Introduction
Age-specific incidence of epithelial ovarian cancer indicates a hormonal etiology and relates it to menopause, a time during the aging process when ovaries fail but the pituitary becomes overactive to produce a greater amount of gonadotropins. There is a 10-fold to 20-fold increase in the follicle-stimulating hormone (FSH) level and a 3-fold to 5-fold increase in the luteinizing hormone (LH) level in blood in menopause. Naturally, gonadotropins, especially follicle stimulating hormone (FSH) and its receptor (FSHR), are implicated as endogenous carcinogens for ovarian cancer.[1,2] Zheng and colleagues[3] demonstrated the presence of FSHR in innocuous postmenopausal ovarian surface epithelium (OSE) from where this cancer originates. This was the first study to provide evidence that OSE is hormonally active. These findings points to the need for new therapies to treat epithelial ovarian cancer encompassing natural and synthetic substances that have both anticancer and hormonal properties.
In studies of the anticancer potential of plants used in folk medicine of Bengal, extracts of plants such as Oroxylum indicum, Moringa oleifera, Aegles marmelos, Hemidesmus indicus, Polyalthia longifolia, and Aphanamixis polystachya could be considered as potential sources of anticancer compounds.[4,5] Among them, Oroxylum indicum has anti-inflammatory and analgesic properties, and Aegles marmelos, with its antibacterial and anti-inflammatory properties, has a role in the treatment of constipation, diarrhea, peptic ulcer, ear infections, and respiratory disorders. However, the only herb that has been shown to play a role in the treatment of female reproductive disorders is Moringa oleifera Lam., whose effectiveness is derived from a combination of antitumor and hormonal properties. Although the name “Shigon” for M oleifera is mentioned in the “Shushruta Sanhita” of India, which was written in the beginning of the first century AD, there is evidence that the cultivation of this tree in India dates back many thousands of years. Moringa oleifera Lam. contains a unique combination of isothiocyanate and glucosinolates. The effectiveness of the moringa plant in treating ovarian cancer became evident after the publication of recent studies demonstrating that benzyl isothiocyanate (BITC) and phenethyl isothiocyanate (PEITC) induce apoptosis in ovarian cancer cells in vitro.[6,7] We knew that isothiocyanates have antitumor activity in cancers of the lung, breast, skin, esophagus, and pancreas, but we did not know that it can also induce apoptosis in ovarian cancer cell in vitro.
Description
Moringa oleifera Lam. is the most widely cultivated species of the monogeneric family Moringaceae (order Brassicales). This family includes 13 species of trees and shrubs distributed in sub-Himalayan ranges of Arabia, India, Madagascar, North Eastern and South Western Africa, and Sri Lanka.[8,9] It contains several phytochemicals, some of which are of high interest because of their medicinal value, as described in the next section. In particular, this plant family is rich in a fairly unique group of glycoside compounds called glucosinolates and isothiocyanates. Their chemical names and structures are shown in Figure 1. Moringa oleifera leaves contain 2 nitrile glycosides, niazirin and niazirinin, and 3 mustard oil glycosides, 4-[(4'-O-acetyl-alpha-L-rhamnosyloxy) benzyl] isothiocyanate, niaziminin A, and niaziminin B, which are reported to be responsible for hypotensive activity. In addition, beta-sitosterol, glycerol-1-(9-octadecanoate), 3-O-(6'-O-oleoyl-beta-D-glucopyranosyl)-beta-sitosterol, and beta-sitosterol-3-O-beta-D-glucopyranoside have also been identified. Most of them have anticancer properties.[10]
Figure 1.
