Abstract
Phospholipid oxidation generates several bioactive aldehydes that remain esterified to the glycerol backbone (‘core’ aldehydes). These aldehydes induce endothelial cells to produce monocyte chemotactic factors and enhance monocyte–endothelium adhesion. They also serve as ligands of scavenger receptors for the uptake of oxidized lipoproteins or apoptotic cells. The biochemical pathways involved in phospholipid aldehyde metabolism, however, remain largely unknown. In the present study, we have examined the efficacy of the three mammalian AKR (aldo-keto reductase) families in catalysing the reduction of phospholipid aldehydes. The model phospholipid aldehyde POVPC [1-palmitoyl-2-(5-oxovaleroyl)-sn-glycero-3-phosphocholine] was efficiently reduced by members of the AKR1, but not by the AKR6 or the ARK7 family. In the AKR1 family, POVPC reductase activity was limited to AKR1A and B. No significant activity was observed with AKR1C enzymes. Among the active proteins, human AR (aldose reductase) (AKR1B1) showed the highest catalytic activity. The catalytic efficiency of human small intestinal AR (AKR1B10) was comparable with the murine AKR1B proteins 1B3 and 1B8. Among the murine proteins AKR1A4 and AKR1B7 showed appreciably lower catalytic activity as compared with 1B3 and 1B8. The human AKRs, 1B1 and 1B10, and the murine proteins, 1B3 and 1B8, also reduced C-7 and C-9 sn-2 aldehydes as well as POVPE [1-palmitoyl-2-(5-oxovaleroyl)-sn-glycero-3-phosphoethanolamine]. AKR1A4, B1, B7 and B8 catalysed the reduction of aldehydes generated in oxidized C16:0-20:4 phosphatidylcholine with acyl, plasmenyl or alkyl linkage at the sn-1 position or C16:0-20:4 phosphatidylglycerol or phosphatidic acid. AKR1B1 displayed the highest activity with phosphatidic acids; AKR1A4 was more efficient with long-chain aldehydes such as 5-hydroxy-8-oxo-6-octenoyl derivatives, whereas AKR1B8 preferred phosphatidylglycerol. These results suggest that proteins of the AKR1A and B families are efficient phospholipid aldehyde reductases, with non-overlapping substrate specificity, and may be involved in tissue-specific metabolism of endogenous or dietary phospholipid aldehydes.
Keywords: aldo-keto reductase, atherosclerosis, electrospray ionization mass spectrometry, lipid peroxidation, 1-palmitoyl-2-(5-oxovaleroyl)-sn-glycero-3-phosphocholine (POVPC), phospholipid aldehyde
Abbreviations: ALDR, aldehyde reductase; 1-alkyl-PAPC, 1-O-hexadecyl-2-arachidonoyl-PC; 1-alkyl-phosphocholine, 1-alkyl-2-arachidonyl-sn-glycero-3-phosphocholine; AKR, aldo-keto reductase; AR, aldose reductase; DTT, dithiothreitol; ESI–MS, electrospray ionization mass spectrometry; FGF-1, fibroblast growth factor 1; 4-HNE, 4-hydroxynonenal; HSD, hydroxysteroid dehydrogenase; LDL, low-density lipoprotein; MVDP, mouse vas deferens protein; PAPA, 1-palmitoyl-2-arachidonoyl-sn-glycero-3-phosphatidic acid; PAPC, 1-palmitoyl-2-arachidonoyl-sn-glycero-3-phosphocholine; PAPG, 1-palmitoyl-2-arachidonoyl-sn-glycero-3-phosphoglycerol; PDHOPC, 1-palmitoyl-2-(5,8-dihydroxyoctenoyl)-sn-glycero-3-phosphocholine; PGPC, 1-palmitoyl-2-glutaryl-phosphocholine; PHHPC, 1-palmitoyl-2-(7-hydroxy-5-heptenoyl)-sn-glycero-3-phosphocholine; PHOOPC, 1-palmitoyl-2-(5-hydroxy-8-oxo-6-octenoyl)-sn-glycero-3-phosphocholine; pPHOOPC, plasmenyl PHOOPC; PHVPC, 1-palmitoyl-2-(5-hydroxyvaleroyl)-sn-glycero-3-phosphocholine; plasmenyl PC, 1-plasmenyl-2-arachidonyl-sn-glycero-3-phosphocholine; POHPC, 1-palmitoyl-2-(7-oxo-5-heptenoyl)-sn-glycero-3-phosphocholine; POHyPC, 1-palmitoyl-2-(7-oxoheptanoyl)-sn-glycero-3-phosphocholine; PONyPC, 1-palmitoyl-2-(9-oxononanoyl)-sn-glycero-3-phosphocholine; POVPC, 1-palmitoyl-2-(5-oxovaleroyl)-sn-glycero-3-phosphocholine; POVPE, 1-palmitoyl-2-(5-oxovaleroyl)-sn-glycero-3-phosphatidylethanolamine; pPAPC, plasmenyl PAPC, 1-O-hexadec-1′-enyl-2-arachidonoyl-phosphocholine
INTRODUCTION
Unsaturated fatty acids of lipoproteins and membrane lipids are highly susceptible to oxidation and oxidative modifications [1]. Although under normal physiological conditions, antioxidant defences prevent excessive oxidation, products of lipid peroxidation accumulate under several pathological states, most notably, atherosclerosis [2] and Alzheimer's disease [3], myocardial ischaemia/reperfusion [4], and heart failure [5]. Products generated from oxidized lipids have also been shown to accumulate in cells undergoing apoptosis [6], indicating that the formation of lipid peroxidation products may be a general feature of oxidative stress or cell injury due to aetiologically unrelated insults.
Non-enzymatic oxidation of lipids results in the formation of a wide range of products. Radical attack at the polyunsaturated centres of fatty acids esterified to cholesterol and phospholipids followed by the addition of oxygen results in the formation of lipid hydroperoxides [1,7] that undergo carbon–carbon cleavage via alkoxyl radicals. After multiple successive reactions, this process results in the formation of short-chain (C3–C12) free aldehydes (unesterified aldehydes) such as 4-HNE (4-hydroxynonenal) and abbreviated fatty acid chains that remain esterified to cholesterol and phospholipids (‘core’ aldehydes). Core phospholipid aldehydes, such as POVPC [1-palmitoyl-2-(5-oxovaleroyl)-sn-glycero-3-phosphocholine] are generated in high quantities in oxidized LDL (low-density lipoprotein) [8]. Similar aldehydes are generated upon the oxidation of linolenic or linoleic acid containing phospholipids [9]. Abundant core phospholipid aldehydes have been detected in atherosclerotic plaques of animal [8] and humans [10] and in the membranes of aged erythrocytes and apoptotic cells [6,11]. Recent studies demonstrate that phospholipid aldehydes are also formed in ischaemic tissue [12], indicating that the generation and reactivity of phospholipid aldehydes may be a general feature of oxidative stress.
Core aldehydes generated from oxidized lipids display high biological activity. Phospholipid aldehydes such as POVPC [8] and the γ-hydroxy,α,β-unsaturated phospholipid aldehyde, PHOOPC [1-palmitoyl-2-(5-hydroxy-8-oxo-6-octenoyl)-sn-glycero-3-phosphocholine] [13], activate the endothelium to bind monocytes and increase the synthesis of pro-inflammatory chemokines, MCP-1 (monocyte chemotactic protein-1) and IL-8 (interleukin-8). Core aldehydes generated in oxidized phospholipids have also been shown to trigger apoptotic signalling [14], stimulate nitric oxide production [15], and serve as pattern recognition ligands in oxLDL [16] and in apoptotic cells [17] for their uptake by macrophages.
We have shown that both free [18,19] as well as core aldehydes [20] are efficiently reduced by the polyol pathway enzyme AR (aldose reductase) (AKR1B1, where AKR stands for aldo-keto reductase) and that catalytic reduction by this enzyme is a significant metabolic fate of POVPC in fibroblasts and monocytes [20]. This is the only enzymatic reduction pathway of POVPC metabolism reported to date. Nevertheless, AR is a member of a large family of phylogenetically conserved oxidoreductases, which are structurally related to each other. Fifteen distinct families of AKRs have been described [21]. These include a variety of ALDRs (aldehyde reductases) (AKR1A/B) and HSDs (hydroxysteroid dehydrogenases) (AKR1C), the β-subunits of the voltage-sensitive potassium channels (AKR6) and aflatoxin reductase (AKR7). The AKRs share overlapping substrate specificity. For instance, steroids are particularly good substrates for the AKR1 family, which also display high activity with aromatic aldehydes. Previous studies have shown that several of the AKRs can catalyse the reduction of lipid peroxidation-derived free aldehydes such as HNE. In addition to AKR1B1 [19], HNE-reducing activity has also been reported with FR-1 [FGF-1 (fibroblast growth factor 1)-regulated protein-1] (AKR1B8; [22]), human ALDR (AKR1A1; [23,24]), and HSDs (AKR1C; [23]). Whether multiple members of the AKR family also catalyse the reduction of core phospholipid aldehydes is not known. Hence, the present study was designed to examine the relative catalytic efficacy of the three mammalian families of AKRs in reducing phospholipid aldehydes of diverse structure. Our results suggest that phospholipid ALDR activity is a unique feature of the AKR1A/B family, which, depending on their level of expression, could regulate the metabolism of phospholipid aldehydes in a tissue-specific manner.
EXPERIMENTAL
Materials
Lipids and phospholipids were purchased from Avanti Polar Lipids (Alabaster, AL, U.S.A.). Sephadex G-25 (PD-10) columns were purchased from Pharmacia Fine Chemical (Uppsala, Sweden). MacroQ ion exchange and Affi-Gel Blue dye affinity reagents were obtained from Bio-Rad (Hercules, CA, U.S.A.). Solvents and other analytical grade reagents were obtained from Sigma Chemical Co.
