter (percentage of patients achieving normal Hb level ≥ 11 g/dL within 8 weeks of treatment) and incidence of ADRs was compared by using chi-square (x2) test. P value < .05 was considered statistically significant. Baseline values were compared to detect homogeneity of the 2 groups.
Results
The study was initiated in September 2003 and completed in October 2004. All of the 100 patients enrolled in the study completed the entire 8 weeks. Data for all 100 patients were included for analysis [see Appendix: Flow Diagram of the Progress Through the Phases of a Randomized Trial (enrollment, intervention, allocation, follow-up, and the data analysis].
Baseline characteristics of all subjects are shown in Table 1. The baseline hematologic parameters including Hb concentration, PCV, MCV, MCH, MCHC, serum ferritin, and serum iron were comparable between the 2 groups (Table 1).
Table 1.
Baseline Characteristics
| Parameters | Ferrous Sulphate (FS) | Iron Polymaltose Complex (IPC) |
|---|---|---|
| No. of patients | 52 | 48 |
| Age, yrs (mean± SD) | 27.54 ± 3.5 | 27.58 ± 4.2 |
| Median age, yrs (range) | 29 (22-35) | 25 (21-35) |
| Weight (kg) (mean ± SD) | 56.83 ± 4.39 | 57.34 ± 3.81 |
| Median weight, kg (range) | 53 (50-62) | 55 (53-61) |
| Mean period of gestation ±SD (wks) | 17.62 ± 3.1 | 18.58 ± 2.8 |
| Median gestation (range) (wks) | 16 (14-27) | 19 (14-24) |
| Vegetarian (%) | 35 (67) | 28 (58) |
| Nonvegetarian (%) | 17(33) | 20 (42) |
| Number of pregnancies | ||
| Primi gravida (%) | 23 (44.2) | 15 (31.3) |
| Second gravida (%) | 16 (30.8) | 20 (41.7) |
| Third gravida (%) | 6 (11.5) | 8 (16.7) |
| Fourth gravida (%) | 6 (11.5) | 4 (8.3) |
| Fifth gravida (%) | 1 (1.9) | 1 (2.1) |
| Urban area residents (%) | 28 (53.8) | 26 (54.2) |
| Rural area residents (%) | 24 (46.2) | 22 (45.8) |
| Hematologic parameters | ||
| Hb (g/dL) (mean ± SD) | 8.39 ± 0.74 | 8.47 ± 0.72 |
| PCV (%) (mean ± SD) | 25 ± 1.66 | 26 ± 2.05 |
| MCV (fL) (mean ± SD) | 74.91 ± 6.9 | 73.41 ± 10.85 |
| MCH (Pg) (mean ±SD) | 22.6 ± 4.27 | 23.35 ± 3.71 |
| MCHC (g/L) (mean ± SD) | 298.75 ± 65.67 | 298.26 ± 68.44 |
| Serum iron (mcg/dL, mean ± SD) | 65.75 ± 21.45 | 67.29 ± 9.12 |
| Serum ferritin (ng/mL, mean ±SD) | 11.38 ± 8.5 | 10.93 ±4.14 |
Hb = hemoglobin; MCH = mean corpuscular hemoglobin; MCHC = mean corpuscular hemoglobin concentration; MCV = mean corpuscular volume; PCV = packed cell volume
In the FS group, 63.5% (33 patients out of 52) achieved Hb > 11 g/dL, whereas in the IPC group, 68.8% (33 of 48 patients) women achieved Hb concentration > 11g/dL, which was the primary end point. Both groups showed a significant increase in Hb level (mean ± SD change from baseline was 2.9 ± 1.08 g/dL in FS group and 2.72 ± 1.55 g/dL in the IPC group (Table 2). The increase in Hb concentration at 4, 6, and 8 weeks of treatment was statistically significant when compared with the baseline value in both groups (Figure 1). Hemoglobin levels started to rise after 2 weeks of treatment, but the increases were significant only after 4 weeks of iron therapy in both groups compared with baseline.
Table 2.