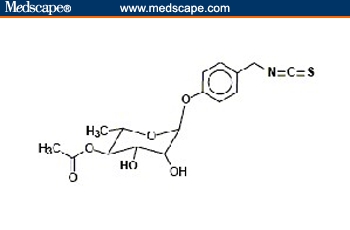
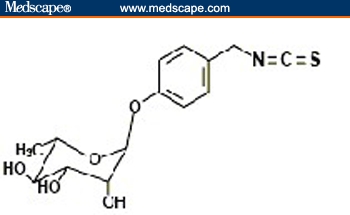
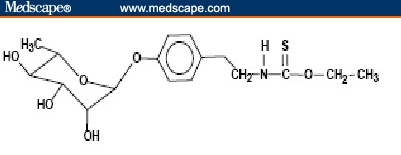
Structures of selected phytochemicals from Moringa oleifera. 4-(4'-O-acetyl-α-L-rhamnopyranosyloxy) benzyl isothiocyanate [A], 4-(-L-rhamnopyranosyloxy) benzyl isothiocyanate [B], and niazimicin [C].
The root bark of Moringa oleifera contains 2 additional alkaloids (total alkaloids, 0.1%), viz. moringine, which is identical to benzylamine, and moringinine, belonging to the sympathomimetic group of bases. In addition, traces of an essential oil with a pungent smell, phytosterol, waxes, and resins are found in the moringa plant, and it contains a rich and rare combination of zeatin, quercetin, beta-sitosterol, caffeoylquinic acid, pterygospermin, and kaempferol. While the entire tree has antitumor activity, the sex hormone-related property is attributable to its root, as folk medicine use has also proven. This plant can be used in patients with diabetes and thyroid disease. The other most common use of its hormonal property, except insulin and thyroid-like property is as emmenagogue and abortifacient.[11]
Relevant Medicinal Value
With regard to the use of the moringa plant in cancer therapy, 4-(4'-O-acetyl-alpha-L-rhamnopyranosyloxy) benzyl isothiocyanate and the related compound niazimicin have been shown to be potent inhibitors of phorbol ester in lymphoblastoid cells. In a mouse study, niazimicin inhibited tumor production. Niaziminin and beta-sitosterol-3-O-beta-D-glucopyranoside have also been shown to be associated with antitumor activity.[10,12] Bharali and colleagues[13] even showed chemopreventive potential of Moringa oleifera drumstick extract against chemical carcinogens via the hepatic pathway.
However, there is currently more research on its antitumor property than its sex hormone-related property.
Along with its antitumor, cancer prevention, and abortifacient properties, benzyl isothiocyanate and the other aforementioned compounds have numerous other properties, such as anti-inflammatory, estrogenic, antiprogestational, hypoglycemic, antiulcer, antihyperthyroidism, hypocholesterolemic, hypotensive, antimicrobial, purgative, and antispasmodic effects.
With regard to the female reproductive system, Moringa oleifera root is shown to have unique estrogenic, antiestrogenic, progestational, and antiprogestational activities.[14] Root-bark yields 2 alkaloids: moringine and moringinine. Moringinine acts as a cardiac stimulant, produces a rise in blood pressure, acts on sympathetic nerve endings as well as smooth muscles throughout the body, and depresses the sympathetic motor fibers of vessels in large doses only. M oleifera confers significant radiation protection as well.
The present author is most interested in the hormonal properties of aqueous extract of Moringa oleifera Lam. root, which may or may not be attributable to benzyl isothiocyanate. Many years ago, this particular property of this miracle tree was shown to cause biochemical and physiologic alterations in the female reproductive organs of cyclic rats.[15] Initially, its administration stimulated the uterine structures, caused metaplastic changes in the cervical epithelium, and provoked considerable cornification in the vaginal epithelium. After more days of treatment, significant inhibition in the general histoarchitecture was observed. Biochemical observations and histologic findings have been correlated with the anti-implantation action of the aqueous extract in light of its hormonal properties. Obviously, these and other experiments can easily explain its use as an abortifacient.[16] However, the active mechanism that produces such effects is still not known, and further study on its female sex hormonal properties is required.
