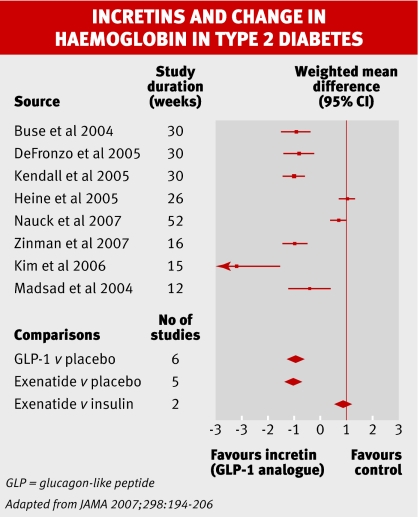
Incretins are gut peptides that help regulate glucose metabolism by augmenting the release of insulin. Since 2005, the US Food and Drug Administration has licensed two new classes of drug exploiting this pathway for treating adults with type 2 diabetes. Glucagon-like peptide 1 (GLP-1) analogues, which mimic the action of one of the incretin peptides, were moderately effective in a recent meta-analysis, reducing concentrations of glycated haemoglobin by about 1% compared with placebo (−0.97%, 95% CI –1.13% to –0.81%). But they made up to 57% of patients feel sick. Patients lost an average of 1.4 kg in weight.
The dipeptidyl peptidase 4 (DPP4) inhibitors, sitagliptin and vildagliptin, which prolong the action of incretin peptides, also worked moderately well compared with placebo, but they seemed to increase risk of infection (risk ratio 1.5, 1.0 to 2.2 for urinary tract infections).
Both new classes of agent looked as effective as other treatments for diabetes in the small number of head to head trials. But the evidence has plenty of gaps, and other trials with active comparators have yet to report. It is still unclear where the new drugs fit into the treatment of most adults with type 2 diabetes, say the authors.