Effects of Ferrous Sulphate (FS) and Iron Polymaltose Complex (IPC) on Hb (g/L), PCV (%), MCV (fl), MCH (Pg), MCHC (g/L), Serum Iron (mcg/dL), and Serum Ferritin (ng/mL)
| Parameters | Baseline (0 wks) | After Treatment (8 wks) | Difference (8 wks – baseline) with 95% CI† | P value | |||
|---|---|---|---|---|---|---|---|
| Hb (g/L) | IPC 8.47 ±0.72 | FS 8.39±0.74 | IPC 11.19±0.67* | FS 11.24±0.61* | IPC Mean ±SD (CI) 2.72 ±0.55 (0.01–5.42) | FS Mean ±SD (CI) 2.9 ±0.46 (0.0–5.8) | .07 |
| PCV (%) | 26 ± 2.05 | 25±1.66 | 34±2.85* | 34±1.90* | 8±1.2 (0.07–9.13) | 9±2.3 (0.09–12.34) | .43 |
| MCV (fl) | 73.41 ± 10.85 | 74.91±6.9 | 90.35±13.46* | 93.61±6.4* | 16.94±12.42 (0.02–33.86) | 18.7±10.4 (0.7–36.7) | .25 |
| MCH (Pg) | 23.35 ± 3.71 | 22.6±4.27 | 30.50±2.0* | 30.25±3.07* | 7.15±2.0 (0.01–14.29) | 7.65±1.6 (0.02–15.28) | .39 |
| MCHC (g/L) | 298.26 ±8.44 | 298.75 ± 5.67 | 326.12±26.17* | 326.72±18.13* | 27.86±15.22 (0.01–55.71) | 27.97±12.25 (0.00–55.94) | .14 |
| Serum iron (mcg/dL) | 67.29 ±9.12 | 65.75±21.45 | 105.61±15.22* | 108.88±42.5* | 38.32±13.21 (0.01–76.63) | 43.13±38.32 (0.13–86.13) | .21 |
| Serum ferritin (ng/mL) | 10.93 ± 4.14 | 11.38±8.5 | 33.52±10.57* | 28.22±10.40* | 22.59±6.8 (0.01–45.17) | 16.84±7.9 (0.02–33.66) | < .001 |
P < .05 vs baseline values (P value is for difference (8 wks – baseline) between the 2 groups)
Figures in parentheses are 95% CI
All values are expressed as mean ± SD
Hb = hemoglobin; MCH = mean corpuscular hemoglobin; MCHC = mean corpuscular hemoglobin concentration; MCV = mean corpuscular volume; PCV = packed cell volume
Figure 1.
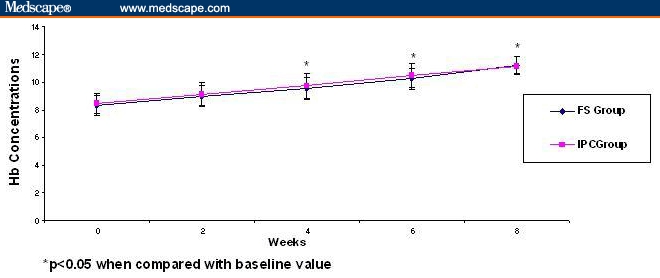
Effect of ferrous sulphate and iron polymaltose complex on hemoglobin concentration (g/dL) at baseline (0 wks), 2 wks, 4 wks, and 8 wks on iron deficiency anemia in pregnant women. Data are expressed as mean ± SD.
After treatment with iron preparations for 8 weeks, PCV values in both the treatment groups showed a rise from 25% (baseline) to 34% (at the end of 8 weeks of treatment) in the FS group and from 26% (baseline) to 34% (at the end of 8 weeks of treatment) in the IPC group (Table 2). The increase in PCV value was statistically significant when baseline vs 8-week values were compared in both groups (Table 2). There was a significant (P < .001) increase in MCV level in both treatment groups at the end of 8 weeks (Table 2).
At the end of 8 weeks of either study treatment, there was a statistically significant increase in both MCH and MCHC value from baseline in both treatment groups (Table 2), but there was no difference between the 2 treatment groups.
Statistically significant increases in the serum iron and serum ferritin levels were observed in both treatment groups (Table 2). The baseline serum iron was 65.75 ± 21.45 mcg/dL in FS group and 67.29 ± 9.12 mcg/dL in the IPC group. In both groups, the serum iron level was increased statistically to 108.88 ± 42.5 mcg/dL in the FS group (P < .001) and to 105.61 ± 15.22 mcg/dL in the IPC group (P < .001). Serum ferritin level was also significantly increased from 11.38 ± 8.5 mcg/dl to 28.22 ± 10.40 mcg/dL (P < .001) in the FS group and from 10.93 ± 4.14 to 33.52 ± 10.57 mcg/dL (P < .001) in the IPC group (Table 2, Figure 2, Figure 3). When compared between the two groups the increase in serum iron was not significantly different (P = .21), but the increase in serum ferritin was significantly higher in IPC group (P < .001) compared with the FS group.
