Abstract
Similarities in physiological roles of LXR (liver X receptors) and co-repressor RIP140 (receptor-interacting protein 140) in regulating energy homoeostasis and lipid and glucose metabolism suggest that the effects of LXR could at least partly be mediated by recruitment of the co-repressor RIP140. In the present study, we have elucidated the molecular basis for regulation of LXR transcriptional activity by RIP140. LXR is evenly localized in the nucleus and neither the N-terminal domain nor the LBD (ligand-binding domain) is necessary for nuclear localization. Both LXR subtypes, LXRα and LXRβ, interact with RIP140 and co-localize in diffuse large nuclear domains. Interaction and co-localization are dependent on the LBD of the receptor. The C-terminal domain of RIP140 is sufficient for full repressive effect. None of the C-terminal NR (nuclear receptor)-boxes is required for the co-repressor activity, whereas the NR-box-like motif as well as additional elements in the C-terminal region are required for full repressive function. The C-terminal NR-box-like motif is necessary for interaction with LXRβ, whereas additional elements are needed for strong interaction with LXRα. In conclusion, our results suggest that co-repression of LXR activity by RIP140 involves an atypical binding mode of RIP140 and a repression element in the RIP140 C-terminus.
Keywords: co-regulatory protein, ligand-binding domain (LBD), liver X receptor (LXR), nuclear receptor, receptor-interacting protein 140 (RIP140)
Abbreviations: AD, activation domain; ATRX, X-linked α thalassaemia mental retardation; DBD, DNA-binding domain; ER, oestrogen receptor; GFP, green fluorescent protein; GLUT4, glucose transporter 4; GST, glutathione S-transferase; HA, haemagglutinin; H12, helix 12; LBD, ligand-binding domain; LXR, liver X receptor; hLXRα, human LXRα; LXRE, LXR responsive element; NR, nuclear receptor; NCoR, NR co-repressor; NLS, nuclear localization signal; RIP140, receptor-interacting protein 140, RT-PCR, real-time PCR; RXR, retinoid X receptor; SMRT, silencing mediator for retinoic acid receptor and thyroid-hormone receptor; TBP, TATA-box-binding protein; TAF-172, TBP-associated factors 172; Ucp1, uncoupling protein 1
INTRODUCTION
The LXR (liver X receptor) is a ligand-inducible transcription factor and member of the NR (nuclear receptor) superfamily [1]. There are two known paralogues, LXRα (NR1H3) and LXRβ (NR1H2). The two subtypes have a high degree of sequence similarity but display different expression patterns. Expression of LXRα is restricted to liver, kidney, adipose tissue, intestine and macrophages whereas LXRβ is ubiquitously expressed [2]. Oxidized cholesterol derivatives, so-called oxysterols, have been shown to bind to and activate the LXRs [3,4]. LXRs bind to target DNA (LXR response element) as a permissive heterodimer with another member of the NR superfamily, the RXR (retinoid X receptor). LXRs have been identified as key players in the regulation of cholesterol homoeostasis, lipid and glucose metabolism as well as immune and inflammatory responses (reviewed in [5,6]). LXRs affect whole-body energy balance. LXRα/β-deficient mice are leaner than wild-type littermates and are also resistant to obesity when fed a high-fat and -cholesterol diet [7–9]. LXR knockout mice have an increased energy expenditure and increased expression in skeletal muscle and adipose tissue of the mitochondrial protein Ucp1 (uncoupling protein 1) that uncouples respiration [7,9]. In accordance, Ucp1 expression is down-regulated by LXR agonist in adipose tissue [10]. LXRs also regulate glucose metabolism. Synthetic LXR agonists improve glucose tolerance in rodent diabetic models [11,12]. LXRs repress genes involved in glucose production, such as PEPCK (phosphoenolpyruvate carboxykinase), and activate genes involved in glucose utilization, such as glucokinase, in hepatocytes [10–13]. In adipocytes, LXRs induce GLUT4 (glucose transporter 4) expression which contributes to increased glucose uptake in the cells [12,14]. Failure of proper regulation of energy balance and lipid and glucose metabolism may lead to a number of diseases, including atherosclerosis, obesity and diabetes. These diseases are considered to be the main causes of mortality in Western society today. LXRs are therefore interesting potential targets for drug treatment of several diseases.
NR-mediated gene transcription involves recruitment to NRs of a large number of co-regulatory proteins that form dynamic multi-protein complexes (reviewed in [15]). Co-regulators function as bridging and/or stabilizing factors, inhibitors of specific protein–protein interactions or modifiers of other proteins with enzymatic activity (i.e. acetylation, methylation, phosphorylation and ubiquitination). These events initiate (co-activators) or inhibit (co-repressors) chromatin remodelling and/or the assembly of the general transcription machinery. RIP140 (receptor-interacting protein 140) is a co-repressor protein that is highly expressed in metabolic tissues such as liver, muscle and adipose tissue and has an important role in regulation of lipid and glucose metabolism (reviewed in [16]). The physiological function of RIP140 is partly similar to that of LXRs. Mice devoid of RIP140 are lean and are resistant to high-fat diet-induced obesity [17]. Adipocytes lacking RIP140 have an increased energy expenditure and elevated expression of proteins involved in energy expenditure such as Ucp1, Cidea [cell-death-inducing DFFA (DNA fragmentation factor-α)-like effector A] and CPT1B (carnitine palmitoyltransferase-1b) [17,18]. Furthermore, RIP140-knockout mice on high-fat diet have an improved glucose tolerance. RIP140-depleted adipocytes have an increased glucose uptake and increased expression of genes involved in glucose uptake and glycolysis such as GLUT4 and hexokinase 2 [19].
RIP140 interacts with many ligand-activated NRs, including LXR [20,21]. RIP140 contains nine NR-boxes (LXXLL-motifs) and one NR-box-like motif (LXXML) [22]. NR-boxes are found in almost all co-activators identified and bind in a ligand-dependent manner to the LBD (ligand-binding domain) of NRs [23]. Furthermore, the NR-box is necessary and sufficient for interaction between the receptor and the co-regulator. The co-repressors NCoR (NR co-repressor) and SMRT (silencing mediator for retinoic acid receptor and thyroid-hormone receptor) contain a related motif, the CoRNR-box (L/IXXI/VI) that mediates the binding to unliganded NRs [24–26]. RIP140 has been suggested to repress gene transcription by competing with co-activators for binding to the receptor [22]. However, RIP140 also has an intrinsic repression activity that is at least partly mediated by recruitment of HDAC (histone deacetylase) proteins and the CtBP (co-repressor C-terminal binding protein) [27–29]. The intrinsic repression activity has been mapped and includes several domains of RIP140 [30–32].
The similarities in physiological roles of LXRs and RIP140 in regulating energy homoeostasis and lipid and glucose metabolism suggest that the effects of LXR could at least partly be mediated by recruitment of the co-repressor RIP140. The aim of the present study was to delineate specific determinants for the repressive effect of RIP140 on LXR-mediated gene transcription. We first demonstrate that both LXR subtypes, LXRα and LXRβ, interact with RIP140 and that RIP140 co-localizes with the LXRs in large nuclear domains. Furthermore, both interaction and co-localization is dependent on the LBD of the receptors. We also show that the main repression activity of RIP140 resides in its C-terminal part and that all NR-boxes within this domain of RIP140 do not need to be intact for the repressive effect of RIP140. Moreover, our results prove the importance of an NR-box-like motif and integrity of the C-terminal domain of RIP140 for obtaining full repression by RIP140 on LXR-mediated gene transcription. Interestingly, we also find LXR subtype differences in binding to RIP140. The NR-box-like motif in RIP140 is necessary and sufficient for the interaction with LXRβ, whereas more than one intact NR-box is needed in order for RIP140 to interact strongly with LXRα.