It appears to be acting on some receptor, most likely the follicle-stimulating hormone receptor (FSHR), because it initially increases estrogenic action when the uterus is enlarged. Then, it may inactivate this receptor locally or with the help of a central mechanism through nerve growth factor (NGF)-mediated pathways.[17] Of interest, the central inhibitory effect of Moringa oleifera root extract and a possible role of neurotransmitters have also been proposed. Dopamine and norepinephrine levels were studied in Holtzman strain adult albino rats. The results revealed that pretreatment with Moringa oleifera inhibited penicillin-induced seizure and markedly reduced locomotor activity. Chronic treatment with Moringa oleifera significantly increased the 5-HT and decreased the dopamine level in the cerebral cortex, midbrain, caudate nucleus, and cerebellum. The norepinephrine level was significantly decreased in the cerebral cortex.[18,19] As dopamine and norepinephrine influence NGF and FSHR through central mechanisms,[17] this result may support and corroborate their possible role in epithelial ovarian cancer. Vascular endothelial growth factor (VEGF) might also be involved via NGF.[20] However, no cell line experiments of the effects of Moringa oleifera on ovarian cancer cells have been reported, and this work is urgently warranted.
Hormonal action may be mediated through the estrogen or progesterone receptor as well. Moringa oleifera inhibits maintenance and growth of reproductive organs. In fact, in rural and tribal areas of the West Bengal province in India, the root of this plant is taken by women, especially prostitutes, as permanent contraception, and it has been shown to totally inactivate or suppress the reproductive system.
Toxicity
Different parts of the plant have different pharmacological actions and toxicity profiles, which have not yet been completely defined. However, several toxicities have been described and are worth mentioning.
The root bark contains 2 alkaloids as well as the toxic hypotensive moringinine. At lower concentrations, it produces a dose-dependent positive inotropic effect, and at higher concentrations, a dose-dependent negative inotropic effect, as was demonstrated in a study using an isolated frog heart. Niazinin A, niazimicin, and niaziminin A+B resulted from bioassay-directed fractionation of the ethanolic extract of Moringa oleifera leaves.[21] Intravenous administration (1-10 mg/kg) produced hypotensive and bradycardiac effects in anesthetized rats and negative inotropic and chronotropic effects in isolated guinea pig atria. The direct depressant action of these compounds exhibited on all of the isolated preparations tested is thought to be responsible for its hypotensive and bradycardiac effects observed in vivo.
The bark of the tree may cause violent uterine contractions that can be fatal.[22] Methanolic extract of Moringa oleifera root was found to contain 0.2% alkaloids. Effects of multiple weekly doses (35, 46, 70 mg/kg) and daily therapeutic (3.5, 4.6, and 7.0 mg/kg) intraperitoneal doses of the crude extract on liver and kidney function and hematologic parameters in mice have been studied. The results indicate that weekly moderate and high doses (> 46 mg/kg body weight) and daily/therapeutic high doses (7 mg/kg) of crude extract affect liver and kidney function and hematologic parameters, whereas a weekly dose (3.5 mg/kg) and low and moderate daily/therapeutic doses (3.5 and 4.6 mg/kg) did not produce adverse effects on liver and kidney function.[23] LD50 and lowest published toxic dose (TDLo) of root bark extract Moringa oleifera Lam. are 500 mg/kg and 184 mg/kg, respectively, when used intraperitoneally in rodents (mice). Changes in clotting factor, changes in serum composition (eg, total protein, bilirubin, cholesterol), along with enzyme inhibition, induction, or change in blood or tissue levels of other transferases have been noted.[24]
However, the interior flesh of the plant can also be dangerous if consumed too frequently or in large amounts. Even though the toxic root bark is removed, the flesh has been found to contain the alkaloid spirochin, which can cause nerve paralysis.[25]
Conclusion
A hormonal etiology of epithelial ovarian cancer has long been suspected, and now the role of FSHR has also been demonstrated.[26] Moringa oleifera can interfere with hormone receptor-related and neoplastic growth-related cytokine pathways via centrally acting mechanisms. It appears to have a tremendous effect on G protein-linked signal transduction system as well. Thus, the effects of Moringa oleifera Lam. in the treatment of epithelial ovarian cancer are worth investigating. The first stage of an experiment using Swiss-strain adult female mice is being completed by the present author to determine the effects of Moringa oleifera Lam. root, and results to date have proven to be similar to those demonstrated by Shukla and colleagues.[15] Further studies will be conducted at the receptor level, in ovarian cancer cell lines, and in experimental (ie, induced) ovarian cancer.