Figure 2.
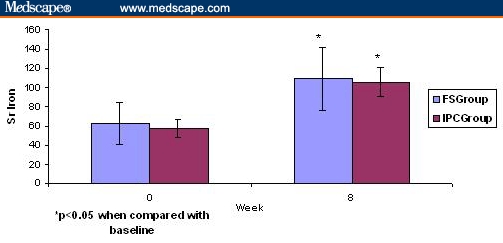
Effects of ferrous sulphate and iron polymaltose complex on serum iron (mcg/dL) at baseline (0 wks) and 8 wks on iron deficiency anemia in pregnant women. Data are expressed as mean ± SD.
Figure 3.
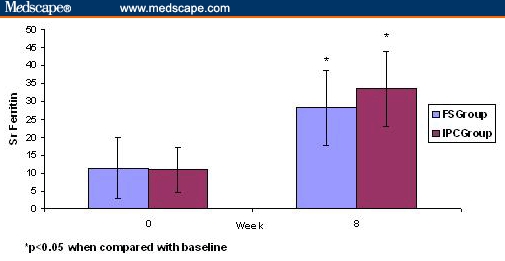
Effects of FS and IPC on serum ferritin (ng/mL) at baseline (0 wks) and 8 wks on iron deficiency anemia in pregnant women. Data are expressed as mean ± SD.
Table 3 shows the incidence of adverse effects experienced during the study. Overall, the adverse effects were more common in the FS group than in the IPC group [41 (78%) in the FS group vs 15 (31%) in the IPC group, P < .001]. The adverse effects experienced were GI intolerance (acidity, gastritis, and heartburn), constipation, metallic taste, diarrhea, and rash. The most common adverse effect was GI intolerance, seen in patients in both the FS and the IPC groups (Table 3). Constipation was the second most common adverse effect seen with both of the drugs (48% in the FS group and 14% in the IPC group).
Table 3.
Comparison of Adverse Events in Ferrous Sulphate (FS) and Iron Polymaltose Complex (IPC) Groups
| Events | FS (n = 52) | IPC (n = 48) | P Value* |
|---|---|---|---|
| No. of patients (%) | No. of patients (%) | ||
| GI intolerance | 34 (65.4%) | 13 (27%) | < .001 |
| Constipation | 25 (48%) | 7 (14.5%) | < .001 |
| Metallic taste | 8 (15.3%) | 1 (2%) | .03 |
| Diarrhea | 2 (3.8%) | 0 | < .05 |
| Rashes | 1 (1.9%) | 0 | < .05 |
| Total | 41 (78%) | 15 (31%) | < .001 |
P value refers to difference between groups with regard to incidence of adverse effect.
GI = gastrointestinal
The compliance rate was higher than 80% in both groups. It was significantly higher (P < .05) in the IPC group (91%) than in the FS group (87%).
The average drug cost was significantly higher for the IPC group (Rs 237.08 ± 47.25) than for the FS group (Rs 169.98 ± 75.51) (P < .001). The cost due to treatment of adverse effects was higher (P < .001) in the FS group (Table 4). So the average total cost (direct + indirect) of treatment of anemia for 8 weeks' duration in the FS group was Rs 1405.12 ± 93.60 and in the IPC group it was Rs 1454.33 ± 73.85; this difference was not statistically significant (Table 4).
Table 4.
Cost (Direct + Indirect) of Iron Therapy in Ferrous Sulphate (FS) and Iron Polymaltose Complex (IPC) Groups (data expressed as Rs* [mean ± SD])
| Cost† | FS (Rs) | IPC (Rs) | Difference between the groups (95% CI)‡ | P Value¶ |
|---|---|---|---|---|
| Total cost | 1405.12 ± 93.60 | 1454.33 ± 73.85 | 49.21 ± 28.12 (17.02–81.4) | .05 |
| Drug cost | 169.98 ± 75.51 | 237.08 ± 47.25 | 67.21 ± 36.15 (42.9–91.3) | < .001 |
| Investigation cost | 711.53 ± 47.08 | 715.62 ± 44.30 | 3.9 ± 2.7 (−4.02–12.2) | .23 |
| ADR-related treatment cost | 13.05 ± 2.08 | 6.84 ± 0.94 | 5.78 ± 1.64 (3–9.42) | < .001 |
| Cost due to hospital visits | 317.30 ± 309.6 | 310.25 ± 245.93 | 6.15 ± 1.7 (2.17–8.65) | .34 |
| Cost due to loss of work | 203.26 ± 35.7 | 213.54 ± 30.83 | 9.29 ± 1.54 (5-13.69) | .19 |
1 US $ = 42 Rs
Direct cost = drug cost + investigation cost + ADR-related treatment cost; indirect cost = cost due to visits + cost due to loss of work
Figures in parentheses are 95% CI.