EXPERIMENTAL
Plasmid constructs
For localization studies, full-length hLXRα (human LXRα) (amino acids 1–447), hLXRα ΔLBD (amino acids 1–214), hLXRα ΔN (amino acids 96–447), hLXRα ΔH12 (where H12 is helix 12) (amino acids 1–436), full-length hLXRβ (amino acids 1–461), hLXRβ ΔLBD (amino acids 1–230), hLXRβ ΔN (amino acids 85–461) and hLXRβ ΔH12 (amino acids 1–450) were generated by PCR and subcloned into the XbaI and XhoI site of the pcDNA3-FLAG plasmid [33]. Plasmid pEGFP-RIP140, expressing GFP (green fluorescent protein)-fused hRIP140, has been described previously [34]. Expression plasmid for transient transfection studies, pSG5-HA-hRIP140, expressing HA (haemagglutinin)-tagged hRIP140, has been described previously [22]. Deletion constructs in the pSG5-HA plasmid, hRIP140/1–281, hRIP140/1–472, hRIP140/431–745, hRIP140/431–1158, and NR-box mutation constructs have been described previously [32]. Deletion constructs in the pSG5-HA plasmid, hRIP140/863–1158, hRIP140/965–1158, hRIP140/747–1060 and hRIP140/747–935, were generated by subcloning PCR fragments into the BglII site of the pSG5-HA plasmid. pSG5-HA-hRIP140 FL mut NR10 were generated using a QuikChange® XL Site-directed Mutagenesis kit (Stratagene) according to the protocol provided by the manufacturer. The reporter plasmids 3xLXRE luciferase reporter, kindly provided by KaroBio AB (Sweden), and RSV (Rous sarcoma virus) β-gal reporter were used. For yeast two-hybrid experiments full-length hLXRα (amino acids 1–447), hLXRα LBD (amino acids 205–447), hLXRα N-terminal domain (amino acids 1–291), hLXRα DBD (DNA-binding domain) (amino acids 96–214), hLXRβ full-length (amino acids 1–461), hLXRβ LBD (amino acids 219–461), hLXRβ N-terminal domain (amino acids 1–258) and hLXRβ DBD (amino acids 85–230) were generated by PCR and subcloned into the SfiI and BamHI site of the pGBKT7 plasmid (Clontech) to create the GAL4 DBD construct. GAL4 AD (activation domain) fusion protein hRIP140/863–1158, hRIP140/965–1158, hRIP140/747–1060, hRIP140/747–935, hRIP140/747–1158 and hRIP140/747–1158 mut NR10 were generated by PCR and subcloned into the SfiI and BamHI site of the pACT2 plasmid (Clontech). GAL4 AD fusion protein hRIP140/560–1158 was isolated in a yeast-two hybrid screen from a pACT2 cDNA library (Clontech).
Confocal microscopy
COS-7 cells were plated on coverslips in six-well plates and, on the following day, transfected with expression plasmids using the FuGENE™ 6 transfection reagent (Roche). Expression plasmid for GFP-fused proteins (100 ng) and expression plasmid for FLAG-tagged proteins (200 ng) were used. On the next day fresh medium was added containing the ligand T0901317 (1 μM) and incubation was carried out overnight. After 24 h, incubated cells were fixed with 3% (w/v) paraformaldehyde in 5% sucrose-PBS rinsed with PBS and permeabilized with PBS containing 0.1% Tween 20 (PBS-T). Cells transfected with FLAG-tagged proteins were blocked with 5% (v/v) goat serum (Jackson ImmunoResearch) in PBS-T for 1 h. Thereafter, the cells were incubated with primary antibody diluted in PBS-T for 1.5 h. Anti-FLAG M5 monoclonal antibody (Sigma) was used at a dilution of 1:200. Cells were then washed in PBS-T and incubated with secondary antibody conjugated with TRITC (tetramethylrhodamine β-isothiocyanate; Jackson ImmunoResearch) diluted at 1:200 in PBS-T for 1 h. Cells were washed and fixed on coverslips with ImmunoMount (Shandon). Subcellular images were determined with a TCSSP Multiband Confocal imaging system (Leica). Fluorescence images for more than 100 transfected cells were studied for each experiment and the images shown are representative for most of the cells studied.
Reporter gene assays
Human hepatoma cells (Huh7) were cultured in Dulbecco's modified Eagle's medium (Invitrogen) containing 10% (v/v) fetal bovine serum, 100 units/ml penicillin and 100 μg/ml streptomycin. On the day before transfection, cells were seeded on to 24-well plates (4×104 cells/well), and on the following day, transfected with 2.5–50 ng of pSG5-HA-RIP140 expression vector, 100 ng of 3xLXRE luciferase reporter and 10 ng of RSV β-gal reporter using Lipofectamine™ 2000 transfection reagent (Invitrogen). To keep vector amount constant in each transfection, appropriate amount of empty pSG5 vector was added. Five hours after transfection, the medium was changed to a medium containing 250 nM T0901317 or DMSO as vehicle and cells were cultured for 24 h. Luciferase and β-galactosidase activities were measured using luciferin and ATP reagents (BioThema) and a Galacto-Star kit (Tropix) respectively in a microplate luminometer (Thermo Electron Corp.). All experiments were repeated at least three times with triplicates in each, and luciferase activity was normalized to β-galactosidase activity.
Yeast two-hybrid assays
For yeast two-hybrid assays different combinations of pGBKT7-GAL4 DBD and pACT2-GAL4 AD fusion constructs (100 ng each) were co-transformed into Saccharomyces cerevisiae, strain Y187 (Clontech), and plated on a medium lacking leucine and tryptophan for selection. Liquid β-galactosidase assay was performed in the presence of 250 nM T0901317 or DMSO as vehicle and β-galactosidase units were calculated according to the protocol provided by the manufacturer (BD Biosciences). All experiments were repeated at least three times with triplicates in each.
Western blotting
Whole cell extracts were prepared from Huh7 cells transfected with HA-tagged RIP140 plasmids. The cell pellet was washed in PBS and resuspended in extraction buffer [10 mM Tris/HCl, pH 7.4, 300 mM NaCl, 1 mM dithiothreitol, 0.1% Igepal CA-630, and Complete™ Mini protein inhibitor cocktail (Roche)], incubated for 15 min on ice and homogenized. After centrifugation (1240 g, 10 min, 4°C) the supernatant was analysed by Western blotting with anti-HA.11 monoclonal antibody (MMS-101P, Nordic Biosite).
RT-PCR (real-time PCR)
For quantification of LXR mRNA levels in Huh7 cells, total RNA was isolated using an EZNA Total RNA kit (Omega Bio-tek) according to the manufacturer's instructions. First-strand cDNA synthesis of RNA (1 μg) was performed using Superscript II reverse transcriptase (Invitrogen) with random hexamers. mRNA expression was quantified using 10 ng cDNA/reaction by SYBR Green (18S as internal standard) quantitative PCR applying the comparative Ct analysis using the Applied Biosystems 7500 RT-PCR. The following primers (5′–3′) were used: LXRα F: CCCCATGACCGACTGATGTT, R: GAGGCTCACCAGTTTCATTAGCAT; LXRβ F: GGAGGACCAGATCGCCCT, R: AGCAGCATGATCTCGATAGTGG.