Methods
Synthesis of phospholipid aldehydes
POVPC was synthesized as described previously [20]. The synthesis of POHyPC [1-palmitoyl-2-(7-oxoheptanoyl)-sn-glycero-3-phosphocholine] and PONyPC [1-palmitoyl-2-(9-oxononanoyl)-sn-glycero-3-phosphocholine] is outlined in Scheme 1. Briefly, for POHyPC synthesis, 25 mmol of ethyl-6-bromocaproate (I), was stirred with dry heptane at −78 °C under nitrogen and mixed drop-wise with 25 mmol of DIBAL-H (di-isobutylaluminium hydride) in hexane. The reaction mixture was stirred for 2 h and acidified with 2 M HCl. The reaction mixture was warmed up to room temperature (25 °C) and the organic phase was separated, washed with water, and dried over anhydrous sodium sulfate and filtered to yield 6-bromohexanal (II; 22 mmol). Trimethyl orthoformate (66 mmol) and p-toluenesulfonic acid (0.2 mmol) were added to a solution of the crude 6-bromohexanal in dry methanol and the mixture was refluxed for 6 h. A 3% solution of KOH in methanol was then added and the mixture was poured on ice-cold saturated sodium bicarbonate solution. The organic phase was separated and the aqueous layer extracted with pentane. The combined organic extracts were dried over sodium sulfate, filtered and distilled under vacuum to yield 6-bromohexanal dimethyl acetal (III; 18 mmol, 70% yield). The 6-bromohexanal dimethyl acetal (5 mmol) was dissolved in DMF (dimethylformamide) and mixed with 10 mmol of potassium cyanide and 0.5 mmol of 18-crown-6 (catalyst). The reaction mixture was stirred for 48 h at room temperature, concentrated and the residue was diluted with water. The aqueous layer was extracted with diethyl ether (3×20 ml), and combined organic extracts were dried over sodium sulfate, filtered and concentrated to yield 7,7-dimethoxyheptanitrile (IV; 3.5 mmol, 65% yield). The 7,7-dimethoxyheptanitrile (3.5 mmol) was mixed with 10 ml of aq. 11 mmol KOH and refluxed for 24 h. The reaction mixture was acidified with 0.1 M HCl in an ice bath and extracted with diethyl ether (3×25 ml). The combined organic extracts were dried over sodium sulfate and filtered. The solvent was removed under vacuum to yield 7,7-dimethoxyheptanoic acid (V; 3 mmol, 85% yield). The NMR spectra of the resultant 7,7-dimethoxyheptanoic acid in CDCl3 are as follows: 1H (500 MHz): δ 4.39–4.37 (1 H, t), 3.33 (6 H, s), 2.38–2.35(2 H, t), 1.67–1.6 (4 H, m), 1.42–37 (4 H, m); 13C NMR (125 MHz): δ 179.6, 104.6, 52.8, 34.1, 32.48, 29.08, 24.8, 24.45. The 7,7-dimethoxy heptanoic acid (0.53 mmol) was coupled with 1-palmitoyl-2-hydroxy-sn-glycereo-3-phosphocholine (0.17 mmol) as described previously [20], to yield 1-palmitoyl-2-(7,7-dimethoxyheptanoyl)-sn-glycero-3-phosphocholine (VI) which was then hydrolysed with Amberlyst-15 resin to generate POHyPC (VII). The 1H NMR characteristics of the resultant POHyPC in CDCl3 at 500 MHz were: δ 9.79 (1 H, s), 5.21 (1 H, m), 4.42–4.35 (3 H, m), 4.16–4.11 (1 H, m), 3.98–3.96 (2 H, m), 3..33–3.31 (2 H, m), 3.23 (9 H, s), 2.45 (2 H, t), 2.38 (2 H, t), 2.28 (2 H, t), 1.61 (8 H, m), 1.25 (30H, m), 0.84 (3 H, t).
Scheme 1. Synthesis of sn-2-substituted phosphatidylcholines.
For PONyPC synthesis, 24 mmol of 8-bromo-1-octanol (VIII) was mixed with of 48 mmol of pyridinium chlorochromate in 20 ml of dichloromethane. The reaction mixture was stirred for 6 h at room temperature and mixed with 40 ml diethyl ether. The reaction mixture was filtered through neutral alumina and concentrated to provide 8-bromo-octanal (IX; 22 mmol, 90% yield). The resultant 8-bromo-octanal was converted into 8-bromo-octanal dimethyl acetal (X), as described for 6-bromohexanal. Cyanation followed by basic hydrolysis of 8-bromo-octanal dimethyl acetal yielded 9,9-dimethoxynonanoic (XII). The NMR spectra of the resultant 9,9-dimethoxynonanoic acid in CDCl3 are as follows: 1H NMR (500 MHz): δ 8.8–8.2 (1 H broad), 4.38 (1 H), 3.38 (6 H), 2.38 (2 H), 1.67 (4 H), 1.39 (8 H). 13C NMR (125 MHz): δ 179.2, 104.70, 52.69, 34.2, 32.5, 29.4, 29.3, 29.1, 29.0, 24.8. The 9,9-dimethoxynonanoic acid was coupled with 1-palmitoyl-2-hydroxy-sn-glycero-3-phosphocholine to yield 1-palmitoyl-2-(9,9-dimethoxynonanoyl)-sn-glycereo-3-phosphocholine (XIII) which was then hydrolysed with Amberlyst-15 resin to generate PONyPC (XIV), as described above for POHyPC. The 1H NMR characteristics of the resultant PONyPC in CDCl3 at 500 MHz were: (CDCl3, 500 MHz): δ 9.79 (1 H, s), 4.32 (8 H, m), 3.23 (9 H, s), 2.4 (6 H, m), 1.61 (8 H, m), 1.25 (30H, m), 0.84 (3 H, t).
POVPE [1-palmitoyl-2-(5-oxovaleroyl)-sn-glycero-3-phosphatidylethanolamine] was synthesized using a slightly modified version of the procedure published previously [25]. Briefly, 2-lyso-phosphatidylethanolamine (XV; 0.22 mmol; Scheme 2) was dissolved in 2.5 ml of 1 M Na2CO3 solution and mixed with 0.23 mmol of 2-trimethylsilylethyl-4-nitrophenylcarbonate. The reaction mixture was stirred under nitrogen for 48 h and filtered through silica gel. The crude product was purified by flash chromatography (15% methanol and chloroform) to afford 1-palmitoyl-2-hydroxy-sn-glycero-3-phosphatidyl-(2-trimethylsilyl-ethoxycarbonylamino)-ethanol (XVI; 0.183 mmol). 1-Palmitoyl-2-hydroxy-sn-glycero-3-phosphatidyl-(2-trimethylsilyl-ethoxycarbonylamino) ethanol was coupled with 5,5-dimethoxy pentanoic acid to yield 1-palmitoyl-2-(5,5-dimethoxy-valeroyl)-sn-glycero-3-phosphatidyl-(2-trimethylsilylethoxycarbonylamino) ethanol (XVII; 0.092 mmol, 50%) as described for POHyPC. The resultant 1-palmitoyl-2-(5,5-dimethoxy-valeroyl)-sn-glycero-3-phosphatidyl-(2-trimethylsilyl-ethoxycarbonylamino)-ethanol (0.063 mmol) was mixed with 0.15 mmol of TBAF (tetrabutyl ammonium fluoride) in THF (tetrahydrofuran) and stirred for 48 h at room temperature. The reaction mixture was concentrated and the crude product purified by flash column chromatography (methanol/chloroform/water; 60:38:2, by vol.) to yield 1-palmitoyl-2-(5,5-dimethoxy-valeroyl)-sn-glycero-3-phosphatidylethanolamine (XVIII, 0.035 mmol, 55%). 1H NMR (500 MHz, CDCl3) δ 8.2 (3 H), 5.23 (1 H), 4.4–3.89 (6 H), 3.3 (6 H), 3.2 (2 H), 2.25 (4 H), 1.8–1.6 (6 H), 1.27 (24H), 0.9 (3 H). The resultant 1-palmitoyl-2-(5,5-dimethoxy-valeroyl)-sn-glycero-3-phosphatidylethanolamine was hydrolysed with Amberlyst-15 resin to yield 1-palmitoyl-2-(5-oxovaleroyl)-sn-glycero-3-phosphatidylethanolamine (XIX; 0.17 mmol, 50%). The 1H NMR characteristics of POVPE in CDCl3 at 500 MHz were: δ 9.71 (1 H), 8.2 (3 H), 5.23 (1 H), 4.32–3.89 (6 H), 3.3 (2 H), 2.54–2.2 (6 H), 1.9 (2 H), 1.60 (2 H), 1.27 (24 H), 0.9 (3 H).
Scheme 2. Synthesis of sn-2-substituted ethanolamine.