P value represents difference in cost between groups
All values are expressed as mean ± SD.
ADR = adverse drug reaction.
Discussion
Anemia associated with pregnancy is a worldwide public health problem. A recent WHO report estimates the anemia prevalence among pregnant women to be 55.9% globally.[22] IDA is the most common form of anemia in women of reproductive age, and it affects approximately 15% of the world's population.[23] When it occurs during pregnancy, anemia has a significant impact on the health of the fetus as well as that of the mother.[2]
The treatment of IDA is directed at replenishing hemoglobin and compensating for the deficit in stored iron by supplying sufficient iron. The iron polymaltose complex has been formulated in such a way that the elemental form is in a nonionic state. This ensures that there is no gastric irritation with iron polymaltose complex. In addition, the high elemental iron content of IPC eliminates the need for frequent dosing and therefore improves compliance.[24]
Other studies have shown similar results to those seen in the present study, but they had limitations. A study by Badhwar and colleagues[25] involving adult patients (both female and male) with IDA demonstrated comparable efficacy and superior bioavailability of IPC over ferrous fumarate. However, this study was conducted in nonpregnant women. A study by Pakar and colleagues[26] also demonstrated efficacy and safety of IPC, in both pregnant and nonpregnant women, but this was an open, uncontrolled trial. In another study, efficacy and tolerability of IPC in IDA during pregnancy in Indian women were evaluated, and IPC was found to result in significantly reduced symptoms of anemia and significant improvements in serum iron, iron binding capacity, and Hb; furthermore, only 8% of patients showed mild GI-related adverse effect[13] However, this study was limited by the absence of a control group. In the study undertaken by Reddy[27] to evaluate the efficacy and safety of IPC in pregnant women with IDA, it was shown that the clinical parameters as well as biochemical parameters showed favorable changes with IPC,[27] but again, this was an open, uncontrolled trial. A study by Jayaram and colleagues[28] also demonstrated the effectiveness and safety of IPC in adult IDA patients (both male and female),[28] but this was an open trial that included both pregnant and nonpregnant patients.
In summary, none of these studies compared compliance and cost of iron preparations, and some of the studies were uncontrolled and open trial and were conducted in nonpregnant women.
In contrast to the aforementioned studies, the present study is a randomized, double-blind, controlled trial involving pregnant women. Compliance and cost were measured in both groups and compared, along with effectiveness and safety; these comparisons were not done in previous studies. To the best of our knowledge, this is the first study that has compared FS and IPC in terms of efficacy, tolerability, compliance, and cost-effectiveness. However, our findings contradict those reported by Mehta;[16] in a case report, he described the ineffectiveness of IPC in 4 pregnant patients with IDA.[16]
Overall, adverse effects were more common in the FS group than in the IPC group (78% vs 31%, P < .001) in the present study. Similar observations were seen in the Indian populations (pregnant women) studied by Reddy[26] and by Rajyadhyaksha.[13]
In the present study, the adverse effects were not severe enough to warrant discontinuation of iron therapy. Increased incidence of adverse effects with FS may be due to release of free radicals, which leads to cell damage and cell death.[8] IPC does not release free radicals, which may account for the observed difference. Reduced incidence of adverse effects will improve patient compliance and ensure regular treatment. In the present study, the compliance rate was higher in the IPC group (91%) than in the FS group (87%), and the improvements seen in all hematologic parameters were the same in both groups.
Since this was a trial in which drugs were provided to the patients by the investigators – and the patients were directly observed by the investigators – these findings cannot be extrapolated to the setting of general practice, where compliance rates may be lower and further reduced by the adverse effects seen with FS.
The pharmacoeconomic analysis shows that the 2 iron preparations are equally cost-effective. In the present study, the drugs (FS or IPC) were given to the patient at no cost, but in our analysis, the cost was calculated as per the retail price. Our institution, where the study was conducted, is a public sector government institute. The consultation fees and investigations fees are subsidized, so the cost calculated in the present study cannot be extrapolated directly to private sector hospitals. Their costs may be higher than those seen in the present study.