RESULTS
LXR is localized in the nucleus
To analyse the intracellular localization of LXRα and LXRβ, we transfected COS-7 cells with plasmids expressing FLAG-tagged LXRα and LXRβ respectively. LXRα and LXRβ were distributed evenly in the nucleus both in the absence and presence of the synthetic LXR agonist ligand T0901317 (LXRα: Figure 1B, panels a and b; the results for LXRβ are identical with those for LXRα and are therefore presented as Supplementary Figure 1 at http://www.BiochemJ.org/bj/405/bj4050031add.htm). The same localization was also found using fluorescent Heteractis crispa (Hc)Red-tagged LXRα and LXRβ (results not shown). To study the influence of different LXR domains on receptor localization we used FLAG-tagged LXR constructs where the N-terminal domain (ΔN), LBD (ΔLBD) or H12 (ΔH12) of LXR α or β was deleted (Figure 1A). Transfection of COS-7 cells with the deletion constructs revealed that LXRα and LXRβ ΔN proteins had a foci-like distribution in the nucleus, while LXRα and LXRβ ΔLBD and ΔH12 proteins were evenly distributed in similarity to full-length LXRα and LXRβ (LXRα: Figure 1B, panels c, e and g; LXRβ: Supplementary Figure 1). The intracellular localization of the deleted LXRα and LXRβ proteins did not change in the presence of the LXR ligand (LXRα: Figure 1B, panels d, f and h; LXRβ: Supplementary Figure 1). Collectively, these results suggest that wild-type LXR is localized in the nucleus and that neither the N-terminal domain nor the LBD is indispensable for nuclear localization of LXRα or LXRβ, indicating the importance of the DBD and the hinge region for nuclear targeting. Furthermore, our results show that removal of the N-terminal domain of LXR changes the intranuclear localization of the receptor, suggesting that this domain is important for formation of the correct receptor structure and/or association with protein complexes that contribute to normal intranuclear localization.
Figure 1. Intracellular localization of LXR.
(A) A schematic picture of the LXR domains used. (B) COS-7 cells were transfected with expression plasmids for FLAG-tagged full-length LXRα and LXRα domains and treated with (+) or without (–) 1 μM T0901317 overnight before visualization of the expressed protein with FLAG-specific antibody using confocal microscopy. The images are representative for 90–100% of the cells studied.
LXRα and LXRβ interact with RIP140
To identify proteins interacting with LXRs in the presence of the synthetic LXR agonist ligand T0901317 we performed yeast two-hybrid screens. Several of the isolated clones from different human cDNA libraries using either LXRα or LXRβ as bait encoded RIP140. These results confirm the interaction between LXRs and RIP140 and indicate a role for RIP140 in LXR-mediated gene regulation. We further verified the interaction between LXR and RIP140 in a GST (glutathione S-transferase)-pull-down analysis with GST-fused LXRα and in vitro translated RIP140. RIP140 interacted with LXRα both in the absence and presence of LXR ligand T0901317 (results not shown).
To elucidate which domains of LXRα and LXRβ interact with RIP140, S. cerevisiae yeast strain Y187 containing an integrated GAL4-responsive lacZ reporter gene was co-transformed with pGBKT7-GAL4 DBD-LXR fusion constructs (Figure 2) and the pACT2-GAL4 AD-RIP140 C-terminus (amino acids 560–1158). Transcriptional activity, as a measurement of interaction, was then assayed using a liquid β-galactosidase assay. Strong interaction was seen between the C-terminus of RIP140 (amino acids 560–1158) and the full-length LXRα and LXRβ proteins respectively in the presence of the synthetic LXR ligand T0901317 (Figure 2). The interaction of RIP140 (amino acids 560–1158) with full-length LXRα was only slightly enhanced by ligand, whereas the interaction with LXRβ was strongly ligand-dependent. The LBDs of LXRα and LXRβ interacted in a ligand-dependent manner with the RIP140 C-terminus whereas no binding of the DBD of either LXR was seen. The N-terminal domain of LXRα interacted weakly with RIP140, both in the presence and absence of ligand, whereas the N-terminal domain of LXRβ did not bind. In summary, the C-terminal part of RIP140 readily binds both LXR subtypes and the LBD is necessary for strong interaction.
Figure 2. Interaction of RIP140 with LXR domains.
(A, B) S. cerevisiae yeast strain Y187 containing an integrated GAL4-responsive lacZ reporter gene was co-transformed with plasmids expressing GAL4 DBD fused to full-length LXRα or LXRβ and LXRα or LXRβ domains and GAL4 AD-RIP140 amino acids 560–1158 in the presence or absence of 250 nM T0901317. β-Galactosidase activity was assayed as a measurement of interaction. The means and standard deviations (n=9) were collected from three independent experiments with triplicates in each.
LXR LBD is necessary for co-localization with RIP140
To investigate whether LXRα and LXRβ co-localize with RIP140, GFP-tagged RIP140 was expressed alone or together with FLAG-tagged LXRα and LXRβ respectively in COS-7 cells. Figure 3(A) shows that GFP–RIP140 is localized in small, discrete foci in the nucleus, as shown previously [32]. The localization of RIP140 was not changed in the presence of LXR ligand T0901317 (Figure 3A). However, co-expression of RIP140 with LXRα in the absence of ligand resulted in redistribution of RIP140 from the small foci to an even distribution (Figure 3B, panels a–c). Moreover, in the presence of LXR ligand, both LXRα and RIP140 were redistributed and co-localized in diffuse large nuclear domains (Figure 3B, panels d–f). The same localization pattern was seen co-expressing LXRβ with RIP140 (see Supplementary Figure 2 at http://www.BiochemJ.org/bj/405/bj4050031add.htm). In summary, the localization of the co-expressed proteins was distinct from the individually expressed proteins. These results suggest that RIP140 and LXR co-localize in the nucleus already in the absence of ligand but that the ligand changes the subnuclear localization of the RIP140–LXR complex.
Figure 3. Intracellular co-localization of LXR and RIP140.
(A) COS-7 cells were transfected with plasmids expressing GFP–RIP140 and treated with (+) or without (–) 1 μM T0901317 overnight before visualization of the protein using confocal microscopy. (B–E) COS-7 cells were co-transfected with plasmids expressing GFP–RIP140 and FLAG–LXRα or FLAG–LXRα domains. The cells were treated with (+) or without (–) 1 μM T0901317 overnight before visualization of the expressed FLAG–LXR protein with FLAG-specific antibody using confocal microscopy. (a, d) The localization of FLAG–LXR protein in red; (b, e) the localization of GFP–RIP140 in green in the same cells. (c, f) The merged images, where co-localization of proteins shows up as yellow. The images shown are representative of 90–100% of the cells studied.