Cloning, expression, and purification of AKRs
Expression constructs for the AKR genes were made by inserting the coding sequence of the cDNA into the NdeI (5′-end) and XhoI (3′-end) sites of pET 28a vector containing a His tag. Corresponding coding sequences were amplified by RT (reverse transcriptase)–PCR from mRNA isolated from murine heart (AKR1B3 and AKR1B8), kidney (AKR1B7 and AKR1C18), liver (AKR1C6 and AKR1C14) and stomach (AKR7A5). The NdeI and XhoI restriction sites were engineered in the PCR primers. The entire length of all the inserts was sequenced and confirmed. Sequence of all the AKRs were identical with their GenBank® accession numbers (Table 1). Although a longer isoform of aflatoxin reductase containing a Golgi localization signal at the N-terminus (GenBank® accession number NM_025337) has been described [26], we used the cytosolic isoform of the protein. The constructs provided expression of AKRs with His tag attached to their N-termini in the BL-21 (DE 3) strain of Escherichia coli. Proteins were purified on a Ni-affinity column as described previously [27]. The catalytic activities of His tag proteins (Table 1) were similar to those of the non-His tag proteins [22,28], and deletion of the His tag by thrombin also did not affect enzyme activities of AKR1B1 and 1B8 (S. Srivastava and A. Bhatnagar, unpublished work). AKR1B1, AKR1A4 and β-subunit of the voltage-sensitive K+ channels (β2; AKR6A) were prepared as described earlier [20,27,29]. Human small intestine reductase (AKR1B10) was cloned into the pET23 expression vector for expression without the His tag. Purification was achieved by chromatography over MacroQ ion exchange and Affi-Gel Blue dye affinity media operated essentially as described previously for other mammalian AKRs [30]. Purity of each protein was established by SDS/PAGE and the catalytic activity was measured with their known substrates as described below.
Table 1. Steady-state kinetic parameters of AKRs.
The enzyme activity was determined in 0.1 M phosphate (pH 7.0) using the indicated substrate and 0.15 mM NADPH at 25 °C. Proteins were reduced with DTT prior to assay. S.D. was <25% for Km and <10% for kcat.
| Proteins | GenBank® accession number | Substrate | Km (μM) | kcat (min−1) | kcat/Km (min−1·μM−1) |
|---|---|---|---|---|---|
| AKR1A4 | NM_021473 | Methylglyoxal | 1100 | 458 | 0.42 |
| AKR1B1 | NM_001628 | Glyceraldehyde | 64 | 32 | 0.43 |
| AKR1B3 | NM_009658 | Glyceraldehyde | 48 | 20.3 | 0.43 |
| AKR1B7 | NM_009731 | p-Nitrobenzaldehyde | 140 | 1.6 | 0.01 |
| AKR1B8 | NM_008012 | Glyceraldehyde | 1300 | 24.4 | .019 |
| AKR1B10 | NM_020299 | Glyceraldehyde | 2000 | 13.6 | 0.007 |
| AKR1C6 | NM_030611 | p-Nitrobenzaldehyde | 37 | 14.6 | 0.39 |
| AKR1C14 | NM_134072 | p-Nitrobenzaldehyde | 172 | 108 | 0.63 |
| AKR1C18 | NM_134066 | p-Nitrobenzaldehyde | 78 | 6.7 | 0.086 |
| AKR7A5 | NM_025337 | Succinic semialdehyde | 6 | 50 | 8.3 |
Enzyme activity measurements
Before each experiment, the stored enzymes were reduced by incubating with 0.1 M DTT (dithiothreitol) at 37 °C for 1 h in 0.1 M Tris/HCl (pH 8.0). Excess DTT was removed by gel filtration on a Sephadex G-25 column (PD-10), pre-equilibrated with nitrogen-saturated 0.1 M potassium phosphate (pH 7.0), containing 1 mM EDTA. To prevent enzyme oxidation, all operations were performed at 4 °C.
Catalytic activity was measured at 37 °C in a 1 ml reaction system containing 0.1 M potassium phosphate buffer (pH 7.0) with 0.15 mM NADPH and various concentrations of substrates. Purity of the phospholipid aldehydes was established by ESI+–MS [electrospray ionization (positive ion mode)–MS] and their concentration was determined by measuring Pi and by measuring their choline content (for phosphatidylcholine-containing aldehydes; [20,31]). Reaction progress was monitored by measuring the decrease in absorbance at 340 nm due to NADPH oxidation using a Varian spectrophotometer (Cary 50 Bio). One unit of the enzyme is defined as the amount of the protein required to oxidize 1 μmol of NADPH per min. The control cuvette contained all components of the mixture except the substrate. Initial velocity was measured at seven to nine different concentrations of the substrates, and steady-state kinetic parameters were calculated by fitting the general Michaelis–Menten equation to the data using a nonlinear iterative fitting procedure [19].
Oxidation, reduction, and analysis of phospholipids
To examine whether AKRs can reduce complex mixtures of phospholipid aldehydes, 50 μg of the arachidonic acid-containing phospholipids: PAPC (1-palmitoyl-2-arachidonoyl-sn-glycero-3-phosphocholine), PAPG (1-palmitoyl-2-arachidonoyl-sn-glycero-3-phosphoglycerol) and PAPA (1-palmitoyl-2-arachidonoyl-sn-glycero-3-phosphatidic acid), as well as 1-alkyl-PC (1-alkyl-2-arachidonyl-sn-glycero-3-phosphocholine) and plasmenyl PC (1-plasmenyl-2-arachidonyl-sn-glycero-3-phosphocholine) were oxidized in air for 24–72 h, and incubated with AKR proteins as described previously [20]. The lipids were extracted and analysed by ESI–MS using a Micromass ZMD 2000 mass spectrometer (Waters–Micromass, Milford, MA, U.S.A.). For positive ionization mode, 2:1 (v/v) methanol/chloroform containing 1% acetic acid was used as the flow injection solvent, whereas, for negative ionization mode, 2:1 methanol/chloroform containing 10 mM ammonium hydroxide was used. Samples were injected into the ESI–MS using a Harvard syringe pump at a flow rate of 10 μl/min. The ESI–MS operating parameters were as follows: capillary voltage 3.38 kV, cone voltage 25 V, extractor voltage 9 V, RF lens voltage 0.9 V, source block and desolvation temperatures 100 and 200 °C respectively. Nitrogen was used as the nebulizer gas at a flow rate of 200 litres/h. Spectra were acquired at a rate of 275 a.m.u. (atomic mass units) per second over the mass range of 2–1000 a.m.u. Spectra were average over a period of 5 min or 100 scans.
RESULTS
Characterization of AKRs
Catalytic activity of AKR proteins was measured with their known substrates (Table 1). The steady-state kinetic parameters of AKR1A, AKR1B and AKR7A proteins with their known substrates are comparable with earlier reports [20,28,29,32,33]. Little is known about the catalytic activity of murine HSDs (AKR1C). We examined the catalytic activity of these proteins with p-nitrobenzaldehyde. As shown in Table 1, the AKR1C proteins show high catalytic activity with p-nitrobenzaldehyde. The steady-state kinetic parameters/catalytic activity of AKR1C proteins are comparable with other mammalian HSDs [34,35].
Catalytic efficiency of AKRs with phospholipid aldehydes
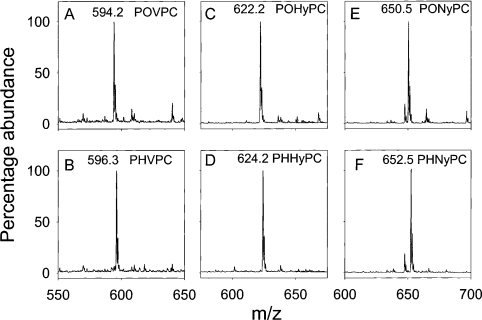
Steady-state kinetic parameters were determined to examine the catalytic efficiency of the three AKR families expressed in mammals (AKR1, AKR6 and AKR7). For the first series of experiments, POVPC was used as the model phospholipid aldehyde because it is one of the most abundant products of oxidation of one of the most common phospholipids (PAPC). POVPC was synthesized as described earlier [20], and its purity was established by ESI+–MS. The ESI+–MS spectrum of POVPC showed a single molecular ion with the expected m/z value of 594 (Figure 1). The reagent POVPC was incubated with several AKRs in the presence of NADPH, and the extent of NADPH oxidation was monitored to calculate the rate of reduction. POVPC was efficiently reduced by human AR (AKR1B1) with Km and kcat values similar to those reported before (Table 2;[20]). In addition to AKR1B1, significant reduction of POVPC was also observed with AKR1B3 (murine AR), ARK1B7 [MVDP (mouse vas deferens protein)], 1B8 (AKR1B8), 1B10 (human small intestine AR) and 1A4 (ALDR). The steady-state kinetic parameters for AKR1B3, 7 and 10 were similar, indicating that these three enzymes catalyse POVPC reduction with equal efficiency. To confirm reduction, the structure of the product generated by AKR1B8 was examined by ESI+–MS. As shown in Figure 1, incubation with AKR1B8 led to the conversion of POVPC ion (m/z 594.2) into an ion of m/z 596.3, consistent with the formation of PHVPC [1-palmitoyl-2-(5-hydroxyvaleroyl)-sn-glycero-3-phosphocholine], the reduced product of POVPC (Figure 1). The reduction of POVPC was much less efficient with AKR1B7 and ARK1A4, but for different reasons. With ARK1B7, the Km POVPC values were high (Table 2). In contrast, even though Km POVPC of ARK1A4 was comparable with the high efficiency AKR1B proteins, the kcat was much lower, suggesting that the catalytic efficiency of this enzyme with POVPC may be lower than the AKR1B family (Table 2). Members of the AKR1C family, namely, AKR1C6 and AKR1C18, showed only traces of activity with POVPC (200 μM), whereas AKR1C14 displayed no catalytic activity with POVPC. Traces of activity were observed when POVPC (200 μM) was tested as a substrate with AKR6A (human Kvβ2), whereas no detectable reduction of POVPC was observed with AKR7A5 (murine aflatoxin reductase). On the basis of these observations, we conclude that only AKR1A and ARK1B proteins are likely to be physiologically significant POVPC reductases.
Figure 1. AKR1B8 catalyses the reduction of phospholipid aldehydes.