IPC is effective in the treatment of iron deficiency anemia in pregnant women. A superior tolerability profile to that of the conventional iron preparation (FS) and an equivalent efficacy profile strongly suggest that it can be considered as a useful, relatively novel, and alternative formulation for the treatment of IDA during pregnancy. However, further studies with large patient populations are required to strengthen the evidence of the present study.
Appendix 1.
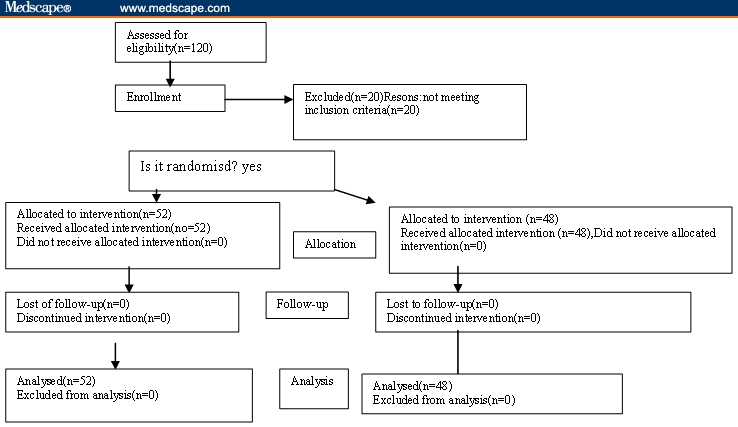
Flow diagram of the progress through the phases of a randomized trial (enrollment, intervention, allocation, follow up and the data analysis).
Footnotes
Readers are encouraged to respond to George Lundberg, MD, Editor of MedGenMed, for the editor's eye only or for possible publication via email: glundberg@medscape.net
Contributor Information
Lekha Saha, Department of Pharmacology, Postgraduate Institute of Medical Education and Research, Chandigarh, India; Email: lekhasaha@rediffmail.com.
Promila Pandhi, Department of Pharmacology, Postgraduate Institute of Medical Education and Research, Chandigarh, India.
Sarala Gopalan, Department of Obstetrics and Gynaecology, Postgraduate Institute of Medical Education and Research, Chandigarh, India.
Samir Malhotra, Department of Pharmacology, Postgraduate Institute of Medical Education and Research, Chandigarh, India.
Pradip kumar Saha, Department of Obstetrics and Gynaecology, Government Medical College and Hospital, Chandigarh, India.
References
- 1.World Health Organization. Technical report series no.503. Geneva, Switzerland: WHO; 1972. Report of a WHO group of experts on nutritional anaemias. [PubMed] [Google Scholar]
- 2.Sharma JB. Nutritional anaemia during pregnancy in non-industrialised countries. In: Studd J, editor. Progress in Obstetrics and Gynaecology. New Delhi: Churchill Livingstone; 2003. pp. 103–122. [Google Scholar]
- 3.Dusch E, Elder L, Achadi E, et al. Women's perceptions of iron deficiency and anemia prevention and control in eight developing countries. Soc Sci Med. 2002;55:529–544. doi: 10.1016/s0277-9536(01)00185-x. [DOI] [PubMed] [Google Scholar]
- 4.Lops VR, Hunter LP, Dixon LR. Anaemia in pregnancy. Am Fam Physician. 1995;94:277–280. [PubMed] [Google Scholar]
- 5.Dugdale M. Anaemia. Obstet Gynecol Clin North Am. 2001;28:363–381. doi: 10.1016/s0889-8545(05)70206-0. [DOI] [PubMed] [Google Scholar]
- 6.Adamson JW. Fauci AS. Kasper DL, et al. Iron deficiency and other hypoproliferative anaemias. In: Braunwald E, editor; Harrison's Principles of Internal Medicine. 15th edition. Mc Graw Hill; 2001. pp. pp 660–66. [Google Scholar]
- 7.Sharma N. Iron absorption: IPC therapy is superior to conventional iron salts. Obstet Gynecol. 2001:515–19. [Google Scholar]
- 8.McCord JM. Iron, free radicals, and oxidative injury. Semin Hematol. 1998;35:5–12. [PubMed] [Google Scholar]
- 9.Geisser P. In vitro studies on interactions of iron salts and complexes with food stuffs and medicaments. Arzneimittelforschung. 1990;40:754–760. [PubMed] [Google Scholar]
- 10.Jacobs P, Johnson G, Wood L. Oral iron therapy in human subjects, comparative absorption between ferrous salts and iron polymaltose. J Med. 1984;15:367–377. [PubMed] [Google Scholar]
- 11.Maxton DG, Thompson RP, Hinder RC. Absorption of iron from ferric hydroxypyranone complexes. Br J Nutr. 1994;71:203–207. doi: 10.1079/bjn19940127. [DOI] [PubMed] [Google Scholar]