To determine which domains of LXR are important for co-localization with RIP140, FLAG-tagged LXR deletion constructs were co-expressed with GFP–RIP140. Co-expression of RIP140 with LXRαΔN and LXRβΔN, both in the absence and presence of ligand, distributed RIP140 to the LXRΔN typical nuclear foci (LXRα: Figure 3C, panels a–f; LXRβ: Supplementary Figure 2), suggesting that the N-terminal domain of LXR is dispensable for co-localization with RIP140. In contrast, co-expression of LXRαΔLBD or LXRβΔLBD did not change the localization of RIP140, neither in the absence nor in the presence of LXR ligand, but rather RIP140 stayed in the small RIP140 characteristic foci (LXRα: Figure 3D, panels a–f; LXRβ: Supplementary Materials, Supplementary Figure 2), showing that the LBD of LXR is necessary for redistribution of RIP140. The conserved H12 in the very C-terminus of NR LBDs has been shown to be important for co-activator binding but not for binding of the co-repressor proteins SMRT and NCoR (reviewed in [35]). Interestingly, RIP140 co-localizes with LXRα ΔH12 and LXRβ ΔH12 in diffuse large nuclear domains in the presence of ligand, in the same way as with full-length LXR (LXRα: Figure 3E, panels a–f; LXRβ: Supplementary Figure 2), suggesting that H12 in LXR is not required for co-localization with RIP140.
In summary, these results show that RIP140 and LXR are redistributed by ligand and co-localize in diffuse large nuclear domains. The LBD is necessary for co-localization, whereas the N-terminal domain is dispensable.
The C-terminal part of RIP140 represses LXR-mediated transcriptional activity
RIP140 has been shown to repress gene activation of an LXRE (LXR responsive element)-regulated reporter gene in monkey kidney cells (BSC40) overexpressing LXRα and RXRα [20]. To determine whether RIP140 can repress gene activation mediated by either LXR subtype, we overexpressed LXRα and LXRβ respectively together with RXRα in COS-7 cells. Increasing amount of a RIP140 expression plasmid showed a dose-dependent repressive effect of both LXRα and LXRβ-mediated gene activation (results not shown). To examine if RIP140 could repress transcriptional activation mediated by endogenous LXR, human hepatoma (Huh7) cells were used.
Quantitative RT-PCR showed that the Huh7 cells express 3.2±0.9-fold higher LXRα mRNA levels compared with LXRβ (value is the mean±S.D. for three independent experiments, n=9). Addition of the LXR ligand T0901317 strongly up-regulated the LXRE-controlled reporter gene activity in the cells (Figure 4B). When cells were transfected with increasing amount of a RIP140-expressing vector and grown in the presence of ligand, a strong dose-dependent repression of the reporter gene activity was observed (Figure 4B). In addition, non-ligand-induced reporter gene activity was repressed, which could be explained by our interaction studies that showed binding between the LXRs and RIP140 even in the absence of ligand. These results demonstrate that RIP140 can repress transcriptional activity mediated by endogenous LXR.
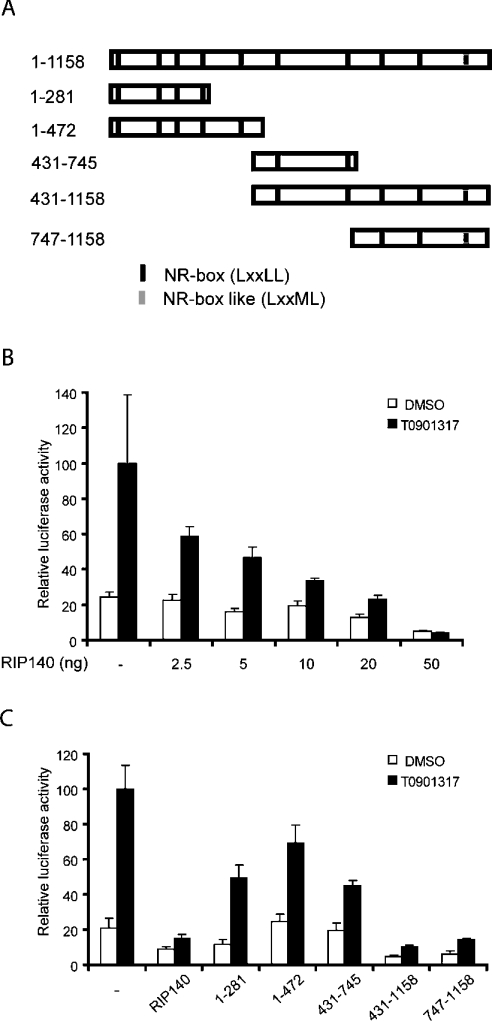
Figure 4. The C-terminal part of RIP140 represses LXR-mediated transcriptional activity.
(A) A schematic picture of the RIP140 domains used. (B) Huh7 cells containing endogenous LXR were transfected with 3xLXRE luciferase reporter plasmid and RSV β-gal reporter plasmid, and increasing amounts of a plasmid expressing RIP140. (C) Huh7 cells were transfected with 3xLXRE luciferase reporter plasmid and RSV β-gal reporter plasmid in the presence (30 ng) or absence of plasmids expressing RIP140 or RIP140 domains. The cells were maintained in the presence of 250 nM T0901317 or DMSO as vehicle control. The values were related to the activity obtained with ligand treatment in the absence of RIP140 protein. The mean and standard deviation (n=3) of a representative experiment is shown.
To obtain further molecular insight into the repressive effect of RIP140, different deletion constructs of RIP140 (Figure 4A) were expressed in Huh7 cells and their influence on endogenous LXR-mediated gene activation was studied. Figure 4(C) shows that the repressive activity of RIP140 mainly resides in the C-terminal part of RIP140 and that the N-terminus and the middle part of RIP140 also have some repressive potential. However, the results suggest that the C-terminal part, amino acids 747–1158, is sufficient for the full repressive effect of RIP140.
C-terminal NR-box-like motif (NR10) is important for full repressive activity of RIP140
The C-terminus of RIP140 (amino acids 747–1158) contains two NR-boxes (LXXLL motifs) and one NR-box-like motif (LXXML). To determine if the NR-boxes are necessary for the repressive activity of RIP140 on LXR-mediated gene activation, we transfected Huh7 cells with vectors expressing RIP140 amino acids 747–1158, intact or mutated in either one NR-box or several NR-boxes in different combinations (Figure 5A). Mutation of the NR-box-like motif NR10 individually (mut NR10) or in combination (mut NR 8,10, NR 9,10 and mut NR 8,9,10) significantly reduced the repressive effect of the C-terminus, whereas mutations in other NR-boxes individually (mut NR8 and mut NR9) or in combination (mut NR 8,9) had no effect (Figure 5C). To verify the importance of the NR-box-like motif NR10 in the context of full-length RIP140, mutation in NR10 was introduced in full-length RIP140 and the repressive effect was studied in Huh7 cells. The results show that mutation of the NR-box-like motif NR10 significantly reduces the repressive activity of full-length RIP140 (Figure 5D). To ensure that the difference was not due to the level of protein expressed, Huh7 cells were transfected with full-length HA-tagged RIP140 or HA-tagged RIP140 mut NR10 (Figure 5B). The expression level of the mutant protein was similar to the wild-type protein supporting that the differences in activity of the two proteins shown in Figure 5(D) reflect that the NR10 motif is important for the repressive function. Thus the results clearly show the importance of the NR-box-like motif for the repressive activity of RIP140 in LXR-mediated transcriptional activation.
Figure 5. C-terminal NR-box-like motif (NR10) is important for full repressive activity of RIP140.