Reagent POVPC, POHyPC or PONyPC (50 μg) were incubated without or with recombinant AKR1B8 (100 μg) and NADPH (150 μM) in 0.1 M potassium phosphate (pH 6.0) for 3 h at 30 °C. The lipids were extracted in chloroform/methanol/water and analysed by ESI+–MS. Mass spectrum shows that reagent POVPC, POHyPC and PONyPC correspond to molecular ions with m/z 594.2 (A), 622.2 (C) and 650.5 (E) respectively, which are increased by 2 Da upon reduction with AKR1B8, indicating the formation of 1-palmitoyl-2-(5-hydroxyvaleroyl) phosphatidylcholine (PHVPC; B; m/z 596.3), 1-palmitoyl-2-(7-hydroxyheptanoyl)-sn-glycero-3-phosphocholine (PHHyPC; D, m/z 624.2) and 1-palmitoyl-2-(9-hydroxynonanoyl)-sn-glycero-3-phosphocholine (PHNyPC; F; m/z 652.5).
Table 2. Steady-state kinetic parameters for the reduction of phospholipid aldehydes by AKRs.
The enzyme activity was determined in 0.1 M phosphate (pH 7.0) using the indicated substrate and 0.15 mM NADPH at 37 °C. Proteins were reduced with DTT prior to assay. NDA, no detectable activity.
Although POVPC is the most abundant aldehyde generated from the oxidation of PAPC, biological membranes contain a mixture of fatty acid side chains of varying chain length and unsaturation; hence phospholipid aldehydes of different chain lengths are generated [9,20]. To determine whether the AKR family differs in the recognition of the chain length of the sn-2 aldehyde, we synthesized the C-7 (POHyPC) and C-9 (PONyPC) analogues of POVPC (see the ‘Methods’ subsection). Structures of these molecules were established by NMR and ESI–MS. The ESI+–MS of reagent POHyPC and PONyPC showed strong molecular ions at m/z 622.2 and 650.5 respectively. Incubation of these compounds with NADPH and AKR1B proteins, e.g. AKR1B8 (Figure 1), resulted in an increase in the m/z values by 2 Da, consistent with the reduction of the ω-aldehydes to their corresponding alcohols. The steady-state kinetic parameters for the reduction of POHyPC and PONyPC are listed in Table 2. All AKR1B proteins reduced POHyPC and PONyPC with a Km of 13–19 μM; however, compared with other AKR1B proteins, AKR1B1 (human AR) displayed a 2-fold higher kcat, resulting in a 1.5–3-fold greater catalytic activity than the murine proteins. The nature of sn-3 substitution did not affect phospholipid aldehyde reduction by the AKR1B family. As shown in Table 2, POVPE was reduced with a catalytic efficiency similar to that that of POVPC, indicating that both phosphatidylcholine and phosphatidylethanolamine family aldehyde derivatives are likely to be reduced with equal efficiency.
Reduction of phospholipid aldehyde mixtures
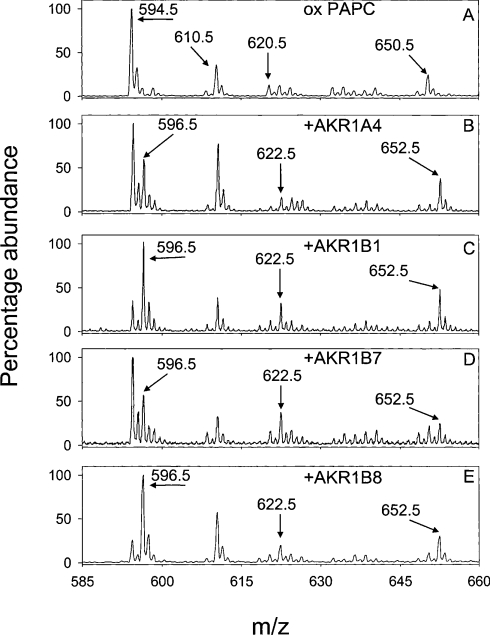
To examine further the efficacy of AKRs in catalysing the reduction of the wide range of lipid peroxidation-derived aldehydes generated by biological phospholipids, we oxidized palmitoyl and arachidonyl (16:0–20:4) phospholipids with different sn-1 or sn-3 linkages. To determine the influence of the sn-1 position, phospholipids with ester (PAPC) or diethyl ether linkages (-alkyl or plasmenyl) were used. To assess the contribution of the charged sn-3 group, phosphatidylglycerol and phosphatidic acid derivatives were used. The phospholipid, PAPC, was oxidized in air for 24 h and then incubated without or with AKR1B8, 1B7, 1A4 or 1B1 and 150 μM NADPH for 3 h at 30 °C. Upon incubation, phospholipids were extracted and analysed by ESI+–MS. As shown before [20], oxidation of PAPC generated multiple phospholipid aldehydes: POVPC (m/z 594.5), POHPC [1-palmitoyl-2-(7-oxo-5-heptenoyl)-sn-glycero-3-phosphocholine; m/z 620.5] and PHOOPC (m/z 650.5) (Figure 2A). The ion at m/z 610.5 is due to the formation of PGPC (1-palmitoyl-2-glutaryl-phosphocholine). Percentage reduction in 5-oxovaleroyl, 7-oxo-5-heptenoyl, and 5-hydroxy-8-oxo-6-octenoyl were monitored for each phospholipid with each of the AKRs. Incubation of oxPAPC (oxidized PAPC) with AKR1B8 and NADPH, for example, resulted in the appearance of new species with m/z values 2 Da higher than the parent phospholipid aldehydes (Figure 2), consistent with the reduction of POVPC, POHPC and PHOOPC to their corresponding alcohols. These ions were assigned to PHVPC (m/z 596.5), PHHPC [1-palmitoyl-2-(7-hydroxy-5-heptenoyl)-sn-glycero-3-phospho-choline; m/z 622.5] and PDHOPC [1-palmitoyl-2-(5, 8-dihydroxyoctenoyl)-sn-glycero-3-phosphocholine]; m/z 652.5]. Similar reduction profiles were observed with ARK1A4 and 1B7.
Figure 2. AKR1 proteins catalyse the reduction of phospholipid aldehydes generated from the oxidation of PAPC.
PAPC (50 μg) was oxidized in air for 24 h and incubated without or with 100 μg of the AKR1 proteins, AKR1A4, 1B1, 1B7 and 1B8, and 150 μM NADPH in 0.1 M potassium phosphate (pH 6.0) for 3 h at 30 °C. The lipids were extracted in methanol/chloroform/water and analysed by ESI+–MS. As shown in (A), oxidation of PAPC generates phospholipid aldehydes at m/z 594, 620 and 650 corresponding to POVPC, POHPC, and PHOOPC respectively and PGPC (m/z 610.5). Incubation of this mixture with the AKR1A4 (B), AKR1B1 (C), AKR1B7 (D) or AKR1B8 (E) resulted in the reduction of these phospholipid aldehydes to their corresponding alcohols, as evinced by a 2 Da increase in their m/z values.
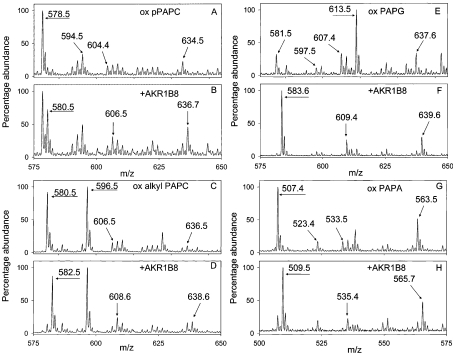
Plasmenyl phospholipids were also reduced by AKRs. Oxidation of pPAPC (plasmenyl PAPC; 1-O-hexadec-1′-enyl-2-arachidonoyl-phosphocholine) led to the formation of pPOVPC (m/z 578.5), pPOHPC (m/z 604.4), pPHOOPC (m/z 634.5) and pPGPC (m/z 594.5; Figure 3A). Incubation of oxidized pPAPC with AKR1B8 and NADPH reduced these phospholipid aldehydes (Figure 3B) yielding new species ascribed to pPHVPC (m/z 580.5), pPHHPC (m/z 606.5) and pPDHOPC (636.7). The corresponding 5-oxovaleroyl, 7-oxo-5-heptenoyl and 5-hydroxy-8-oxo-6-octenoyl (m/z 580.5, 606.5 and 636.5; Figure 3C) aldehydes derived from oxidized 1-alkyl-PC (1-alkyl-2-arachidonyl-sn-glycero-3-phosphocholine) were also reduced by the AKRs to their corresponding alcohols (m/z 582.5, 608.6 and 638.6). The strong molecular ion at m/z 596.5 is due to alkyl PGPC (Figures 3C and 3D). Changes in representative spectra of aldehyde phospholipids due to AKR reduction are shown in Figure 3.
Figure 3. AKR1B8 catalyses the reduction of aldehydes generated from the oxidation of pPAPC, 1-alkyl-PAPC (1-O-hexadecyl-2-arachidonoyl-phosphocholine), PAPG and PAPA.