Footnotes
Readers are encouraged to respond to the author at ckbose@hotmail.com or to Paul Blumenthal, MD, Deputy Editor of MedGenMed, for the editor's eyes only or for possible publication via email: pblumen@stanford.edu
References
- 1.Biskind MS, Biskind GS. Development of tumours in the rat ovary after transplantation into the spleen. Proc Soc Exp Biol Med. 1944;55:176–179. [Google Scholar]
- 2.Choi JH, Choi KC, Auersperg N, Leung PC. Gonadotropins upregulate the epidermal growth factor receptor through activation of mitogen-activated protein kinases and phosphatidyl-inositol-3-kinase in human ovarian surface epithelial cells. Endocr Relat Cancer. 2005;12:407–421. doi: 10.1677/erc.1.00896. [DOI] [PubMed] [Google Scholar]
- 3.Zheng W, Magid MS, Kramer EE, Chen YT. Follicle-stimulating hormone receptor is expressed in human ovarian surface epithelium and fallopian tube. Am J Pathol. 1996;148:47–53. [PMC free article] [PubMed] [Google Scholar]
- 4.Costa-Lotufo LV, Khan MT, Ather A, et al. Studies of the anticancer potential of plants used in Bangladeshi folk medicine. J Ethnopharmacol. 2005;99:21–30. doi: 10.1016/j.jep.2005.01.041. [DOI] [PubMed] [Google Scholar]
- 5.Lampronti I, Khan MT, Bianchi N, et al. Bangladeshi medicinal plant extracts inhibiting molecular interactions between nuclear factors and target DNA sequences mimicking NF-kappaB binding sites. Med Chem. 2005;1:327–333. doi: 10.2174/1573406054368684. [DOI] [PubMed] [Google Scholar]
- 6.Kalkunte S, Swamy N, Dizon DS, Brard L. Benzyl isothiocyanate (BITC) induces apoptosis in ovarian cancer cells in vitro. J Exp Ther Oncol. 2006;5:287–300. [PubMed] [Google Scholar]
- 7.Satyan KS, Swamy N, Dizon DS, Singh R, Granai CO, Brard L. Phenethyl isothiocyanate (PEITC) inhibits growth of ovarian cancer cells by inducing apoptosis: role of caspase and MAPK activation. Gynecol Oncol. 2006;103:261–270. doi: 10.1016/j.ygyno.2006.03.002. [DOI] [PubMed] [Google Scholar]
- 8.Fahey JW. Moringa oleifera: a review of the medical evidence for its nutritional, therapeutic, and prophylactic properties. Part 1. Trees for Life Journal. 2005;1:5. [Google Scholar]
- 9.Rajangam J, Azahakia R, Manavalan S, Thangaraj T, Vijayakumar A. The Miracle Tree/The Multiple Attributes of Moringa. Dakar: CWS; 2001. Muthukrishan N. Status of production and utilisation of Moringa in Southern India; pp. 45–47. [Google Scholar]
- 10.Guevara AP, Vargas C, Sakurai H, et al. An antitumor promoter from Moringa oleifera Lam. Mutat Res. 1999;440:181–188. doi: 10.1016/s1383-5718(99)00025-x. [DOI] [PubMed] [Google Scholar]
- 11.Hsu R, Midcap S, Arbainsyah M, de Witte L. International Course on Economic Botany, National Herbarium Leiden. The Netherlands: Moringa oleifera: medicinal and socio-economic uses. September 2006. [Google Scholar]
- 12.Murakami A, Kitazono Y, Jiwajinda S, Koshimizu K, Ohigashi H. Niaziminin, a thiocarbamate from the leaves of Moringa oleifera, holds a strict structural requirement for inhibition of tumor-promoter-induced Epstein-Barr virus activation. Planta Med. 1998;64:319–323. doi: 10.1055/s-2006-957442. [DOI] [PubMed] [Google Scholar]