(A) A schematic picture of the RIP140 mutations used. (B) The expression levels of transfected HA-tagged full-length and NR10 mutant RIP140 in Huh7 cells were compared. The values on the left are molecular sizes in kDa. (C) Huh7 cells containing endogenous LXR were transfected with 3xLXRE luciferase reporter plasmid and RSV β-gal reporter plasmid in the presence (10 ng) or absence of plasmids expressing the C-terminal domain of RIP140 (amino acids 747–1158) or proteins containing mutations of the indicated NR-boxes in the context of RIP140/747–1158. (D) Huh7 cells were transfected with 3xLXRE luciferase reporter plasmid and RSV β-gal reporter plasmid in the presence (10 ng) or absence of plasmids expressing full-length RIP140, full-length RIP140 containing a mutation of NR10, the C-terminal domain of RIP140 (amino acids 747–1158) or the C-terminal domain containing a mutation of NR10. The cells were maintained in the presence of 250 nM T0901317 or DMSO as vehicle control. The values were related to the activity obtained with ligand treatment in the absence of RIP140 protein. The mean and standard deviation (n=3) of a representative experiment is shown.
The integrity of the C-terminal part of RIP140 is needed for full repressive activity
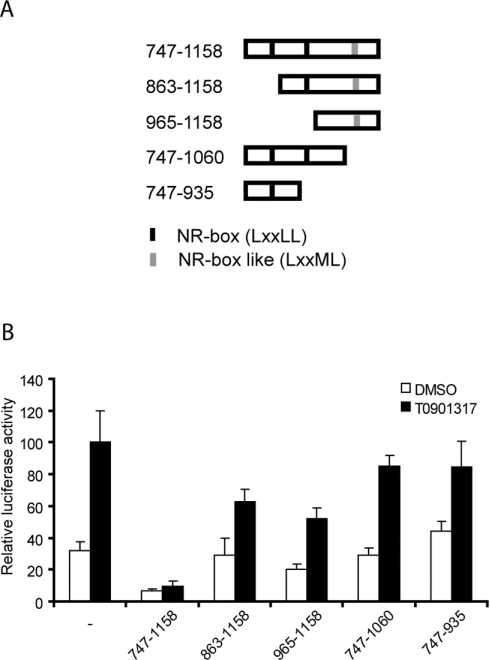
To determine whether the NR-box-like motif NR10 is the only determinant for repression in RIP140 C-terminus, deletion constructs of RIP140 C-terminus were tested for their repressive potential on LXRE-controlled reporter gene activity in Huh7 cells (Figure 6A). All deletion constructs containing the NR-box-like motif NR10 showed repression capability (Figure 6B, see constructs 747–1158, 863–1158 and 965–1158). In contrast, deletion of the region containing the NR-box-like motif NR10 abolished the repression activity (Figure 6B, see constructs 747–1060 and 747–935), confirming the role of the motif for repression. Moreover, deletion of amino acids 747–862 also reduced the repressive activity (Figure 6B, see construct 863–1158), demonstrating that the NR-box motif is not sufficient for full repression. In summary, our results suggest that the presence of the NR-box-like motif NR10 and the integrity of the C-terminal region are needed for full repressive activity.
Figure 6. The integrity of the C-terminal part is needed for full repressive activity.
(A) A schematic picture of the RIP140 domains used. (B) Huh7 cells containing endogenous LXR were transfected with 3xLXRE luciferase reporter plasmid and RSV β-gal reporter plasmid in the presence (10 ng) or absence of plasmids expressing RIP140 domains. The cells were maintained in the presence of 250 nM T0901317 or DMSO as vehicle control. The values were related to the activity obtained with ligand treatment in the absence of RIP140 protein. The mean and standard deviation (n=3) of a representative experiment is shown.
RIP140 interacts differently with the LXR subtypes
To clarify the relative importance of the two LXR subtypes for RIP140's repressive activity on LXR-mediated gene transcription, a yeast two-hybrid experiment was performed where the determinants for RIP140's interaction with LXRα and LXRβ respectively were identified. The C-terminal domain of RIP140 (amino acids 747–1158) interacts with LXRβ in a ligand-enhanced manner (Figure 7B), while LXRα interacted ligand independently (Figure 7A). The deletion constructs containing the NR-box-like motif NR10 interacted with LXRβ (Figure 7B, see constructs 863–1158 and 965–1158). Interestingly, the deletion constructs lacking the motif (747–1060 and 747–935) or containing a mutation of the motif (mut NR10) did not bind to LXRβ (Figure 7B). In contrast, LXRα interacted with construct 747–1060, which lacks the NR-box-like motif NR10, and with the mut NR10 construct. The interactions were, however, weak compared with the 747–1158 construct (Figure 7B). Constructs containing just one NR-box motif, 965–1158 or 747–935, had a total loss of binding towards LXRα. Taken together these results indicate that LXRα and LXRβ interact differently with RIP140 C-terminal domain. The NR-box-like motif NR10 is necessary for interaction with LXRβ whereas, in the case of LXRα, our results suggest that more than one NR-box is needed to obtain any binding; for full binding capacity the NR-box-like motif must be present.
Figure 7. Interaction of LXRα and LXRβ with different RIP140 constructs.
(A, B) S. cerevisiae yeast strain Y187 containing an integrated GAL4-responsive lacZ reporter gene was co-transformed with plasmids expressing GAL4 DBD-LXRα (A) or LXRβ (B) and GAL4 AD-RIP140 constructs in the presence or absence of 250 nM T0901317. β-Galactosidase activity was assayed as measurement of interaction. The means and standard deviations (n=9) were collected from three independent experiments with triplicates in each.
DISCUSSION
In the present study we have characterized the molecular basis for repression of LXR-regulated transcription by RIP140 that could contribute to the physiological role of LXRs in control of energy homoeostasis and lipid and glucose metabolism.
We have shown that both LXRα and LXRβ readily bind RIP140 and that the interaction is ligand-dependent with LXRβ and almost ligand-independent with LXRα. A recent study described a weaker affinity of the co-repressors NCoR and SMRT to unliganded LXRα than to LXRβ [36], which together with our results could imply that LXRα has already in the absence of ligand adopted a conformation that preferably binds NR-box-containing co-regulators.
An important regulatory mechanism for protein function is specific intracellular localization. Some NRs, such as the glucocorticoid receptor, are translocated into the nucleus upon ligand binding, whereas others are present within the nucleus both in the absence and presence of ligand. The subnuclear localization of NRs can also be regulated by ligand or by formation of protein complexes. We observed a nuclear localization of LXRα and LXRβ both in the absence and presence of the agonist ligand T0901317. Mapping studies show that neither the N-terminal domain nor the LBD is necessary for nuclear localization, suggesting that the DBD and hinge region are important for this to occur. Our results support a recent study that identified an NLS (nuclear localization signal) within LXR hinge region [37]. The study also identified an NLS in the N-terminal domain of LXRs [37]. Interestingly, our results show that while the N-terminally deleted LXRs were still localized in the nucleus, the subnuclear localization had changed from an even pattern to nuclear foci. Although the significance of the altered localization is unknown it could reflect changes in composition of receptor protein complexes affecting nuclear distribution.
We have previously demonstrated that a redistribution of nuclear RIP140 and the glucocorticoid receptor correlates with co-repression of glucocorticoid receptor activity by RIP140 [32]. In the present study we observed a ligand-induced redistribution of LXR and RIP140 to diffuse large nuclear domains. Similar findings have also been reported for the androgen receptor [38]. Although the functional significance of the nuclear localization pattern is unclear it might reflect formation of new multiprotein complexes.