Aliquots of pPAPC, 1-alkyl-PAPC, PAPG and PAPA (50 μg each) were oxidized in air for 24–72 h and resuspended in 0.1 M potassium phosphate buffer (pH 6.0) containing 0.15 mM NADPH and either left untreated or incubated with 100 μg of recombinant AKR1B8 for 3 h at 30 °C. The phospholipids were extracted and analysed by ESI–MS. Mass spectrum shows that oxidation of pPAPC (A) resulted in the formation of phospholipid aldehydes, pPOVPC (m/z 578.5), pPOHPC (m/z 604.4) and pPHOOPC (m/z 634.5), and pPGPC (m/z 594.5). The phospholipid aldehydes were reduced by AKR1B8 (B) to pPHVPC (m/z 580.5), pPHHPC (606.5) and pPDHOPC (636.7). Likewise, oxidation of 1-alkyl-PC (C) resulted in the formation of alkyl POVPC (m/z 580.5), alkyl PGPC (m/z 596.5), alkyl POHPC (m/z 606.5) and alkyl PHOOPC (m/z 636.5); oxidation of PAPG (E) resulted in the formation of POVPG (m/z 581.5), PGPG (m/z 597.5), POHPG (m/z 607.4) and PHOOPG (m/z 637.6), and oxidation of PAPA (G) resulted in the formation of POVPA (m/z 507.4), PGPA (m/z 523.4), POHPA (m/z 533.5) and PHOOPA (m/z 563.5). AKR1B8 efficiently catalysed the reduction of phospholipid aldehydes generated from the oxidation of 1-alkyl-PAPC (D), PAPG (F) and PAPA (H) to their corresponding alcohols, as evident by an increase of 2 Da in their m/z values. Oxidation of PAPG also resulted in the formation of a strong ion with m/z 613.5 (n=9), which disappeared upon reduction. Structural identity of this peak has not yet been established.
For C-5 aldehydes with acyl linkage at the sn-1 position, PAPA and PAPG were reduced more effectively by the AKRs than PAPC, indicating that in complex mixtures the choline head groups are likely to be the least preferred substrates (Figures 3E–3H, Table 3). Similarly, ether linkages in the sn-1 position tended to decrease the catalytic efficiency of reduction, suggesting that these lipids may be some of the poorest AKR substrates. The only exception was AKR1B8, which was more active with alkyl PC than with PAPC. Since alkyl lipids are particularly rich in the brain and heart, AKR1B8 may represent an efficient mechanism for their reduction in these tissues. In general, compared with other AKRs, AKR1B8 was most effective in catalysing the reduction of PAPC, alkyl and plasmenyl PC as well as PAPG. AKR1B1 on the other hand was more effective with PAPA than with any other phospholipid. For most head groups with C-5, AKR1A4 was the least active enzyme tested, indicating that this enzyme may not be efficient at catalysing the reduction of short-chain phospholipid aldehydes.
Table 3. AKRs catalyse the reduction of phospholipid aldehydes of diverse structures.
Arachidonic acid containing phospholipids, PAPC, pPAPC, 1-alkyl-PAPC, PAPG and PAPA (50 μg each), were oxidized in air for 24–72 h and incubated with 100 μg of protein and 150 μM NADPH in 0.1 M potassium phosphate (pH 6.0) at 30 °C for 3 h. Phospholipids were extracted and analysed by ESI–MS, as described in the Experimental section. The three main aldehydes generated from arachidonic acid oxidation are shown for each head group and enzyme. Percentage reduction was calculated from the decrease in the intensity of the parent aldehyde and the corresponding increase in the intensity of the alcohol ion ±S.D.
| Reduction (%) | |||||||
|---|---|---|---|---|---|---|---|
| Proteins | sn-2 Chain | Parent phospholipids… | PAPC | pPAPC | 1-alkyl-PAPC | PAPG | PAPA |
| AKR1A4 | 5-Oxovaleroyl | 36.8±3.9 | 28.2±7.4 | 31.2±4.3 | 90.6±1.6 | 53.9±18.8 | |
| 7-Oxo-5-heptenoyl | 57.8±5.3 | 42.3±4.9 | 69.1±2.8 | 63.3±16.4 | 57.8±22.7 | ||
| 5-Hydroxy-8-oxo-6-octenoyl | 78.5±2.2 | 61.1±7.6 | 58.7±3.5 | 80.5±4.2 | 74.0±17.0 | ||
| AKR1B1* | 5-Oxovaleroyl | 63.6±14.1 | 50.5±8.5 | 65.5±14.5 | 66.4±6.6 | 96.2±2.5 | |
| 7-Oxo-5-heptenoyl | 43.8±1.1 | 38.3±3.6 | 48.6±1.4 | 70.5±0.5 | 82.5±4.2 | ||
| 5-Hydroxy-8-oxo-6-octenoyl | 74.0±4.1 | 66.3±6.5 | 42.3±2.7 | 62.9±1.8 | 69.3±4.8 | ||
| AKR1B7 | 5-Oxovaleroyl | 42.5±10.3 | 57.0±6.4 | 73.3±7.6 | 77.5±4.3 | 73.1±7.6 | |
| 7-Oxo-5-heptenoyl | 71.2±8.9 | 34.8±10.9 | 75.0±2.5 | 73.8±6.2 | 74.7±4.6 | ||
| 5-Hydroxy-8-oxo-6-octenoyl | 63.7±11.4 | 76.5±4.5 | 63.5±0.9 | 68.5±6.8 | 83.7±2.9 | ||
| AKR1B8 | 5-Oxovaleroyl | 63.9±13.4 | 38.5±6.9 | 83.1±1.7 | 94.9±1.3 | 73.6±13.0 | |
| 7-Oxo-5-heptenoyl | 50.3±4.1 | 40.9±3.2 | 39.9±8.2 | 86.3±3.0 | 57.2±9.1 | ||
| 5-Hydroxy-8-oxo-6-octenoyl | 54.4±13.1 | 58.3±5.0 | 41.3±4.6 | 83.3±3.7 | 66.9±6.3 | ||
| *From [20]. | |||||||
The AKRs also catalysed the reduction of 7-oxo-heptenoyl phospholipid derivatives (Table 3). With this group of aldehydes, however, the plasmenyl derivatives were reduced with the least efficiency. In comparison, the alkyl groups were reduced to a greater extent especially with 1A4 and 1B7 AKRs. Significantly, most AKRs were moderately effective with C-7 aldehydes, suggesting that depending on their extent of expression and tissue abundance, any of these AKRs could participate in C-7 aldehyde metabolism (Table 3). A similar profile was evident from the reduction of 5-hydroxy-8-oxo-6-octenoyl phospholipids. In this case, the alkyl PC phospholipids were consistently good AKR substrates. The reduction of plasmenyl PC was not effective either, and for both plasmenyl PC and alkyl PC, ARK1B7 was the most efficient catalyst, whereas AKR1B8 was the least effective. Highest extent of reduction with this class of aldehydes was observed with AKR1B8 with PAPG, suggesting that glycerol at the sn-3 position facilitates the reduction of C-8 phospholipid aldehydes by AKR1B8. We observed the appearance of a strong molecular ion at m/z 613.5 upon oxidation of PAPG. This peak disappears upon reduction with AKRs, but the chemical identity of this peak has yet not been established.
In general, AKR1B1 and 1B8 behaved similarly; displaying a marked preference for short-chain aldehydes with ester linkages at the sn-1 position. Nevertheless, there were significant differences. AKR1B1 seemed to prefer the negatively charged head group of PAPA, whereas ARK1B8 seemed to accommodate alkyl linkages at the sn-1 position better than ARK1B1. The AKR1B7 and AKR1A4, on the other hand, seem to have similar catalytic preferences. Both these proteins were, in general, more active with longer chain aldehydes. For AKR1A4, the catalytic efficacy seems to increase with increasing chain length, regardless of the head group, indicating that the enzyme may be a significant participant in the metabolism of long-chain or substituted aldehydes. Taken together, these results demonstrate that AKRs are capable of reducing a wide range of phospholipid aldehydes of different linkages at the sn-1 and different substituents at the sn-3 position. Significantly, distinct substrate preferences were identified which indicate that the proteins may have unique, non-overlapping functions in phospholipid aldehyde reduction and metabolism.
DISCUSSION
The results of the present study show that several members of the AKR superfamily catalyse the reduction of structurally diverse phospholipid aldehydes. Previous studies from our laboratory have demonstrated that human AR (AKR1B1) is an efficient catalyst of POVPC reduction [20]. Our current findings extend and expand this observation by demonstrating comparable reductive capabilities of other AKRs. Our results also show that in addition to C-5 aldehydes, the AKR1A and 1B proteins catalyse the reduction of sn-2 C-7 and C-9 aldehydes as well as phospholipid aldehydes with different sn-1 and sn-3 substitutions. Collectively, these observations establish phospholipid ALDR activity as a unique feature of the AKR1A/B family, and suggest that multiple AKRs may be involved in the metabolism of phospholipid aldehydes and in regulating the biological effects of phospholipid aldehydes in tissues in which these enzymes are expressed.
The AKR superfamily comprises seven distinct clusters of proteins that are distributed across all phyla examined. To date more than 100 AKR proteins from bacteria, yeast, plants, invertebrates and vertebrates have been identified [21]. Mammalian AKRs are distributed between three major families, AKR1, AKR6 and AKR7. The AKR1 family is the most diverse – consisting of several ALDRs and HSDs. The AKR6 family is a class of three different proteins that represent the ancillary subunits of the voltage-sensitive potassium channels of the Kv1 and the Kv4 family. The AKR7 family members are aflatoxin reductases. These enzymes have been shown to be involved in a variety of tissue-specific roles ranging from glucose reduction to steroid metabolism. No general family-specific function has been assigned to AKRs, although on the basis of their wide substrate specificity it appears that in addition to catalysing the reduction of aldehyde metabolites these enzymes may also be involved in the general detoxification of environmental aldehydes or aldehydes generated endogenously from lipid peroxidation. This view is further supported by the results presented here showing that several members of the AKR1 family can catalyse the reduction of lipid peroxidation-derived phospholipid aldehydes.