- 12.Jacobs P, Wormald LA, Gregory MC. Absorption of iron polymaltose and ferrous sulphate in rats and humans – a comparative study. S Afr Med J. 1979;26:1065–1072. [PubMed] [Google Scholar]
- 13.Rajadhyaksha GC, Shahani S, Pawar D. Evaluation of efficacy and tolerability of iron polymaltose complex tablets in iron deficiency anaemia during pregnancy. JAMA India – the Physician's Update. 2000;3:53–55. [Google Scholar]
- 14.Reddy PSN, Adsul BB, Gandewar K, Korde KM, Desai A. Evaluation of efficacy and safety of iron polymaltose complex and folic acid (Mumfer) vs iron formulation (ferrous fumarate) in female patients with anemia. J Indian Med Assoc. 2001;99:154–155. [PubMed] [Google Scholar]
- 15.Jacobs P, Fransman D, Coghlan P. Comparative bioavailability of ferric polymaltose and ferrous sulphate in iron deficient blood donors. J Clin Apheresis. 1993;8:89–95. doi: 10.1002/jca.2920080207. [DOI] [PubMed] [Google Scholar]
- 16.Mehta BC. Iron hydroxide polymaltose complex –cause of persistent anemia at delivery. Indian J Med Sci. 2001;55:616–620. [PubMed] [Google Scholar]
- 17.Nielsen P, Gabbe EE, Fischer R, Heinrich HC. Bioavailability of iron from oral ferric polymaltose in humans. Arzneimittelforschung. 1990;40:754–760. [PubMed] [Google Scholar]
- 18.Drabkin DL, Austin JH. Spectrophotometric studies: spectrometric constants for common haemoglobin derivatives in human, dog and rabbit blood. J Biol Chem. 1932;198:719–725. [Google Scholar]
- 19.England JM, Walford DM, Waters DAW. Re-assessment of the reliability of the haematocrit. Br J Haematol. 1972;23:247–256. doi: 10.1111/j.1365-2141.1972.tb03477.x. [DOI] [PubMed] [Google Scholar]
- 20.Tietz NW. In: Textbook of Clinical Chemistry. Tietz NW, editor. Philadelphia, Pa: WB Saunders Co.; 1986. pp. 1578–1583. [Google Scholar]
- 21.White D, Kramer D, Johnson G, Dick F, Hamilton H. Estimation of serum ferritin by using enzyme immunoassay method. Am J Clin Pathol. 1986;72:346–351. [Google Scholar]
- 22.World Health Organization. The prevalence of anaemia in women: a tabulation of available information. Geneva, Switzerland: WHO; 1992. [Google Scholar]
- 23.World Health Organization. Report of working group on iron deficiency anaemia. 1992;1:17–20. [Google Scholar]
- 24.Schwartze WJ, III, Thurnau GR. Iron deficiency anaemia in pregnancy. Clin Obstet Gynaecol. 1995;38:443–454. doi: 10.1097/00003081-199509000-00004. [DOI] [PubMed] [Google Scholar]
- 25.Badhwar VR, Rao S, Fonseca MM. Comparative efficacy and safety of iron polymaltose+folic acid and oral ferrous fumarate in the treatment of adult patients with iron deficiency anemia. Indian Med Gazette. 2003;136:296–301. [Google Scholar]
- 26.Patkar VD, Patkar S, Khandeparker PS, Dingankar NS, Shetty RS. Evaluation of efficacy and tolerability of iron (III) – hydroxide polymaltose complex tablets in the treatment of iron deficiency anaemia in women. Indian Med Gazette. 2001;135:306–309. [Google Scholar]
- 27.Reddy PS, Adsul BB, Gandewar K, Desai A. Mumfer (iron polymaltose complex) in the management of anaemia in pregnancy – an Indian study. J Indian Med Assoc. 2000;98:343–346. [PubMed] [Google Scholar]
- 28.Jayaram S, Khandeparkar P, Dingankar NS, Shetty RS. Efficacy and tolerability of iron polymaltose complex in the treatment of iron deficiency anaemia. Indian Med Gazette. 2002;134:234–237. [Google Scholar]