Our yeast two-hybrid data and co-localization studies show that the LXR LBD is necessary for interaction with RIP140. RIP140 has nine NR-boxes and one NR-box-like motif. NR-box motif LXXLL mediates the interaction of numerous co-activators, such as p160 family of co-activators, TRAP220 (thyroid-hormone-receptor-associated protein 220) and PGC-1 (peroxisome-proliferator-activated receptor γ co-activator-1), with NR LBDs. Crystal structures have shown that agonist-induced conformational change in the NR LBD leads to formation of a recognition surface for the NR-box consisting of helices 3–5 and H12 (reviewed in [39]). Thus the conserved H12 in the very C-terminus of NR LBDs is important for co-activator binding. The co-repressors NCoR and SMRT contain a related motif, the CoRNR-box (L/IXXI/VI) that mediates the binding to unliganded NRs [24–26]. The CoRNR-box-binding site overlaps partially with the NR-box-binding site on NR LBDs. However, H12 is not needed for CoRNR-box binding (reviewed in [40]). Our results show that the co-localization of RIP140 in nuclear domains with LXRs is independent of the presence of H12. Interestingly, we have identified a CoRNR-box motif in the C-terminus of RIP140 (amino acids 1042–1046, IKWVI) that, in addition to the NR-boxes, might contribute to receptor binding.
We and others have identified several RIP140 domains with intrinsic repression activity [30–32]. To fully understand the mechanism of RIP140-mediated repression, the activity of RIP140 on NR-regulated genes must also be studied. Here we show that the C-terminus of RIP140 (amino acids 747–1158) is sufficient for repression of LXR-mediated transcription in Huh7 cells. Previous studies have also pointed to the role of this region for repression of GR (glucocorticoid receptor)- and AR (androgen receptor)-regulated genes [32,38]. The C-terminus contains two NR-boxes (NR8 and NR9) and one NR-box-like motif (NR10). Our results show that mutation of the NR-box-like motif (LYYML) dramatically reduces the repressive potential. Since mutation of the NR-box-like motif also abolished binding to LXRβ and strongly reduced binding to LXRα, the main role of this motif in co-repression is to interact with the receptor. The NR-box-like motif has also been shown to be important for ligand-induced binding of RIP140 to the retinoic acid receptor [28]. In contrast, the main determinant for co-repression of GR activity is NR8 and mutation of the NR-box-like motif NR10 does not have any effect [32]. Our repression study using deletion constructs of the C-terminal part of RIP140 confirms the importance of the NR-box-like motif for repression of LXR-mediated transcriptional activation, but also shows that in order to achieve full repressive activity the integrity of the whole C-terminal RIP140 is needed. When elucidating the role of the two LXR subtypes in RIP140-mediated co-repression, we demonstrate the importance of RIP140 NR-box-like motif for interaction with LXRβ while for LXRα more than one NR-box seems to be important for binding. The clear differences in NR-box preferences of LXRα and LXRβ for binding to RIP140 suggest that the two LXR subtypes have different affinity and/or interaction dynamics. The requirement for more than one NR-box for LXRα binding suggests that individual NR-boxes only have weak affinity for LXRα. For sufficient affinity several NR-boxes are needed for binding and rebinding. Our results suggest that the different biological functions of the two LXR subtypes are not only due to subtype-specific expression patterns and availability of co-regulators, but also to different binding dynamics of a co-regulator such as RIP140 to the receptor subtypes.
To further address the importance of NR-box-like motifs, the LXXML motif was scanned using the ScanProsite tool [41] against Swiss-Prot/TrEMBL databases [42]. Proteins found were then further scanned for LXXLL motifs. A selection of identified proteins are shown in Table 1. The LXXML motif was found in the orphan NR DAX-1 [43] that contains two NR-box-like motifs and one NR-box. Human DAX-1 (dosage-sensitive sex reversal adrenal hypoplasia congenita X chromosome gene 1) interacts with the NRs ERα (oestrogen receptor α) and ERβ through these motifs and functions as an NR-box-containing co-repressor in the presence of ligand [44]. Moreover, the cofactor PSU1 (mRNA decapping protein 2) contains several atypical NR-boxes with the conserved sequence LXXΦL (Φ=hydrophobic), including four LXXML motifs, and interacts with several NRs in a ligand-dependent manner [45]. Furthermore, this motif scan revealed that the chromatin remodelling protein ATRX (X-linked a thalassaemia mental retardation) which is a member of the Snf2/Swi2 family has one LXXML motif and two LXXLL motifs that are highly conserved [46]. The human TBP (TATA-binding protein)-associated factor, TAF-172 (TBP-associated factor 172), contains two LXXML motifs and 11 LXXLL motifs [47]. Both the ATRX and the TAF-172 protein are tightly coupled with the transcriptional machinery and it could be speculated that they bind NRs. In summary, the alignments/database scanning show that NR-box-like motif is highly conserved in RIP140 species, implying an important role for this motif in RIP140 function. Furthermore, this motif is found in proteins with different functions, which could also suggest a role for the NR-box-like motifs in other biological contexts.
Table 1. Database scanning for LXXML motifs.
Proteins identified using ScanProsite tool against Swiss-Prot/TrEMBL databases with LXXML motif as search pattern. Proteins with LXXML motifs were further scanned for LXXLL motifs.
Our results on the role of the C-terminal NR-boxes and NR-box-like motif in RIP140 clearly demonstrate that the determinants for RIP140 interaction and thus co-repression differ between NRs. RIP140 seems to interact with LXRβ in an atypical manner which does not require the determinants for co-activator binding such as the LXXLL motif and H12. On the other hand, our further mapping of the RIP140 C-terminal domain showed that the NR-box-like motif (NR10) is not sufficient to mediate co-repression. A construct containing NR10 but with a deletion of amino acids 747–862 had reduced co-repressive activity. Thus the integrity of the C-terminal domain (amino acids 747–1158) is also necessary to mediate full co-repression. The function of amino acids 747–862 in mediating co-repression is not yet understood but might involve the formation of so far unidentified repressive protein complexes.
RIP140 is an atypical co-repressor protein that can interact with ligand-activated NRs and mediate the ligand-induced repression of regulated genes. Importantly, ligand-activated LXR represses genes involved in energy, lipid and carbohydrate metabolism in liver and adipose tissue [10]. In the present study we have identified RIP140 as a co-repressor for LXRs and characterized the molecular basis for repression. Further studies will identify which LXR regulated gene networks involve the co-repression activity of RIP140. The important role of both LXRs and RIP140 in regulation of pathways related to development of human metabolic disease emphasizes the significance of understanding of RIP140-mediated repression of LXR-regulated genes for future development of therapeutics.
Online data
Acknowledgments
This work was supported by grants from the Swedish Science Council.
DECLARATION OF INTEREST
Jan-Åke Gustafsson is a cofounder, shareholder, Deputy Board Member, research grant recipient and consultant of KaroBio AB.