We found that AKR1B and 1A proteins were efficient catalysts for the reduction of several ‘core’ aldehydes generated during the oxidation of phospholipids that are abundant in mammalian lipoproteins and cell membranes. No significant POVPC reduction was observed with the AKR1C family. This is surprising because previous studies have shown that AKR1C1 catalyses the reduction of the lipid peroxidation-derived free aldehyde, HNE, with a catalytic efficiency similar to the AKR1B family but far exceeding that of AKR1A1 [23]. Reasons for the inability of AKR1C proteins to catalyse the reduction of large phospholipid aldehydes are not clear, but may relate to the differences in the architecture of the active sites of AKR1A, B and C families. Our previous molecular modelling studies with AR (AKR1B1) suggest that the sn-2 chain of POVPC is well accommodated in the deep crevice of the β-barrel, and that the residues Trp20, Val47, Tyr48, Trp79, His110, Trp111, Phe121, Phe122, Trp219 and Leu300 are within 3 Å (1 Å=0.1 nm) of POVPC bound to the AR active site [20]. These studies also suggested that there might be hydrophilic interactions between the active site of the enzyme and sn-1 and the phospholipid head group, or sn-2 substituents and a potential hydrogen-bond interaction between phosphate oxygen and Nϵ1 of Trp20 [20]. Sequence alignments suggest that the tryptophan residue corresponding to Trp20 is present in AKR1B8, B7 and A4, which reduces POVPC, but was absent from AKR1C and AKR7A proteins that were inactive. Moreover, Phe121 and Phe121, which could potentially participate in hydrophobic interactions with POVPC, are conserved in active enzymes (AKR1B7, B8 and A4) but not in the inactive (AKR1C and 7A5) AKRs, suggesting that there may be specific differences within the active site of these enzymes that may allow them to discriminate between free and phospholipid aldehydes and that unique features of the AKR1A and B active sites may be optimized and conserved for phospholipid aldehyde reduction. Further studies are required to assess the contribution of individual active site residues in phospholipid binding and catalysis by the ARK1A and B families.
No catalytic reduction of POVPC was observed with AKR7A5 protein. The AKR7 proteins catalyse the reduction of dicarbonyl-containing compounds such as aflatoxin B1-dialdehyde [36]. The rat protein (AKR7A1) displays low activity with α,β-unsaturated carbonyl compounds [36]; however, in comparison with other AKRs, the active site of AKR7 proteins contains several charged amino acids that may prevent the binding of bulky hydrophobic molecules such as phospholipids [37]. Moreover, the active site is smaller and less open. The mouse protein (AKR7A5) in particular [38] has bulky residues at the active site that restrict the size of the substrate-binding pocket, which could, in part, explain the inability of this protein to catalyse POVPC reduction.
Although we observed only traces of enzyme activity with AKR6A, it is possible that in vivo the enzyme may be involved in the reduction of phospholipid aldehydes. Recent work by Weng et al. [39] shows that the AKR6A has weak catalytic activity with 4-carboxybenzaldehyde. The overall rate of catalysis, however, is slow, and at the lower limit of our spectrophotometric assay. Nonetheless, the Kvβ2 active site does contain a tryptophan residue corresponding to Trp20 of AR, suggesting that the protein may be capable of binding (if not reducing) POVPC. In contrast with AKR6 and AKR7 families, robust POVPC reductase activity was observed with the AKR1A and AKR1B proteins and, therefore, we focused on these enzymes as they are likely to be the most significant AKRs involved in the metabolism and detoxification of POVPC.
The AKR1A and AKR1B families are widely distributed in several tissues. The most well-studied member of these families, AKR1B1 or human AR, is present in high abundance in most tissues with the exception of liver. Particularly high AR expression levels have been detected in heart, skeletal muscle, lens and brain [40,41], suggesting that in these tissues this may be an important route of POVPC metabolism. AR is also expressed in monocytes [20], macrophages [42], neutrophils [43] and the endothelium [44], indicating that AR may be the most relevant metabolic pathway in tissues that are most likely to be exposed to oxidized lipoproteins or apoptotic cells. In contrast with cardiovascular tissues, the levels of AR are low in the liver and the renal cortex. In these tissues ALDR is expressed at higher levels than AR [45]. The substrate specificities of AR and ALDR are, however, different [46]. Unlike AR, ALDR does not reduce glucose and it is much less efficient in reducing lipid peroxidation-derived free aldehydes such as HNE than either AKR1B1 or AKR1C1 [23,24]. In the present study, murine ALDR, AKR1A3, was found to reduce POVPC and many other phospholipid aldehydes. Significantly, the substrate specificity of AKR1A4 was found to be different from the AKR1B group of enzymes, and ARK1A3 displayed higher efficiency with long-chain sn-2 aldehydes such as the 5-hydroxy-8-oxo-6-octenoyl derivatives, suggesting that ALDR may have a specific role in reducing phospholipid aldehydes particularly in the kidney, brain and liver where it is expressed in high abundance and that it may be particularly effective with long-chain phospholipid aldehydes.
Phospholipid ALDR activity was also observed with AKR1B7 and AKR1B8. The AKR1B7 or the MVDP is expressed mainly in the adrenal glands, although low levels are also present in eye, intestine, seminal vesicles, kidney, liver, testis and lung [47]. In addition to mice, it is also expressed in human, guinea-pig, rat and rabbit adrenal glands [48]. The enzyme displays high affinity for isocaproaldehyde, suggesting that it may be involved in removing the cholesterol cleavage product and preventing its toxicity [49]. The protein also reduces HNE with high affinity and HNE is the best endogenous substrate of the enzyme identified to date, indicating that detoxification of lipid peroxidation products may be an additional role of the enzyme [48]. This view is further reinforced by our current observations that MVDP catalyses the reduction of POVPC and related phospholipid aldehydes.
Efficient phospholipid ALDR activity was also observed with AKR1B8. This protein was first identified as a delayed-early gene product induced by FGF-1 in NIH 3T3 cells [50], indicating that it might have a potentially important role in regulating cell growth and differentiation. In adult mice, the protein is abundant in the testis, heart, adrenal gland and ovary [47]. Previous studies show that like AKR1B1, AKR1B8 also catalyses the reduction of HNE and related aldehydes [22]. This property and the Nrf2 (nuclear factor-erythroid 2 p45 subunit-related factor 1)-dependent regulation of the AKR1B8 gene [51] suggests that AKR1B8 may be a significant component of antioxidant defence mechanisms that protect against injurious electrophiles such as the aldehydic products of lipid peroxidation.
Similar to other members of the AKR1B family, AKR1B10 also displayed high activity with a wide range of aldehyde phospholipids. This protein is similar (82% homology) to AKR1B8 and is expressed primarily in the small intestine and in the colon in humans; although low levels of ARK1B10 mRNA are present also in the liver [40]. The AKR1B10 has been reported to be dramatically overexpressed in 84% of non-small cell lung carcinoma and nearly 30% of adenocarcinoma [52]. Smoking has been found to be an independent variable responsible for AKRB10 overexpression [52]. Kinetic studies show that AKR1B10 is efficient at catalysing the reduction of aliphatic and aromatic aldehydes [40]. Our observation that AKR1B10 reduces C-5, C-7 and C-9 phosphocholine aldehydes and C-5 phosphatidylethanolamine aldehyde suggests that the robust activity of this protein with multiple aldehydes may be of significance in regulating oxidative stress and apoptosis in rapidly growing cells. Moreover, the high expression of this protein in the small intestine and colon suggest that it may be involved in the removal and detoxification of lipid peroxidation-derived aldehydes ingested as food.
Several pathological conditions increase lipid peroxidation. Products of lipid peroxidation, such as lipid hydroperoxide or free aldehydes such as HNE and acrolein, are highly reactive and toxic [7]. Phospholipid aldehydes, in contrast, display low toxicity, but due to their unique structural features trigger specific signalling events. Phospholipid aldehydes such as POVPC activate endothelial cells to bind monocytes [8] and increase the production of monocyte chemotactic factors, mediated in part by the activation of the cAMP/R-Ras/PI3K (phosphoinositide 3-kinase) [53], c-Src kinase–STAT3 (signal transducer and activator of transcription 3) [54] and endothelial nitric oxide synthase [15]. Reasons for such specificity remain poorly understood; however, chemical reduction of POVPC has been shown to abolish the ability of the phospholipid to enhance monocyte binding [55], indicating that the alcohol product of POVPC is not biologically active. Hence, reduction by AKRs could regulate the biological activities of phospholipid aldehydes. Furthermore, because some of the biological effects of phospholipids may be mediated by the binding of these phospholipids to intracellular receptors such as PPARα (peroxisome-proliferator-activated receptor α) [56], AKR-catalysed reduction, by controlling intracellular levels of the bioactive phospholipid aldehydes, could regulate receptor activation. Further studies are required to dissect out how AKRs regulate individual, tissue-specific signalling mechanisms triggered by phospholipid aldehydes and how these enzymes participate in the overall metabolism of these aldehydes in relation to other metabolic transformations such as those due to phospholipases.
Acknowledgments
This work was supported in part by NIH (National Institutes of Health; Bethesda, MD, U.S.A.) grants HL65618, ES 011594 (to S. S.), EY02687, EY05856 (to J. M. P.) and HL59378, and ES11860 (to A. B.), and a grant from Philip Morris.