- 13.Bharali R, Tabassum J, Azad MR. Chemomodulatory effect of Moringa oleifera, Lam, on hepatic carcinogen metabolising enzymes, antioxidant parameters and skin papillomagenesis in mice. Asian Pac J Cancer Prev. 2003;4:131–139. [PubMed] [Google Scholar]
- 14.Shukla S, Mathur R, Prakash AO. Antifertility profile of the aqueous extract of Moringa oleifera roots. J Ethnopharmacol. 1988;22:51–62. doi: 10.1016/0378-8741(88)90230-9. [DOI] [PubMed] [Google Scholar]
- 15.Shukla S, Mathur R, Prakash AO. Biochemical and physiological alterations in female reproductive organs of cyclic rats treated with aqueous extract of Moringa oleifera Lam. Acta Eur Fertil. 1988;19:225–232. [PubMed] [Google Scholar]
- 16.Nath D, Sethi N, Singh RK, Jain AK. Commonly used Indian abortifacient plants with special reference to their teratologic effects in rats. J Ethnopharmacol. 1992;36:147–154. doi: 10.1016/0378-8741(92)90015-j. [DOI] [PubMed] [Google Scholar]
- 17.Bose CK. Role of nerve growth factor, follicle stimulating hormone receptor and epithelial ovarian cancer. RBM Online. 2005;11:194–197. doi: 10.1016/s1472-6483(10)60958-3. [DOI] [PubMed] [Google Scholar]
- 18.Ray K, Hazrai R, Guha D. Central inhibitory effect of Moringa oleifera root extract: possible role of neurotransmitters. Indian J Exp Biol. 2003;41:1279–1284. [PubMed] [Google Scholar]
- 19.Ray K, Hazra R, Debnath PK, Guha D. Role of 5-hydroxytryptamine in Moringa oleifera induced potentiation of pentobarbitone hypnosis in albino rats. Indian J Exp Biol. 2004;42:632–635. [PubMed] [Google Scholar]
- 20.Selman A, Yazigi R, Moyano L, Weinstein-Oppenheimer C, Lara HE, Romero C. Nerve growth factor and its high-affinity receptor trkA participate in the control of vascular endothelial growth factor expression in epithelial ovarian cancer. Gynecol Oncol. 2007 doi: 10.1016/j.ygyno.2006.07.007. in press. [DOI] [PubMed] [Google Scholar]
- 21.Faizi S, Siddiqui BS, Saleem R, Siddiqui S, Aftab K, Gilani AH. Isolation and structure elucidation of new nitrile and mustard oil glycosides from Moringa oleifera and their effect on blood pressure. J Nat Prod. 1994;57:1256–1261. doi: 10.1021/np50111a011. [DOI] [PubMed] [Google Scholar]
- 22.Bhattacharya J, Guha G, Bhattacharya B. Powder microscopy of bark–poison used for abortion: moringa pterygosperma gaertn. J Indian Forensic Sci. 1978;17:47–50. [PubMed] [Google Scholar]
- 23.Mazumder UK, Gupta M, Chakrabarti S, Pal D. Evaluation of hematological and hepatorenal functions of methanolic extract of Moringa oleifera Lam root treated mice. Indian J Exp Biol. 1999;37:612–614. [PubMed] [Google Scholar]
- 24.Woodard D, Stuart R. The Vermont Safe Information Resources, Inc. Material Safety Data Sheet collection. Available at: http://hazard.com/msds/tox/f/q77/q479.html. Accessed January 27, 2007.
- 25.Morton JF. The Horseradish Tree, Moringa Pterygosperma (Moringaceae) – a boon to arid lands? Economic Botany. 1991;45:318–333. [Google Scholar]
- 26.Choi J-H, Choi K-C, Auersperg N, Leung PCK. Overexpression of follicle-stimulating hormone receptor activates oncogenic pathways in preneoplastic ovarian surface epithelial cells. J Clin Endocrinol Metab. 2004;89:5508–5516. doi: 10.1210/jc.2004-0044. [DOI] [PubMed] [Google Scholar]