References
- 1.Robinson-Rechavi M., Escriva Garcia H., Laudet V. The nuclear receptor superfamily. J. Cell Sci. 2003;116:585–586. doi: 10.1242/jcs.00247. [DOI] [PubMed] [Google Scholar]
- 2.Repa J. J., Mangelsdorf D. J. The role of orphan nuclear receptors in the regulation of cholesterol homeostasis. Annu. Rev. Cell Dev. Biol. 2000;16:459–481. doi: 10.1146/annurev.cellbio.16.1.459. [DOI] [PubMed] [Google Scholar]
- 3.Janowski B. A., Willy P. J., Devi T. R., Falck J. R., Mangelsdorf D. J. An oxysterol signalling pathway mediated by the nuclear receptor LXR alpha. Nature. 1996;383:728–731. doi: 10.1038/383728a0. [DOI] [PubMed] [Google Scholar]
- 4.Lehmann J. M., Kliewer S. A., Moore L. B., Smith-Oliver T. A., Oliver B. B., Su J. L., Sundseth S. S., Winegar D. A., Blanchard D. E., Spencer T. A., Willson T. M. Activation of the nuclear receptor LXR by oxysterols defines a new hormone response pathway. J. Biol. Chem. 1997;272:3137–3140. doi: 10.1074/jbc.272.6.3137. [DOI] [PubMed] [Google Scholar]
- 5.Steffensen K. R., Gustafsson J. A. Putative metabolic effects of the liver X receptor (LXR) Diabetes. 2004;53(Suppl. 1):S36–S42. doi: 10.2337/diabetes.53.2007.s36. [DOI] [PubMed] [Google Scholar]
- 6.Zelcer N., Tontonoz P. Liver X receptors as integrators of metabolic and inflammatory signaling. J. Clin. Invest. 2006;116:607–614. doi: 10.1172/JCI27883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gerin I., Dolinsky V. W., Shackman J. G., Kennedy R. T., Chiang S. H., Burant C. F., Steffensen K. R., Gustafsson J. A., MacDougald O. A. LXRbeta is required for adipocyte growth, glucose homeostasis, and beta cell function. J. Biol. Chem. 2005;280:23024–23031. doi: 10.1074/jbc.M412564200. [DOI] [PubMed] [Google Scholar]
- 8.Juvet L. K., Andresen S. M., Schuster G. U., Dalen K. T., Tobin K. A., Hollung K., Haugen F., Jacinto S., Ulven S. M., Bamberg K., et al. On the role of liver X receptors in lipid accumulation in adipocytes. Mol. Endocrinol. 2003;17:172–182. doi: 10.1210/me.2001-0210. [DOI] [PubMed] [Google Scholar]
- 9.Kalaany N. Y., Gauthier K. C., Zavacki A. M., Mammen P. P., Kitazume T., Peterson J. A., Horton J. D., Garry D. J., Bianco A. C., Mangelsdorf D. J. LXRs regulate the balance between fat storage and oxidation. Cell Metab. 2005;1:231–244. doi: 10.1016/j.cmet.2005.03.001. [DOI] [PubMed] [Google Scholar]
- 10.Stulnig T. M., Steffensen K. R., Gao H., Reimers M., Dahlman-Wright K., Schuster G. U., Gustafsson J. A. Novel roles of liver X receptors exposed by gene expression profiling in liver and adipose tissue. Mol. Pharmacol. 2002;62:1299–1305. doi: 10.1124/mol.62.6.1299. [DOI] [PubMed] [Google Scholar]
- 11.Cao G., Liang Y., Broderick C. L., Oldham B. A., Beyer T. P., Schmidt R. J., Zhang Y., Stayrook K. R., Suen C., Otto K. A., et al. Antidiabetic action of a liver X receptor agonist mediated by inhibition of hepatic gluconeogenesis. J. Biol. Chem. 2003;278:1131–1136. doi: 10.1074/jbc.M210208200. [DOI] [PubMed] [Google Scholar]
- 12.Laffitte B. A., Chao L. C., Li J., Walczak R., Hummasti S., Joseph S. B., Castrillo A., Wilpitz D. C., Mangelsdorf D. J., Collins J. L., et al. Activation of liver X receptor improves glucose tolerance through coordinate regulation of glucose metabolism in liver and adipose tissue. Proc. Natl. Acad. Sci. U.S.A. 2003;100:5419–5424. doi: 10.1073/pnas.0830671100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schuster G. U., Johansson L., Kietz S., Stulnig T. M., Parini P., Gustafsson J. A. Improved metabolic control by depletion of liver X receptors in mice. Biochem. Biophys. Res. Commun. 2006;348:176–182. doi: 10.1016/j.bbrc.2006.07.044. [DOI] [PubMed] [Google Scholar]
- 14.Dalen K. T., Ulven S. M., Bamberg K., Gustafsson J. A., Nebb H. I. Expression of the insulin-responsive glucose transporter GLUT4 in adipocytes is dependent on liver X receptor alpha. J. Biol. Chem. 2003;278:48283–48291. doi: 10.1074/jbc.M302287200. [DOI] [PubMed] [Google Scholar]
- 15.Rosenfeld M. G., Lunyak V. V., Glass C. K. Sensors and signals: a coactivator/corepressor/epigenetic code for integrating signal-dependent programs of transcriptional response. Genes Dev. 2006;20:1405–1428. doi: 10.1101/gad.1424806. [DOI] [PubMed] [Google Scholar]
- 16.Christian M., White R., Parker M. G. Metabolic regulation by the nuclear receptor corepressor RIP140. Trends Endocrinol. Metab. 2006;17:243–250. doi: 10.1016/j.tem.2006.06.008. [DOI] [PubMed] [Google Scholar]
- 17.Leonardsson G., Steel J. H., Christian M., Pocock V., Milligan S., Bell J., So P. W., Medina-Gomez G., Vidal-Puig A., White R., Parker M. G. Nuclear receptor corepressor RIP140 regulates fat accumulation. Proc. Natl. Acad. Sci. U.S.A. 2004;101:8437–8442. doi: 10.1073/pnas.0401013101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Christian M., Kiskinis E., Debevec D., Leonardsson G., White R., Parker M. G. RIP140-targeted repression of gene expression in adipocytes. Mol. Cell. Biol. 2005;25:9383–9391. doi: 10.1128/MCB.25.21.9383-9391.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Powelka A. M., Seth A., Virbasius J. V., Kiskinis E., Nicoloro S. M., Guilherme A., Tang X., Straubhaar J., Cherniack A. D., Parker M. G., Czech M. P. Suppression of oxidative metabolism and mitochondrial biogenesis by the transcriptional corepressor RIP140 in mouse adipocytes. J. Clin. Invest. 2006;116:125–136. doi: 10.1172/JCI26040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Miyata K. S., McCaw S. E., Meertens L. M., Patel H. V., Rachubinski R. A., Capone J. P. Receptor-interacting protein 140 interacts with and inhibits transactivation by, peroxisome proliferator-activated receptor alpha and liver-X-receptor alpha. Mol. Cell. Endocrinol. 1998;146:69–76. doi: 10.1016/s0303-7207(98)00196-8. [DOI] [PubMed] [Google Scholar]
- 21.Teboul M., Enmark E., Li Q., Wikstrom A. C., Pelto-Huikko M., Gustafsson J. A. OR-1, a member of the nuclear receptor superfamily that interacts with the 9-cis-retinoic acid receptor. Proc. Natl. Acad. Sci. U.S.A. 1995;92:2096–2100. doi: 10.1073/pnas.92.6.2096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Treuter E., Albrektsen T., Johansson L., Leers J., Gustafsson J. A. A regulatory role for RIP140 in nuclear receptor activation. Mol. Endocrinol. 1998;12:864–881. doi: 10.1210/mend.12.6.0123. [DOI] [PubMed] [Google Scholar]
- 23.Heery D. M., Kalkhoven E., Hoare S., Parker M. G. A signature motif in transcriptional co-activators mediates binding to nuclear receptors. Nature. 1997;387:733–736. doi: 10.1038/42750. [DOI] [PubMed] [Google Scholar]