References
- 1.Porter N. A., Caldwell S. E., Mills K. A. Mechanisms of free radical oxidation of unsaturated lipids. Lipids. 1995;30:277–290. doi: 10.1007/BF02536034. [DOI] [PubMed] [Google Scholar]
- 2.Glass C. K., Witztum J. L. Atherosclerosis. the road ahead. Cell. 2001;104:503–516. doi: 10.1016/s0092-8674(01)00238-0. [DOI] [PubMed] [Google Scholar]
- 3.Montine T. J., Neely M. D., Quinn J. F., Beal M. F., Markesbery W. R., Roberts L. J., Morrow J. D. Lipid peroxidation in aging brain and Alzheimer's disease. Free Radical Biol. Med. 2002;33:620–626. doi: 10.1016/s0891-5849(02)00807-9. [DOI] [PubMed] [Google Scholar]
- 4.Shinmura K., Bolli R., Liu S. Q., Tang X. L., Kodani E., Xuan Y. T., Srivastava S., Bhatnagar A. Aldose reductase is an obligatory mediator of the late phase of ischemic preconditioning. Circ. Res. 2002;91:240–246. doi: 10.1161/01.res.0000029970.97247.57. [DOI] [PubMed] [Google Scholar]
- 5.Srivastava S., Chandrasekar B., Bhatnagar A., Prabhu S. D. Lipid peroxidation-derived aldehydes and oxidative stress in the failing heart: role of aldose reductase. Am. J. Physiol. Heart Circ. Physiol. 2002;283:H2612–H2619. doi: 10.1152/ajpheart.00592.2002. [DOI] [PubMed] [Google Scholar]
- 6.Chang M. K., Binder C. J., Miller Y. I., Subbanagounder G., Silverman G. J., Berliner J. A., Witztum J. L. Apoptotic cells with oxidation-specific epitopes are immunogenic and proinflammatory. J. Exp. Med. 2004;200:1359–1370. doi: 10.1084/jem.20031763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Esterbauer H., Schaur R. J., Zollner H. Chemistry and biochemistry of 4-hydroxynonenal, malonaldehyde and related aldehydes. Free Radical Biol. Med. 1991;11:81–128. doi: 10.1016/0891-5849(91)90192-6. [DOI] [PubMed] [Google Scholar]
- 8.Watson A. D., Leitinger N., Navab M., Faull K. F., Horkko S., Witztum J. L., Palinski W., Schwenke D., Salomon R. G., Sha W., et al. Structural identification by mass spectrometry of oxidized phospholipids in minimally oxidized low density lipoprotein that induce monocyte/endothelial interactions and evidence for their presence in vivo. J. Biol. Chem. 1997;272:13597–13607. doi: 10.1074/jbc.272.21.13597. [DOI] [PubMed] [Google Scholar]
- 9.Podrez E. A., Poliakov E., Shen Z., Zhang R., Deng Y., Sun M., Finton P. J., Shan L., Gugiu B., Fox P. L., et al. Identification of a novel family of oxidized phospholipids that serve as ligands for the macrophage scavenger receptor CD36. J. Biol. Chem. 2002;277:38503–38516. doi: 10.1074/jbc.M203318200. [DOI] [PubMed] [Google Scholar]
- 10.Ravandi A., Babaei S., Leung R., Monge J. C., Hoppe G., Hoff H., Kamido H., Kuksis A. Phospholipids and oxophospholipids in atherosclerotic plaques at different stages of plaque development. Lipids. 2004;39:97–109. doi: 10.1007/s11745-004-1207-5. [DOI] [PubMed] [Google Scholar]
- 11.Prescott S. M., Zimmerman G. A., Stafforini D. M., McIntyre T. M. Platelet-activating factor and related lipid mediators. Annu. Rev. Biochem. 2000;69:419–445. doi: 10.1146/annurev.biochem.69.1.419. [DOI] [PubMed] [Google Scholar]
- 12.Gao S., Zhang R., Greenberg M. E., Sun M., Chen X., Levison B. S., Salomon R. G., Hazen S. L. Phospholipid hydroxyalkenals, a subset of recently discovered endogenous CD36 ligands, spontaneously generate novel furan-containing phospholipids lacking CD36 binding activity in vivo. J. Biol. Chem. 2006;281:31298–31308. doi: 10.1074/jbc.M604039200. [DOI] [PubMed] [Google Scholar]
- 13.Subbanagounder G., Deng Y., Borromeo C., Dooley A. N., Berliner J. A., Salomon R. G. Hydroxy alkenal phospholipids regulate inflammatory functions of endothelial cells. Vascul. Pharmacol. 2002;38:201–209. doi: 10.1016/s1537-1891(02)00170-2. [DOI] [PubMed] [Google Scholar]
- 14.Loidl A., Sevcsik E., Riesenhuber G., Deigner H. P., Hermetter A. Oxidized phospholipids in minimally modified low density lipoprotein induce apoptotic signaling via activation of acid sphingomyelinase in arterial smooth muscle cells. J. Biol. Chem. 2003;278:32921–32928. doi: 10.1074/jbc.M306088200. [DOI] [PubMed] [Google Scholar]
- 15.Gharavi N. M., Baker N. A., Mouillesseaux K. P., Yeung W., Honda H. M., Hsieh X., Yeh M., Smart E. J., Berliner J. A. Role of endothelial nitric oxide synthase in the regulation of SREBP activation by oxidized phospholipids. Circ. Res. 2006;98:768–776. doi: 10.1161/01.RES.0000215343.89308.93. [DOI] [PubMed] [Google Scholar]
- 16.Horkko S., Bird D. A., Miller E., Itabe H., Leitinger N., Subbanagounder G., Berliner J. A., Friedman P., Dennis E. A., Curtiss L. K., et al. Monoclonal autoantibodies specific for oxidized phospholipids or oxidized phospholipid–protein adducts inhibit macrophage uptake of oxidized low-density lipoproteins. J. Clin. Invest. 1999;103:117–128. doi: 10.1172/JCI4533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chang M. K., Bergmark C., Laurila A., Horkko S., Han K. H., Friedman P., Dennis E. A., Witztum J. L. Monoclonal antibodies against oxidized low-density lipoprotein bind to apoptotic cells and inhibit their phagocytosis by elicited macrophages: evidence that oxidation-specific epitopes mediate macrophage recognition. Proc. Natl. Acad. Sci. U.S.A. 1999;96:6353–6358. doi: 10.1073/pnas.96.11.6353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Srivastava S., Chandra A., Wang L. F., Seifert W. E., Jr, DaGue B. B., Ansari N. H., Srivastava S. K., Bhatnagar A. Metabolism of the lipid peroxidation product, 4-hydroxy-trans-2-nonenal, in isolated perfused rat heart. J. Biol. Chem. 1998;273:10893–10900. doi: 10.1074/jbc.273.18.10893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Srivastava S., Watowich S. J., Petrash J. M., Srivastava S. K., Bhatnagar A. Structural and kinetic determinants of aldehyde reduction by aldose reductase. Biochemistry. 1999;38:42–54. doi: 10.1021/bi981794l. [DOI] [PubMed] [Google Scholar]
- 20.Srivastava S., Spite M., Trent J. O., West M. B., Ahmed Y., Bhatnagar A. Aldose reductase-catalyzed reduction of aldehyde phospholipids. J. Biol. Chem. 2004;279:53395–53406. doi: 10.1074/jbc.M403416200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jez J. M., Bennett M. J., Schlegel B. P., Lewis M., Penning T. M. Comparative anatomy of the aldo-keto reductase superfamily. Biochem. J. 1997;326:625–636. doi: 10.1042/bj3260625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Srivastava S., Harter T. M., Chandra A., Bhatnagar A., Srivastava S. K., Petrash J. M. Kinetic studies of FR-1, a growth factor-inducible aldo-keto reductase. Biochemistry. 1998;37:12909–12917. doi: 10.1021/bi9804333. [DOI] [PubMed] [Google Scholar]
- 23.Burczynski M. E., Sridhar G. R., Palackal N. T., Penning T. M. The reactive oxygen species- and Michael acceptor-inducible human aldo-keto reductase AKR1C1 reduces the alpha,beta-unsaturated aldehyde 4-hydroxy-2-nonenal to 1,4-dihydroxy-2-nonene. J. Biol. Chem. 2001;276:2890–2897. doi: 10.1074/jbc.M006655200. [DOI] [PubMed] [Google Scholar]
- 24.O'Connor T., Ireland L. S., Harrison D. J., Hayes J. D. Major differences exist in the function and tissue-specific expression of human aflatoxin B1 aldehyde reductase and the principal human aldo-keto reductase AKR1 family members. Biochem. J. 1999;343:487–504. [PMC free article] [PubMed] [Google Scholar]
- 25.Gugiu B. G., Salomon R. G. Total syntheses of bioactive oxidized ethanolamine phospholipids. Org. Lett. 2003;5:2797–2799. doi: 10.1021/ol034729z. [DOI] [PubMed] [Google Scholar]
- 26.Kelly V. P., Sherratt P. J., Crouch D. H., Hayes J. D. Novel homodimeric and heterodimeric rat gamma-hydroxybutyrate synthases that associate with the Golgi apparatus define a distinct subclass of aldo-keto reductase 7 family proteins 1. Biochem. J. 2002;366:847–861. doi: 10.1042/BJ20020342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liu S. Q., Jin H., Zacarias A., Srivastava S., Bhatnagar A. Binding of pyridine nucleotide coenzymes to the β-subunit of the voltage-sensitive K+ channel. J. Biol. Chem. 2001;276:11812–11820. doi: 10.1074/jbc.M008259200. [DOI] [PubMed] [Google Scholar]
- 28.Ramana K. V., Dixit B. L., Srivastava S., Balendiran G. K., Srivastava S. K., Bhatnagar A. Selective recognition of glutathiolated aldehydes by aldose reductase. Biochemistry. 2000;39:12172–12180. doi: 10.1021/bi000796e. [DOI] [PubMed] [Google Scholar]
- 29.Barski O. A., Papusha V. Z., Kunkel G. R., Gabbay K. H. Regulation of aldehyde reductase expression by STAF and CHOP. Genomics. 2004;83:119–129. doi: 10.1016/s0888-7543(03)00213-1. [DOI] [PubMed] [Google Scholar]
- 30.Petrash J. M., Harter T. M., Devine C. S., Olins P. O., Bhatnagar A., Liu S., Srivastava S. K. Involvement of cysteine residues in catalysis and inhibition of human aldose reductase. Site-directed mutagenesis of Cys-80, -298 and -303. J. Biol. Chem. 1992;267:24833–24840. [PubMed] [Google Scholar]
- 31.Zacarias A., Bolanowski D., Bhatnagar A. Comparative measurements of multicomponent phospholipid mixtures by electrospray mass spectroscopy: relating ion intensity to concentration. Anal. Biochem. 2002;308:152–159. doi: 10.1016/s0003-2697(02)00209-9. [DOI] [PubMed] [Google Scholar]
- 32.Hinshelwood A., McGarvie G., Ellis E. Characterisation of a novel mouse liver aldo-keto reductase AKR7A5. FEBS Lett. 2002;523:213–218. doi: 10.1016/s0014-5793(02)02982-4. [DOI] [PubMed] [Google Scholar]
- 33.Martin H. J., Breyer-Pfaff U., Wsol V., Venz S., Block S., Maser E. Purification and characterization of akr1b10 from human liver: role in carbonyl reduction of xenobiotics. Drug Metab. Dispos. 2006;34:464–470. doi: 10.1124/dmd.105.007971. [DOI] [PubMed] [Google Scholar]
- 34.Kuchinke W., Barski O., Watanabe K., Hayaishi O. A lung type prostaglandin F synthase is expressed in bovine liver: cDNA sequence and expression in E. coli. Biochem. Biophys. Res. Commun. 1992;183:1238–1246. doi: 10.1016/s0006-291x(05)80323-7. [DOI] [PubMed] [Google Scholar]
- 35.Pawlowski J. E., Penning T. M. Overexpression and mutagenesis of the cDNA for rat-liver 3-alpha-hydroxysteroid dihydrodiol dehydrogenase – role of cysteines and tyrosines in catalysis. J. Biol. Chem. 1994;269:13502–13510. [PubMed] [Google Scholar]
- 36.Ellis E. M., Hayes J. D. Substrate specificity of an aflatoxin-metabolizing aldehyde reductase. Biochem. J. 1995;312:535–541. doi: 10.1042/bj3120535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kozma E., Brown E., Ellis E. M., Lapthorn A. J. The crystal structure of rat liver AKR7A1. A dimeric member of the aldo-keto reductase superfamily. J. Biol. Chem. 2002;277:16285–16293. doi: 10.1074/jbc.M110808200. [DOI] [PubMed] [Google Scholar]
- 38.Zhu X., Lapthorn A. J., Ellis E. M. Crystal structure of mouse succinic semialdehyde reductase AKR7A5: structural basis for substrate specificity. Biochemistry. 2006;45:1562–1570. doi: 10.1021/bi051610k. [DOI] [PubMed] [Google Scholar]
- 39.Weng J., Cao Y., Moss N., Zhou M. Modulation of voltage-dependent Shaker family potassium channels by an aldo-keto reductase. J. Biol. Chem. 2006;281:15194–15200. doi: 10.1074/jbc.M513809200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cao D., Fan S. T., Chung S. S. Identification and characterization of a novel human aldose reductase-like gene. J. Biol. Chem. 1998;273:11429–11435. doi: 10.1074/jbc.273.19.11429. [DOI] [PubMed] [Google Scholar]
- 41.Grimshaw C. E., Mathur E. J. Immunoquantitation of aldose reductase in human tissues. Anal. Biochem. 1989;176:66–71. doi: 10.1016/0003-2697(89)90273-x. [DOI] [PubMed] [Google Scholar]
- 42.Brown K. E., Broadhurst K. A., Mathahs M. M., Kladney R. D., Fimmel C. J., Srivastava S. K., Brunt E. M. Immunodetection of aldose reductase in normal and diseased human liver. Histol. Histopathol. 2005;20:429–436. doi: 10.14670/HH-20.429. [DOI] [PubMed] [Google Scholar]
- 43.Boland O. M., Blackwell C. C., Clarke B. F., Ewing D. J. Effects of ponalrestat, an aldose reductase inhibitor, on neutrophil killing of Escherichia coli and autonomic function in patients with diabetes mellitus. Diabetes. 1993;42:336–340. doi: 10.2337/diab.42.2.336. [DOI] [PubMed] [Google Scholar]
- 44.Ramana K. V., Bhatnagar A., Srivastava S. K. Inhibition of aldose reductase attenuates TNF-α-induced expression of adhesion molecules in endothelial cells. FASEB J. 2004;18:1209–1218. doi: 10.1096/fj.04-1650com. [DOI] [PubMed] [Google Scholar]
- 45.Barski O. A., Gabbay K. H., Bohren K. M. Characterization of the human aldehyde reductase gene and promoter. Genomics. 1999;60:188–198. doi: 10.1006/geno.1999.5915. [DOI] [PubMed] [Google Scholar]
- 46.Barski O. A., Gabbay K. H., Grimshaw C. E., Bohren K. M. Mechanism of human aldehyde reductase: characterization of the active site pocket. Biochemistry. 1995;34:11264–11275. doi: 10.1021/bi00035a036. [DOI] [PubMed] [Google Scholar]
- 47.Lau E. T., Cao D., Lin C., Chung S. K., Chung S. S. Tissue-specific expression of two aldose reductase-like genes in mice: abundant expression of mouse vas deferens protein and fibroblast growth factor-regulated protein in the adrenal gland. Biochem. J. 1995;312:609–615. doi: 10.1042/bj3120609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Martinez A., Aigueperse C., Val P., Dussault M., Tournaire C., Berger M., Veyssiere G., Jean C., Lefrancois M. A. Physiological functions and hormonal regulation of mouse vas deferens protein (AKR1B7) in steroidogenic tissues. Chem. Biol. Interact. 2001;130–132:903–917. doi: 10.1016/s0009-2797(00)00244-1. [DOI] [PubMed] [Google Scholar]
- 49.Lefrancois-Martinez A. M., Tournaire C., Martinez A., Berger M., Daoudal S., Tritsch D., Veyssiere G., Jean C. Product of side-chain cleavage of cholesterol, isocaproaldehyde, is an endogenous specific substrate of mouse vas deferens protein, an aldose reductase-like protein in adrenocortical cells. J. Biol. Chem. 1999;274:32875–32880. doi: 10.1074/jbc.274.46.32875. [DOI] [PubMed] [Google Scholar]
- 50.Donohue P. J., Alberts G. F., Hampton B. S., Winkles J. A. A delayed-early gene activated by fibroblast growth factor-1 encodes a protein related to aldose reductase. J. Biol. Chem. 1994;269:8604–8609. [PubMed] [Google Scholar]
- 51.Rangasamy T., Cho C. Y., Thimmulappa R. K., Zhen L., Srisuma S. S., Kensler T. W., Yamamoto M., Petrache I., Tuder R. M., Biswal S. Genetic ablation of Nrf2 enhances susceptibility to cigarette smoke-induced emphysema in mice. J. Clin. Invest. 2004;114:1248–1259. doi: 10.1172/JCI21146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Fukumoto S., Yamauchi N., Moriguchi H., Hippo Y., Watanabe A., Shibahara J., Taniguchi H., Ishikawa S., Ito H., Yamamoto S., et al. Overexpression of the aldo-keto reductase family protein AKR1B10 is highly correlated with smokers’ non-small cell lung carcinomas. Clin. Cancer Res. 2005;11:1776–1785. doi: 10.1158/1078-0432.CCR-04-1238. [DOI] [PubMed] [Google Scholar]
- 53.Cole A. L., Subbanagounder G., Mukhopadhyay S., Berliner J. A., Vora D. K. Oxidized phospholipid-induced endothelial cell/monocyte interaction is mediated by a cAMP-dependent R-Ras/PI3-kinase pathway. Arterioscler. Thromb. Vasc. Biol. 2003;23:1384–1390. doi: 10.1161/01.ATV.0000081215.45714.71. [DOI] [PubMed] [Google Scholar]
- 54.Yeh M., Gharavi N. M., Choi J., Hsieh X., Reed E., Mouillesseaux K. P., Cole A. L., Reddy S. T., Berliner J. A. Oxidized phospholipids increase interleukin 8 (IL-8) synthesis by activation of the c-src/signal transducers and activators of transcription (STAT)3 pathway. J. Biol. Chem. 2004;279:30175–30181. doi: 10.1074/jbc.M312198200. [DOI] [PubMed] [Google Scholar]
- 55.Subbanagounder G., Leitinger N., Schwenke D. C., Wong J. W., Lee H., Rizza C., Watson A. D., Faull K. F., Fogelman A. M., Berliner J. A. Determinants of bioactivity of oxidized phospholipids. Specific oxidized fatty acyl groups at the sn-2 position. Arterioscler. Thromb. Vasc. Biol. 2000;20:2248–2254. doi: 10.1161/01.atv.20.10.2248. [DOI] [PubMed] [Google Scholar]
- 56.Lee H., Shi W., Tontonoz P., Wang S., Subbanagounder G., Hedrick C. C., Hama S., Borromeo C., Evans R. M., Berliner J. A., Nagy L. Role for peroxisome proliferator-activated receptor α in oxidized phospholipid-induced synthesis of monocyte chemotactic protein-1 and interleukin-8 by endothelial cells. Circ. Res. 2000;87:516–521. doi: 10.1161/01.res.87.6.516. [DOI] [PubMed] [Google Scholar]