- 24.Hu X., Lazar M. A. The CoRNR motif controls the recruitment of corepressors by nuclear hormone receptors. Nature. 1999;402:93–96. doi: 10.1038/47069. [DOI] [PubMed] [Google Scholar]
- 25.Nagy L., Kao H. Y., Love J. D., Li C., Banayo E., Gooch J. T., Krishna V., Chatterjee K., Evans R. M., Schwabe J. W. Mechanism of corepressor binding and release from nuclear hormone receptors. Genes Dev. 1999;13:3209–3216. doi: 10.1101/gad.13.24.3209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Perissi V., Staszewski L. M., McInerney E. M., Kurokawa R., Krones A., Rose D. W., Lambert M. H., Milburn M. V., Glass C. K., Rosenfeld M. G. Molecular determinants of nuclear receptor-corepressor interaction. Genes Dev. 1999;13:3198–3208. doi: 10.1101/gad.13.24.3198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Vo N., Fjeld C., Goodman R. H. Acetylation of nuclear hormone receptor-interacting protein RIP140 regulates binding of the transcriptional corepressor CtBP. Mol. Cell. Biol. 2001;21:6181–6188. doi: 10.1128/MCB.21.18.6181-6188.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wei L. N., Farooqui M., Hu X. Ligand-dependent formation of retinoid receptors, receptor-interacting protein 140 (RIP140), and histone deacetylase complex is mediated by a novel receptor-interacting motif of RIP140. J. Biol. Chem. 2001;276:16107–16112. doi: 10.1074/jbc.M010185200. [DOI] [PubMed] [Google Scholar]
- 29.Wei L. N., Hu X., Chandra D., Seto E., Farooqui M. Receptor-interacting protein 140 directly recruits histone deacetylases for gene silencing. J. Biol. Chem. 2000;275:40782–40787. doi: 10.1074/jbc.M004821200. [DOI] [PubMed] [Google Scholar]
- 30.Castet A., Boulahtouf A., Versini G., Bonnet S., Augereau P., Vignon F., Khochbin S., Jalaguier S., Cavailles V. Multiple domains of the receptor-interacting protein 140 contribute to transcription inhibition. Nucleic Acids Res. 2004;32:1957–1966. doi: 10.1093/nar/gkh524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Christian M., Tullet J. M., Parker M. G. Characterization of four autonomous repression domains in the corepressor receptor interacting protein 140. J. Biol. Chem. 2004;279:15645–15651. doi: 10.1074/jbc.M313906200. [DOI] [PubMed] [Google Scholar]
- 32.Tazawa H., Osman W., Shoji Y., Treuter E., Gustafsson J. A., Zilliacus J. Regulation of subnuclear localization is associated with a mechanism for nuclear receptor corepression by RIP140. Mol. Cell. Biol. 2003;23:4187–4198. doi: 10.1128/MCB.23.12.4187-4198.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bavner A., Matthews J., Sanyal S., Gustafsson J. A., Treuter E. EID3 is a novel EID family member and an inhibitor of CBP-dependent co-activation. Nucleic Acids Res. 2005;33:3561–3569. doi: 10.1093/nar/gki667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zilliacus J., Holter E., Wakui H., Tazawa H., Treuter E., Gustafsson J. A. Regulation of glucocorticoid receptor activity by 14-3-3-dependent intracellular relocalization of the corepressor RIP140. Mol. Endocrinol. 2001;15:501–511. doi: 10.1210/mend.15.4.0624. [DOI] [PubMed] [Google Scholar]
- 35.Nagy L., Schwabe J. W. Mechanism of the nuclear receptor molecular switch. Trends Biochem. Sci. 2004;29:317–324. doi: 10.1016/j.tibs.2004.04.006. [DOI] [PubMed] [Google Scholar]
- 36.Albers M., Blume B., Schlueter T., Wright M. B., Kober I., Kremoser C., Deuschle U., Koegl M. A novel principle for partial agonism of liver X receptor ligands. Competitive recruitment of activators and repressors. J. Biol. Chem. 2006;281:4920–4930. doi: 10.1074/jbc.M510101200. [DOI] [PubMed] [Google Scholar]
- 37.Prufer K., Boudreaux J. Nuclear localization of liver X receptor alpha and beta is differentially regulated. J. Cell. Biochem. 2007;100:69–85. doi: 10.1002/jcb.21006. [DOI] [PubMed] [Google Scholar]
- 38.Carascossa S., Gobinet J., Georget V., Lucas A., Badia E., Castet A., White R., Nicolas J. C., Cavailles V., Jalaguier S. Receptor-interacting protein 140 is a repressor of the androgen receptor activity. Mol. Endocrinol. 2006;20:1506–1518. doi: 10.1210/me.2005-0286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Savkur R. S., Burris T. P. The coactivator LXXLL nuclear receptor recognition motif. J. Pept. Res. 2004;63:207–212. doi: 10.1111/j.1399-3011.2004.00126.x. [DOI] [PubMed] [Google Scholar]
- 40.Nettles K. W., Greene G. L. Ligand control of coregulator recruitment to nuclear receptors. Annu. Rev. Physiol. 2005;67:309–333. doi: 10.1146/annurev.physiol.66.032802.154710. [DOI] [PubMed] [Google Scholar]
- 41.Hulo N., Bairoch A., Bulliard V., Cerutti L., De Castro E., Langendijk-Genevaux P. S., Pagni M., Sigrist C. J. The PROSITE database. Nucleic Acids Res. 2006;34:D227–D230. doi: 10.1093/nar/gkj063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Boeckmann B., Bairoch A., Apweiler R., Blatter M. C., Estreicher A., Gasteiger E., Martin M. J., Michoud K., O'Donovan C., Phan I., et al. The SWISS-PROT protein knowledgebase and its supplement TrEMBL in 2003. Nucleic Acids Res. 2003;31:365–370. doi: 10.1093/nar/gkg095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zanaria E., Muscatelli F., Bardoni B., Strom T. M., Guioli S., Guo W., Lalli E., Moser C., Walker A. P., McCabe E. R., et al. An unusual member of the nuclear hormone receptor superfamily responsible for X-linked adrenal hypoplasia congenita. Nature. 1994;372:635–641. doi: 10.1038/372635a0. [DOI] [PubMed] [Google Scholar]
- 44.Zhang H., Thomsen J. S., Johansson L., Gustafsson J. A., Treuter E. DAX-1 functions as an LXXLL-containing corepressor for activated estrogen receptors. J. Biol. Chem. 2000;275:39855–39859. doi: 10.1074/jbc.C000567200. [DOI] [PubMed] [Google Scholar]
- 45.Gaudon C., Chambon P., Losson R. Role of the essential yeast protein PSU1 in transcriptional enhancement by the ligand-dependent activation function AF-2 of nuclear receptors. EMBO J. 1999;18:2229–2240. doi: 10.1093/emboj/18.8.2229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Picketts D. J., Higgs D. R., Bachoo S., Blake D. J., Quarrell O. W., Gibbons R. J. ATRX encodes a novel member of the SNF2 family of proteins: mutations point to a common mechanism underlying the ATR-X syndrome. Hum. Mol. Genet. 1996;5:1899–1907. doi: 10.1093/hmg/5.12.1899. [DOI] [PubMed] [Google Scholar]
- 47.Chicca J. J., II, Auble D. T., Pugh B. F. Cloning and biochemical characterization of TAF-172, a human homolog of yeast Mot1. Mol. Cell. Biol. 1998;18:1701–1710. doi: 10.1128/mcb.18.3.1701. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.